Significance
CO2 is a greenhouse gas. Synthesis of liquid fuel using CO2 and H2 is promising for the sustainability of mankind. The reported technologies usually proceed via CO intermediate, which needs high temperature, and tend to cause low selectivity. Direct hydrogenation of CO2 to liquid fuel, not via CO, is a challenging issue. In this work, we designed a Co6/MnOx nanocatalyst that could successfully avoid the CO route. The reaction could proceed at 200 °C, which is much lower than those reported so far. The selectivity of the liquid fuel in total products reached 53.2 C-mol%, which is among the highest reported to date.
Keywords: CO2 hydrogenation, liquid fuel, paraffin, lower temperature, reaction pathway
Abstract
Synthesis of liquid fuels (C5+ hydrocarbons) via CO2 hydrogenation is very promising. Hydrogenation of CO2 to liquid hydrocarbons usually proceeds through tandem catalysis of reverse water gas shift (RWGS) reaction to produce CO, and subsequent CO hydrogenation to hydrocarbons via Fischer–Tropsch synthesis (FTS). CO2 is a thermodynamically stable and chemically inert molecule, and RWGS reaction is endothermic and needs a higher temperature, whereas FTS reaction is exothermic and is thermodynamically favored at a lower temperature. Therefore, the reported technologies have some obvious drawbacks, such as high temperature, low selectivity, and use of complex catalysts. Herein we discovered that a simple Co6/MnOx nanocatalyst could efficiently catalyze CO2 hydrogenation. The reaction proceeded at 200 °C, which is much lower than those reported so far. The selectivity of liquid hydrocarbon (C5 to C26, mostly n-paraffin) in total product could reach 53.2 C-mol%, which is among the highest reported to date. Interestingly, CO was hardly detectable during the reaction. The in situ Fourier transform infrared characterization and 13CO labeling test confirmed that the reaction was not via CO, accounting for the eminent catalytic results. This report represents significant progress in CO2 chemistry and CO2 transformation.
Transformation of CO2 into value-added chemicals has attracted much attention in past decades (1–5). Liquid hydrocarbons (more than five carbon atoms, i.e., C5+) are widely used as liquid fuels, which are currently produced from fossil resources, especially petroleum. The growing demand for liquid fuels and gradual depletion of petroleum lead to the urgent need for their production from renewable resources. Hydrogen gas is a clean reductant, which can be produced from water by artificial photosynthesis or by electricity from renewable energy such as wind and solar energy, and this area is developing rapidly (6, 7). Moreover, CO2 hydrogenation into liquid hydrocarbons is an interesting topic in chemistry (8–15). However, at the present stage, it is still a great challenge. Firstly, CO2 is a fully oxidized, thermodynamically stable and chemically inert molecule. Secondly, the difficulty of CO2 adsorption on a catalyst surface may result in a low C/H ratio at catalytic sites, favoring methane formation and inhibiting the chain growth probability (8, 9). To date, most reports of CO2 hydrogenation have been focusing on C1 or short-chain products, such as CO, HCOOH, CH3OH, CH4, and C2 to C4 olefins, while studies on producing liquid (C5+) hydrocarbons are rarely reported (8–11).
Hydrogenation of CO2 to liquid hydrocarbons usually proceeds through tandem catalysis of reverse water gas shift (RWGS) reaction and subsequent CO hydrogenation to hydrocarbons via Fischer–Tropsch synthesis (FTS) (8, 9). The RWGS reaction is endothermic and needs a higher temperature, whereas the FTS reaction is exothermic and favors a lower temperature. Hence this reaction path has inherent drawbacks. Firstly, complicated catalysts are usually required to tailor the cascade reactions, and a high temperature (usually above 300 °C) is often needed to drive these reactions. Secondly, the equilibrium of the RWGS reaction limits the pressure of CO generated in situ, which restricts the selectivity of liquid hydrocarbon in subsequent FTS reaction. Hence, an additional reforming catalyst is usually required to obtain good catalytic results. Thirdly, considerable CO is inevitably retained in the final product, which usually occupies about 20 to 45% of the total product. Although, in several cases, methanol acted as an intermediate to produce liquid hydrocarbon from CO2 hydrogenation at high temperature, considerable CO byproduct also existed in the final product. Iron-based catalysts, which could catalyze both RWGS and FTS reactions, were predominantly used as a catalyst for CO2 hydrogenation to longer-chain hydrocarbons, especially olefins (16, 17). Very recently, significant progress has been made in hydrogenating CO2 into gasoline fuel. It was found that, in a flow reactor, Na−Fe3O4/HZSM-5 multifunctional catalyst (14) and In2O3/HZSM-5 bifunctional catalyst (15) showed excellent performance in converting CO2 and H2 into liquid fuel at about 320 °C. In the reaction, CO was an intermediate and/or considerable byproduct. Obviously, a novel catalyst for liquid fuel synthesis via CO2 hydrogenation that can avoid CO is highly desirable.
In a previous paper, we developed a route to synthesize acetic acid via methanol hydrocarboxylation over a homogeneous catalyst, where CO was almost undetectable and CO2 directly participated in forming carboxyl of the acetic acid (5). Here we report the high-selective synthesis of liquid hydrocarbons via CO2 hydrogenation at a lower temperature using a simple Co6/MnOx nanocatalyst. Interestingly, very little CO was detected in the product. Further study indicates that hydrogenation of CO2 to form C5+ paraffins did not proceed through the CO pathway.
Results and Discussion
Catalyst Structure.
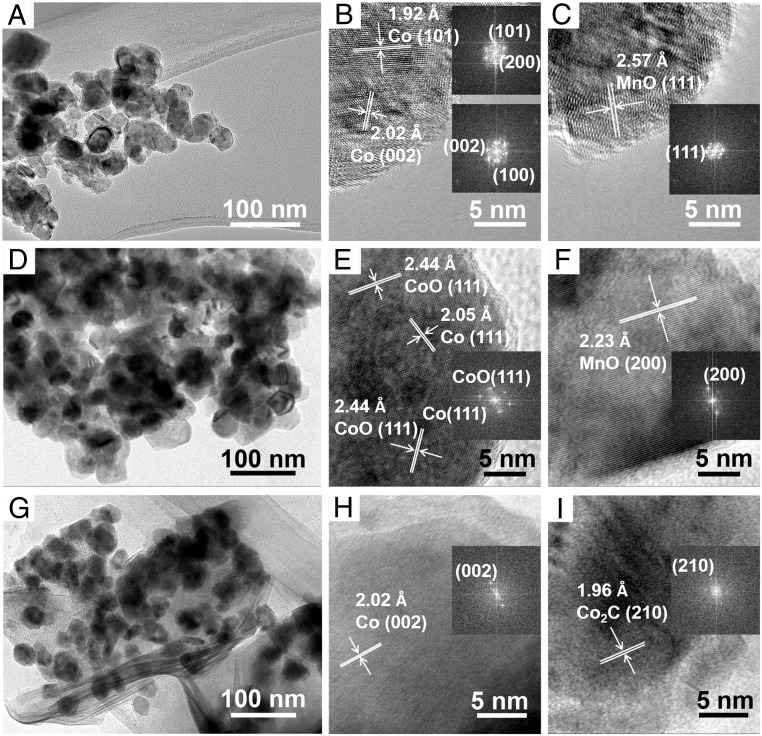
The Co6/MnOx nanocatalyst was prepared by a coprecipitation method. The transmission electron microscope (TEM) image shows that the size of the catalyst particles was mostly in the range of 25 nm to 40 nm (Fig. 1A). The N2 adsorption test revealed that the Brunauer–Emmett–Teller surface area of the piled catalyst was only 49.4 m2/g (SI Appendix, Fig. S1), indicating that it was not a porous material. The metallic Co (Co0) catalyst and the Co−Mn bimetallic catalysts fabricated by the above method were all spherical-like, and the sizes of the bimetallic catalysts were slightly larger (SI Appendix, Fig. S2). In the TEM images of Co6/MnOx, the lattice spacings of 1.92 and 2.02 Å (Fig. 1B) corresponded to the (101) plane and the (002) plane of Co0, and the lattice spacing of 2.57 Å (Fig. 1C) could be assigned to the (111) plane of MnO. The X-ray diffraction (XRD) pattern also reveals two kinds of crystal phases, i.e., Co0 bulk phase and MnO, in the catalyst (SI Appendix, Fig. S3). It is known that cobalt oxides can be easily reduced to metallic Co by H2 (18). The temperature-programmed reduction (TPR) analysis proved that the Mn atoms could evidently retard the reduction of Co oxides because strong interaction existed between Co−Mn atoms (SI Appendix, Fig. S4). The X-ray photoelectron spectroscopy (XPS) characterization suggests that Co2+ and Mn3+ were on the surface of the catalyst (SI Appendix, Fig. S5 A–C). The elementary mapping shows that Co, Mn, and O atoms were well dispersed in the catalyst (SI Appendix, Fig. S6). The inductively coupled plasma optical emission spectroscopy (ICP-OES) and XPS analysis demonstrated that compositions of the catalysts and catalyst surface were consistent with the design values (SI Appendix, Table S1). In brief, the Co6/MnOx consisted of Co0 and MnO crystallites, and strong interaction existed between the atoms.
Fig. 1.
TEM images of Co6/MnOx and fast Fourier transform patterns (Insets): the fresh catalyst (A−C); the catalyst after CO2 hydrogenation (D−F), and the catalyst after CO hydrogenation (G−I).
Catalytic Performance.
The target reaction was effectively catalyzed by Co6/MnOx in squalane solvent at milder conditions (Table 1). Liquid hydrocarbons (C5 to C26) were the major product, and their selectivity in the total product was as high as 53.2 C-mol% (Table 1, entry 1). The alkane products were all linear alkanes (n-paraffins). The byproducts were mostly C1 to C4 hydrocarbons. The C atoms in the hydrocarbons were all from CO2, because no product was observed without the reactant CO2 (entry 2). The reaction temperature was 200 °C, which is much lower than those reported in the literature using a flow reactor. The detailed product distribution (SI Appendix, Fig. S7 A and B) indicated that a high proportion of n-paraffins, especially C5+ n-paraffins, was obtained in the reaction. The chain length distribution of the hydrocarbon products followed the Anderson−Schulz−Flory (ASF) statistics (SI Appendix, Fig. S7C). The chain growth probability (α) was 0.78, which is consistent with a large portion of longer-chain products. Interestingly, the amount of CO was very small (0.4 C-mol%). Further studies also showed that CO was hardly detectable during the whole reaction, which will be discussed in the following paragraphs. We would like to mention that the above results were collected using a batch reactor, where CO2 and H2 reacted on suspending catalyst particles under stirring. The product generated remained in the reactor during the reaction. While in a flow reactor, reactant gases of constant pressure passed through the packed catalyst bed, and the products generated were simultaneously taken out of the reactor by flowing gas.
Table 1.
CO2 hydrogenation using different catalysts
| Entry | Catalyst | Solvent | Selectivity (C-mol%) | Activity (mmolCO2·gcat−1·h−1) | |||
| CO | C1–4 | C5+ | ROH | ||||
| 1* | Co6/MnOx | squalane | 0.4 | 46.4 | 53.2 | 0 | 15.1 |
| 2† | Co6/MnOx | squalane | — | — | — | — | 0 |
| 3 | Co0 | squalane | 0.6 | 80.7 | 18.7 | 0 | 1.7 |
| 4 | CoO | squalane | 0 | 0 | 0 | 0 | 0 |
| 5 | Co3O4 | squalane | 0 | 0 | 0 | 0 | 0 |
| 6 | Mn | squalane | 0 | 0 | 0 | 0 | 0 |
| 7 | MnO | squalane | 0 | 0 | 0 | 0 | 0 |
| 8 | MnO2 | squalane | 0 | 0 | 0 | 0 | 0 |
| 9 | Co14/MnOx | squalane | 0.5 | 58.5 | 41.0 | 0 | 6.6 |
| 10 | Co10/MnOx | squalane | 0.5 | 47.2 | 52.3 | 0 | 11.2 |
| 11 | Co2/MnOx | squalane | 0.4 | 76.2 | 23.4 | 0 | 13.3 |
| 12 | Co6/ZnOx | squalane | 0.1 | 80.7 | 19.2 | 0 | 4.8 |
| 13 | Co6/AlOx | squalane | 0.1 | 94.2 | 5.7 | 0 | 4.1 |
| 14 | Co6/CeOx | squalane | 0.4 | 89.8 | 9.8 | 0 | 6.3 |
| 15 | Co6/MnOx | — | 0.7 | 60.9 | 38.4 | 0 | 11.3 |
| 16 | Co6/MnOx | cyclohexane | 0.4 | 48.3 | 51.3 | 0 | 14.7 |
| 17 | Co6/MnOx | benzene | 0.5 | 95.3 | 4.2 | 0 | 7.9 |
| 18 | Co6/MnOx | DMI | 0.6 | 87.6 | 6.8 | 5.0 | 2.6 |
| 19 | Co6/MnOx | water | 0.3 | 92.9 | 0 | 6.8 | 3.2 |
Reaction conditions: 20 mg of catalyst, 1.0 mL of solvent (squalane), 200 °C, 15 h, initial pressure 8 MPa (CO2/H2 = 1).
The conversion of CO2 was 15.3%, and the selectivity of paraffin in the total hydrocarbons was 78.1 C-mol%; moreover, the selectivity of paraffin in the C5+ products was 67.2 C-mol%.
No CO2 was added. The error of activity was ±1.0 mmolCO2·gcat−1·h−1 and the error of selectivity was ±1.5 C-mol%, which were obtained by three repeated experiments.
To optimize the catalyst, we tested the Co0 and its oxides (CoO, Co3O4) in CO2 hydrogenation. The results revealed that Co0 could catalyze the synthesis of C5+ hydrocarbons at very low activity and C5+ selectivity, and the largest compound contained only 16 C atoms (Table 1, entry 3), whereas the Co oxides could not catalyze the reaction at all (entries 4, 5). We also used the metallic Mn and its oxides (MnO, MnO2), but the reaction did not occur either (entries 6 to 8). Thus, we deduce that Co was the major catalyst, and Mn was the promoter. After testing the Co-Mn bimetallic catalysts of different atom ratios, we found that Co6/MnOx showed the best performance (entries 1, 9 to 11). We also tested other Co based bimetallic catalyst prepared by a similar method, including Co6/ZnOx, Co6/AlOx, and Co6/CeOx, but the results were poor (entries 12 to 14). Thus, Co6/MnOx was appropriate for the reaction.
The solvent was also important for the reaction. Without solvent, the reaction could take place at lower activity and selectivity (Table 1, entry 15). Both squalane and cyclohexane as solvent could effectively improve the catalytic performance, and selectivity in squalane was slightly higher (entries 1, 16). We also tested benzene and found that the solvent aromaticity inhibited the reaction (entry 17). When we used polar solvents, such as 1,3-Dimethyl-2-imidazolidinone (DMI) and water, both reaction activity and selectivity were much lower (entries 18, 19). Interestingly, minor methanol and ethanol were observed. In short, squalane solvent and Co6/MnOx catalyst were a suitable combination for the reaction.
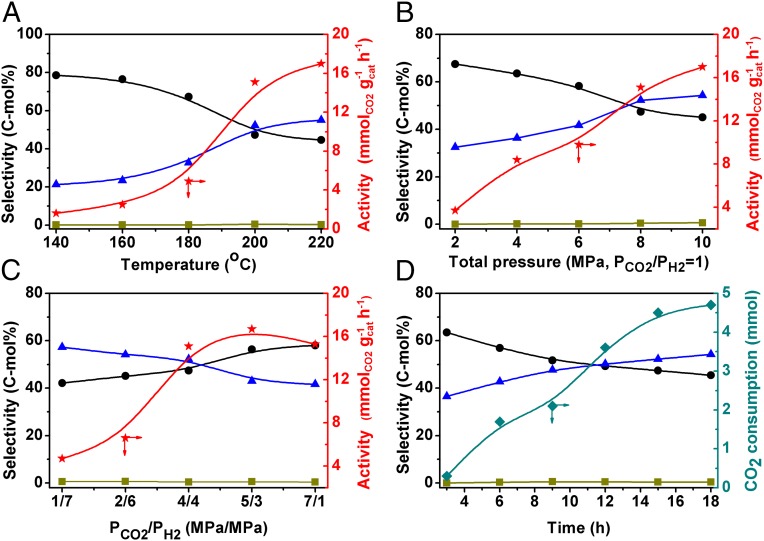
Fig. 2A shows the effect of reaction temperature upon the reaction activity and selectivity. The C5+ products began to form at 140 °C. When the temperature was gradually elevated to 200 °C, the reaction rate and C5+ selectivity increased remarkably. The contribution of catalytic activity from 200 °C to 220 °C was not as significant as that from 180 °C to 200 °C. In addition, the increase of C5+ selectivity also became slower from 200 °C to 220 °C, suggesting that 200 °C was a suitable temperature. At this temperature, we studied the influence of the total pressure at an equal proportion of CO2 and H2 (Fig. 2B). Both the activity and C5+ selectivity were enhanced markedly with the elevating total pressure, while they changed slowly when the pressure exceeded 8 MPa. We further fixed the total pressure at 8 MPa and studied the influence of CO2/H2 ratio on the reaction (Fig. 2C). The activity rose rapidly with increasing CO2 pressure, which became less evident when the ratio of CO2/H2 reached 4/4. On the other hand, the C5+ selectivity decreased with elevating CO2 pressure, and the rate of decrease became faster after the CO2/H2 ratio was larger than 4/4. Thus, 4 MPa of CO2/4 MPa of H2 was the best for the reaction. Fig. 2D illustrates the time course of the reaction. The CO2 was consumed continuously during the reaction, and the C5+ selectivity rose gradually with reaction time. The GC analysis also verified that the chain length of the products increased with increasing reaction time. It is noteworthy that, at all above conditions, CO was hardly detectable.
Fig. 2.
Effect of reaction conditions [(A) reaction temperature, (B) total pressure, (C) CO2/H2 ratio, and (D) reaction time] on the CO2 hydrogenation using 20 mg of Co6/MnOx and 1 mL of squalane. Other conditions: (A) 4 MPa of CO2 and 4 MPa of H2, 15 h; (B) PCO2/PH2 = 1, 200 °C, 15 h; (C) initial pressure 8 MPa, 200 °C, 15 h; and (D) 4 MPa of CO2 and 4 MPa of H2, 200 °C. Symbols: triangle, C5+ selectivity; circle, C1–4 selectivity; square, CO selectivity; star, activity; diamond, CO2 consumption.
The reusability of the catalyst is shown in SI Appendix, Fig. S8A. A trend of slight decrease in selectivity and activity was observed. The TEM, N2 adsorption, XRD, and XPS characterization also showed that the catalyst changed slightly after the recycling test (Fig. 1 and SI Appendix, Figs. S1, S3, and S5). In the hot filtration test, no product was generated after the catalyst was filtered out, indicating that the catalyst worked in a heterogeneous mode (SI Appendix, Fig. S8B). It should be mentioned that industrial use of a heterogeneous catalyst usually operates in a flow reactor. Further work should be done to optimize operation conditions using a flow reactor for practical application.
Reaction Mechanism.
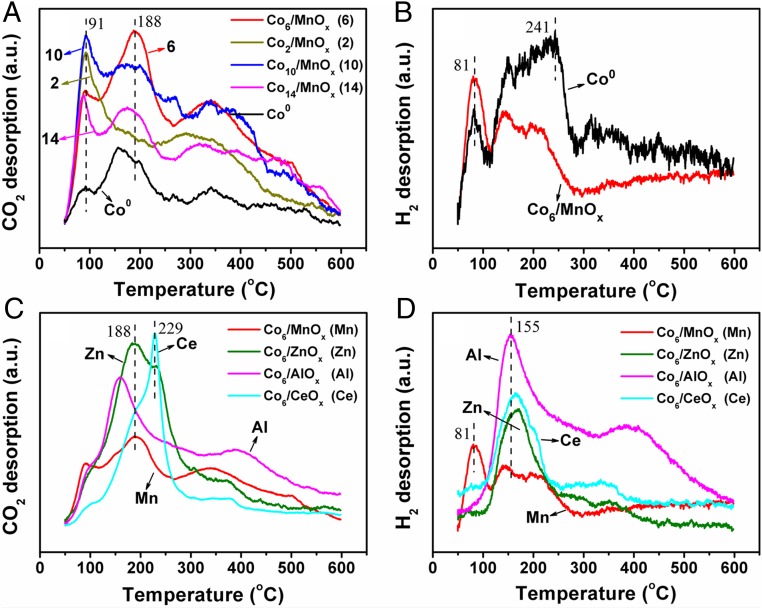
Co0 is a well-known FTS catalyst using syngas, but it is generally regarded as a methanation catalyst in CO2 hydrogenation (9, 16). This can be ascribed to its weak CO2 adsorption and strong H2 adsorption ability, which results in high H/C ratio on the catalyst surface. The high H/C ratio inhibited the chain growth process and favored methane generation (19). Inspired by this, we tested the temperature-programmed desorption of CO2 (CO2-TPD) of Co0 catalyst and the Co−Mn catalysts. The results demonstrated that the amount of CO2 adsorbed on the Co−Mn catalysts was much higher than that on Co0 (Fig. 3A). In addition, CO2 adsorption on Co6/MnOx was the highest in all of the catalysts, especially near the reaction temperature. The CO2-TPD data were consistent with the catalytic results. The CO2 desorbed at a higher temperature (e.g., 188 °C) directly correlated with the C5+ selectivity. The H2 adsorption ability of the catalyst was also crucial. The H2-TPD analysis revealed that the amount of H2 adsorbed on Co6/MnOx was remarkably lower than that on the Co0 catalyst, especially at higher temperature (150 °C to 250 °C; Fig. 3B). The above results verified that the C/H ratio on Co6/MnOx must be much higher than that on Co0, accounting for the exceptional behavior of Co6/MnOx. We would like to mention that competitive adsorption of the reactant gases (e.g., CO2, H2) on the catalyst surface existed at reaction conditions. We further conducted the CO2-TPD and H2-TPD characterization of other Co-based bimetallic catalysts (Co6/ZnOx, Co6/AlOx, Co6/CeOx), as shown in Fig. 3 C and D. The results demonstrated that addition of Zn, Al, and Ce promoters excellently accelerated the CO2 adsorption, but could not suppress or even promote the H2 adsorption on the catalyst, while addition of Mn promoter to Co catalyst not only remarkably enhanced the CO2 adsorption but also significantly suppressed the H2 adsorption. This is the reason Mn is the appropriate promoter for producing long-chain paraffins. The TPD data could also explain why the C5+ selectivity over Co6/ZnOx was higher than those over Co6/AlOx and Co6/CeOx (Table 1, entries 12 to 14).
Fig. 3.
CO2-TPD (A and C) and H2-TPD (B and D) data of the catalysts. The TPD signals have been normalized to the mass of the tested samples.
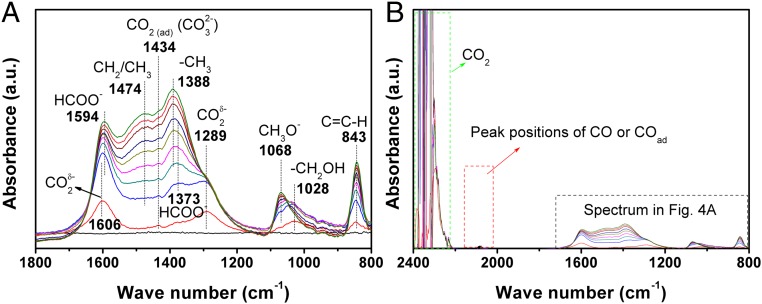
We characterized Co6/MnOx catalyst pretreated with H2 at 200 °C by the semi-in situ XPS method. The result revealed that, at reaction condition, Co0/Co2+ coexisted on the catalyst surface and Mn3+ was in situ reduced to Mn2+ (SI Appendix, Fig. S5 D–F). The outstanding performance of Co6/MnOx may be due to the synergy of the surface Co0, Co2+, and Mn2+ atoms during the reaction. The semi-in situ XPS study also manifested that the CO2 adsorption was improved when the temperature increased from 50 °C to 150 °C (SI Appendix, Fig. S9). At 200 °C, the adsorbed CO2 was reduced to hydrocarbons by the H atoms preadsorbed on the catalyst surface. To detect the intermediates of the reaction, we further conducted an in situ Fourier transform infrared (FTIR) study of CO2 adsorption on the pretreated catalyst at 200 °C. The result of the in situ FTIR study is shown in Fig. 4. It can be seen that CO2 was adsorbed on the catalyst surface and gradually reduced to CH2/CH3, via CO2δ−, HCOO−, −CH2OH, and/or CH3O− intermediates (Fig. 4A). The weak peak at 1,434 cm−1 is assigned to asymmetrically adsorbed CO2 (CO32−). At 2 min, it was quickly reduced to CO2δ− by H atoms on the catalyst surface, as can be seen from the peaks at 1,289 and 1,606 cm−1. At 2.5 min, it was observed that formate (HCOO−) species began to form by reducing CO2δ−. The peaks at 1,373 and 1,594 cm−1 correspond to symmetric and asymmetric OCO stretches of HCOO−. The HCOO− was further reduced to primary alcohol group (-CH2OH, 1,028 cm−1) and/or methoxy group (CH3O−, 1,068 cm−1). Finally, the −CH2OH/CH3O− were reduced to CH2 group (1,474 cm−1) and/or CH3 group (1,388 cm−1). The broad peak around 1,474 cm−1 revealed the coexistence of the CH2 and CH3 groups. No carboxylic acid, alcohol, or ether was detected in the reaction product, indicating that the HCOO−, −CH2OH, and/or CH3O− intermediates were finally converted into CH2 and/or CH3. With the consumption of HCOO−, −CH2OH, and/or CH3O− species, the peaks of CH2 and CH3 became more and more remarkable, which further proved such conversion. The peak at 843 cm−1 can be attributed to out-of-plane bending vibration of C−H in alkenes. The assignment of the above peaks agrees well with that in the literature (20). No gaseous CO or adsorbed CO (COad) was observed (Fig. 4B). The FTIR study suggested that the reaction did not proceed through the CO pathway.
Fig. 4.
In situ FTIR spectra [(A) key intermediates and (B) wide band] recorded during the CO2 adsorption on Co6/MnOx. The catalyst was pretreated with H2 at 200 °C for 2 h before the adsorption test. The black straight line at the bottom was the baseline recorded before CO2 adsorption. The spectra were recorded after the CO2 was introduced to the catalyst, and the time sequence, from bottom to top, was 2, 2.5, 4.5, 7.5, 15, 25, 35, 45, and 55 min.
Only a very little CO was detected during the reaction over Co6/MnOx (Fig. 2), which agreed with the very low RWGS ability of the Co catalyst (9, 16). Moreover, addition of Mn to Co-based catalyst may accelerate the dissociation and disproportionation of CO (21). To investigate the contribution of the trace CO in the C5+ hydrocarbons synthesis, we conducted the reaction using CO (0.5 MPa, 4 MPa) instead of CO2. It was observed that the reaction rate was much lower, and little C5+ product was observed (SI Appendix, Table S2, entries 1, 2). The products were mostly C1–C4 hydrocarbons with a small amount of methanol and ethanol, which were different from those in CO2 hydrogenation. The CO-TPD and H2-TPD data indicate that addition of Mn promoter inhibited the adsorption of CO and H2 on the Co catalyst, especially at the reaction temperature (200 °C) (Fig. 3B and SI Appendix, Fig. S10). Furthermore, most of the Co atoms on the catalyst surface were blocked by the carbon deposit after the CO hydrogenation (SI Appendix, Fig. S11). The TEM and XPS data also suggested that CO altered the Co/Mn valence and resulted in surface reconstruction (Fig. 1 and SI Appendix, Fig. S5). These facts help to explain the poor result of CO hydrogenation over Co6/MnOx. We further conducted a 13CO labeling test by adding a small amount of 13CO in the reactant gases (i.e., 0.2 MPa of 13CO, 3.8 MPa of CO2, 4 MPa of H2). The gas chromatography mass spectrometry (GC-MS) spectra of this test are shown in SI Appendix, Fig. S12. As expected, both 13CO and CO2 could generate the target paraffins, whereas the 13C of 13CO did not enter the products generated from CO2, and the CO2 did not take part in the products formed by 13CO either. This phenomenon revealed that the CO2 and 13CO formed long-chain paraffins separately and independently in the reaction. There is a strong possibility that hydrogenation of CO2 and CO proceeded on segregated and different sites of the catalyst. Otherwise, the 13CH2 monomer from 13CO and the CH2 monomer from CO2 would enter the same paraffin molecule. The surface segregation of the cobalt catalyst during the FTS reaction has been reported elsewhere (22). The results of the 13CO labeling test further confirmed that hydrogenation of CO2 to produce paraffins over Co6/MnOx did not proceed via CO. We also used CH3OH or HCOOH, instead of CO2, to react with H2, but the results were poor (SI Appendix, Table S2, entries 3 and 4). This may be because the dissociative adsorption of formic acid and methanol on the catalyst surface was difficult at the reaction condition, which was necessary for further transformation.
Based on all of the results above, we proposed the possible reaction mechanism. Firstly, CO2 and H2 adsorbed on the Co6/MnOx surface, where the Mn promoter enhanced the CO2 adsorption and weakened the H2 adsorption. Secondly, the adsorbed CO2 was reduced to CH2 and CH3 by H atoms on the catalyst, via CO2δ−, HCOO−, −CH2OH, and/or CH3O− intermediates. The CH2 is known as the principle monomer in FTS reactions, while the CH3 species are common starters for chains to grow (22). Finally, the liquid hydrocarbons were formed by chain growth steps with CH2 and CH3. The largest molecule in the product had 26 C atoms, and the product distribution followed the ASF statistics (α = 0.78), indicating that the chain growth steps are very similar to those of the well-known Co0 catalyzed FTS reaction (22). The presence of Co0 atoms on the Co6/MnOx surface at reaction condition further supported this mechanism. The major mechanistic contribution of this work is direct formation of CH2/CH3 in CO2 hydrogenation, not via CO. Obviously, the parts of the pathway related to FTS are consistent with the well-established Fischer−Tropsch chemistry (e.g., the monomer, the chain growth process, and the ASF distribution of products).
Concluding Remarks
In summary, we discovered a simple Co6/MnOx nanocatalyst that could efficiently catalyze CO2 hydrogenation to normal C5+ hydrocarbons at a lower temperature. Co was the major catalyst, while Mn promoter enhanced CO2 adsorption and weakened H2 adsorption on the catalyst. During the reaction, CO2 adsorbed on the catalyst and was reduced by H atoms to CH2 monomer and CH3 species, via CO2δ−, HCOO−, −CH2OH, and/or CH3O− intermediates. The liquid hydrocarbons were generated by further chain growth steps, which are similar to those of the well-known Co0 catalyzed FTS reaction. The reaction could proceed by avoiding CO, which opens an avenue for liquid fuel synthesis and CO2 transformation.
Materials and Methods
Catalyst Preparation.
The Co−Mn bimetallic catalysts were prepared by a coprecipitation method. In a typical procedure, Co(NO3)2·6H2O (0.873 g, 3.0 mmol) and Mn(NO3)2·4H2O (0.125 g, 0.5 mmol) were dissolved in 40 mL of water and were stirred for 1 h. Then the solution was added into 100 mL of Na2CO3 solution (0.5 mol/L) within 1 h of stirring. After stirring for another 3 h, the solid was centrifuged and washed with 500 mL of water. It was dried at 110 °C for 10 h and calcined at 400 °C for 3 h, and was reduced under hydrogen flow at 400 °C for 1 h. Finally, the catalyst was passivated with a low content of oxygen (e.g., 1%) in N2 at room temperature for 30 min, and Co6/MnOx was obtained. The catalysts with Co/Mn molar ratios of 2, 10, and 14 were also prepared using a similar method. Co6/ZnOx, Co6/AlOx, and Co6/CeOx were also fabricated by the above method, using Co(NO3)2·6H2O to react with Zn(NO3)2·6H2O, Al(NO3)3·9H2O, and Ce(NO3)3·6H2O, respectively. Furthermore, the metallic Co catalyst (Co0) was synthesized by a similar method, during which Co(NO3)2·6H2O (0.873 g, 3.0 mmol) was used as precursor. The source and specification of the chemicals used in this work are given in SI Appendix. The catalysts fabricated above had good air stability.
Catalyst Characterization.
The as-prepared catalysts were characterized by N2 adsorption, XRD, XPS, TEM, ICP-OES, H2-TPR, and TPD. More experimental details are provided in SI Appendix.
In Situ Characterization.
The semi-in situ XPS measurement was aided by a special vacuum seal sample unit to transfer the sample prepared in the glove box. To study the CO2 adsorption, Co6/MnOx was pretreated with 4 MPa of H2 at 200 °C for 2 h. The reactor was cooled, and the H2 was released and replaced with 1 MPa of CO2 three times. Then the reactor was charged with 2 MPa of CO2 and kept at the specified temperature for 1 h. Finally, the reactor was cooled to room temperature, and the gases were released. The sample was prepared in a glove box, and transferred to the sample chamber of an XPS spectrometer using the sample unit. To reduce the loss of adsorbates during the XPS analysis, the sample chamber was kept at ultra-low temperature using liquid nitrogen.
The in situ FTIR spectra were recorded with a Nicolet FTIR Spectrometer 6700 equipped with a liquid-nitrogen-cooled narrow-band mercury–cadmium–telluride detector and diffuse reflectance infrared Fourier transform spectroscopy optics. The details of the apparatus and operation procedure were described elsewhere (23). To get clear spectra, the black Co6/MnOx and KBr powders were mixed and ground together. Then the mixture was pressed into the sample cup. At 200 °C, the sample was firstly purged with N2 for 2 h, then it was treated with H2 for 2 h. After further purging the sample with N2 for 1 h, CO2 was introduced to the sample, and the CO2 adsorption began. All of the above experiments were conducted at ambient pressure. The flow rate of the above gases was 200 mL/min. A background spectrum was recorded before CO2 adsorption. Spectra were collected as difference spectra with the background spectrum. The surface products were shown as positive bands, while loss of surface species was shown as negative bands. In this way, the formation and disappearance of the surface species during the process can be clearly recorded.
CO2 Hydrogenation.
The CO2 hydrogenation was conducted in a 16-mL stainless steel batch reactor with a Teflon lining. In a typical experiment, the catalyst (20 mg) and solvent (1.0 mL) were added in the reactor under air. The reactor was sealed, and the air was removed by flushing with 1 MPa of CO2 three times. Then, 4 MPa of CO2 and 4 MPa of H2 were charged at room temperature. The reaction was performed at 200 °C for the desired time. Then the reactor was cooled in an ice-water bath, and the gas was released slowly and collected for analysis in an HP 4890B GC with thermal conductivity detector. The liquid products were analyzed in an HP 7890B GC with flame ionization detector using toluene as an internal standard. The liquid products were identified using GC-MS (Agilent-7890B-5977A) as well as by comparing the retention times with the standards in the GC traces. The yields of the products were calculated from the GC data. The conversion of CO2 was calculated from the amount of reacted CO2 divided by the total CO2 added to the reactor. The total amount of CO2 charged into the reactor could be obtained by weighing, and the reacted CO2 was known from the amount of total carbon in the product.
The Recycling Test.
The solid catalyst was separated and washed with cyclohexane (5 × 3 mL) and dried under vacuum at 60 °C for 10 h. Then the catalyst was used directly for the next run.
The Hot Filtration Test.
The hot filtration test was conducted at typical reaction conditions. After 6 h, the catalyst was filtered, and the solution was analyzed by GC. The reaction in the resulting solution proceeded for another 9 h, and then the reaction solution was analyzed again by GC.
Supplementary Material
Acknowledgments
We thank Shengrui Tong and Kaihui Xia for the in situ FTIR test; Zhijuan Zhao, Xiaoyu Zhang, and Baolong Qu for XPS analysis; and Yang Sun and Xiang Hao for XRD characterization. This work is supported by the National Natural Science Foundation of China (Grants 21875262, 21533011, 21733011, and 21706152), National Key Research and Development Program of China (Grant 2017YFA0403102), Beijing Municipal Science & Technology Commission (Grant Z181100004218004), and the Chinese Academy of Sciences (Grant QYZDY-SSW-SLH013).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821231116/-/DCSupplemental.
References
- 1.He M., Sun Y., Han B., Green carbon science: Scientific basis for integrating carbon resource processing, utilization, and recycling. Angew. Chem. Int. Ed. Engl. 52, 9620–9633 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Aresta M., Carbon Dioxide as Chemical Feedstock (Wiley-VCH, Weinheim, 2010). [Google Scholar]
- 3.Liu Q., Wu L., Jackstell R., Beller M., Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 6, 5933 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Graciani J., et al. , Catalysis. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 345, 546–550 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Qian Q., Zhang J., Cui M., Han B., Synthesis of acetic acid via methanol hydrocarboxylation with CO2 and H2. Nat. Commun. 7, 11481 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X. B., Tung C. H., Wu L. Z., Semiconducting quantum dots for artificial photosynthesis. Nat. Rev. Chem. 2, 160–173 (2018). [Google Scholar]
- 7.Cano M. H., Agbossou K., Kelouwani S., Dube Y., Experimental evaluation of a power management system for a hybrid renewable energy system with hydrogen production. Renew. Energy 113, 1086–1098 (2017). [Google Scholar]
- 8.Wang W., Wang S., Ma X., Gong J., Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 40, 3703–3727 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Prieto G., Carbon dioxide hydrogenation into higher hydrocarbons and oxygenates: Thermodynamic and kinetic bounds and progress with heterogeneous and homogeneous catalysis. ChemSusChem 10, 1056–1070 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Klankermayer J., Wesselbaum S., Beydoun K., Leitner W., Selective catalytic synthesis using the combination of carbon dioxide and hydrogen: Catalytic chess at the interface of energy and chemistry. Angew. Chem. Int. Ed. Engl. 55, 7296–7343 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Álvarez A., et al. , Challenges in the greener production of formates/formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes. Chem. Rev. 117, 9804–9838 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jessop P. G., Joó F., Tai C. C., Recent advances in the homogeneous hydrogenation of carbon dioxide. Coord. Chem. Rev. 248, 2425–2442 (2004). [Google Scholar]
- 13.He Z. H., et al. , Water-enhanced synthesis of higher alcohols from CO2 hydrogenation over a Pt/Co3O4 catalyst under milder conditions. Angew. Chem. Int. Ed. 55, 737–741 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Wei J., et al. , Directly converting CO2 into a gasoline fuel. Nat. Commun. 8, 15174 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao P., et al. , Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 9, 1019–1024 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Dorner R. W., Hardy D. R., Williams F. W., Willauer H. D., Heterogeneous catalytic CO2 conversion to value-added hydrocarbons. Energy Environ. Sci. 3, 884–890 (2010). [Google Scholar]
- 17.Choi Y. H., et al. , Carbon dioxide Fischer-Tropsch synthesis: A new path to carbon-neutral fuels. Appl. Catal. B 202, 605–610 (2017). [Google Scholar]
- 18.Batley G. E., Ekstrom A., Johnson D. A., Studies of topochemical heterogeneous catalysis: 3. Catalysis of the reduction of metal oxides by hydrogen. J. Catal. 34, 368–375 (1974). [Google Scholar]
- 19.Visconti C. G., et al. , Fischer–Tropsch synthesis on a Co/Al2O3 catalyst with CO2 containing syngas. Appl. Catal. A 355, 61–68 (2009). [Google Scholar]
- 20.Wang L., et al. , Selective hydrogenation of CO2 to ethanol over cobalt catalysts. Angew. Chem. Int. Ed. Engl. 57, 6104–6108 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Zhao Z., et al. , Insight into the formation of Co@Co2C catalysts for direct synthesis of higher alcohols and olefins from syngas. ACS Catal. 8, 228–241 (2018). [Google Scholar]
- 22.Schulz H., Major and minor reactions in Fischer–Tropsch synthesis on cobalt catalysts. Top. Catal. 26, 73–85 (2003). [Google Scholar]
- 23.Tong S. R., Wu L. Y., Ge M. F., Wang W. G., Pu Z. F., Heterogeneous chemistry of monocarboxylic acids on α-Al2O3 at different relative humidities. Atmos. Chem. Phys. 10, 7561–7574 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.