Significance
Temporal and spatial variation in precipitation affect the functional composition of biological communities and ecosystems. Belowground, these changes disrupt the fragile balance between root herbivores, which are major constraints of ecosystem primary production, and their predators. We provide evidence that droughts and deluges alter the functional composition of soil nematode communities depending on the long-term mean annual precipitation (MAP) along a gradient from arid to moist grasslands. The abundance of root-feeding nematodes increased under drought following reductions in the number of predators. These responses increased in magnitude along the MAP gradient, demonstrating that climate change can tip the nematode predator-prey balance and result in higher abundance of root herbivores with potentially important implications for mesic grasslands.
Keywords: climate change, nematodes, soil fauna, precipitation
Abstract
Precipitation changes among years and locations along gradients of mean annual precipitation (MAP). The way those changes interact and affect populations of soil organisms from arid to moist environments remains unknown. Temporal and spatial changes in precipitation could lead to shifts in functional composition of soil communities that are involved in key aspects of ecosystem functioning such as ecosystem primary production and carbon cycling. We experimentally reduced and increased growing-season precipitation for 2 y in field plots at arid, semiarid, and mesic grasslands to investigate temporal and spatial precipitation controls on the abundance and community functional composition of soil nematodes, a hyper-abundant and functionally diverse metazoan in terrestrial ecosystems. We found that total nematode abundance decreased with greater growing-season precipitation following increases in the abundance of predaceous nematodes that consumed and limited the abundance of nematodes lower in the trophic structure, including root feeders. The magnitude of these nematode responses to temporal changes in precipitation increased along the spatial gradient of long-term MAP, and significant effects only occurred at the mesic site. Contrary to the temporal pattern, nematode abundance increased with greater long-term MAP along the spatial gradient from arid to mesic grasslands. The projected increase in the frequency of extreme dry years in mesic grasslands will therefore weaken predation pressure belowground and increase populations of root-feeding nematodes, potentially leading to higher levels of plant infestation and plant damage that would exacerbate the negative effect of drought on ecosystem primary production and C cycling.
Soil food webs play a key role in supporting the provision of ecosystem services in grasslands (1), which cover about one quarter of the Earth’s land. Climate change poses a threat to grassland ecosystem functioning due to, among other factors, changes in rainfall regimes that are projected to increase the occurrence of extreme precipitation events (2, 3). However, our ability to predict how grasslands will respond to these changes is currently restricted by our limited understanding of how more frequent extreme events will impact the trophic structure and food-web interactions in soil communities, which ultimately govern ecosystem processes such as decomposition, nutrient cycling, and carbon cycling (4, 5).
Soil fauna are essential to carbon cycling processes in grasslands (4, 5), where 60–90% of net primary productivity occur belowground as roots (6–8). Nematodes, the most abundant and functionally diverse group of fauna inhabiting grassland soils globally (9), are a major constraint of primary production as their direct and indirect interactions with the root system alter plant uptake of water and nutrients and create abnormalities in root morphology and/or physiology (10). Root-feeding nematodes may consume more plant biomass than do their more conspicuous aboveground counterparts in grasslands (11, 12). They are in turn consumed by carnivorous nematodes that have evolved special features for ingesting nematode prey (including root feeders, bacterivorous and fungivorous nematodes (13)), thus forming a complex trophic structure (14). Although all nematodes rely on soil water for movement and activity, larger sized predators have been suggested to depend more on thicker water films around soil particles for their activity than smaller nematodes in lower trophic groups (15). Thus, predator nematodes may be especially vulnerable to changes in water availability compared with lower trophic levels. Experiments in aquatic and terrestrial environments indicated that abiotic stress has greater effects on higher trophic levels that cascade down to affect lower levels in the trophic pyramid (16, 17). Therefore, quantifying relationships between nematode trophic groups and water availability varying in time and space is of pivotal importance for understanding the fundamental functioning of ecosystems and for predicting how expected precipitation change will affect grassland ecosystems.
Evidence suggests that nematode responses to more extreme precipitation regimes from year to year include changes in community trophic structure that tip the predator-prey balance (18–20). However, these nematode responses to temporal changes in precipitation may vary across different ecosystems since nematode communities from arid grasslands exhibit a resistance to water stress (21–23) not found in mesic grasslands (24). One reason is that moisture reductions in arid environments over short (a few hours) or long periods (days) can induce nematodes to enter anhydrobiosis. In this survival state, nematodes are uncoupled from ecosystem processes until soil moisture becomes favorable for biotic activity again (21, 22). Furthermore, the abundance of topsoil predators in arid ecosystems is very low due to the small primary production that limits the basis of the trophic pyramid (23).
Across spatial gradients, variation in precipitation that occurs among regions has been shown to indirectly structure nematode communities through the positive relationship between long-term mean annual precipitation (MAP) and ecosystem primary production that impacts resource supply for soil food webs (24–26). This spatial MAP–primary productivity relationship is usually much steeper than that of productivity responses to temporal changes in precipitation at one site (27, 28). In space, species composition changes along MAP gradients reflecting long-term evolutionary processes (29). Communities made up of species adapted to long-term specific precipitation patterns have the largest potential to utilize this resource. Through time and in one site, responses to year-to-year changes in precipitation are constrained to mostly physiological adaptations of existing communities and therefore are smaller than responses to changes in MAP along a spatial gradient.
Our aim was to quantify how temporal and spatial changes in precipitation affect nematode trophic groups. We present three hypotheses. (i) Through time and in a single location, total nematode abundance decreases with greater growing-season precipitation due to increased abundance of predators that consume and limit the abundance of nematodes lower in the trophic structure. (ii) The magnitude of these nematode responses to temporal changes in precipitation increases with long-term MAP from deserts to mesic grasslands. There is evidence showing that arid ecosystems have higher disturbance resistance and naturally smaller abundance of nematode predators than moist ecosystems, supporting this hypothesis (23, 24). (iii) Through space and along a gradient of precipitation, total nematode abundance increases with MAP along a spatial gradient accompanying increased resources supply from arid to mesic grasslands. The increase in resources may overshadow the effect of growing predation pressure. Evidence of much larger increases in resource availability (i.e., primary production) across spatial compared with temporal precipitation gradients supports this hypothesis (27, 28), which yields the opposite pattern expected with the temporal gradient. We tested these hypotheses by manipulating growing-season precipitation regimes to develop a temporal gradient of received precipitation in three sites, which are located along a spatial MAP gradient from arid to semiarid and mesic grasslands.
Results
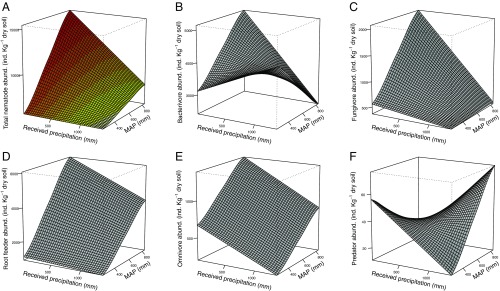
Our results showed divergent responses of nematode populations to spatial and temporal changes in precipitation. Total nematode abundance increased across a spatial gradient of MAP from desert to humid grasslands (Fig. 1A). In contrast, total nematode abundance decreased with the amount of precipitation received in each site resulting from differences in years and treatments. This negative effect of received precipitation on total nematodes was restricted to the highest MAP site, and resulted from the strong negative responses of microbivores and root feeders (Fig. 1 B–D) that were not fully compensated by a positive response of low-abundance predators (Fig. 1F). For all trophic groups, statistical models showed weaker nematode responses to changes in received water at low than at high MAP (Fig. 1). Within-site models confirmed these differential responses of nematodes to precipitation received in different years and treatments depending on the MAP level. We observed negative responses to increasing precipitation of all trophic groups but positive responses of predators at the mesic grassland. Nevertheless, we did not detect any significant effect of received precipitation on nematode populations at both the semiarid and arid grasslands (SI Appendix, Fig. S1).
Fig. 1.
Fitted relationships between nematode population abundance and precipitation in space and time. Changes in precipitation through space (z axis), are represented by the mean annual precipitation (MAP) across three sites. Changes in precipitation through time within sites (x axis, Received precipitation) resulted from a combination of variability between years and rainfall manipulation treatments. (A) Total abundance of nematodes (PReceived precip. = 0.0507, PMAP = 0.002, PInteraction = 0.008, r2 = 67.85%); (B) bacterivores (PReceived precip. = 0.001, PMAP = 0.486, PInteraction = 0.014, r2 = 67.17%); (C) fungivores (PReceived precip. = 0.070, PMAP < 0.001, PInteraction = 0.079, r2 = 66.43%); (D) root feeders (PReceived precip. < 0.001, PMAP < 0.001, PInteraction = 0.005, r2 = 50.62%); (E) omnivores (PReceived precip. = 0.059, PMAP < 0.001, PInteraction = 0.909, r2 = 14.13%); and (F) predators (PReceived precip. = 0.004, PMAP = 0.447, PInteraction = 0.607, r2 = 98.66%) explained by received growing-season precipitation and long-term MAP. For all tests n = 240.
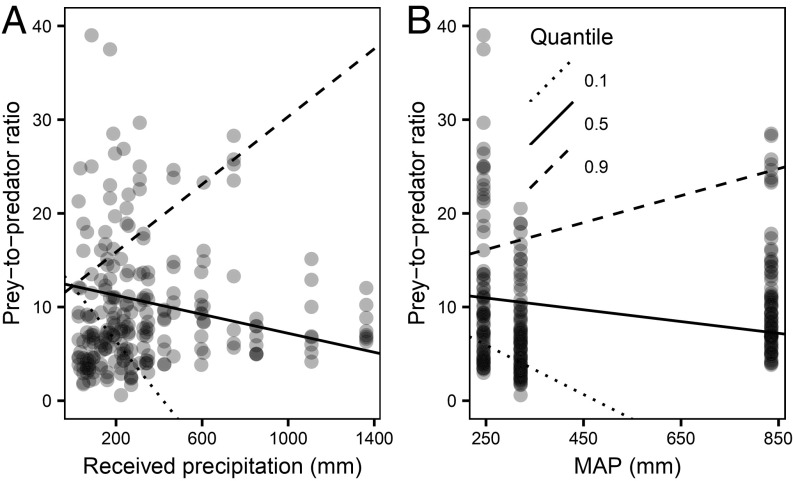
The slope describing the relationship between received precipitation in each site and number of nematode preys per predator varied among the different quantiles of the prey-to-predator ratio (Fig. 2A). Negative effects of received precipitation were revealed at medium to lower prey-to-predator ratios associated with the center and lower quantiles (slope τ0.1 = −0.03, pτ0.1 < 0.001, slope τ0.5 = −0.005, pτ0.5 = 0.009), and a positive effect occurred at higher ratios associated to the upper quantile (slopeτ0.9 = 0.02, pτ0.9 < 0.001). In other words, greater received precipitation decreased the number of preys per predator in communities with medium to higher proportional abundances of predator nematodes (lower ratios), as those observed at more mesic environments (Fig. 1 and SI Appendix, Fig. S1). Conversely, in communities with low proportional abundances of predator nematodes (higher prey-to-predator ratios), such as found in arid environments (Fig. 1 and SI Appendix, Fig. S1), precipitation increased the number of preys per predator. When fitting received precipitation onto the ordination plot (Fig. 3A), predators were linked to greater received growing-season precipitation at the highest MAP site (npMANOVA: F = 9.07, r2 = 10.54%, P = 0.001).
Fig. 2.
Number of nematode prey (bacterivorous, fungivorous, and root-feeding nematodes) per predator (predaceous and omnivorous nematodes) by precipitation levels. (A) Prey-to-predator ratio as a function of received growing-season precipitation for all of the sites combined (n = 240). (B) Prey-to-predator ratio as a function of long-term mean annual precipitation (MAP) across a regional gradient (n = 240). Solid trend lines track the center of the distribution (50th percentiles) of the relationship between precipitation regressors and prey-to-predator ratio estimated by mixed-effect quantile estimates (Received precipitation: slope τ0.5 = −0.005, pτ0.5 = 0.009; MAP: slope τ0.5 = −0.006, pτ0.5 = 0.006). Dotted and dashed trend lines represent the 10th (Received precipitation: slope τ0.1 = −0.03, pτ0.1 < 0.001; MAP: slope τ0.1 = −0.03, pτ0.1 < 0.001) and 90th percentiles (Received precipitation: slopeτ0.9 = 0.02, pτ0.9 < 0.001; MAP: slope τ0.9 = 0.014, pτ0.9 < 0.001) of the distribution, respectively.
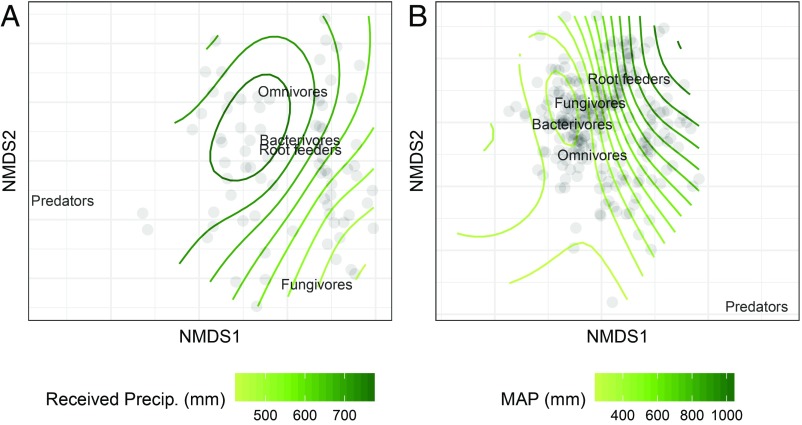
Fig. 3.
Nematode community trophic composition across precipitation gradients. (A) Nonmetric multidimensional scaling plot of nematode trophic groups as a function of received growing-season precipitation at the mesic site (n = 80) (Bray–Curtis). (B) Nonmetric multidimensional scaling plot of nematode trophic groups as a function of the long-term mean annual precipitation (MAP, n = 240) (Bray–Curtis).
Combined, these results provide strong evidence that lower and higher nematode trophic levels inversely respond to temporal changes in received growing-season precipitation, and that the magnitude of these responses increases spatially with greater long-term MAP. The observed increase in total nematode abundance along a regional MAP gradient was driven by positive responses across all nematode trophic groups (Fig. 1), with stronger statistical support for fungivores (P < 0.001), root feeders (P < 0.001), and omnivores (P < 0.001). Despite this positive MAP effect on trophic groups, the number of preys per predator was negatively related to the regional MAP gradient (Fig. 2B), indicating a proportionally greater positive effect of increasing MAP on predators compared with lower trophic levels. The trophic structure of nematode communities changed along the MAP gradient (npMANOVA: F = 242.23, r2 = 50.41%, P = 0.001). When performing environmental fitting of MAP onto the ordination plot, root feeders were associated with higher MAP and omnivores to lower MAP values (Fig. 3B).
Discussion
As hypothesized, total nematode abundance decreased with greater received growing-season precipitation. However, this negative effect of received precipitation only occurred at higher MAP levels and became null at the lower end of the regional MAP gradient. These results aligned with our first and second hypotheses and resulted from the negative effects of precipitation, received in each year and treatment, on the abundance of all nematode trophic groups, except for predators. These were the least abundant feeding groups overall and responded positively to increases in received precipitation at high MAP. Therefore, temporal variations in water availability in more mesic grasslands inversely affected the abundance at lower and higher trophic levels. A possible explanation is that increased moisture strengthened top-down control by carnivorous nematodes on root feeder and microbivore populations. This phenomenon occurred only in mesic ecosystems, where predators are naturally more numerous and positively affected by greater growing-season precipitation. Analysis of the effects of received precipitation on nematode prey-to-predator ratios showed that the slope of this relationship varied from positive (for nematode communities with fewer predators) to negative (for communities with medium to high predator density). These results suggest that increases in precipitation through time in more mesic grassland ecosystems, where predators are naturally more numerous, constrain root feeders and microbivores by promoting their nematode consumers. Predaceous nematodes are known to be highly responsive to environmental changes (24, 30). In contrast to mesic environments, these findings indicate a less influential role of predation on the mechanism through which water availability controls nematode communities in xeric environments, suggesting that in drier ecosystems populations of nematodes are predominantly controlled by resource availability rather than predation.
In contrast with the temporal pattern, the abundance of nematodes increased with greater long-term MAP along a regional gradient from arid to mesic grasslands. This is in agreement with studies showing increased densities of soil nematodes in response to higher MAP at regional to continental scales (24–26, 31). Evidence shows that, at these large spatial scales, climate characteristics such as precipitation become more important than inherent properties of the soil substrate in explaining variations in soil fauna communities that occur across ecosystem types (32, 33). Water availability directly controls nematode movement and reproduction by limiting the soil water films on which nematodes depend (21), and indirectly affects community size and structure by controlling root production and carbon availability at the base of the food web (18). MAP is strongly correlated with net primary production (27), and the slopes of this spatial relationship are usually considerably steeper than those of temporal precipitation-productivity relationships (27, 28). Up to two-thirds of the increase in ecosystem primary production along spatial MAP gradients has been attributed to differences in long-term adapted plant communities and not to direct responses to increased precipitation (34), whereas vegetation does not differ appreciably over time in temporal gradients. Therefore, the total input of energy and organic matter for nematode populations increase more along MAP gradients than along temporal precipitation gradients. Higher in the trophic chain, these direct and indirect MAP effects resonate through the population of predaceous nematode species. In our study, root feeders showed the strongest positive response to MAP in absolute terms, becoming the dominant feeding group at the highest MAP levels (Figs. 1D and 3A), whereas the positive response of predators was proportionally stronger than that of lower trophic levels (Fig. 2B). These patterns are consistent with findings of a previous cross-site study (24). Therefore, we argue that the amelioration of microhabitat conditions and resource availability account for the positive effect of long-term MAP on the abundance of all nematode trophic groups.
Projections point to more extreme precipitation regimes caused by global warming and amplification of the hydrological cycle (35). Our data indicate that, under extreme dry conditions, declines in the population of predator nematodes in mesic grasslands will reduce predation of root-feeding nematodes and increase their abundance in soil. In contrast, in xeric ecosystems, responses of nematode communities to changes in water availability will be weaker.
This interaction among temporal and spatial changes in precipitation structuring nematode communities has not been previously reported and advances our understanding of water controls on nematodes. This is likely to have ecosystem-wide repercussions, because root herbivory by soil nematodes can be a major factor controlling overall net biomass production in grasslands (10, 11). Low levels of root herbivory associated to the feeding activity of microbivores may increase soil nutrient concentration and root growth in grasses (36, 37). However, increased populations of root-feeding nematodes lead to high levels of plant infestation and plant damage that reduce 43–88% of belowground biomass (38), a major compartment of net primary production in grasslands that may become even more important for carbon storage with more extreme droughts (39–41). Thus, the direct effect of drought in mesic grasslands will be enhanced by the indirect effect through changes in the nematode food-web structure. Although other soil biota, not considered here, may also play an important role in determining ecosystem-level responses to changes in precipitation, our results suggest that weakened predation pressure and increased populations of root feeders in response to reduced water availability can potentially enhance the negative effect of drought on ecosystem primary production and C cycling. These unanticipated consequences of climate change for grasslands may challenge the predictions of increasing plant biomass allocation belowground in mesic ecosystems under drought.
Materials and Methods
Site Descriptions and Experimental Design.
We experimentally manipulated growing-season precipitation regimes in 2016 and 2017 in North American desert grassland, semiarid shortgrass steppe, and mesic tallgrass prairie, and analyzed the trophic structure of soil nematode communities in each of the experimental years. The sites span a regional precipitation gradient along the Great Plains’ grassland biome and vary in other climatic characteristics, soil types, and vegetation composition (Table 1). The arid site is a desert grassland in the Jornada Basin Long-Term Ecological Research (LTER) site in Southern New Mexico. In 2016 and 2017 the site received 212 and 290 mm of precipitation, respectively. The long-term mean annual precipitation is 245 mm. The semiarid site is a shortgrass steppe located in Northern Colorado at the Semiarid Grasslands Research Center, formerly Shortgrass Steppe LTER. Mean annual precipitation is 321 mm, and the site received 207 and 291 mm in 2016 and 2017, respectively. The mesic site, a tallgrass prairie, was located in Eastern Kansas at the Konza Prairie LTER. Average annual precipitation is 835 mm, with 991 and 726 mm in 2016 and 2017, respectively. We carried out all experiments in areas protected from cattle grazing. See Table 1 for detailed information about each site.
Table 1.
Site characteristics for the Jornada Basin LTER, NM (Arid), Semiarid Grasslands Research Center, CO (Semiarid), and Konza Prairie LTER, KS (Mesic)
| Site characteristic | Arid | Semiarid | Mesic |
| Geographic location | |||
| Latitude | 32°33′N | 40°50′N | 39°4′N |
| Longitude | 106°49′W | 104°45′W | 96°34′W |
| Ecosystem type | Desert grassland | Semiarid shortgrass | Mesic tallgrass |
| Climate | |||
| MAP (mm)a | 245 | 321 | 835 |
| Mean growing season precipitation (mm)a | 105 | 204 | 428 |
| MAT (°C)a | 14.7 | 8.4 | 12.5 |
| Soil | |||
| Textureb | Fine sandy loam | Fine sandy loam | Silty clay loam |
| Soil moisture (%)c | 0.42–3.00 | 1.51–4.65 | 14.39–32.81 |
| Vegetation | |||
| ANPP (g m−2 year−1) | 201.1 | 52.8 | 382.5 |
| Dominant species | Black grama (Bouteloua eriopoda; Torr.) | Blue grama (Bouteloua gracilis; (Willd. Ex. Kunth) Lag. Ex Griffiths) | Big bluestem (Andropogon gerardii; Vitman) |
| Little bluestem (A. scoparius; | |||
| (Michx.) Nash) | |||
| Yellow indiangrass (Sorghastrum nutans; (L.) Nash) |
Obtained from NOAA climate data from Las Cruces, NM, Nunn, CO, and Manhattan, KS.
Obtained from Soil Survey USDA (https://websoilsurvey.sc.egov.usda.gov/App/WebSoilSurvey.aspx).
Range of soil moisture values reflect measurements taken at the two sampling events in 2016 and 2017.
At each site, experimental plots were established in a relatively flat area of ∼0.3 ha where the composition of vegetation represented that of the larger ecosystem. We manipulated rainfall by using rainout shelters that intercept the incoming precipitation that drained into a temporary storage tank, and automatically transferred the water to irrigation plots through a solar-powered pumping system (42). We imposed five levels of growing-season precipitation manipulation: large and small water reduction, large and small water addition, and an ambient control. These precipitation levels correspond to the first and 10th percentile of long-term precipitation for the rainfall reduction treatments and to the 90th and 99th percentile of long-term precipitation for the rainfall addition treatments at each site following current recommendations for manipulative experiments (43). Therefore, we reduced incoming precipitation relative to the control by 80% and 50% in the arid site, by 70 and 40% in the semiarid site and 60 and 30% in the mesic site. We added precipitation simulating an increase of 150% and 180%, 140 and 170% and 130 and 160% in those three sites. In this way, treatments across sites had drought/deluges that were similar relative to their historic record although the percentages of precipitation added and subtracted varied (see ref. 44 for the rationale of the design). We had rain gauges adjacent to each site where we were manipulating precipitation. We calculated growing-season received precipitation in each treatment by multiplying incoming precipitation by the percent reduction or addition associated with each treatment. We had eight replicates at each site, totaling 40 plots of 5 m by 2.5 m size per site. Treatments were randomly allocated to plots, which were at least 5 m apart. The automated rainfall manipulation system was in place from April to September in both experimental years.
Soil Collection.
We collected soil samples at the end of the 2016 and 2017 growing seasons. Within each experimental plot, we used a 2.5-cm diameter soil corer to collect four subsamples (one in each of the plot’s quadrants) from the top 10 cm soil from directly beneath the dominant vegetation type, cleaning all equipment with alcohol wipes between plots to avoid cross-contamination. We pooled the subsamples together to form one composite sample per plot and mixed soil gently. The same plots and sampling scheme were used in both years, avoiding the previous year disturbance on the second sampling event.
Bags containing the soil samples were immediately placed inside coolers containing ice packets for transportation, returned to laboratories at Colorado State University, stored at 4 °C, and soil nematodes were extracted within 2 d.
Nematode Extraction and Counting.
Nematodes in soil samples were extracted from 100-g soil aliquots using Baermann funnels (45), from which nematodes in water were removed daily for 3 d, and stored at 4 °C. Nematodes were counted and identified using an inverted microscope (Olympus CKX41, 200× magnification) within 5 d of extraction to five different trophic groups: bacterivores, fungivores, root feeders, omnivores, and predators (13). Standardized nematode population abundances were calculated as number of individuals per kg of soil (corrected to oven-dried weight equivalent). Gravimetric soil water content (wt/wt) and oven-dry weight equivalents were determined for each sample from mass loss of soils heated to 105 °C for 72 h.
Statistical Analyses.
We generated statistical models for the abundance of total nematodes and each individual trophic group using received growing-season precipitation, long-term MAP, and their interaction. We used linear mixed effects models with a plot-level random effect term to account for the interdependency that stems from having repeated measurements per plot. Residual variance of abundance differed between MAP levels (i.e., sites), but this was accounted for using a special variance structure (R function “varIdent” in nlme library (46)). For each model, the conditional r2 (that of the whole model, including the random effect) was calculated following Nakagawa and Schielzeth (47). For the predator nematode models, we accounted for a large number of zero counts and over-dispersion by using a quasi-Poisson distribution.
We also examined the effects of precipitation on the ratio of preys (the sum of root-feeding, bacterivorous, and fungivorous nematodes) to predator (the sum of omnivores and predators) as a proxy for presumed top-down pressure on the nematode population. We categorized omnivores as predators for this analysis because many species from the order Dorylaimida are known to feed on nematodes (13). To test whether MAP and received growing-season precipitation affected the upper, medium, and lower limits of the prey-to-predator ratio, we used a mixed-effect modification of quantile regression (48, 49) with 10%, 50%, and 90% quantiles of the prey-to-predator ratio as the response variable.
To visualize major patterns structuring the nematode community, we performed ordination on group composition with nonmetric multidimensional scaling (NMDS), using Bray-Curtis dissimilarity matrix of the nematode trophic structure data, on which we overlaid MAP data to explore the role of annual precipitation. We then tested for the effects of MAP by running nonparametric multivariate analysis of variance (npMANOVA) on the dissimilarity matrix. The same procedure was followed to visualize and test the effects of received precipitation on community structure, but in this case we limited the analyses to the highest MAP level (mesic site) since the univariate analyses had revealed no effects of received precipitation at other MAP levels.
All analyses were conducted using the software R, version 3.2.2 (50), and packages nlme (51), piecewiseSEM (52), lqmm (49), vegan (53), and ggplot2 (54).
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation under grant number DEB-1456631 and DEB-1456597 to D.H.W. and O.E.S. This work was also supported by DEB-1235828. We thank the staff at the Jornada Basin LTER, Semi-arid Grassland Research Center, Central Plains Experimental Range, and Konza Prairie Biological Station for site-based assistance.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1900572116/-/DCSupplemental.
References
- 1.de Vries F. T., et al. , Soil food web properties explain ecosystem services across European land use systems. Proc. Natl. Acad. Sci. U.S.A. 110, 14296–14301 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fay P. A., Kaufman D. M., Nippert J. B., Carlisle J. D., Harper C. W., Changes in grassland ecosystem function due to extreme rainfall events: Implications for responses to climate change. Glob. Change Biol. 14, 1600–1608 (2008). [Google Scholar]
- 3.Gherardi L. A., Sala O. E., Enhanced precipitation variability decreases grass- and increases shrub-productivity. Proc. Natl. Acad. Sci. U.S.A. 112, 12735–12740 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford M., et al. , Impacts of soil faunal community composition on model grassland ecosystems. Science 298, 615–618 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Wagg C., Bender S. F., Widmer F., van der Heijden M. G. A., Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. U.S.A. 111, 5266–5270 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Titlyanova A. A., Romanova I. P., Kosykh N. P., Mironycheva-Tokarev N., Pattern and process in above-ground and below-ground components of grassland ecosystems. J. Veg. Sci. 10, 307–320 (1999). [Google Scholar]
- 7.Sims P., Singh J., The structure and function of ten western North American grasslands: III. Net primary production, turnover and efficiencies of energy capture and water use. J. Ecol. 66, 573–597 (1978). [Google Scholar]
- 8.Potter C. S., et al. , Terrestrial ecosystem production: A process model based on global satellite and surface data. Global Biogeochem. Cycles 7, 811–841 (1993). [Google Scholar]
- 9.Bardgett R. D., Cook R., Yeates G. W., Denton C. S., The influence of nematodes on below-ground processes in grassland ecosystems. Plant Soil 212, 23–33 (1999). [Google Scholar]
- 10.Neher D. A., Ecology of plant and free-living nematodes in natural and agricultural soil. Annu. Rev. Phytopathol. 48, 371–394 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Ingham R. E., Detling J. K., Effects of root-feeding nematodes on aboveground net primary production in a North American grassland. Plant Soil 121, 279–281 (1990). [Google Scholar]
- 12.Stanton N. L., The underground in grasslands. Annu. Rev. Ecol. Syst. 19, 573–589 (1988). [Google Scholar]
- 13.Yeates G. W., Bongers T., De Goede R. G. M., Freckman D. W., Georgieva S. S., Feeding habits in soil nematode families and genera-an outline for soil ecologists. J. Nematol. 25, 315–331 (1993). [PMC free article] [PubMed] [Google Scholar]
- 14.Yeates G. W., Wardle D. A., Nematodes as predators and prey: Relationships to biological control and soil processes. Pedobiologia (Jena) 40, 43–50 (1996). [Google Scholar]
- 15.Wallace H. R., The dynamics of nematode movement. Annu. Rev. Phytopathol. 6, 91–114 (1968). [Google Scholar]
- 16.Menge B. A., Sutherland J. P., Community regulation: Variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Am. Nat. 130, 730–757 (1987). [Google Scholar]
- 17.Preisser E. L., Strong D. R., Climate affects predator control of an herbivore outbreak. Am. Nat. 163, 754–762 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Todd T., Blair J., Milliken G., Effects of altered soil-water availability on a tallgrass prairie nematode community. Appl. Soil Ecol. 13, 45–55 (1999). [Google Scholar]
- 19.Stevnbak K., et al. , Suppression of soil decomposers and promotion of long-lived, root herbivorous nematodes by climate change. Eur. J. Soil Biol. 52, 1–7 (2012). [Google Scholar]
- 20.Ruan W., et al. , The response of soil nematode community to nitrogen, water, and grazing history in the inner Mongolian steppe, China. Ecosystems (N. Y.) 15, 1121–1133 (2012). [Google Scholar]
- 21.Freckman D. W., Whitford W. G., Y. Steinberger, Effect of irrigation on nematode population dynamics and activity in desert soils. Biol Fertil Soils 3–3, 3–10 (1987). [Google Scholar]
- 22.Moorhead D. L., Freckman D. W., Reynolds J. F., Whitford W. G., A simulation- model of soil nematode population-dynamics–Effects of moisture and temperature. Pedobiologia (Jena) 30, 361–372 (1987). [Google Scholar]
- 23.Vandegehuchte M. L., et al. , Responses of a desert nematode community to changes in water availability. Ecosphere 6, art44 (2015). [Google Scholar]
- 24.Sylvain Z. A., et al. , Soil animal responses to moisture availability are largely scale, not ecosystem dependent: Insight from a cross-site study. Glob. Change Biol. 20, 2631–2643 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Nielsen U. N., et al. , Global-scale patterns of assemblage structure of soil nematodes in relation to climate and ecosystem properties. Glob. Ecol. Biogeogr. 23, 968–978 (2014). [Google Scholar]
- 26.Chen D., et al. , Regional-scale patterns of soil microbes and nematodes across grasslands on the Mongolian plateau: Relationships with climate, soil, and plants. Ecography 38, 622–631 (2015). [Google Scholar]
- 27.Sala O. E., Gherardi L. A., Reichmann L., Jobbágy E., Peters D., Legacies of precipitation fluctuations on primary production: Theory and data synthesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 3135–3144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huxman T. E., et al. , Convergence across biomes to a common rain-use efficiency. Nature 429, 651–654 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Lauenroth W. K., Sala O. E., Long-term forage production of North American shortgrass steppe. Ecol. Appl. 2, 397–403 (1992). [DOI] [PubMed] [Google Scholar]
- 30.Franco A. L. C., et al. , Nematode exclusion and recovery in experimental soil microcosms. Soil Biol. Biochem. 108, 78–83 (2017). [Google Scholar]
- 31.Song D., et al. , Large-scale patterns of distribution and diversity of terrestrial nematodes. Appl. Soil Ecol. 114, 161–169 (2017). [Google Scholar]
- 32.Ettema C., Wardle D. A., Spatial soil ecology. Trends Ecol. Evol. 17, 177–183 (2002). [Google Scholar]
- 33.Blankinship J. C., Niklaus P. A., Hungate B. A., A meta-analysis of responses of soil biota to global change. Oecologia 165, 553–565 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Gaitán J. J., et al. , Vegetation structure is as important as climate for explaining ecosystem function across Patagonian rangelands. J. Ecol. 102, 1419–1428 (2014). [Google Scholar]
- 35.Knapp A. K., et al. , Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience 58, 811–821 (2008). [Google Scholar]
- 36.Gebremikael M. T., Steel H., Buchan D., Bert W., De Neve S., Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions. Sci. Rep. 6, 32862 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bardgett R. D., Denton C. S., Cook R., Below-ground herbivory promotes soil nutrient transfer and root growth in grassland. Ecol. Lett. 2, 357–360 (1999). [Google Scholar]
- 38.Scott J., French N., Leetham J., “Patterns of consumption in grasslands” in Perspectives in Grassland Ecology, French N., Ed. (Springer, New York, 1979), pp. 89–105. [Google Scholar]
- 39.Hui D., Jackson R. B., Geographical and interannual variability in biomass partitioning in grassland ecosystems: A synthesis of field data. New Phytol. 169, 85–93 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Xu X., Sherry R. A., Niu S., Li D., Luo Y., Net primary productivity and rain-use efficiency as affected by warming, altered precipitation, and clipping in a mixed-grass prairie. Glob. Change Biol. 19, 2753–2764 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Poorter H., Nagel O., The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant Physiol. 27, 1191 (2000). [Google Scholar]
- 42.Gherardi L. A., Sala O. E., Automated rainfall manipulation system: A reliable and inexpensive tool for ecologists. Ecosphere 4, art18 (2013). [Google Scholar]
- 43.Knapp A. K., et al. , Characterizing differences in precipitation regimes of extreme wet and dry years: Implications for climate change experiments. Glob. Change Biol. 21, 2624–2633 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Knapp A. K., et al. , Pushing precipitation to the extremes in distributed experiments: Recommendations for simulating wet and dry years. Glob. Change Biol. 23, 1774–1782 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Hooper D. J. “Extraction of free-living stages from soil” in Laboratory Methods for Work with Plant and Soil Nematodes, Ed. Southey JF. (Ministery of Agriculture, Fisheries and Food, London, ed. 6, 1970), pp. 5–30. [Google Scholar]
- 46.Pinheiro J., Bates D., DebRoy S., Sarkar D, nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3, 1-117. https://cran.r-project.org/web/packages/nlme/citation.html. Accessed 4 June 2019.
- 47.Nakagawa S., Schielzeth H., A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142 (2013). [Google Scholar]
- 48.S Cade B.., Noon B. R., A gentle introduction to quantile regression for ecologists. Front. Ecol. Environ. 1, 412–420 (2003). [Google Scholar]
- 49.Geraci M., Linear quantile mixed models: The lqmm package for laplace quantile regression. J. Stat. Softw. 57, 1–29 (2014).25400517 [Google Scholar]
- 50.R Core Team , R: A Language and Environment for Statistical Computing (Version 3.1.2, R Foundation for Statistical Computing, Vienna, Austria, 2014).
- 51.Bates D., Maechler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 52.Bartoń K., MuMIn: Multi-Model Inference. R package Version 1.40.0. https://cran.r-project.org/package=MuMIn. Accessed 4 June 2019.
- 53.Oksanen J., et al. , Community Ecology Package. R package Version 2.3-4. https://cran.r-project.org/package=vegan. Accessed 4 June 2019.
- 54.Wickham H., ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag, New York, 2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.