Significance
The evolutionary origins of novel traits that define animal phyla have puzzled biologists for centuries. The possible antecedent of one such novelty that draws extensive discussion is that of the chordate central nervous system, which exists in a dorsoventrally inverse orientation relative to other bilaterally symmetric animals. Studies of chordates’ closest relatives, hemichordates and echinoderms, may provide insight into chordate origins. Using hemichordate and sea urchin larvae, we found that BMP signaling controls dorsoventral and neural patterning. Furthermore, alterations of BMP levels result in pronounced morphological changes reminiscent of a proposed intermediate stage in the emergence of the chordate body plan. Thus, our study provides an example of how molecular tinkering may lead to evolution of phylum-level novelties.
Keywords: chordate origins, indirect-developing hemichordates, dorsoventral patterning, neural patterning, BMP
Abstract
A defining feature of chordates is the unique presence of a dorsal hollow neural tube that forms by internalization of the ectodermal neural plate specified via inhibition of BMP signaling during gastrulation. While BMP controls dorsoventral (DV) patterning across diverse bilaterians, the BMP-active side is ventral in chordates and dorsal in many other bilaterians. How this phylum-specific DV inversion occurs and whether it is coupled to the emergence of the dorsal neural plate are unknown. Here we explore these questions by investigating an indirect-developing enteropneust from the hemichordate phylum, which together with echinoderms form a sister group of the chordates. We found that in the hemichordate larva, BMP signaling is required for DV patterning and is sufficient to repress neurogenesis. We also found that transient overactivation of BMP signaling during gastrulation concomitantly blocked mouth formation and centralized the nervous system to the ventral ectoderm in both hemichordate and sea urchin larvae. Moreover, this mouthless, neurogenic ventral ectoderm displayed a medial-to-lateral organization similar to that of the chordate neural plate. Thus, indirect-developing deuterostomes use BMP signaling in DV and neural patterning, and an elevated BMP level during gastrulation drives pronounced morphological changes reminiscent of a DV inversion. These findings provide a mechanistic basis to support the hypothesis that an inverse chordate body plan emerged from an indirect-developing ancestor by tinkering with BMP signaling.
In 1977, Jacob proposed that evolution does not produce novelties from scratch but instead operates by tinkering with what already exists (1). Specific mechanisms of how molecular tinkering might produce phylum-level body plan novelties remain largely unknown, however. The phylum Chordata is unique in possessing a dorsally located central nervous system (CNS) consisting of a hollow neural tube that is initially specified as a neural plate in the dorsal ectoderm (2, 3). Specification of the chordate neural plate is intimately linked to dorsoventral (DV) patterning, as both these processes depend on the dorsal inhibition of BMP signaling by antagonists, such as Chordin, which are secreted from the organizer during gastrulation (4, 5). BMP signaling is a conserved mechanism for DV patterning across diverse bilaterians (6); however, in chordates, BMP activity is high on the ventral side, where the mouth is located, while in other bilaterians, BMP signaling is active on the dorsal side (7, 8). This difference suggests that a DV axis inversion occurred in the lineage leading to the chordates (2), but the mechanistic basis for the inversion and its possible links to the evolutionary origin of the dorsal CNS remain elusive.
Chordates belong to deuterostomes, within which the two other phyla, Echinodermata and Hemichordata, form a clade called Ambulacraria, the closest group to the chordates (9). Thus, echinoderms and hemichordates may exhibit ancestral features that are useful for understanding chordate origins (Fig. 1A). Echinoderms have evolved a unique pentaradially symmetric body, although the larvae remain bilaterally symmetric (10). DV patterning of echinoderm sea urchin embryos is similar to that of other nonchordate bilaterians, depending on high BMP activity on the dorsal side (11). Ectopic activation of BMP signaling suppresses neurogenesis along the ciliary band (12), an embryonic ectodermal region positioned between the dorsal and ventral ectoderm (13). On the other hand, hemichordates retain a bilaterally symmetric body throughout their lives, and the adults have dorsal and ventral solid nerve cords, in addition to an elaborated epidermal nerve net that is concentrated anteriorly (14).
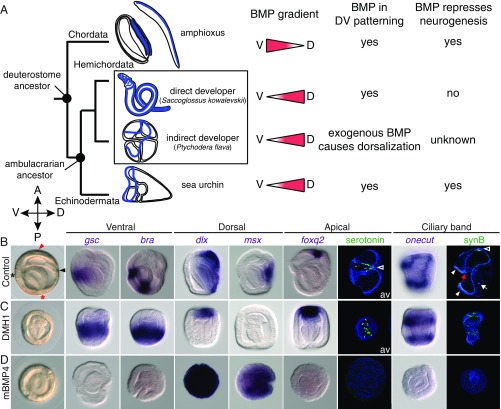
Fig. 1.
BMP controls DV patterning and represses neurogenesis in the indirect-developing hemichordate. (A) Deuterostome phylogeny shows nervous systems (blue) of Chordata (amphioxus embryo and adult as representatives), direct- and indirect-developing hemichordates, and the indirect-developing sea urchin embryo. Directions of BMP gradients (red triangles) along the dorsal (D) and ventral (V) axes and their roles in DV patterning and neural development are indicated for each lineage. (B–D) Controls cultured with DMSO or 125 ng/mL BSA (B), and embryos treated with 2 μM DMH1 (C) or 125 ng/mL mBMP4 (D). The treatments were performed after fertilization, and the embryos were fixed at the gastrula stage for in situ hybridization with ventral, dorsal, apical, and ciliary band marker genes. Except the tornaria larvae stained for serotonin, which are viewed from the apical surface (av), all other images are side views, with the directions of the anterior (A), posterior (P), dorsal (D), and ventral (V) axes indicated. Positions of the mouth (black asterisk), the anus (red asterisk), the hydropore opening from the dorsally extended mesoderm (black arrowhead), and the apical region (red arrowhead) are indicated in the control gastrula. The nervous system of tornaria larva includes serotonergic neurons in the apical ectoderm (empty arrowheads) and the neurons distributed along the ciliary band (white arrowheads). Some neuronal cells are also found in the pharynx (red arrow) and near the sphincter between the midgut and hindgut (white arrow). Neurons in the apical ectoderm and the ciliary band were visualized at the tornaria stage by immunostaining with anti-serotonin and anti-synB antibodies, respectively. The data represent the phenotypes of most samples (>95%).
The possible homology between the chordate neural tube and the hemichordate adult nervous system has been discussed extensively. A study of the direct-developing hemichordate Saccoglossus kowalevskii revealed that the opposing expression of BMP on the dorsal midline and Chordin on the ventral midline regulates DV patterning (15). However, neural gene expression is not repressed by exogenous BMP treatments, suggesting that BMP signaling is not involved in the initial segregation of epidermal and neural ectoderm in this organism, although BMP may still pattern neural cell fates within its diffuse nervous system (15). This result suggests the possibility that centralization of the nervous system was evolved in the chordate lineage, leaving uncertainty as to whether the hemichordate adult nervous system could be homologous to the chordate CNS.
While direct-developing hemichordates develop directly into juveniles that resemble the adult form, embryos of indirect-developing hemichordates develop through tornaria larval stages that are morphologically similar to those of echinoderm larvae with a ciliary band nervous system (10, 14). Due to this similarity, in 1894, Garstang proposed that chordates may have evolved from the dipleurula-type larva, with fusion of the ciliary bands at the dorsal midline creating the characteristic neural tube of the chordates (16). His evolutionary scenario was modified and elaborated several times in the 1920s, arriving at the idea that the ancestral dipleurula larva metamorphosed into a sessile adult, similar to an adult ascidian (17). Garstang’s hypothesis has been disputed because it is not supported by modern molecular data suggesting that a DV inversion occurred in the chordate ancestor (3, 18, 19). Moreover, molecular phylogenomic data indicate that amphioxus, but not tunicates, occupies the basal position of chordates, suggesting that the chordate ancestor was motile rather than sedentary (20, 21).
To incorporate DV inversion into the chordate evolutionary model, Nielsen modified Garstang’s 1894 idea to propose that instead of moving dorsally, the ciliary bands fused ventrally to form the chordate neural tube; coincidently, the mouth would have moved dorsally, allowing the dipleurula larva to evolve into chordates (19). This modified hypothesis has been unexamined for 20 y, and it is unclear what specific changes in developmental programs would be required to drive chordate evolution from an indirect-developing ancestor.
To explore the Garstang/Nielsen hypothesis and gain insight into the origins of the DV inversion and the CNS in the chordate lineage, we investigated the DV and neural patterning mechanisms of an indirect-developing hemichordate, Ptychodera flava. We discovered that in P. flava, similar to chordates and echinoderms, BMP signaling controls DV patterning and represses neurogenesis. Furthermore, transient overactivation of BMP signaling during gastrulation blocks mouth formation and centralizes the nervous system to the ventral ectoderm in both hemichordate and sea urchin larvae. These morphological changes are similar to a proposed intermediate stage leading to the emergence of chordates with a dorsally located CNS in an inverse DV body axis relative to their ancestors. Moreover, the resulting mouthless neurogenic ventral ectoderm displays a medial-to-lateral organization similar to that of the chordate neural plate. These findings strongly suggest that the deuterostome ancestor was an indirect developer, and that tinkering with BMP signals is sufficient to drive morphological changes that might have led to the emergence of chordates. This study represents an example of how molecular tinkering may account for the evolutionary origins of novelties, and our results provide important insight into the possible origin of chordates.
Results
BMP Signaling Controls DV Patterning and Represses Neurogenesis in P. flava.
As early as the blastula stage of the P. flava embryo, expression of chordin in the ventral ectoderm is opposite the dorsal BMP-active domain, as monitored by immunostaining with an antibody against phospho-Smad1/5/8, a BMP signaling downstream effector (SI Appendix, Fig. S1A). Previous studies in P. flava also revealed that the bmp2/4 and chordin genes are expressed in the dorsal and ventral ectoderm, respectively, and that ectopic BMP signaling is sufficient to dorsalize the embryo (22, 23).
To determine the endogenous functions of BMP signaling, we treated P. flava zygotes with BMP signaling inhibitors, either LDN or DMH1, and observed the morphology of embryos at the gastrula stage, when the DV axis is normally distinguishable by dorsal extension of the mesoderm and ventral bending of the gut (Fig. 1B). The expression of several previously published and newly identified DV-marker genes was also examined (SI Appendix, Figs. S1B and S2). Inhibition of BMP signaling disrupted DV morphological characteristics, caused expansion of the ventral gene (gsc, bra, chordin, foxa, nkx2.1, and nkx2.2) expression domains, and reduced dorsal gene (dlx, msx, bmp2/4, and tbx2/3) expression (Fig. 1C and SI Appendix, Fig. S3A). At the tornaria stage, DMH1 treatment resulted in a circumferential mouth and the expanded expression of stomodeal genes (gsc, bra, and foxa) (SI Appendix, Fig. S3B). Intriguingly, in these embryos, expression domains of the genes marking the two neurogenic regions, the apical ectoderm (foxq2, sfrp1/5, and t-brain) and the ciliary bands (onecut and foxg), were expanded (Fig. 1 B and C and SI Appendix, Fig. S3A). Accordingly, the DV organization of serotonergic neurons in the apical ectoderm and the ciliary band neurons, labeled by anti-synaptotagmin B (synB), was disrupted (Fig. 1 B and C), supporting the notion that BMP signaling is required for both DV and neural patterning in P. flava embryos. Conversely, elevation of BMP activity caused opposite effects on DV gene expression, while repressing neurogenesis (Fig. 1D and SI Appendix, Fig. S3A).
We also examined the expression of elav, which codes for an RNA-binding protein expressed in neurons of many bilaterians and the adult P. flava nervous system (24). During P. flava embryogenesis, elav is expressed in cells scattered in several ectodermal domains, including the apical region and the ciliary band, as well as in some mesodermal cells (SI Appendix, Fig. S3C). In addition to its conserved role as a pan-neural marker, mesodermal expression is also observed in sea urchin and sea star embryos (25, 26). Similar to the aforementioned apical and ciliary band markers, the ectodermal expression of elav was expanded when BMP signaling was inhibited and disappeared when BMP signaling was elevated (SI Appendix, Fig. S3C). In those treated embryos, gastrulation occurred normally, suggesting that BMP does not act on whole-body anteroposterior patterning. These results demonstrate that in the indirect-developing hemichordate, BMP signaling controls DV patterning and represses neurogenesis, similar to what is seen in many bilaterians, including chordates and echinoderms (Fig. 1A). Given that the ambulacrarian larval nervous system, but not the hemichordate adult nervous system, has regulatory features similar to the chordate CNS, the deuterostome ancestor may have been an indirect developer with a ciliary band nervous system.
Transient Overactivation of BMP Activity During P. flava and Strongylocentrotus purpuratus Gastrulation.
We next examined the role of BMP signaling during P. flava gastrulation, a stage comparable to that at which the chordate neural plate is specified from the dorsal ectoderm. Transient overactivation of BMP signaling for 4 h (from 24 to 28 h postfertilization) during gastrulation resulted in loss of the mouth, but otherwise the DV axis of the embryo was still recognizable by observing the mesoderm extended to the dorsal side (Fig. 2 and SI Appendix, Fig. S4). The mouthless tornaria larva survived in seawater for at least 10 d (SI Appendix, Fig. S5). Consistent with the lack of a mouth opening, stomodeal expression of bra, foxa, and gsc, as well as pharyngeal muscles, were absent (Fig. 2). On the other hand, the blastoporal expression of bra and the endodermal expression of foxa were not affected, and the expression of several dorsal genes, such as dlx, tbx2/3, and bmp2/4, was expanded toward the ventral ectoderm (Fig. 2 A and B).
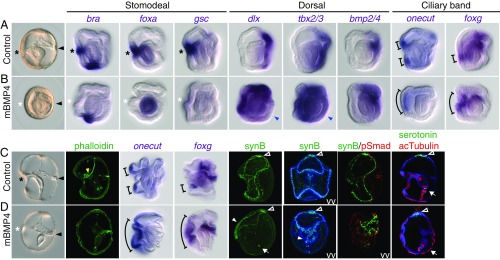
Fig. 2.
Transient overactivation of BMP activity blocks mouth formation and concentrates the larval nervous system ventrally. (A and C) Controls cultured with 125 ng/mL BSA. (B and D) Embryos treated with 125 ng/mL mBMP4. The treatments were performed for 4 h during gastrulation (from 24 to 28 h postfertilization), then washed out. Embryos were fixed at the late gastrula (A and B) or tornaria (C and D) stage for in situ hybridization and immunostaining. BMP activity was monitored using an anti-phospho-Smad1/5/8 (pSmad) antibody. The black asterisks and arrowheads indicate the mouth and the hydropore openings, respectively. The white asterisks denote the disappearance of the mouth and stomodeal gene expression. The blue arrowheads indicate the weaker signal of dlx and tbx2/3 in the most dorsal ectoderm. The yellow arrowhead indicates the pharyngeal muscles in the control tornaria larva. The brackets indicate the expression domains of the onecut and foxg genes in the ventral ectoderm. In mBMP4-treated embryos, synB signals in the postoral ciliary band were concentrated on the ventral side (white arrowheads), while the distributions of the serotonergic neurons (empty arrowheads) and the neurons in the sphincter (arrows) were similar to those of controls. All images are side views (ventral to the left) unless indicated otherwise. VV, ventral view (viewed from the mouth opening side with left and right sides shown). The data represent the phenotypes of most samples (>95%).
Interestingly, we observed a restriction of onecut and foxg expression patterns, which normally label the ciliary bands, to the ventral ectoderm, along with a concentration of the distribution of the postoral ciliary band neurons in the ventral ectoderm (Fig. 2). The presence of the neuronal population (synB+ cells) was correlated with the restricted BMP-negative domain (Fig. 2 C and D). Therefore, BMP signals are able to pattern the larval nervous system of the indirect-developing hemichordate.
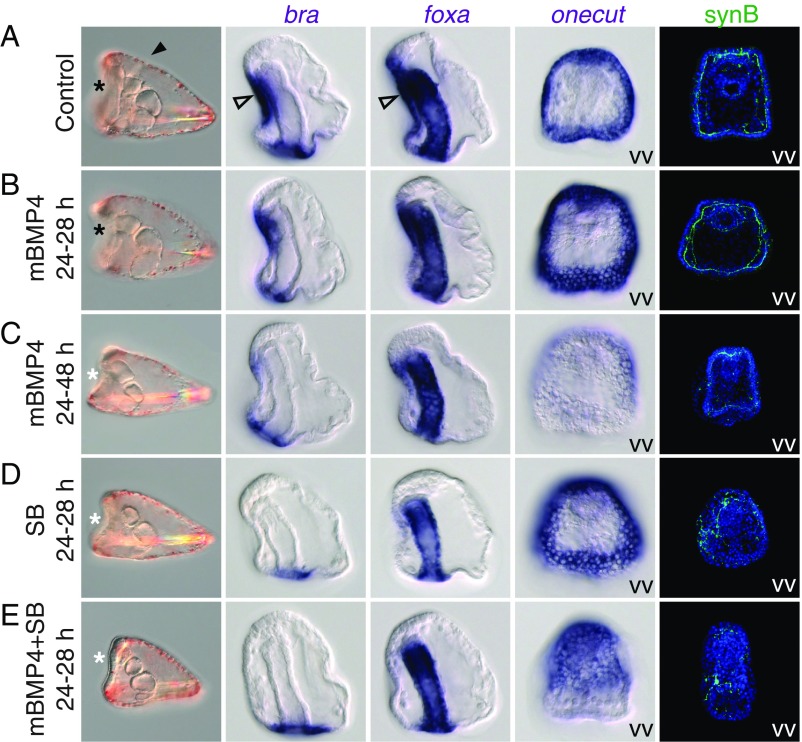
We then performed similar experiments in the sea urchin (Strongylocentrotus purpuratus) embryos. Treatment of sea urchin embryos with mBMP4 for 4 h during early gastrulation (from 24 to 28 h postfertilization) did not affect mouth opening, although the expression domain of onecut, a marker for ciliary band (27), was expanded (Fig. 3 A and B). Extension of the treatment period to 24 h (from 24 to 48 h postfertilization) resulted in loss of the mouth and weakening of stomodeal gene (bra and foxa) expression, while onecut expression was expanded but weaker in the ventral ectoderm (Fig. 3C). In sea urchin embryos, Nodal signaling is known to pattern the DV axis by activating the expression of several ventral genes, including chordin, bmp2/4, and bra (28, 29). It has been reported that inhibition of Nodal signaling with 10 μM SB431542 (SB) for 4 h alone can inhibit bra expression in the stomodeal region (30). Thus, we performed the same treatment and observed that this short treatment with SB was sufficient to block mouth formation and expand the onecut expression domain (Fig. 3D). The treatment also partially concentrated the ciliary band nervous system in the ventral ectoderm (synB in Fig. 3D). Cotreatment of SB and mBMP4 for 4 h blocked mouth formation and further centralized the nervous system to the ventral ectoderm; onecut expression was also expanded and covered the whole ventral ectoderm (Fig. 3E). Similar to the mouthless tornaria larva, the mouthless pluteus larva also survived in seawater for at least 10 d (SI Appendix, Fig. S6). These phenotypic changes are similar to those observed in the hemichordate embryo on transient overactivation of BMP signaling, suggesting that BMP-mediated patterning of larval nervous systems is a conserved trait in Ambulacraria, and that this trait could have already been present in an indirect-developing deuterostome ancestor.
Fig. 3.
Inhibition of mouth formation and ventral concentration of the nervous system in the sea urchin larvae. (A) Control S. purpuratus embryos cultured with 250 ng/mL BSA or DMSO. (B) Embryos treated with 250 ng/mL mBMP for 4 h during early gastrulation (from 24 to 28 h postfertilization). (C) Embryos treated with 250 ng/mL mBMP for 24 h (from 24 to 48 h postfertilization). (D) Embryos treated with 10 μM SB431542 (SB), a Nodal signaling inhibitor, for 4 h alone. (E) Cotreatment with SB and mBMP for 4 h blocked mouth formation and further centralized the nervous system to the ventral ectoderm. The first and fifth columns display the pluteus larval stage (4 d postfertilization), and the second, third, and fourth columns are embryos at the gastrula stage (2 d postfertilization). All images are side views unless indicated otherwise. VV, ventral view (viewed from the mouth opening side). The black asterisk denotes a mouth opening, indicating the ventral side; the white asterisk, lack of a mouth opening. The arrowhead denotes an opening of the hydropore, indicating the dorsal side, and the empty arrowhead denotes the ectodermal, stomodeal expression of bra and foxa. The data represent the phenotypes of most samples (>95%).
Medial-to-Lateral Organization of the Hemichordate and Sea Urchin Ectoderm.
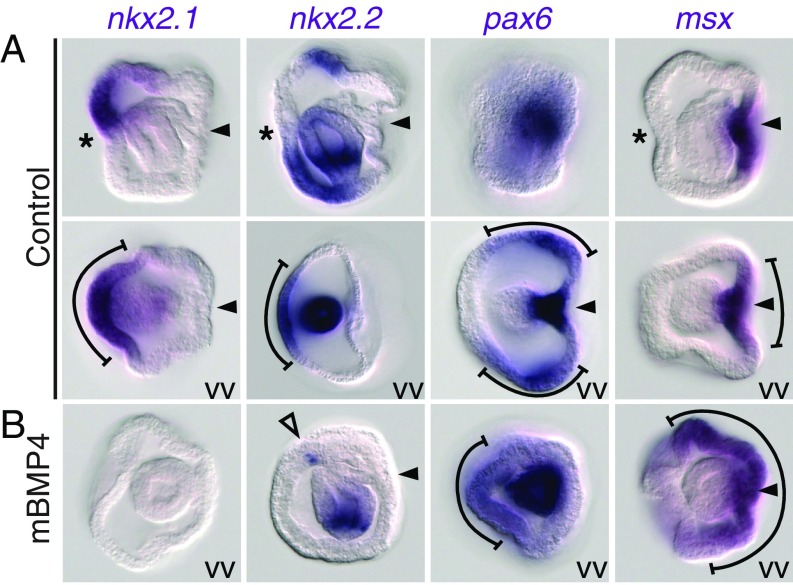
The chordate neural plate is patterned by expression of a set of conserved transcription factors in a medial-to-lateral organization mediated by the BMP signaling gradient (31). As such, nkx2.1/nkx2.2 genes are expressed in the most medial region, pax6+ cells are located in the mediolateral region, and msx transcripts are detected in the lateral neural plate and the ventral epidermal ectoderm (32). We found that in the P. flava embryo, orthologs of these genes are expressed in a ventral-to-dorsal organization, with the nkx2.1/nkx2.2 genes expressed in the ventral ectoderm, pax6 expressed in the lateral ectoderm, and msx expressed in the most dorsal ectoderm (Fig. 4A and SI Appendix, Fig. S1). When BMP signaling was transiently overactivated during gastrulation, the ectodermal expression of nkx2.1 and nkx2.2 was mostly undetected, with only a few nkx2.2+ cells present in the ventral ectoderm (Fig. 4B). At a lower concentration of mBMP4, weaker expression of nkx2.1 and nkx2.2 was detected in the ventral ectoderm (SI Appendix, Fig. S7). Pax6 expression converged to the ventromedial region, and the msx expression domain expanded to cover the lateral and dorsal ectoderm (Fig. 4B).
Fig. 4.
Medial-to-lateral organization of the ventral neurogenic ectoderm in P. flava embryos. Shown are expression patterns of nkx2.1, nkx2.2, pax6, and msx in controls (A) and in embryos treated with mBMP4 for 4 h during gastrulation (B). Asterisks and arrowheads indicate the mouth and hydropore openings, respectively. The brackets indicate the ectodermal expression domains. The empty arrowhead indicates the residual ectodermal expression of nkx2.2. Images are either side views or vegetal views (VV). Ventral is to the left in all images.
Similarly, the ventral-to-dorsal organization of the ectoderm is also seen in sea urchin embryos (Fig. 5A), except that instead of pax6, sea urchin pax2/5/8 is expressed in the lateral ectoderm (33). When BMP signaling was transiently overactivated and Nodal signaling was transiently blocked during gastrulation, the ventral ectodermal expression of nkx2.1 and nkx2.2 was undetectable or decreased (Fig. 5B). Similar to the changes in pax6 and msx expression in the P. flava embryo, the sea urchin pax2/5/8 expression domain converged to the ventral region, and the msx expression domain in the dorsal ectoderm expanded (Fig. 5B). Therefore, the mouthless, neurogenic ventral ectoderm of the hemichordate and sea urchin embryos exhibited a medial-to-lateral organization similar to that of the chordate neural plate.
Fig. 5.
Medial-to-lateral organization of the ventral neurogenic ectoderm in S. purpuratus embryos. Shown are expression patterns of nkx2.1, nkx2.2, pax2/5/8, and msx in controls (A) and in embryos treated with mBMP4 and SB for 4 h during gastrulation (B). The ventral ectodermal expression of nkx2.1 (black asterisk) disappeared (white asterisk) after drug treatment. The vegetal-ventral ectodermal expression of nkx2.2 (black arrowhead) also weakened on treatment (blank arrowhead). The brackets indicate the ectodermal expression domains. The first two columns display the late gastrula stage (2 d postfertilization, side views), while the last two columns are embryos at the pluteus larval stage (3 d postfertilization, vegetal views). Ventral is to the left in all images.
Discussion
Here we present evidence that BMP signals pattern the DV axis and repress neurogenesis in the indirect-developing hemichordate P. flava. Moreover, BMP signaling controls the ectodermal, ventral-to-dorsal organization of nkx2.1/nkx2.2, pax6, and msx expression domains in P. flava embryos. In sea urchin embryos, pax2/5/8 instead of pax6 is expressed in the lateral ectoderm. Given that bilaterian pax6 and pax2 are derived from a common ancestral gene, possibly homologous to the single Cnidarian paxb gene (34), and that the two genes are functionally interchangeable (35), the lateral ectodermal domains, marked by pax6 expression in the embryos of P. flava and another indirect-developing hemichordate (36) and by pax2/5/8 expression in sea urchin embryos, may be equivalent (Fig. 6). Our present results and previous studies show that similar patterning mechanisms are used in echinoderm and hemichordate larvae (23, 33, 36–38), suggesting that the common ancestor of Ambulacraria was an indirect developer with a larval stage. Moreover, because the ambulacrarian larval nervous system and the chordate CNS are similarly regulated (both repressed by BMP signaling), we propose that the deuterostome ancestor was also an indirect developer that metamorphosed into a worm-like, motile adult, similar to an indirect-developing enteropneust.
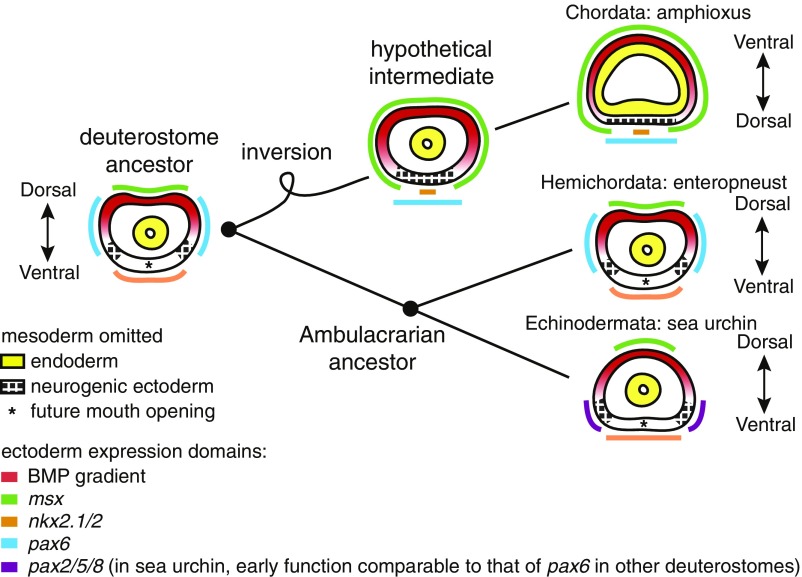
Fig. 6.
Scenario for evolution of the chordate body plan. All diagrams depict cross-sections of the gastrula stage of extant deuterostome species and their hypothetical ancestors. Enhanced BMP activity during gastrulation could have resulted in loss of the ancestral mouth and centralization of neurogenic ectoderm on the ancestral ventral side of a hypothetical intermediate to chordates. Opening of a new mouth on the ancestral dorsal side could account for the inverse DV orientation of chordates relative to the ambulacraria. The colored lines outside the embryos indicate the ectodermal expression domains of transcription factors (33, 50, 60–62). Note that the sea urchin pax6 gene has no ectodermal expression at the gastrula stage, although another Pax factor gene, pax2/5/8, is expressed in the lateral ectoderm, marking a subdomain of the presumptive ciliary band (33, 63). Asterisks indicate the sites of future mouth openings. Based on the similarity of the larval patterning mechanisms between the hemichordates and sea urchins, we propose that the deuterostome ancestor was an indirect developer with a ciliary band nervous system. During early chordate evolution, changes in the BMP level during gastrulation could have occurred, resulting in the loss of the larval mouth and centralization of the neurogenic ectoderm on the ancestral ventral side devoid of BMP activity. A new mouth opening on the ancestral dorsal side could then redefine an inverse DV orientation relative to the Ambulacraria.
The phenotype that we observed in the hemichordate and sea urchin larvae on transient overactivation of BMP signals (i.e., loss of the mouth and ventrally centralized neurogenic ectoderm) suggests a possible mechanism explaining how chordates could have evolved from an indirect-developing ancestor. These morphological changes are reminiscent of Nielsen’s hypothesis (modified from Garstang’s 1894 idea) that the chordate CNS evolved from fusion of the postoral ciliary band on the ventral side (19). Importantly, the phenotype that we observed differs from Nielsen’s hypothesis with regard to the fate of the larval mouth. Instead of moving from the ventral to the dorsal side, as Nielsen proposed, we suggest that the ancestor mouth was lost as the ciliary band nervous system became ventralized. Because DV axes are defined by the position of the mouth opening, loss of the original mouth and replacement by a new mouth would redefine the DV orientation.
Several lines of evidence support the idea that chordates evolved a new mouth (39), and it has been proposed that a gill slit or a coelomic hydropore, both of which are located on the dorsal side of extant hemichordates, is homologous to the amphioxus mouth (40, 41). Molecular and paleontological evidence support the idea that pharyngeal gill slits were present in the deuterostome ancestor (42, 43). Although gill slits of indirect-developing enteropneusts form at later larval stages or after metamorphosis (14, 44), our observation that these mouthless larvae could survive for days without food in their digestive tracts (SI Appendix, Figs. S5 and S6) suggests an opportunity for mouthless larvae to use gill slits as an alternative mouth. Moreover, the chordate ancestor could have evolved a faster developmental process to shorten the period of a nonfeeding larval stage. Cases in which organisms have adopted such a developmental strategy can be seen in extant basal chordates; for example, ascidian tadpole larvae do not feed but quickly metamorphose into the adult form (45), and the Branchiostoma floridae amphioxus forms its mouth and gill slits within 2 d postfertilization (46). Thus, we hypothesize that changes in BMP levels during gastrulation resulted in loss of the deuterostome ancestral mouth, which was presumably homologous to the ambulacrarian mouth, and the use of one of the dorsal openings (perhaps a pharyngeal gill slit) as a new mouth would have allowed the chordate ancestor to take on an inverse DV orientation relative to Ambulacraria and the deuterostome ancestor (Fig. 6).
Our results also show that the medial-to-lateral organization of the mouthless, ventral neuroectoderm resembles that of the chordate neural plate, and on further acquisition of a neurulation mechanism, this hypothetical chordate ancestor would be poised to evolve a hollow neural tube. Lacalli and West (47) reported a distinct nerve cell type with apical processes present in both the ciliary bands of ambulacrarian larvae and the neural tube of amphioxus, suggesting that the chordate neural tube could have evolved through fusion and internalization of the ciliary band. This idea is further supported by the observation that while in sea urchin and P. flava, foxg and onecut transcripts mark the ciliary band, the amphioxus orthologs are expressed in the central nervous system; foxg transcript is detected in the cerebral vesicle (i.e., anterior widening of the amphioxus neural tube) (48), while onecut is expressed in the posterior cerebral vesicle and pairs of neurons in the neural tube (SI Appendix, Fig. S8).
One possible explanation for the evolution of neurulation in the chordate ancestor may be suggested by the gene expression patterns at the borders of the neural ectoderm. In both Xenopus and amphioxus, msx expression is initially detected in the ventral ectoderm, extending to the edges of the neural plate of the late gastrula embryos; at the neurula stage, chordate msx expression is then restricted to the neural plate edges as the ventral expression decreases (49, 50). In P. flava and S. purpuratus embryos with transient overactivation of BMP signaling, we observed expansion of msx expression but not its restriction to the edges of the ventral ectoderm (Figs. 4B and 5B). Given that disruption of mouse msx impairs neurulation (51, 52), the restriction of msx expression at the neural plate edges may be essential for the evolution of neurulation in the chordate lineage.
In conclusion, we propose that tinkering with the BMP level in the indirect-developing deuterostome ancestor may have driven seemingly dramatic morphological changes, leading to the origin of chordates. This study thus provides an example illustrating how the emergence of phylum-level novelties may occur by molecular tinkering, as proposed by Jacob (1) more than 40 y ago. We anticipate that further studies will reveal other genetic changes, either occurring with or independent of DV inversion, that elucidate the emergence of other chordate novelties, such as the notochord and segmented somites.
Methods
Biological Materials.
P. flava adults were collected from Penghu Islands, Taiwan. Spawning was induced, and embryos were cultured at 23 °C (53). S. purpuratus adults were obtained from Pat Leahy (Corona del Mar, CA). Fertilization and embryo cultures were carried out at 15 °C. BMP signaling perturbation was performed by treating embryos with inhibitors or recombinant proteins. The concentrations of the reagents used in this study were as follows: DMH1, 2 μM (Sigma-Aldrich), LDN-193189, 2 μM (Stemgent), and mouse BMP4, 250, 125, 62.5 or 31.25 ng/mL (R&D Systems). Solvents (DMSO or 0.1% BSA) were added as controls.
Molecular Cloning.
All genes investigated in this study were cloned by PCR, and the primers were designed based on the transcriptome or genome sequences (SI Appendix, Table S1) (42, 54). The cDNA sequences have been deposited in GenBank (SI Appendix, Table S2).
In Situ Hybridization and Immunostaining.
The primers used to construct the clones for probe synthesis are listed in SI Appendix, Table S1. In situ hybridization and immunostaining were performed as described previously (55–57). The primary antibodies used in this study were anti-serotonin antibody (1:1,000; Sigma-Aldrich), monoclonal anti-synaptotagmin B (58, 59) (1e11, 1:20; Developmental Studies Hybridoma Bank), and rabbit anti–phospho-Smad1/5/8 (1:200; Cell Signaling Technology). The nuclei were counterstained with Hoechst 33342 (1:1,000; Invitrogen). The embryos were imaged using a Leica TCS-SP5 AOBS inverted confocal system.
Supplementary Material
Acknowledgments
We thank L. Holland and M. Martindale for discussions, N. Holland and S. Schneider for critical reading of the manuscript, M. Calkins for English editing, and the staff at the core facility and the Marine Research Station of the Institute of Cellular and Organismic Biology, Academia Sinica for technical assistance. This study was supported by the Ministry of Science and Technology, Taiwan (Grant MOST-107-2321-B-001-017 to Y.-H.S. and Grant MOST-105-2628-B-001-003-MY3 to J.-K.Y.) and the Academia Sinica intramural fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data have been submitted to GeneBank, https://www.ncbi.nlm.nih.gov/genbank (accession nos. MH782152 for Ptychodera flava Gsc, MH782154 for P. flava Foxg, MH782156 for P. flava Pax6, MH782155 for P. flava Nkx2.2, MH782157 for P. flava Onecut, MH782153 for P. flava Tbx2/3, and MK679618 for Branchiostoma floridae Onecut).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1901919116/-/DCSupplemental.
References
- 1.Jacob F., Evolution and tinkering. Science 196, 1161–1166 (1977). [DOI] [PubMed] [Google Scholar]
- 2.Holland N. D., Holland L. Z., Holland P. W., Scenarios for the making of vertebrates. Nature 520, 450–455 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Lowe C. J., Clarke D. N., Medeiros D. M., Rokhsar D. S., Gerhart J., The deuterostome context of chordate origins. Nature 520, 456–465 (2015). [DOI] [PubMed] [Google Scholar]
- 4.De Robertis E. M., Kuroda H., Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 20, 285–308 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J. K., et al. , Axial patterning in cephalochordates and the evolution of the organizer. Nature 445, 613–617 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Bier E., De Robertis E. M., BMP gradients: A paradigm for morphogen-mediated developmental patterning. Science 348, aaa5838 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Arendt D., Nübler-Jung K., Inversion of dorsoventral axis? Nature 371, 26 (1994). [DOI] [PubMed] [Google Scholar]
- 8.De Robertis E. M., Sasai Y., A common plan for dorsoventral patterning in Bilateria. Nature 380, 37–40 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Dunn C. W., Giribet G., Edgecombe G. D., Hejnol A., Animal phylogeny and its evolutionary implications. Annu. Rev. Ecol. Evol. Syst. 45, 371–395 (2014). [Google Scholar]
- 10.McClay D. R., Evolutionary crossroads in developmental biology: Sea urchins. Development 138, 2639–2648 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapraz F., Besnardeau L., Lepage T., Patterning of the dorsal-ventral axis in echinoderms: Insights into the evolution of the BMP-chordin signaling network. PLoS Biol. 7, e1000248 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaguchi S., Yaguchi J., Angerer R. C., Angerer L. M., Burke R. D., TGFβ signaling positions the ciliary band and patterns neurons in the sea urchin embryo. Dev. Biol. 347, 71–81 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angerer L. M., Yaguchi S., Angerer R. C., Burke R. D., The evolution of nervous system patterning: Insights from sea urchin development. Development 138, 3613–3623 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Röttinger E., Lowe C. J., Evolutionary crossroads in developmental biology: Hemichordates. Development 139, 2463–2475 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Lowe C. J., et al. , Dorsoventral patterning in hemichordates: Insights into early chordate evolution. PLoS Biol. 4, e291 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garstang W., Preliminary note on a new theory of the phylogeny of the Chordata. Zool. Anz. 17, 122–125 (1894). [Google Scholar]
- 17.Holland N. D., Walter Garstang: A retrospective. Theory Biosci. 130, 247–258 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Lacalli T. C., Protochordate body plan and the evolutionary role of larvae: Old controversies resolved? Can. J. Zool. 83, 216–224 (2005). [Google Scholar]
- 19.Nielsen C., Origin of the chordate central nervous system and the origin of chordates. Dev. Genes Evol. 209, 198–205 (1999). [DOI] [PubMed] [Google Scholar]
- 20.Bourlat S. J., et al. , Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature 444, 85–88 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Delsuc F., Tsagkogeorga G., Lartillot N., Philippe H., Additional molecular support for the new chordate phylogeny. Genesis 46, 592–604 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Röttinger E., DuBuc T. Q., Amiel A. R., Martindale M. Q., Nodal signaling is required for mesodermal and ventral but not for dorsal fates in the indirect developing hemichordate, Ptychodera flava. Biol. Open 4, 830–842 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Röttinger E., Martindale M. Q., Ventralization of an indirect developing hemichordate by NiCl2 suggests a conserved mechanism of dorso-ventral (D/V) patterning in Ambulacraria (hemichordates and echinoderms). Dev. Biol. 354, 173–190 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Nomaksteinsky M., et al. , Centralization of the deuterostome nervous system predates chordates. Curr. Biol. 19, 1264–1269 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Garner S., et al. , Neurogenesis in sea urchin embryos and the diversity of deuterostome neurogenic mechanisms. Development 143, 286–297 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Yankura K. A., Koechlein C. S., Cryan A. F., Cheatle A., Hinman V. F., Gene regulatory network for neurogenesis in a sea star embryo connects broad neural specification and localized patterning. Proc. Natl. Acad. Sci. U.S.A. 110, 8591–8596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barsi J. C., Davidson E. H., cis-regulatory control of the initial neurogenic pattern of onecut gene expression in the sea urchin embryo. Dev. Biol. 409, 310–318 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Duboc V., Röttinger E., Besnardeau L., Lepage T., Nodal and BMP2/4 signaling organizes the oral-aboral axis of the sea urchin embryo. Dev. Cell 6, 397–410 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Su Y. H., Gene regulatory networks for ectoderm specification in sea urchin embryos. Biochim. Biophys. Acta 1789, 261–267 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Li E., Materna S. C., Davidson E. H., New regulatory circuit controlling spatial and temporal gene expression in the sea urchin embryo oral ectoderm GRN. Dev. Biol. 382, 268–279 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizutani C. M., Bier E., EvoD/Vo: The origins of BMP signalling in the neuroectoderm. Nat. Rev. Genet. 9, 663–677 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland L. Z., et al. , Evolution of bilaterian central nervous systems: A single origin? Evodevo 4, 27 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saudemont A., et al. , Ancestral regulatory circuits governing ectoderm patterning downstream of Nodal and BMP2/4 revealed by gene regulatory network analysis in an echinoderm. PLoS Genet. 6, e1001259 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozmik Z., et al. , Role of Pax genes in eye evolution: A cnidarian PaxB gene uniting Pax2 and Pax6 functions. Dev. Cell 5, 773–785 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Carbe C., et al. , An allelic series at the paired box gene 6 (Pax6) locus reveals the functional specificity of Pax genes. J. Biol. Chem. 288, 12130–12141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez P., Uhlinger K. R., Lowe C. J., The adult body plan of indirect developing hemichordates develops by adding a Hox-patterned trunk to an anterior larval territory. Curr. Biol. 27, 87–95 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Chang Y. C., et al. , Regulatory circuit rewiring and functional divergence of the duplicate admp genes in dorsoventral axial patterning. Dev. Biol. 410, 108–118 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Yankura K. A., Martik M. L., Jennings C. K., Hinman V. F., Uncoupling of complex regulatory patterning during evolution of larval development in echinoderms. BMC Biol. 8, 143 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christiaen L., et al. , Evolutionary modification of mouth position in deuterostomes. Semin. Cell Dev. Biol. 18, 502–511 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Holland N. D., Formation of the initial kidney and mouth opening in larval amphioxus studied with serial blockface scanning electron microscopy (SBSEM). Evodevo 9, 16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaji T., Reimer J. D., Morov A. R., Kuratani S., Yasui K., Amphioxus mouth after dorso-ventral inversion. Zoological Lett. 2, 2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simakov O., et al. , Hemichordate genomes and deuterostome origins. Nature 527, 459–465 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gee H., Across the Bridge: Understanding the Origin of the Vertebrates (Univ of Chicago Press, 2018). [Google Scholar]
- 44.Gonzalez P., Jiang J. Z., Lowe C. J., The development and metamorphosis of the indirect developing acorn worm Schizocardium californicum (Enteropneusta: Spengelidae). Front. Zool. 15, 26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemaire P., Smith W. C., Nishida H., Ascidians and the plasticity of the chordate developmental program. Curr. Biol. 18, R620–R631 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stokes M. D., Holland N. D., Embryos and larvae of a lancelet, Branchiostoma floridae, from hatching through metamorphosis: Growth in the laboratory and external morphology. Acta Zool. 76, 105–120 (1995). [Google Scholar]
- 47.Lacalli T. C., West J. E., A distinctive nerve cell type common to diverse deuterostome larvae: Comparative data from echinoderms, hemichordates, and amphioxus. Acta Zool. 74, 1–8 (1993). [Google Scholar]
- 48.Toresson H., Martinez-Barbera J. P., Bardsley A., Caubit X., Krauss S., Conservation of BF-1 expression in amphioxus and zebrafish suggests evolutionary ancestry of anterior cell types that contribute to the vertebrate telencephalon. Dev. Genes Evol. 208, 431–439 (1998). [DOI] [PubMed] [Google Scholar]
- 49.Maeda R., et al. , Xmsx-1 modifies mesodermal tissue pattern along dorsoventral axis in Xenopus laevis embryo. Development 124, 2553–2560 (1997). [DOI] [PubMed] [Google Scholar]
- 50.Yu J. K., Meulemans D., McKeown S. J., Bronner-Fraser M., Insights from the amphioxus genome on the origin of vertebrate neural crest. Genome Res. 18, 1127–1132 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colas J. F., Schoenwolf G. C., Towards a cellular and molecular understanding of neurulation. Dev. Dyn. 221, 117–145 (2001). [DOI] [PubMed] [Google Scholar]
- 52.Foerst-Potts L., Sadler T. W., Disruption of Msx-1 and Msx-2 reveals roles for these genes in craniofacial, eye, and axial development. Dev. Dyn. 209, 70–84 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Lin C. Y., Tung C. H., Yu J. K., Su Y. H., Reproductive periodicity, spawning induction, and larval metamorphosis of the hemichordate acorn worm Ptychodera flava. J. Exp. Zoolog. B Mol. Dev. Evol. 326, 47–60 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Chen S. H., et al. , Sequencing and analysis of the transcriptome of the acorn worm Ptychodera flava, an indirect developing hemichordate. Mar. Genomics 15, 35–43 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Ikuta T., et al. , Identification of an intact ParaHox cluster with temporal colinearity but altered spatial colinearity in the hemichordate Ptychodera flava. BMC Evol. Biol. 13, 129 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo Y. J., Su Y. H., Opposing nodal and BMP signals regulate left-right asymmetry in the sea urchin larva. PLoS Biol. 10, e1001402 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu T. M., Luo Y. J., Yu J. K., BMP and Delta/Notch signaling control the development of amphioxus epidermal sensory neurons: Insights into the evolution of the peripheral sensory system. Development 139, 2020–2030 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Nakajima Y., Humphreys T., Kaneko H., Tagawa K., Development and neural organization of the tornaria larva of the Hawaiian hemichordate, Ptychodera flava. Zool. Sci. 21, 69–78 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Nakajima Y., Kaneko H., Murray G., Burke R. D., Divergent patterns of neural development in larval echinoids and asteroids. Evol. Dev. 6, 95–104 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Glardon S., Holland L. Z., Gehring W. J., Holland N. D., Isolation and developmental expression of the amphioxus Pax-6 gene (AmphiPax-6): Insights into eye and photoreceptor evolution. Development 125, 2701–2710 (1998). [DOI] [PubMed] [Google Scholar]
- 61.Takacs C. M., et al. , Expression of an NK2 homeodomain gene in the apical ectoderm defines a new territory in the early sea urchin embryo. Dev. Biol. 269, 152–164 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Venkatesh T. V., Holland N. D., Holland L. Z., Su M. T., Bodmer R., Sequence and developmental expression of amphioxus AmphiNk2-1: Insights into the evolutionary origin of the vertebrate thyroid gland and forebrain. Dev. Genes Evol. 209, 254–259 (1999). [DOI] [PubMed] [Google Scholar]
- 63.McIntyre D. C., Seay N. W., Croce J. C., McClay D. R., Short-range Wnt5 signaling initiates specification of sea urchin posterior ectoderm. Development 140, 4881–4889 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.