Significance
Studying individual differences in gender and sexual orientation provides insight into how early-life biology shapes brain and behavior. The literature identifies multiple biodevelopmental influences on male sexual orientation, but these influences are generally studied individually, and the potential for association or interaction between them remains largely unexplored. We hypothesized that distinct biodevelopmental pathways correspond to specific subgroups of nonheterosexual men. We present evidence that nonheterosexual men can be categorized into at least four subgroups based on established biomarkers, and these biodevelopmental pathways differentially relate to gender expression and personality traits. These findings indicate individual differences in biodevelopmental pathways of male sexual orientation. They also illustrate the value of latent profile analyses for studying individual differences.
Keywords: male sexual orientation, latent profile analysis, familiality, fraternal birth order, handedness
Abstract
Several biological mechanisms have been proposed to influence male sexual orientation, but the extent to which these mechanisms cooccur is unclear. Putative markers of biological processes are often used to evaluate the biological basis of male sexual orientation, including fraternal birth order, handedness, and familiality of same-sex sexual orientation; these biomarkers are proxies for immunological, endocrine, and genetic mechanisms. Here, we used latent profile analysis (LPA) to assess whether these biomarkers cluster within the same individuals or are present in different subgroups of nonheterosexual men. LPA defined four profiles of men based on these biomarkers: 1) A subgroup who did not have these biomarkers, 2) fraternal birth order, 3) handedness, and 4) familiality. While the majority of both heterosexual and nonheterosexual men were grouped in the profile that did not have any biomarker, the three profiles associated with a biomarker were composed primarily of nonheterosexual men. We then evaluated whether these subgroups differed on measures of gender nonconformity and personality that reliably show male sexual orientation differences. The subgroup without biomarkers was the most gender-conforming whereas the fraternal birth order subgroup was the most female-typical and agreeable, compared with the other profiles. Together, these findings suggest there are multiple distinct biodevelopmental pathways influencing same-sex sexual orientation in men.
Sex differences have been found widely in the human brain (1–5) and in behavior (6–9), and these differences are thought to contribute to the sex bias in a myriad of neurological conditions (10–13). Sexual orientation has often been examined as a model for understanding mechanisms related to sex-differentiated aspects of brain and behavior (14). In humans, the possible biological basis of male sexual orientation is often studied via putative markers of biological processes (i.e., biomarkers), such as the number of older brothers, handedness, and familiality of same-sex sexual orientation. These biomarkers relate to immunological, endocrine, and genetic mechanisms (15, 16). However, few studies evaluate these developmental markers within the same individuals. Thus, it is unclear to what extent these biomarkers cluster within the same individuals and/or map onto subgroups of nonheterosexual men. Clustering of biomarkers could point to an additive effect of multiple biological events during development. By contrast, each of the various biomarkers might better map onto particular subgroups of nonheterosexual men, which would suggest that multiple distinct biodevelopmental pathways influence same-sex sexual orientation in men. The present study used latent profile analysis (LPA) to evaluate whether subgroups of nonheterosexual men emerged based on three well-established biomarkers associated with male sexual orientation: Familiality, handedness, and fraternal birth order. Furthermore, we evaluated whether the subgroups defined by LPA differed in terms of gender nonconformity and personality, which are domains previously associated with sexual orientation differences in men (17–20). Doing so provided insight into possible differences in behavioral phenotypes between nonheterosexual male subgroups, which may indicate how these markers might differentially affect the development of male- and female-typical traits.
A well-established biomarker of sexual orientation is familiality of male same-sex sexual orientation. Same-sex sexual orientation clusters in families (21–28), twin studies show greater sexual orientation concordance among monozygotic than dizygotic twins (29–34), and molecular genetic studies have identified candidate genes associated with sexual orientation (35–37). As such, genetic mechanisms appear to at least partially influence male same-sex sexual orientation. The heritability of male sexual orientation is estimated at ∼0.32 (15), and the associated genetic factors appear to be inherited from both the maternal and paternal lines given that gay men, compared with heterosexual men, have more gay male family members in both their maternal (22–25, 27) and paternal lines (21, 25, 26, 28). Consistent with this familiality research, male sexual orientation has been associated with genes on the X chromosome and autosomal chromosomes (23, 38), with the largest studies finding associations with Xq28 (23, 36, 38), the sonic hedgehog gene on chromosome 7 (35, 37), and the pericentromeric region of chromosome 8 (35–37). These genetic mechanisms are thought to affect sexual differentiation of the brain and behavior, either through genes related to sociosexual behaviors (e.g., Xq28: AVPR2 and CNGA2; Chromosome 8: NPBWR1) or by interaction with androgenic mechanisms (e.g., androgen receptor is X-linked) (39).
A second well-studied biomarker of sexual orientation is handedness. Although the biological underpinnings of handedness are not yet clear, increasing evidence suggests that handedness is a marker of cerebral lateralization determined prenatally by genetic, immunological, and endocrine mechanisms and/or by developmental instability (40–42). The higher prevalence of non−right-handedness among men compared with women suggests that handedness is a developmental biomarker of brain sexual differentiation (43). A large body of evidence indicates that non−right-handedness is more common among gay men than among heterosexual men, suggesting that at least some proportion of gay men owe their same-sex sexual orientation to developmental mechanisms underlying handedness (for a review and metaanalysis, see refs. 44–54, but see refs. 55 and 56). Specifically, it is estimated that men have 20% greater odds of non−right-handedness than women (43), and gay men have 34% greater odds of being non−right-handed than heterosexual men (44).
A third well-established biomarker of sexual orientation is the fraternal birth order effect (57). Across a diverse range of cultures and sample types, studies have shown that older brothers increase the odds of androphilia in later-born males. The maternal immune hypothesis is the best-developed explanation of the fraternal birth order effect. It argues that male antigens enter maternal circulation during the gestation and birthing of male offspring, promoting an immune response to these male-specific antigens that increases with each successive male fetus gestated; thus, with each successive pregnancy with a male fetus, the odds increase that these maternal antibodies will affect sexual differentiation of the brain and behavior, including sexual preferences (58, 59). Supporting a prenatal mechanism for the fraternal birth order effect is the finding that birth weight is lower in gay than in heterosexual men with older brothers (e.g., ref. 60). A recent study by Bogaert et al. (61) reported direct evidence for the maternal immune hypothesis: Increased antibodies against the Y-protein NLGN4Y were found in mothers of gay men and children assigned male at birth who experienced gender dysphoria (who are likely to exhibit androphilia as adults) (62, 63), especially those with older brothers. It is estimated that 14.8 to 48% of gay men owe their sexual orientation to the fraternal birth order effect (64, 65).
Although the aforementioned biomarkers of male sexual orientation have been studied rather extensively in isolation, little research has investigated the relationships among them. A few studies considered both birth order and handedness, finding that the fraternal birth order effect is only related to sexual orientation in right-handed gay men (i.e., not mixed or left-handed men) (45, 51, 53, 54). These results are consistent with the possibility that different male sexual orientation biomarkers delineate distinct biodevelopmental pathways and the existence of subgroups of gay men. However, whether the familial nature of male sexual orientation overlaps with fraternal birth order and/or handedness or is associated with yet another distinct subgroup of gay men remains unclear. For example, although the fraternal birth order effect and non−right-handedness appear to apply to subgroups of gay men, non−right-handedness and familiality of same-sex sexual orientation might overlap, as both biomarkers are thought to be influenced by genetic factors (21–24, 26, 40).
In the present study, we used a multivariate LPA approach to evaluate whether sexual orientation subgroups of men would emerge based on these well-established biomarkers of sexual orientation: Handedness, fraternal birth order, and familiality of nonheterosexual orientation. LPA involves the formation of profiles (i.e., subgroups) based on patterns of similarities among individuals on the various indicator variables (66), or, in this study, the biomarkers; LPA was done irrespective of sexual orientation. Thus, subgroups are defined solely based on their variance on the biomarkers, allowing us to test whether biomarkers cooccur within the same individuals vs. are independently present among distinct subgroups. Once subgroups were defined, we assessed whether the distribution of heterosexual and nonheterosexual men differed across these subgroups. We also evaluated the meaningfulness of the defined subgroups on measures of gender nonconformity and personality—measures on which gay and heterosexual men typically differ.
Results and Discussion
Latent Profiles, Developmental Biomarkers, and Sexual Orientation.
The LPA indicated that a four-profile model was the best fit for the data, as decided by the Bootstrap likelihood ratio (BLRT) (i.e., a significant P value indicated that the four-profile model fit better than the three-profile model), and the size of the profiles (i.e., the five-profile model starts to delineate small groups with <3% of our sample, whereas all profiles in the four-profile model are near or greater than the predetermined 5% cutoff for profile size; see SI Appendix, Table S1). Please see Table 1 for descriptive statistics for each biomarker and for sexual orientation measures; Table 2 shows the number and percentage of heterosexual and nonheterosexual men per latent profile.
Table 1.
Descriptive statistics for biomarkers and sexual orientation (SO) measures: Mean (M), SD, sample size (N), and % missing data (% missing) based on the full sample size (N = 827)
| Variable | N | M | SD | % missing |
| Fraternal birth order | 600 | 0.27 | 0.21 | 27.44 |
| Handedness | 826 | 0.18 | 0.24 | 0.12 |
| Familiality | 334 | 0.05 | 0.12 | 59.61 |
| SO self-identification | 580 | 2.77 | 1.79 | 29.75 |
| SO attraction | 578 | 2.78 | 1.74 | 30.12 |
| SO behavior | 562 | 2.77 | 1.81 | 32.04 |
Note that possible values for each biomarker (fraternal birth order, handedness, and familiality) all range from 0 to 1. Higher FBO values indicate a greater proportion of older brothers, higher handedness values indicate greater non−right-handedness, and higher familiality values indicate a greater proportion of gay/bisexual male family members. Self-identification of SO was coded as follows: 0 = heterosexual, 2 = bisexual/other, and 4 = gay. Attraction and behavior (Likert scale measures of sexual orientation) range from 0 to 4; participants who reported 0 on these scales were classified as heterosexual, whereas participants with values 1 to 4 were grouped together to make the nonheterosexual group. See SI Appendix, Sexual Orientation for comparisons of participants with values of 1, 2, 3, and 4. See SI Appendix, Figs. S1 and S2 for frequency distribution of participants by sexual orientation responses. Data were found to be missing at random (for details, see SI Appendix, Missing Data).
Table 2.
Number and percentage of heterosexual and nonheterosexual men per latent profile
| Sexual orientation | Profile 1 | Profile 2 | Profile 3 | Profile 4 | Total | ||||||
| Measure | Group | n | % | n | % | n | % | n | % | n | % |
| Identity | Heterosexual | 119 | 73 | 29 | 17.8 | 8 | 4.9 | 7 | 4.3 | 163 | 28.1 |
| Nonheterosexual | 264 | 63.3 | 88 | 21.1 | 46 | 11 | 19 | 4.6 | 417 | 71.9 | |
| Attraction | Heterosexual | 106 | 72.6 | 25 | 17.1 | 8 | 5.5 | 7 | 4.8 | 146 | 25.3 |
| Nonheterosexual | 275 | 63.7 | 92 | 21.3 | 46 | 10.6 | 19 | 4.4 | 432 | 74.7 | |
| Behavior | Heterosexual | 119 | 73.5 | 26 | 16.1 | 10 | 6.2 | 7 | 4.3 | 162 | 28.8 |
| Nonheterosexual | 250 | 62.5 | 89 | 22.3 | 43 | 10.8 | 18 | 4.5 | 400 | 71.2 | |
Note that χ2 analyses indicated that the distribution of heterosexual and nonheterosexual men significantly differed between profile 1 (i.e., no biomarker subgroup) and the combined profiles 2, 3, and 4 (i.e., subgroups with a biomarker); due to small sample sizes, profiles 2 to 4 were combined for this analysis. Although the distribution is in the predicted direction for each profile, χ2 analyses with uncombined profiles indicated that the distribution of nonheterosexual men compared with the distribution of heterosexual men reached statistical significance when comparing profiles 1 and 3 (i.e., all other profile comparisons P > 0.05). We also used χ2 to compare the correspondence of the three measures of sexual orientation and found that all three variables corresponded highly: Self-identification and sexual attraction, χ2(1) = 473.03, P < 0.001 (ϕ = 0.91), self-identification and sexual behavior, χ2(1) = 485.48, P < 0.001 (ϕ = 0.94), and sexual attraction and behavior, χ2(1) = 467.17, P < 0.001 (ϕ = 0.92).
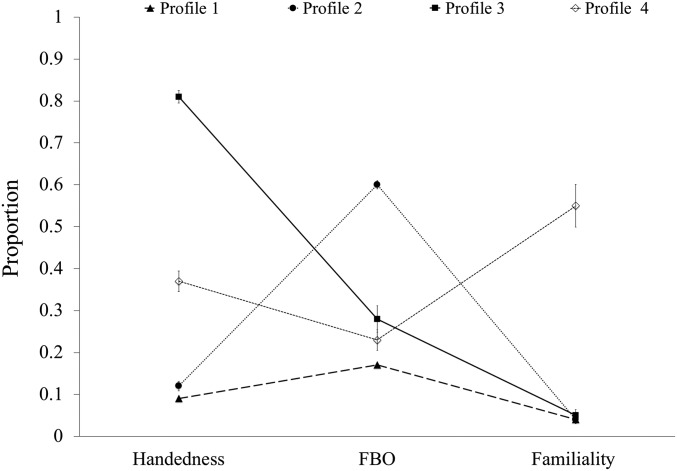
The four latent profiles differed significantly on all biomarkers: Handedness, H (3, 826) = 293.85, P < 0.001; fraternal birth order, H (3, 600) = 276.93, P < 0.001; and familiality, H (3, 334) = 49.56, P < 0.001 (Fig. 1). We also tested whether differences between profiles on familiality were driven by maternal and/or paternal line relatives; we found latent profiles differed for both, H (3, 420) = 10.79, P = 0.013 and H (3, 386) = 52.75, P < 0.001, respectively. Each profile can be succinctly defined based on the biomarkers: Profile 1 did not display evidence of elevation on any of the biomarkers studied, profile 2 displayed a high proportion of older brothers, profile 3 displayed elevated non−right-handedness, and profile 4 displayed elevated gay/bisexual male familiality.
Fig. 1.
LPA: Four-profile model by developmental biomarkers (means ± SEM). Profile 1 did not display elevations on any of the biomarkers; this profile contained the majority of the heterosexual and nonheterosexual men. Profile 2 consisted primarily of nonheterosexual men who reported a higher proportion of older brothers [i.e., fraternal birth order (FBO)]. Profile 3 consisted primarily of nonheterosexual men who reported large degrees of non−right-handedness. Profile 4 consisted primarily of nonheterosexual men who displayed high degrees of gay/bisexual male familiality. FBO is represented by proportion of older brothers. Note that handedness scores are a proportion of left-handedness; a handedness score of zero indicated the use of the right hand for all tasks on the Edinburgh questionnaire, whereas a score of 1 indicated use of the left hand for all tasks on the Edinburgh questionnaire. Familiality scores are a proportion of biological male family members with same-sex sexual orientation in their maternal and paternal family (i.e., gay or bisexual).
As expected, the distribution of participants based on sexual orientation was unequal for profile 1 (i.e., the group that did not display any developmental markers) compared with profiles 2 to 4 (i.e., groups that displayed increases in the developmental markers typical among gay male samples). Specifically, more nonheterosexual than heterosexual men were distributed across profiles 2 to 4, regardless of the way sexual orientation was defined [self-identification: χ2(1, 580) = 4.49, P = 0.034; attraction: χ2(1, 578) = 3.5, P = 0.061; behavior: χ2(1, 562) = 5.66, P = 0.017].
Together, on the basis of these well-established developmental biomarkers of male sexual orientation, LPA defined four profiles. Across these profiles, the distribution of heterosexual vs. nonheterosexual men was nonrandom, with nonheterosexual men being more likely than heterosexual men to belong to profiles showing elevations on the sexual orientation biomarkers. A key finding was that profiles 2 to 4, which were predominantly composed of nonheterosexual men, showed unique biomarker profiles in which there were elevations on one type of biomarker in particular. That said, some profiles consisted of a small subset of participants (e.g., profile 4, n = 26) and showed only a small nonsignificant difference in the distribution of heterosexual and nonheterosexual men (Table 2). As such, testing whether these findings can be replicated in a larger sample will be important. Nevertheless, the overall pattern presented here indicates that each biomarker maps onto a subgroup of same-sex−oriented men, as opposed to clustering within the same subset of individuals. In terms of developmental implications, these findings support the hypothesis that there are subgroups of gay men who might owe their sexual orientation to different biological mechanisms.
Of note is that a large number of nonheterosexual men were classified as belonging to profile 1, which showed no elevations with respect to the biomarkers investigated. There are at least three possible, non-mutually exclusive explanations for why this was the case. First, the biomarkers investigated here serve as proxies for biological mechanisms that are thought to influence male sexual orientation. Some of the profile 1 nonheterosexual men might owe their sexual orientation to these mechanisms, but the proxies are not present or reported. For example, Bogaert et al. (61) found elevated anti-male antibodies in mothers of gay men without older brothers, suggesting such mechanisms might apply to other gay men without older brothers. In the same vein, a large proportion of participants in profile 1 did not report on one or more of the biomarkers (SI Appendix, Table S3); thus, many of these nonheterosexual men may have a biomarker but may not have reported it. Second, recent literature proposed mechanisms related to alternate (epi)genetic or maternal immune processes (59, 67–69). These processes and/or some alternate biodevelopmental processes yet to be proposed may apply to a portion of the profile 1 nonheterosexual men. For example, profile 1 nonheterosexual men are more right-handed than the other groups and report a lower proportion of older brothers than do heterosexual men as well as compared with those in profiles 2 to 4 (SI Appendix, Subgroups of Nonheterosexual Men Differ from Heterosexual Men on Developmental Markers); perhaps mechanisms related to extreme right-handedness and only children/firstborns influence the development of nonheterosexual men in profile 1, as previously proposed (49, 68). Third, psychosocial and cognitive developmental factors have been proposed and might apply. Although evidence in support of such factors has been limited (15, 70), one recent study found that past same-sex behavior was more common among individuals who attended single-sex schools (71). In any case, findings pertaining to the nonheterosexual men in the other profiles provide evidence for distinct biodevelopmental pathways influencing same-sex sexual orientation in men.
Importantly, aspects of our data aligned with previously well-established observations. The fraternal birth order effect has been estimated to account for sexual orientation in ∼14.8 to 48% of gay men (64, 65); in the current sample, 14.15% of the nonheterosexual men belonged to the fraternal birth order profile (i.e., profile 2). Furthermore, in line with previous reports that 10 to 22% of nonheterosexual men are non−right-handed, the LPA defined a non−right-handed profile (i.e., profile 3) consisting of 11% of the present study’s nonheterosexual sample. We also found that the fraternal birth order group (i.e., profile 4) consisted of primarily right-handed participants, in line with the finding that the fraternal birth order effect only appears to influence sexual orientation in right-handed men (45). Given the consistencies between our sample and previously reported large data sets and metaanalyses, it seems unlikely that our current findings might in some way be attributable to sample bias or characteristics unique to our sample.
As proposed by Blanchard et al. (46), the fraternal birth order effect might exist only among right-handed nonheterosexual men because the respective mechanisms associated with fraternal birth order and handedness counteract each other. For example, one hypothesis underlying non−right-handedness is increased androgen exposure. The hyperandrogenization associated with non−right-handedness could perhaps then counteract demasculinization and/or feminization actions proposed by the maternal immune hypothesis/fraternal birth order effect (57). Alternatively, as proposed by Blanchard and Lippa (48), the combination of non−right-handedness and the fraternal birth order effect may be toxic such that it could predispose individuals to conditions that would make them less likely to participate in research (i.e., intellectual disability or severe mental illness) or that end in the termination of a pregnancy (i.e., spontaneous abortion). Regardless of the mechanisms underlying this interaction, the present results reinforce that fraternal birth order and handedness effects associated with male sexual orientation are nonoverlapping and pertain to distinct subgroups of nonheterosexual men.
This study addresses whether familiality of same-sex sexual orientation is related to the fraternal birth order and/or handedness effects. Familiality defined a distinct subgroup of nonheterosexual men, but this subgroup also displayed somewhat increased non−right-handedness compared with profile 1 (i.e., the group that showed no evidence of biomarkers). This finding may be attributed to genetic factors that exert some influence on handedness and also on sexual orientation. At the same time, handedness and familiality biomarkers nevertheless defined separate subgroups of nonheterosexual men, possibly because handedness is also thought to be influenced by immunological and endocrine factors.
Of note, one might question whether profiles 3 and 4 nonheterosexual men were also somewhat affected by the fraternal birth order effect, as these profiles showed an increase in proportion of older brothers compared with profile 1. However, profiles 3 and 4 did not differ from the expected population value (based on a stable population) for proportion of older brothers (SI Appendix, The Fraternal Birth Order Effect by Latent Profiles Compared to the Expected Population Mean). Only profile 2, the subgroup defined as the fraternal birth order profile, had a significantly higher proportion of older brothers compared with the expected population value.
Gender Nonconformity and Personality Differences across Latent Profiles.
One concern with all mixture models is the possibility that a mixture will be estimated where none exists (e.g., ref. 72). Thus, to further ascertain the usefulness of the identified profiles, we compared them on gender (non)conformity and personality measures for which men of varying sexual orientations typically differ (17–20). Kruskal−Wallis tests revealed significant differences between latent profiles on gender (non)conformity and personality measures: The Recalled Childhood Gender Identity/Gender Role Questionnaire (RCGI) H (3, 549) = 9.4, P = 0.024; Bem femininity (73), H (3, 571) = 10.77, P = 0.013; masculine occupational preferences, H (3, 572) = 8.07, P = 0.045; and agreeableness, H (3, 570) = 11.73, P = 0.008 (Table 3 and SI Appendix, Table S10). The gender (non)conformity measures indicated that the profile that did not display any biomarkers (i.e., profile 1) was the most gender-conforming, followed by the familiality subgroup. The fraternal birth order subgroup (i.e., profile 2) was the most female-typical but did not show any evidence of decreased adult male typicality, suggesting that the processes underlying the fraternal birth order effect did not influence masculinization but rather increased the development of female-typical traits. Conversely, the handedness subgroup (i.e., profile 3) showed evidence of decreased male typicality but did not differ on adulthood female typicality. On the Big Five personality traits, profiles only differed on agreeableness. Specifically, profile 2, the fraternal birth order subgroup, scored the highest on agreeableness; this score was significantly higher than those of profiles 1 and 3. These differences between profiles remained when removing heterosexual men from the analyses (SI Appendix, Table S8). No other differences between profiles were found on the gender (non)conformity or personality measures. Together, these results indicate that the subgroups delineated by biomarkers in the present study map onto different behavioral phenotypes, further supporting that the subgroups defined by LPA are meaningful.
Table 3.
Means and SDs for outcome variables by latent profile
| Outcome variables | Profile 1 | Profile 2 | Profile 3 | Profile 4 |
| Recalled Childhood Gender Identity/Role | 3.88 (0.60)*,† | 3.78 (0.55)‡ | 3.72 (0.57)‡ | 3.71 (0.60) |
| Bem masculinity | 48.66 (8.67) | 48.56 (8.53) | 49.89 (7.35) | 50.72 (9.03) |
| Bem femininity | 52.78 (9.15)* | 55.19 (8.25)‡,† | 50.17 (9.52)* | 53.20 (8.91) |
| Feminine occupational preference | 17.87 (6.17)* | 19.42 (5.82)‡ | 17.81 (6.14) | 18.72 (5.37) |
| Masculine occupational preference | 19.55 (7.49)† | 19.52 (6.69) | 17.08 (7.64)‡,§ | 22.40 (7.38)† |
| Extroversion | 6.36 (2.15) | 6.43 (2.09) | 6.49 (2.22) | 6.64 (1.85) |
| Agreeableness | 7.01 (1.70)* | 7.46 (1.53)‡,† | 6.57 (1.68)* | 6.80 (1.93) |
| Conscientiousness | 7.04 (1.69) | 7.32 (1.54) | 7.23 (1.57) | 7.13 (1.80) |
| Neuroticism | 5.76 (2.15) | 5.92 (2.08) | 5.51 (2.15) | 5.16 (2.23) |
| Openness | 7.39 (1.79) | 7.32 (1.72) | 7.87 (1.73) | 7.00 (1.80) |
Significantly different from profile 2, P < 0.05.
Significantly different from profile 3, P < 0.05.
Significantly different from profile 1, P < 0.05.
Significantly different from profile 4, P < 0.05.
Conclusions
Using LPA, the present study found evidence to suggest that nonheterosexual men are heterogeneous with respect to biomarkers of processes hypothesized to influence male sexual orientation development. As such, the biomarkers investigated here appear to each map onto different subgroups of nonheterosexual men, rather than cluster within the same individuals. The subgroups defined in this study appear to be differentially influenced on measures of masculinity and femininity, providing insight into how these markers might differentially affect the development of typical masculine and feminine traits. Understanding how personality and gender-(non)conforming traits are related to developmental biomarkers may provide insight into the aspects of development (masculinization/demasculinization or feminization/defeminization) related to these biomarkers. Lastly, this study illustrates the value of an LPA approach to advancing the understanding of sexual orientation development.
Methods
Participants.
A total of 827 participants were included in the analyses. These participants were recruited via Facebook advertisements between August and November 2015 and at the June 2015 Toronto Pride Festival. The study was approved and conducted in accordance with the guidelines of the University of Toronto’s research ethics board, and informed consent was obtained from all participants. See SI Appendix for further details.
Measures.
Sexual orientation was defined with three measures: Self-identification, attraction, and behavior. Specifically, participants were asked whether they identified as heterosexual, gay, bisexual, or other (i.e., identity). Self-identified heterosexuals were classified as heterosexual, and all others were classified as nonheterosexual. Second, for attraction, participants were asked, “In the last 12 mo, I have felt sexually attracted to... 0- Only females, never to males” to “4- only males, never females.” Lastly, for behavior, participants were asked, “In the last 12 mo, I have had sexual experience with... 0- Only females, never with males”, to “4- only males, never females.” There was also an option to indicate they did not have any feelings of attraction or experience sexual behavior. No participants indicated lack of sexual attraction; however, 11 participants indicated they did not have sexual experience in the last year and, thus, were excluded from analyses involving sexual experience. For statistical comparisons between heterosexual and nonheterosexual men, individuals with a score of 0 were classified as heterosexual and all others were classified as nonheterosexual (i.e., both for attraction and behavior scores). Thus, statistical comparisons based on sexual orientation were performed for each of the three sexual orientation classification methods.
Handedness was assessed using the 10-item Edinburgh inventory. Answers were provided on a five-point Likert scale: 0-always right to 4-always left. Responses were summed, and proportion scores were calculated by dividing each participant’s sum by 40, resulting in scores ranging from 0 (i.e., always uses the right hand) to 1 (i.e., always uses the left hand). Fraternal birth order was measured by asking participants whether they had any biological siblings with whom they shared the same mother (full siblings and/or half-siblings with the same mother). If they indicated “yes,” they were then asked how many older brothers, older sisters, younger brothers, and younger sisters they have with the same mother. Proportion of older brothers was calculated as in Blanchard (74): (number of older brothers + 0.25)/(total number of siblings + 1). Proportion of familial gay/bisexual men among participants’ male relatives was calculated by dividing the number of gay and bisexual men (uncles and male cousins) reported in the maternal and paternal line by the total number of biological male family members (uncles and male cousins) reported in both maternal and paternal lines. All gender (non)conformity and personality scales are described in the SI Appendix (73, 75–78).
Statistical Analyses.
LPAs were conducted using Mplus Version 7.11 (79). LPA was chosen as our clustering method because it provides various diagnostics such as the Bayesian information criterion (BIC) statistic, which is useful in determining number of profiles, whereas other methods do not give such information (80). Full information maximum likelihood estimation for missing data was used for the LPA (81), and the randomness of missing values was evaluated using IBM SPSS version 24 missing values analysis (Little’s MCAR) with estimation maximization (i.e., EM method) to ensure there were no systematic relationships between the missing values and any other values—the data for the biomarkers (i.e., fraternal birth order, handedness, and familiality) as well as for all of the outcome measures (i.e., RCGI scale, Bem masculinity, Bem femininity, feminine occupational preferences, masculine occupational preferences, and the Big Five measures) were included in this missing values analysis.
Three indicator variables were used to determine latent profiles: Handedness, proportion of older brothers, and proportion of male relatives who were gay/bisexual. These indicator variables were transformed into z scores for latent analyses. Model fit was examined for one through five profile solutions, and, for each model, the BLRT, BIC, and entropy values were examined (82). A nonsignificant BLRT P value suggests that a model with one fewer latent profiles is a better model fit (82). Smaller BIC values indicate that the model fit is better for the data (82). Entropy values range from 0 to 1, with values closer to 1 indicating greater overall accuracy of the classification (83). The BLRT and the size of the profiles were the primary indicators used to identify the best model (i.e., a profile with less than ∼5% of the sample size is not ideal) (84, 85).
The following analyses were all performed using SPSS version 24. The Kruskal−Wallis test was the inferential test chosen to compare profiles, due to unequal sample sizes. Latent profiles were first compared on the developmental markers (i.e., Handedness, fraternal birth order, and familiality). Due to the small number of self-identified bisexual individuals, and individuals who identified their attraction/behavior between 1 and 3 on the Likert scale [i.e., not exclusively heterosexual, some attraction and/or sexual experience (i.e., behavior) with the same sex], we evaluated whether these groups differed from exclusively gay men in their distribution across profiles (SI Appendix). Results indicated that these groups did not differ in their distribution across profiles, and thus, for all analyses reported here, we compared nonheterosexual with heterosexual men. The χ2 tests with Yates’s correction were used to evaluate the distribution of heterosexual and nonheterosexual participants (based on self-identification, sexual attraction, and sexual behavior) in the profile that displayed the relative absence of the focal developmental biomarkers (i.e., profile 1) compared with profiles that displayed elevated presence of one or more developmental biomarkers (i.e., profiles 2, 3, and 4). Kruskal−Wallis tests were also used to compare latent profiles on outcome variables, including the RCGI, Bem masculinity, Bem femininity, feminine occupational preferences, masculine occupational preferences, and the Big Five personality traits. Mann−Whitney U tests were used for post hoc analyses for significant omnibus effects (alpha set at P < 0.05). The effect sizes for all latent profile comparisons are reported in SI Appendix, Table S10.
Supplementary Material
Acknowledgments
We thank all our volunteers who assisted in participant recruitment at the Toronto Pride Festival (Mary Loka, Lindsay Melhuish, Phil Nguyen, Lauren Oakley, Martha Pokarowski, Firyal Ramzan, Anna Takagi, and Robert Wither). The research reported in this article was supported by a postdoctoral fellowship and a doctoral Canada Graduate Scholarship from the National Sciences and Engineering Research Council of Canada (NSERC) and an Ontario Graduate Scholarship to A.S.-G., as well as by NSERC Discovery Grants to D.A.M. and D.P.V.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809920116/-/DCSupplemental.
References
- 1.Guadalupe T., et al. , Human subcortical brain asymmetries in 15,847 people worldwide reveal effects of age and sex. Brain Imaging Behav. 11, 1497–1514 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Hemmen J., et al. , Sex differences in white matter microstructure in the human brain predominantly reflect differences in sex hormone exposure. Cereb. Cortex 27, 2994–3001 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Ingalhalikar M., et al. , Sex differences in the structural connectome of the human brain. Proc. Natl. Acad. Sci. U.S.A. 111, 823–828 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruigrok A. N., et al. , A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 39, 34–50 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritchie S. J., et al. , Sex differences in the adult human brain: Evidence from 5216 UK biobank participants. Cereb. Cortex 28, 2959–2975 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eagly A. H., Wood W., The origins of sex differences in human behavior: Evolved dispositions versus social roles. Am. Psychol. 54, 408–423 (1999). [Google Scholar]
- 7.Ellis L., et al. , Sex Differences: Summarizing More than a Century of Scientific Research (Psychology Press, 2013). [Google Scholar]
- 8.Greenberg D. M., Warrier V., Allison C., Baron-Cohen S., Testing the empathizing-systemizing theory of sex differences and the extreme male brain theory of autism in half a million people. Proc. Natl. Acad. Sci. U.S.A. 115, 12152–12157 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hines M., Sex-related variation in human behavior and the brain. Trends Cogn. Sci. 14, 448–456 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bale T. L., Epperson C. N., Sex differences and stress across the lifespan. Nat. Neurosci. 18, 1413–1420 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bale T. L., Epperson C. N., Sex as a biological variable: Who, what, when, why and how. Neuropsychopharmacology 42, 386–396 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joel D., McCarthy M. M., Incorporating sex as a biological variable in neuropsychiatric research: Where are we now and where should we be? Neuropsychopharmacology 42, 379–385 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S. K., Sex as an important biological variable in biomedical research. BMB Rep. 51, 167–173 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balthazart J., Sex differences in partner preferences in humans and animals. Philos. Trans R. Soc. Lond. B Biol. Sci., 371, 20150118 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey J. M., et al. , Sexual orientation, controversy, and science. Psychol. Sci. Public Interest 17, 45–101 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Breedlove S. M., Prenatal influences on human sexual orientation: Expectations versus data. Arch. Sex. Behav. 46, 1583–1592 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lippa R. A., Sexual orientation and personality. Annu. Rev. Sex Res. 16, 119–153 (2005). [PubMed] [Google Scholar]
- 18.Lippa R. A., Sex differences and sexual orientation differences in personality: Findings from the BBC internet survey. Arch. Sex. Behav. 37, 173–187 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Zheng L., Hart T. A., Zheng Y., The relationship between intercourse preference positions and personality traits among gay men in China. Arch. Sex. Behav. 41, 683–689 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Zucker K. J., et al. , The recalled childhood gender identity/gender role questionnaire:Psychometric properties. Sex Roles 54, 469–483 (2006). [Google Scholar]
- 21.Bailey J. M., et al. , A family history study of male sexual orientation using three independent samples. Behav. Genet. 29, 79–86 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Camperio-Ciani A., Corna F., Capiluppi C., Evidence for maternally inherited factors favouring male homosexuality and promoting female fecundity. Proc. Biol. Sci. 271, 2217–2221 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamer D. H., Hu S., Magnuson V. L., Hu N., Pattatucci A. M., A linkage between DNA markers on the X chromosome and male sexual orientation. Science 261, 321–327 (1993). [DOI] [PubMed] [Google Scholar]
- 24.Rahman Q., et al. , Maternal inheritance and familial fecundity factors in male homosexuality. Arch. Sex. Behav. 37, 962–969 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Semenyna S. W., VanderLaan D. P., Petterson L. J., Vasey P. L., Familial patterning and prevalence of male androphilia in Samoa. J. Sex Res. 54, 1077–1084 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Schwartz G., Kim R. M., Kolundzija A. B., Rieger G., Sanders A. R., Biodemographic and physical correlates of sexual orientation in men. Arch. Sex. Behav. 39, 93–109 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Vanderlaan D. P., Forrester D. L., Petterson L. J., Vasey P. L., The prevalence of fa’afafine relatives among Samoan gynephilic men and fa’afafine. Arch. Sex. Behav. 42, 353–359 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Vanderlaan D. P., Vokey J. R., Vasey P. L., Is transgendered male androphilia familial in non-Western populations? The case of a Samoan village. Arch. Sex. Behav. 42, 361–370 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Alanko K., et al. , Common genetic effects of gender atypical behavior in childhood and sexual orientation in adulthood: A study of Finnish twins. Arch. Sex. Behav. 39, 81–92 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Bailey J. M., Dunne M. P., Martin N. G., Genetic and environmental influences on sexual orientation and its correlates in an Australian twin sample. J. Pers. Soc. Psychol. 78, 524–536 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Kendler K. S., Thornton L. M., Gilman S. E., Kessler R. C., Sexual orientation in a U.S. national sample of twin and nontwin sibling pairs. Am. J. Psychiatry 157, 1843–1846 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Kirk K. M., Bailey J. M., Dunne M. P., Martin N. G., Measurement models for sexual orientation in a community twin sample. Behav. Genet. 30, 345–356 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Långström N., Rahman Q., Carlström E., Lichtenstein P., Genetic and environmental effects on same-sex sexual behavior: A population study of twins in Sweden. Arch. Sex. Behav. 39, 75–80 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Santtila P., et al. , Potential for homosexual response is prevalent and genetic. Biol. Psychol. 77, 102–105 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Mustanski B. S., et al. , A genomewide scan of male sexual orientation. Hum. Genet. 116, 272–278 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Sanders A. R., et al. , Genome-wide scan demonstrates significant linkage for male sexual orientation. Psychol. Med. 45, 1379–1388 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Wang B., et al. , Association analysis between the tag SNP for sonic hedgehog rs9333613 polymorphism and male sexual orientation. J. Androl. 33, 951–954 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Hu S., et al. , Linkage between sexual orientation and chromosome Xq28 in males but not in females. Nat. Genet. 11, 248–256 (1995). [DOI] [PubMed] [Google Scholar]
- 39.Arning L., et al. , Handedness and the X chromosome: The role of androgen receptor CAG-repeat length. Sci. Rep. 5, 8325 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paracchini S., Scerri T., “Genetics of human handedness and laterality” in Lateralized Brain Functions Rogers L., G. Vallortigara, Eds. (Springer, 2017), pp. 523–552. [Google Scholar]
- 41.Ellis L., Skorska M. N., Bogaert A. F., Handedness, sexual orientation, and somatic markers for prenatal androgens: Are southpaws really that gay? Laterality 22, 157–180 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Yeo R. A., Gangestad S. W., Daniel W. F., Hand preference and developmental instability. Psychobiology 21, 161–168 (1993). [Google Scholar]
- 43.Papadatou-Pastou M., Martin M., Munafò M. R., Jones G. V., Sex differences in left-handedness: A meta-analysis of 144 studies. Psychol. Bull. 134, 677–699 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Lalumière M. L., Blanchard R., Zucker K. J., Sexual orientation and handedness in men and women: A meta-analysis. Psychol. Bull. 126, 575–592 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Blanchard R., Review and theory of handedness, birth order, and homosexuality in men. Laterality 13, 51–70 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Blanchard R., Cantor J. M., Bogaert A. F., Breedlove S. M., Ellis L., Interaction of fraternal birth order and handedness in the development of male homosexuality. Horm. Behav. 49, 405–414 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Blanchard R., Lippa R. A., Birth order, sibling sex ratio, handedness, and sexual orientation of male and female participants in a BBC internet research project. Arch. Sex. Behav. 36, 163–176 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Blanchard R., Lippa R. A., The sex ratio of older siblings in non-right-handed homosexual men. Arch. Sex. Behav. 37, 970–976 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Bogaert A. F., Blanchard R., Crosthwait L. E., Interaction of birth order, handedness, and sexual orientation in the Kinsey interview data. Behav. Neurosci. 121, 845–853 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Lippa R. A., Handedness, sexual orientation, and gender-related personality traits in men and women. Arch. Sex. Behav. 32, 103–114 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Kishida M., Rahman Q., Fraternal birth order and extreme right-handedness as predictors of sexual orientation and gender nonconformity in men. Arch. Sex. Behav. 44, 1493–1501 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Swift-Gallant A., Coome L. A., Monks D. A., VanderLaan D. P., Handedness is a biomarker of variation in anal sex role behavior and recalled childhood gender nonconformity among gay men. PLoS One 12, e0170241 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valenzuela C. Y., Sexual orientation, handedness, sex ratio and fetomaternal tolerance-rejection. Biol. Res. 43, 347–356 (2010). [PubMed] [Google Scholar]
- 54.Xu Y., Zheng Y., Fraternal birth order, handedness, and sexual orientation in a Chinese population. J. Sex Res. 54, 10–18 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Cohen K. M., Relationships among childhood sex-atypical behavior, spatial ability, handedness, and sexual orientation in men. Arch. Sex. Behav. 31, 129–143 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Mustanski B. S., Chivers M. L., Bailey J. M., A critical review of recent biological research on human sexual orientation. Annu. Rev. Sex Res. 13, 89–140 (2002). [PubMed] [Google Scholar]
- 57.Blanchard R., Fraternal birth order, family size, and male homosexuality: Meta-analysis of studies spanning 25 years. Arch. Sex. Behav. 47, 1–15 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Bogaert A. F., Skorska M., Sexual orientation, fraternal birth order, and the maternal immune hypothesis: A review. Front. Neuroendocrinol. 32, 247–254 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Blanchard R., A possible second type of maternal-fetal immune interaction involved in both male and female homosexuality. Arch. Sex. Behav. 41, 1507–1511 (2012). [DOI] [PubMed] [Google Scholar]
- 60.Blanchard R., Ellis L., Birth weight, sexual orientation and the sex of preceding siblings. J. Biosoc. Sci. 33, 451–467 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Bogaert A. F., et al. , Male homosexuality and maternal immune responsivity to the Y-linked protein NLGN4Y. Proc. Natl. Acad. Sci. U.S.A. 115, 302–306 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green R., The “Sissy Boy Syndrome” and the Development of Homosexuality (Yale University Press, 1987). [Google Scholar]
- 63.Wallien M. S., Cohen-Kettenis P. T., Psychosexual outcome of gender-dysphoric children. J. Am. Acad. Child Adolesc. Psychiatry 47, 1413–1423 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Blanchard R., Bogaert A. F., Proportion of homosexual men who owe their sexual orientation to fraternal birth order: An estimate based on two national probability samples. Am. J. Hum. Biol. 16, 151–157 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Cantor J. M., Blanchard R., Paterson A. D., Bogaert A. F., How many gay men owe their sexual orientation to fraternal birth order? Arch. Sex. Behav. 31, 63–71 (2002). [DOI] [PubMed] [Google Scholar]
- 66.Magnusson D., The person approach: Concepts, measurement models, and research strategy. New Dir. Child Adolesc. Dev. 101, 3–23 (2003). [DOI] [PubMed] [Google Scholar]
- 67.Ngun T. C., Vilain E., The biological basis of human sexual orientation: Is there a role for epigenetics? Adv. Genet. 86, 167–184 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Skorska M. N., Blanchard R., VanderLaan D. P., Zucker K. J., Bogaert A. F., Gay male only-children: Evidence for low birth weight and high maternal miscarriage rates. Arch. Sex. Behav. 46, 205–215 (2017). [DOI] [PubMed] [Google Scholar]
- 69.VanderLaan D. P., Blanchard R., Wood H., Garzon L. C., Zucker K. J., Birth weight and two possible types of maternal effects on male sexual orientation: A clinical study of children and adolescents referred to a gender identity service. Dev. Psychobiol. 57, 25–34 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Rahman Q., Wilson G., Born gay: The psychobiology of human sexual orientation. Pers. Individ. Dif. 34, 1337–1382 (2003). [Google Scholar]
- 71.Li G., Wong W. I., Single-sex schooling: Friendships, dating, and sexual orientation. Arch. Sex. Behav. 47, 1025–1039 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Bauer D. J., Curran P. J., Overextraction of latent trajectory classes: Much ado about nothing? Reply to Rindskopf (2003), Muthen (2003) and Cudeck & Henly (2003). Psychol. Methods 8, 384–393 (2003). [DOI] [PubMed] [Google Scholar]
- 73.Bem S. L., The measurement of psychological androgyny. J. Consult. Clin. Psychol. 42, 155–162 (1974). [PubMed] [Google Scholar]
- 74.Blanchard R., Detecting and correcting for family size differences in the study of sexual orientation and fraternal birth order. Arch. Sex. Behav. 43, 845–852 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Cullen J. M., Wright L. W. Jr, Alessandri M., The personality variable openness to experience as it relates to homophobia. J. Homosex. 42, 119–134 (2002). [DOI] [PubMed] [Google Scholar]
- 76.Lippa R., Gender-related individual differences and the structure of vocational interests: The importance of the people-things dimension. J. Pers. Soc. Psychol. 74, 996–1009 (1998). [DOI] [PubMed] [Google Scholar]
- 77.Lippa R. A., Gender differences in personality and interests: When, where, and why? Soc. Personal. Psychol. Compass 4, 1098–1110 (2010). [Google Scholar]
- 78.Rammstedt B., John O. P., Measuring personality in one minute or less: A 10-item short version of the big five inventory in English and German. J. Res. Pers. 41, 203–212 (2007). [Google Scholar]
- 79.Muthén L. K., Muthén B., Mplus Statistical Modeling Software: Release 7.0 (Muthén & Muthén, Los Angeles, CA, 2012). [Google Scholar]
- 80.Magidson J., Vermunt J., Latent class models for clustering: A comparison with K-means. Can. J. Mark. Res. 20, 36–43 (2002). [Google Scholar]
- 81.Enders C. K., Bandalos D. L., The relative performance of full information maximum likelihood estimation for missing data in structural equation models. J. Struct. Equ. Model. 8, 430–457 (2001). [Google Scholar]
- 82.Nylund K. L., Asparouhov T., Muthén B. O., Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. J. Struct. Equ. Model. 14, 535–569 (2007). [Google Scholar]
- 83.Geiser C., Data Analysis with Mplus (Guilford Press, 2012). [Google Scholar]
- 84.Collins L. M., Lanza S. T., Latent Class and Latent Transition Analysis: With Applications in the Social, Behavioral, and Health Sciences (John Wiley, 2010). [Google Scholar]
- 85.Muthén B., Muthén L. K., Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcohol. Clin. Exp. Res. 24, 882–891 (2000). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.