Significance
Cytomegalovirus (CMV) is a herpesvirus that causes severe disease in infants and immunocompromised people. Development of a vaccine to control the spread of CMV has been a decades-long goal. Most vaccine strategies target glycoprotein B (gB), a viral glycoprotein used for entry into host cells. We have instead targeted viral interleukin-10 (vIL-10), an immunomodulatory protein. We investigated whether these two approaches combined are capable of controlling horizontal transmission of CMV in a nonhuman primate model. Our results demonstrated that, while vaccination against gB was not protective, neutralizing antibodies against vIL-10 significantly reduced CMV transmission and disrupted host immunity when infection did occur. These results demonstrate that vIL-10 is an important target for prevention of CMV disease.
Keywords: cytomegalovirus, rhesus cytomegalovirus, viral IL-10, IL-10, vaccine
Abstract
Human cytomegalovirus (HCMV) causes severe disease in infants and immunocompromised people. There is no approved HCMV vaccine, and vaccine development strategies are complicated by evidence of both persistent infection and reinfection of people with prior immunity. The greatest emphasis has been placed on reducing transmission to seronegative pregnant women to prevent vertical transmission and its potentially severe sequelae. Increasing evidence suggests that the earliest host–HCMV interactions establish conditions for viral persistence, including evasion of host immune responses to the virus. Using a nonhuman primate model of HCMV infection, we show that rhesus macaques immunized against viral interleukin-10 (IL-10) manifest delayed rhesus cytomegalovirus (RhCMV) acquisition and altered immune responses to the infection when it does occur. Among animals with the greatest antiviral IL-10–neutralizing activity, the timing of RhCMV seroconversion was delayed by an average of 12 weeks. After acquisition, such animals displayed an antibody response to the new infection, which peaked as expected after 2 weeks but then declined rapidly. In contrast, surprisingly, vaccination with glycoprotein B (gB) protein had no discernible impact on these outcomes. Our results demonstrate that viral IL-10 is a key regulator of successful host immune responses to RhCMV. Viral IL-10 is, therefore, an important target for vaccine strategies against cytomegalovirus (CMV). Furthermore, given the immunoregulatory function of viral IL-10, targeting this protein may prove synergistic with other vaccine therapies and targets. Our study also provides additional evidence that the earliest host–CMV interactions can have a significant impact on the nature of persistent infection.
A hallmark of infection with human cytomegalovirus (HCMV), a beta-herpesvirus, is shedding of infectious virions in bodily fluids, such as saliva, urine, tears, cervical secretions, and semen. Viral shedding continues well past primary infection and recurs after periods of viral latency or after reinfection with a divergent strain (1). Horizontal transmission of HCMV is the result of repeated exposures of mucosal epithelium to infectious virions contained in these fluids (2). These aspects of the HCMV natural history illustrate one of the major challenges facing vaccine development: protection from repeated mucosal exposures to potentially antigenic variants from people shedding infectious virus.
Primary HCMV infection in immune-competent people is associated with mild, transient, or subclinical outcomes, and clinical symptoms of persistent infection are virtually absent. However, HCMV is associated with profound disease in both immunocompromised people and infants; therefore, a vaccine to prevent HCMV infection is a public health priority (3). Due to the severe pathologic sequalae of congenital HCMV (4), prevention of congenital transmission is a critical goal for vaccine development (5, 6). A vaccine designed to disrupt horizontal transmission could reduce congenital disease by reducing the risk of HCMV infection of seronegative women during pregnancy in addition to reducing the risk of reinfection of seropositive women with divergent strains.
Importantly for HCMV vaccine development, while immunity to HCMV seems to provide protection from HCMV end organ disease, it does not necessarily protect from reinfection with antigenically divergent strains (7). Because horizontal transmission can occur despite prior immunity to HCMV, it is not clear if vaccine strategies designed to recapitulate immune responses observed during natural infection can provide robust protection against HCMV transmission. Nonetheless, the most successful vaccination strategy to date utilizes recombinant HCMV glycoprotein B (gB) to recapitulate immune responses observed during HCMV infection and has achieved 43–50% efficacy in clinical studies (8, 9). Anti-gB–neutralizing responses develop early in natural infection and are persistent (10). It is not clear whether vaccination against gB will be sufficient to reduce the incidence of HCMV. Thus, alternative vaccine targets and/or additional vaccination strategies will likely be required. While gB is required for viral entry into fibroblasts, likely serving as a fusion protein (11), the pentameric complex (gH/gL, UL128, UL130, and UL131) is also required for entry into epithelial cells (12). Recent vaccine approaches have focused on eliciting neutralizing antibody responses to this complex as well (13).
We have developed a nonhuman primate model of HCMV pathogenesis, persistence, and immunity to test vaccine strategies (14). While cytomegaloviruses (CMVs) are highly adapted to their respective host species, rhesus cytomegalovirus (RhCMV) shares many key features with HCMV, including genomic structure and encoded immunomodulatory genes [such as viral interleukin-10 (vIL-10)], as well as similar pathogenesis and observed host immune responses (reviewed in ref. 14). Previous work from our group demonstrated that vaccination with RhCMV gB using DNA or modified vaccinia virus Ankara (MVA) elicited immune responses that were associated with reduced viral replication and inflammation at the site of later subcutaneous RhCMV challenge (15) as well as reduced viral shedding in saliva (16). A recombinant gB protein prime/boost strategy has not yet been tested in macaques. We also previously investigated targeting the UL111A open reading frame, which encodes an ortholog of the host interleukin-10 (IL-10) gene (17). Our studies showed that vaccination with a nonfunctional recombinant vIL-10 protein (18) reduced viral replication at the site of subcutaneous inoculation and also reduced shedding in saliva and urine (19, 20). The impact of these vaccine strategies on efficiency of horizontal transmission (i.e., after repeated, low-dose mucosal exposure) has not been determined.
In this study, we tested two vaccine strategies that could work synergistically to reduce the rate of horizontal RhCMV transmission: (i) vaccination with recombinant gB to interfere with mucosal RhCMV acquisition and (ii) vaccination with recombinant vIL-10 to reduce viral shedding. We tested these strategies in the context of an experimental design allowing true horizontal transmission between animals assigned to the study.
Results
Vaccination with Recombinant RhCMV Antigens Elicits Host Responses.
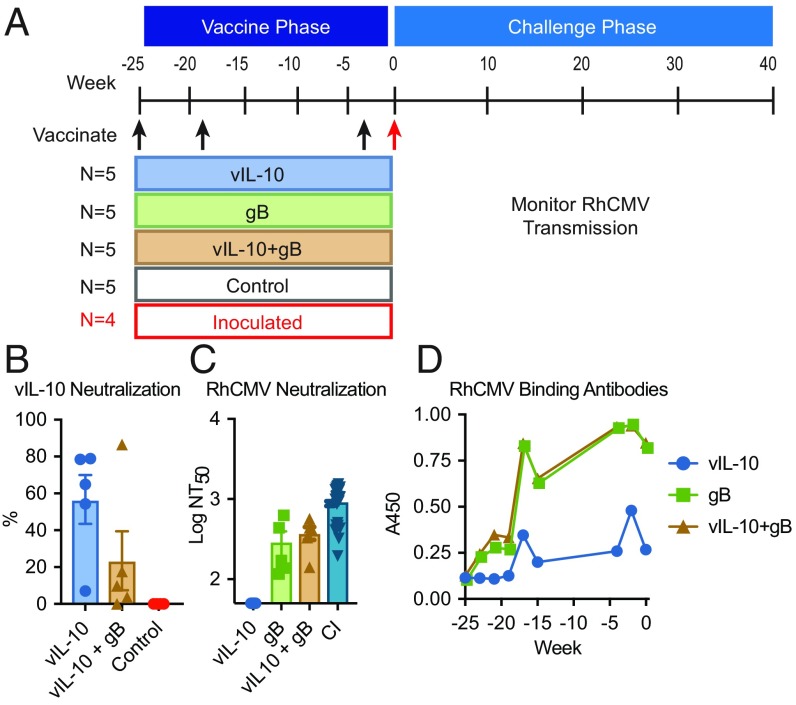
To test our vaccine strategies for protection against repeated mucosal exposures, 24 RhCMV-seronegative macaques were cohoused in an outdoor enclosure that would allow horizontal RhCMV transmission in the challenge phase of the experiment as a result of interactions between these highly social animals. After a stabilization period, the animals were randomly assigned to five groups. Three groups were vaccinated with a prime-boost protocol utilizing modified recombinant gB, vIL-10, or both proteins, while the other two groups remained unvaccinated (Fig. 1A). Vaccinated macaques were immunized at weeks −25, −19, and −4 relative to introduction of challenge virus. The recombinant vIL-10 immunogens used are biologically inactive proteins, unable to engage the host cellular interleukin-10 (cIL-10) response pathway (18). Antibodies elicited by this immunogen do not cross-react with host cIL-10 (19). To provide a horizontal source of challenge virus, four unvaccinated macaques in the enclosure were inoculated subcutaneously at week 0 with 105 plaque-forming units of RhCMV isolate UCD52. This low-passage clinical-type isolate encodes a UL/b′ region, retains tropism for both epithelial and endothelial cells in culture, and sheds persistently in saliva and urine (21).
Fig. 1.
Vaccination with recombinant gB, vIL-10, or both proteins elicited binding and neutralizing antibody responses. (A) Experimental design and timeline. Three groups of five RhCMV-uninfected macaques were immunized three times with modified recombinant vIL-10, modified recombinant gB, or both. (B) Neutralizing antibody responses to recombinant vIL-10 were assessed in macaques vaccinated with either vIL-10 alone or with both vIL-10 and gB. The assay was completed in duplicate. (C) Plasma samples were assessed for RhCMV-neutralizing antibody responses using a cell culture-based assay. NT50 antibody titers are shown. A panel of macaques chronically infected with RhCMV (CI; dark blue inverted triangles) from the CNPRC was identified by ELISA. These animals were also assessed for RhCMV-neutralizing antibodies; n = 5 for each vaccine treatment group, and n = 26 for the CI group. Symbols indicate individual macaques, the boxes indicate the group mean, and the error bars display SEM of the assay completed in triplicate. (D) Plasma samples were assessed for RhCMV binding antibodies to the vaccine strain by ELISA. The assay was completed in duplicate, and the lines display the median A450 for each group.
Macaques vaccinated with modified recombinant vIL-10 protein, either alone or in combination with gB, were assessed for the development of systemic antibody responses capable of neutralizing vIL-10 in a bioassay (Fig. 1B) (18); 9 of 10 vaccinated macaques developed detectable vIL-10–neutralizing antibody responses. One macaque vaccinated with both vIL-10 and gB failed to develop vIL-10–neutralizing antibody responses. Indeed, inclusion of gB with vIL-10 vaccination reduced vIL-10–specific neutralizing responses compared with administration of modified vIL-10 alone, although this difference was not statistically significant. We also observed that these macaques could be separated into two discrete populations defined by their neutralizing antibody responses: weak responders with below 20% vIL-10 neutralization and strong responders above 50%. Four of five animals immunized with vIL-10 alone were strong responders, whereas only one of five immunized with both gB and vIL-10 was a strong responder.
We next assessed the macaques for antibodies capable of neutralizing a fibroblast-adapted strain of RhCMV (68–1 EGFP) (22). Vaccination with gB elicited neutralizing antibody responses in all 10 macaques, with peak 50% virus neutralization titer (NT50) ranging from 1:138 to 1:631 (Fig. 1C). No RhCMV-neutralizing antibodies were detected in animals receiving vIL-10 immunogen alone. For comparison of these responses with neutralizing antibody titers developed during persistent RhCMV infection, we collected blood samples from a group of 26 chronically infected macaques. Titers in these samples ranged from 1:196 to 1:1,477; thus, neutralizing antibody titers in gB-vaccinated macaques overlapped with the lower titers observed in the chronically infected macaques, similar to our previous studies (15, 16).
Binding antibody responses to RhCMV proteins were measured in plasma samples from the vaccinated macaques using lysate from telomerase-immortalized fibroblasts infected with RhCMV strain 68–1 (Fig. 1D). All vaccinated macaques developed detectable antibody responses, although responses observed in animals vaccinated with vIL-10 alone were lower than those receiving gB.
RhCMV Horizontal Transmission Occurred in All Experimental Groups.
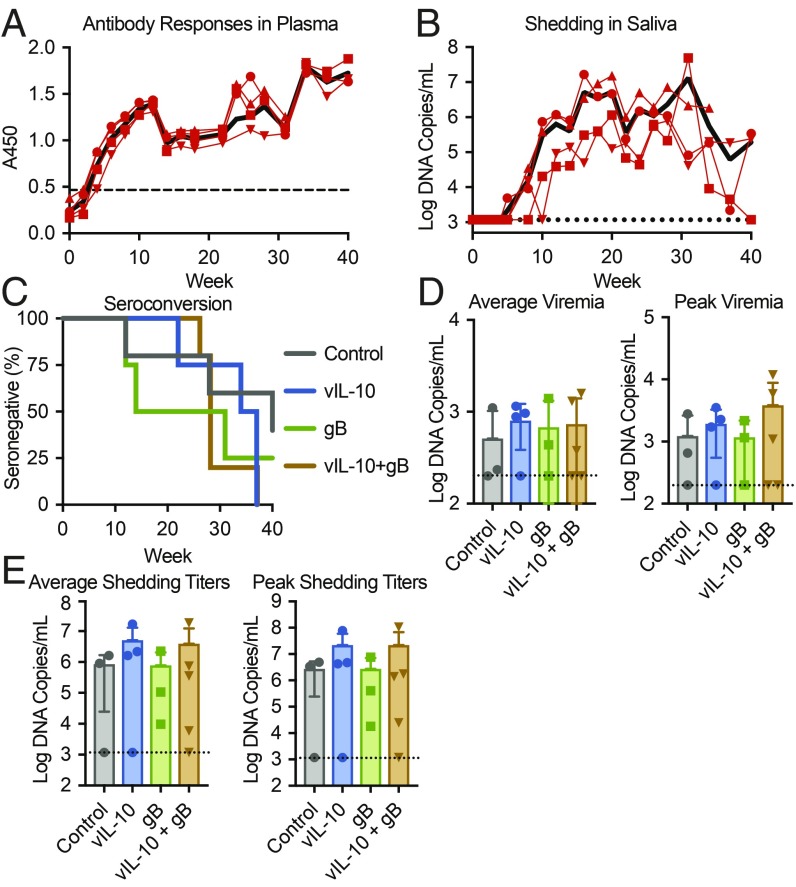
To initiate the challenge phase of the study, four unvaccinated animals in the enclosure were inoculated subcutaneously with RhCMV isolate UCD52 (week 0). All of the experimentally inoculated macaques seroconverted by 4 wk postinoculation and shed virus in saliva by 12 wk (Fig. 2 A and B). Antibody responses in these inoculated animals continued to increase throughout the study in all cases. Shedding of RhCMV in saliva peaked on average at week 24 postinfection (average peak titer = 2.06 × 107 DNA copies per milliliter; median peak titer = 1.60 × 107 copies per milliliter) and continued in most cases through the end of the study at week 40.
Fig. 2.
Analysis of RhCMV UCD52 transmission. (A and B) Four unvaccinated macaques, cohoused with the vaccinated macaques, were inoculated with RhCMV UCD52 at week 0. (A) Plasma samples from the inoculated macaques were analyzed by ELISA in duplicate for UCD52 to determine seroconversion. (B) RhCMV shedding in saliva in the inoculated macaques was monitored by qPCR analysis for RhCMV DNA in triplicate. Symbols and thin lines indicate individual macaques, the black lines display the group average, and the dashed lines indicate either (A) the cutoff value for ELISA seroconversion or (B) the limit of detection for qPCR analysis. (C) Survival plot for vaccinated macaques displaying the rates of seroconversion to RhCMV pp65, a nonvaccine antigen. Binding antibodies were measured in plasma by ELISA and used to determine rates of horizontal transmission. The assays were completed in duplicate. (D) RhCMV DNA was quantified in plasma samples by qPCR in triplicate reactions to assess systemic viral replication and dissemination. The bars display the average virus load post-RhCMV seroconversion or the mean peak viremia for each group, and the error bars display SEM. (E) RhCMV DNA was measured in oral swabs by qPCR in triplicate to quantify viral shedding in saliva. The bars display the average virus titers in saliva post-RhCMV seroconversion or the mean peak viremia for each group, and the error bars display SEM.
Horizontal transmission to the vaccinated macaques was followed by assessing development of binding antibodies to RhCMV pp65, a nonvaccine antigen. A Cox regression analysis determined that there was no significant difference in the rate of seroconversion between treatment groups (Fig. 2C). Unexpectedly, two control (unvaccinated) macaques remained seronegative at termination of the study, which was 40 wk after virus introduction.
RhCMV DNA in plasma and oral swab samples was measured by qPCR to determine if vaccination reduced systemic virus replication or shedding in saliva. There was no significant difference in either the average plasma viral loads postseroconversion or peak plasma viral DNA levels between groups (Fig. 2D), suggesting that vaccination had no impact on virus replication or viral dissemination. The average or peak virus detected in saliva also did not differ between vaccination groups (Fig. 2E). Together, these data demonstrate that the macaques did not vary between vaccine treatment groups in terms of RhCMV acquisition, systemic dissemination, or shedding.
Anti–vIL-10 Antibody Responses Delay RhCMV Acquisition.
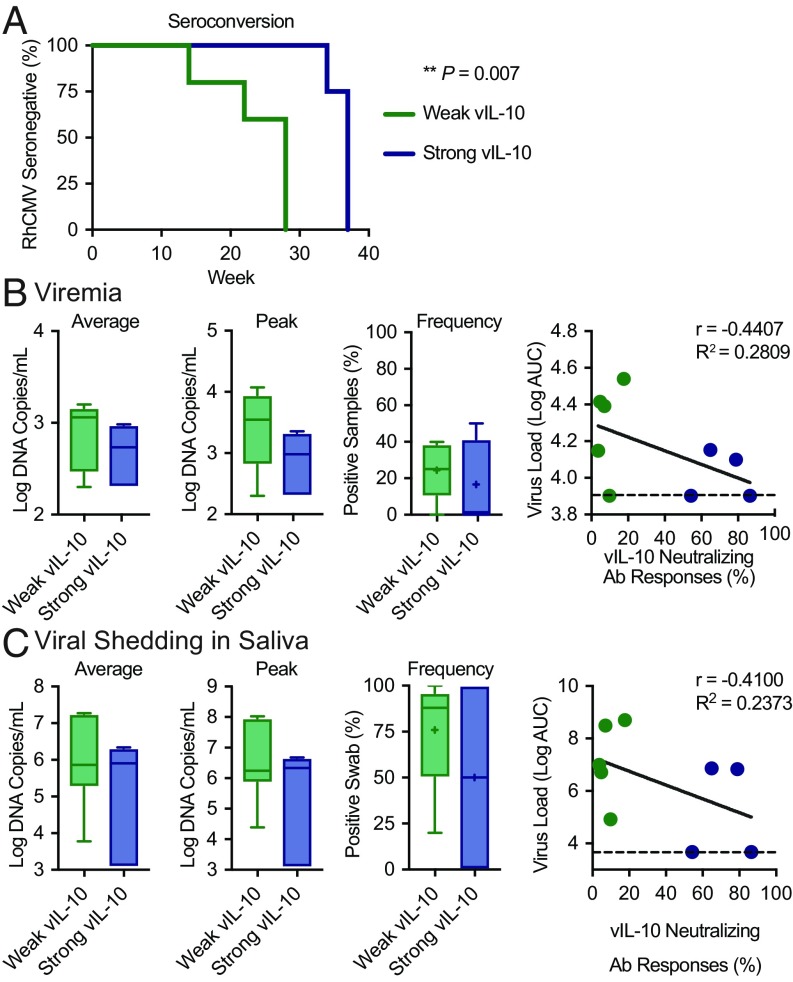
Based on the observation that macaques vaccinated with either vIL-10 or vIL-10 plus gB were stratified into two discrete populations [weak vIL-10 responders (below 20% vIL-10 neutralization titer) vs. strong vIL-10 responders (above 50% vIL-10 neutralization titer)] (Fig. 1B), we investigated whether the strength of these pre-RhCMV exposure responses impacted RhCMV acquisition, viremia, or shedding. We first assessed whether strong vIL-10 vaccine-induced neutralizing antibody responses impacted RhCMV transmission. Macaques with the strongest vIL-10–neutralizing antibody responses manifested a 12-wk delay in the timing of RhCMV seroconversion on average compared with macaques that developed weaker vIL-10–neutralizing antibody responses (Fig. 3A) (acquisition at average week 36 vs. 24 and median week 37 vs. 28). A Cox regression analysis demonstrated that this delay in RhCMV seroconversion was statistically significant (χ2 = 7.2 on one degree of freedom, P = 0.007).
Fig. 3.
Horizontal transmission of RhCMV was compared between vaccinees that developed strong vaccine-induced vIL-10–neutralizing antibody responses (blue; n = 5) and those that developed only weak vIL-10–neutralizing antibody responses from vaccination (green; n = 5). (A) RhCMV pp65 seroconversion was compared between the two groups of responders in a survival plot. The results of a Cox regression analysis are displayed. The control unvaccinated animals are not included in this analysis, because two did not seroconvert. The impact of these responses on viremia (B) and viral shedding in saliva (C) postseroconversion was assessed by analyzing samples longitudinally by qPCR for RhCMV DNA. Box and whisker plots display the results: the horizontal lines indicate the median, the + symbols indicate the average, the boxes indicate the interquartile range, and the stems represent 1.5 times the value of this range. Scatterplots compare the average AUCs throughout the study with vaccine-mediated vIL-10–neutralizing antibody responses. Linear regression analysis for each plot is also displayed (solid black lines). The dashed lines indicate the limit of detection by qPCR.
We next assessed whether viremia was impacted by vaccine-induced vIL-10 immune responses. Comparisons of average viremia, peak viremia, and the percentage of RhCMV qPCR-positive plasma samples postseroconversion determined no significant difference between the macaques with strong vIL-10 responses and those with weak vIL-10–neutralizing antibody responses (Fig. 3B, unpaired t tests). Anti–vIL-10 responses were also not significantly associated with a reduced area under the curve (AUC) for virus levels in plasma throughout the challenge phase, although a trend was observed (Fig. 3B). These data suggest that, on infection, the strength of vaccine-induced vIL-10 immune responses did not have a significant impact on viral replication or dissemination.
We also assessed the impact of vaccine-induced vIL-10 antibody responses on RhCMV shedding in saliva. We compared average shedding titers, peak shedding titers, and the percentage of qPCR-positive oral swab samples postseroconversion between animals with strong and weak vaccine-mediated vIL-10 responses (Fig. 3C, unpaired t tests). There were no significant differences in any of these comparisons (unpaired t tests). Additionally, strong vIL-10 responses were not significantly correlated with reduced total RhCMV levels in saliva samples over the course of the study (Fig. 3C).
Together, these data demonstrate that vaccination with recombinant vIL-10 elicited neutralizing antibody responses in a subset of macaques that significantly delayed the timing of RhCMV seroconversion. After infection, however, we found no significant impact on viral replication, dissemination, or shedding in saliva.
Strong vIL-10–Neutralizing Vaccine Responses Disrupt Long-Term RhCMV Immunity.
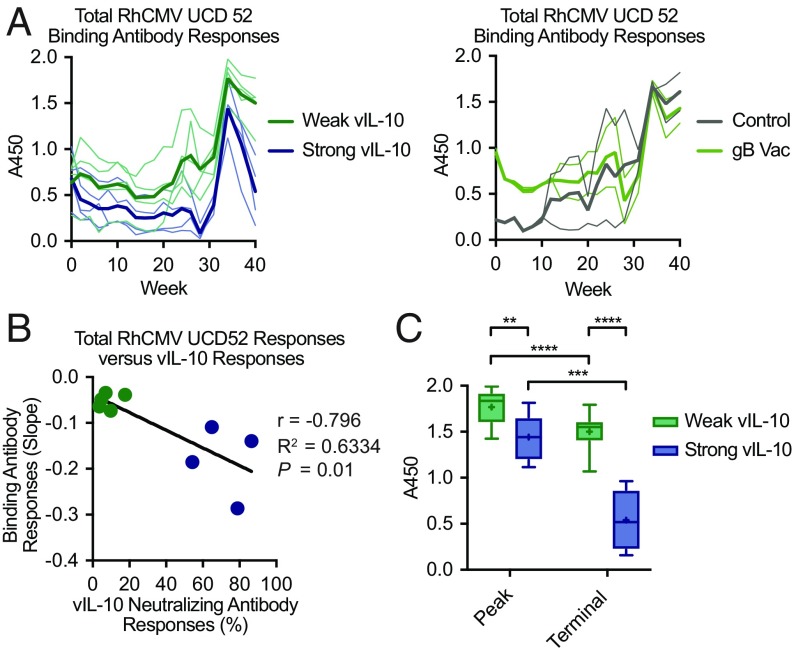
RhCMV infection normally stimulates stable, sustained binding antibody responses (Fig. 2A) (15). Unexpectedly, we observed a marked decline in binding antibody responses to RhCMV over the last 6 wk of the experiment (weeks 34–40) among macaques that developed strong vaccine-induced vIL-10–neutralizing antibody responses (n = 4) but not in those that developed only weak responses from vaccination (n = 5) (Fig. 4A). No such declines were seen in unvaccinated and subcutaneously inoculated macaques (Fig. 2A), unvaccinated control macaques (Fig. 4A), or macaques vaccinated with gB alone (Fig. 4A). Furthermore, the strengths of vIL-10–neutralizing antibody responses were significantly correlated with the slope of the decline in systemic long-term RhCMV binding antibody responses (Fig. 4B) (r = −0.7959, P = 0.01).
Fig. 4.
Systemic binding antibody responses to total RhCMV UCD52 antigen were compared between macaques that manifested strong vaccine-induced vIL-10–neutralizing antibody responses (blue; n = 4) and those that developed only weak responses (green; n = 5). (A) Longitudinal analyses of UCD52 antibody responses in plasma during the challenge phase of the study were analyzed by ELISA in duplicate. Thin lines display individual macaques within each group, while the bold lines represent mean responses. (B) The slope of the line representing UCD52 binding antibody responses over the final 6 wk of the study (weeks 34–40) was determined for each macaque and compared using a scatterplot. A correlation analysis demonstrated that increased strength of vIL-10–neutralizing antibody responses was associated with a greater decline in RhCMV binding antibody responses. The points represent individual macaques, and the solid line displays a linear regression analysis trendline. (C) Peak and terminal (week 40) RhCMV UCD52 antibody responses in strong and weak vIL-10 vaccine responders were compared. The boxplots with Tukey analysis display the group medians (horizontal lines), the group averages (+ symbols), the interquartile ranges (boxes), and 1.5 times the value of the ranges (stems). The results of t tests comparing these responses are also displayed. **P = 0.0006; ***P = 0.0002; ****P < 0.0001.
Next, we assessed peak and terminal (week 40) antibody responses in these macaques (Fig. 4C). The observed responses between these two groups differed both at peak (P = 0.006, t test) and at the end of the study (P < 0.0001, t test). We also observed differences within each group of macaques. While total RhCMV binding antibody responses dropped from peak to terminal samples in both strong and weak vIL-10 vaccine responders (P = 0.0002 and P < 0.0001, respectively; t tests), the difference between peak and terminal responses was greater among strong vIL-10 responders (mean of differences = −0.902 vs. −0.268, respectively). Together, these data demonstrated that vaccine-induced vIL-10–neutralizing antibody responses at the time of primary acquisition/exposure to RhCMV altered the long-term pattern of systemic immune responses to RhCMV infection.
Discussion
HCMV utilizes a broad repertoire of immunomodulatory strategies to manipulate host immunity in favor of viral persistence in addition to permitting reinfection in people with prior immunity to HCMV. Epithelial cells that line mucosal surfaces are important portals of viral entry and egress that play a key role in viral dissemination between hosts. To prevent HCMV infection, many HCMV vaccine strategies have focused on eliciting immunity to the viral envelope glycoproteins, particularly gB, used for viral entry into target cells. We additionally pursued vaccination with inactive recombinant vIL-10, genetically engineered to prevent binding to the cIL-10 receptor. In previous work, we found that vaccination with this protein reduced viral replication at the site of inoculation and reduced viral shedding (18–20). In this study, we investigated the hypothesis that combining these vaccine approaches would synergistically protect from horizontal RhCMV transmission in the macaque model by reducing the risk of RhCMV acquisition and also reducing viral shedding. We also designed a challenge protocol, introducing RhCMV to our study cohort through experimental inoculation of a small subgroup, which more closely recapitulates the repeated natural challenges to which humans are exposed.
Surprisingly, we found no significant protection in macaques vaccinated with recombinant gB (Fig. 2 C–E), although the vaccine elicited robust binding and neutralizing antibody responses comparable with those observed in naturally infected macaques (Fig. 1C). It is unclear why the anti-gB immunity that was elicited failed to protect the macaques from horizontal acquisition of challenge RhCMV from RhCMV-shedding cohorts. Two recent studies have suggested that neutralizing antibody responses are less central to efficacy of gB vaccination than nonneutralizing antibodies (23, 24). Previous studies demonstrated reduced viremia in macaques vaccinated with a gB DNA prime and either inactivated whole-virus boost (15) or gB MVA boost (16). In this study, we utilized a modified recombinant gB protein for priming and boosted with gB/Addavax as an adjuvant. Although comparable levels of both binding and neutralizing antibodies were elicited in those previous studies and in this study, there were important differences in the experimental designs. While our previous studies also utilized gB with a transmembrane domain deletion (15, 16), the gB in this study had an additional mutation in the furin protease cleavage site. Although the intent of this mutation was to stabilize the antigen, recent studies have suggested that this nonauthentic form of gB might present different epitopes and/or elicit different antiviral immunity than an authentic, prefusion form of gB (23, 24). The adjuvant that we used here, Addavax, is a squalene-based, oil-in-water formulation based on MF59, which was the adjuvant used in the human clinical gB vaccination study (8). Oil-in-water emulsion-based adjuvants have been demonstrated to elicit strong antibody responses, particularly against influenza virus (25) and recently against HIV in nonhuman primates (26). The antibody responses elicited in our study were strong, although not protective, supporting use of this adjuvant to encourage high-titer responses. Our studies also differed in the route of RhCMV challenge. While both of the previous studies utilized subcutaneous inoculation, our study used a horizontal transmission model involving repeated exposures to mucosal tissue from cohoused animals persistently shedding after experimental inoculation.
In contrast to the lack of protection from gB vaccination, vaccination with vIL-10 resulted in immune responses that, when sufficiently robust, were partially protective against horizontal RhCMV challenge. These responses were associated in this study with nonsignificant trends to reduced viremia and viral shedding in saliva after infection, echoing a previous study that found significant reductions in these parameters after subcutaneous challenge (20). The challenge model is a significant difference between the two studies. Our statistical survival analysis was based on a continuous challenge model (Cox’s proportional hazards model). It is possible, however, that, as the infection spread throughout our cohort, the risk of infection increased as additional animals began shedding virus. This would result in a greater exposure risk for the uninfected macaques, and therefore, the level of protection that we report here might be an underestimate of the vaccine efficacy. These considerations highlight the fact that our continuous challenge model makes it impossible to measure efficacy of vaccination against vIL-10 in the conventional way (i.e., as a reduction in the per challenge risk of CMV acquisition). Vaccine efficacy on this basis might be high if challenges were effectively very frequent (i.e., if vIL-10 vaccination prevented many potential transmission events each day). The potential clinical impact of human vaccination against vIL-10 depends on this per challenge risk reduction and on the rate of effective challenges occurring (e.g., in childcare centers).
We hypothesized that vIL-10 vaccination might mitigate horizontal transmission in a population by reducing shedding. Our finding that vIL-10 vaccination reduced RhCMV acquisition was somewhat surprising. Importantly, this protection was dependent on the development of strong vIL-10–neutralizing antibody responses, as vaccinees that developed only weak responses from vaccination were not protected (Fig. 3A). A reduction in vIL-10 at the site of infection due to the presence of strong vIL-10–neutralizing antibody responses could significantly alter innate immunity by reducing activation of the host cIL-10 pathway and thereby, restricting viral replication in a manner similar to that observed in vIL-10 knockout RhCMV infection (27). It is also possible that reduced IL-10 signaling at the time of challenge leads to enhanced T cell responses capable of controlling a nascent infection as has been demonstrated for lymphocytic choriomeningitis virus (28, 29). Indeed, subcutaneous inoculation of macaques with vIL-10 knockout, fibroblast-tropic RhCMV (strain 68–1) demonstrated alterations in both innate and adaptive immune responses relative to inoculation with the parenteral strain of RhCMV (27). In that study, the absence of vIL-10 resulted in both enhanced T cell responses and increased inflammatory cell infiltrates at the site of inoculation. Because activation of the cIL-10 pathway results in immune suppression and contributes to viral persistence (30), neutralizing antibody responses to vIL-10 could limit persistence by allowing host control of a nascent mucosal infection at the portal of entry.
Our finding that strong vIL-10–neutralizing antibody responses disrupt the development of long-term RhCMV antibody responses has not been previously reported. This result was not observed in any of the unvaccinated macaques subcutaneously inoculated with UCD52 (Fig. 2A), any of the macaques vaccinated with gB alone, or unvaccinated controls (Fig. 4A). We also have not observed declining antibody responses in any of our previous studies of RhCMV isolates that are competent for vIL-10 production (15, 31). The nonfunctional, recombinant version of vIL-10 used in our study does not bind to host cIL-10 receptors and also lacks functional activity (18, 19). Previous studies have demonstrated that the neutralizing antibody responses elicited from vaccination with these proteins do not cross-react with host IL-10 protein. It is, therefore, unlikely that our observations are the result of any direct impact of the immunogen on host IL-10 signaling. Of note, while two strong vIL-10 responders did not shed virus in saliva, two persistently shed virus through the end of the study. Thus, loss of systemic antibody responses to UCD52 was not due to uniform viral clearance, although it remains possible that neutralization of vIL-10 allows relative host control of infection that would attenuate antibody responses over time. It is also possible that an immunomodulatory effect of UCD52 replication itself triggers antibody decline among animals with strong vIL-10–neutralizing responses.
Circulating vIL-10 protein can be detected in peripheral blood (PB) of HCMV-infected people (32); furthermore, the level of vIL-10 in PB correlates with an increased level of cIL-10 (32). While the impact of cIL-10 on T cells, dendritic cells, and macrophages is not entirely clear (reviewed in ref. 33), its role in B cell proliferation and differentiation into plasma cells as well as its impact on immunoglobulin class switching are well established (34–37). The disruption in long-term antibody responses to RhCMV observed in macaques with strong vIL-10–neutralizing antibody responses (Fig. 4) could, therefore, be the result of decreased IL-10 signaling during RhCMV infection and reduced B cell proliferation, differentiation, and antibody production. Future studies will investigate this possibility.
In this study, we found that strong neutralizing antibody responses in a subset of vaccinated macaques reduced horizontal acquisition of RhCMV and significantly altered long-term immunity after those macaques became infected. Our findings suggest that immunization against vIL-10 could be an important immunomodulatory component of an effective CMV vaccine. In addition, our results have implications for ongoing investigations of CMV as a vaccine delivery vector, as the recipients of such vaccines will differ in their preexisting immunity to vIL-10 (38). Our experimental model of horizontal CMV transmission is also a significant advance in the nonhuman primate modeling of HCMV. Additional development and testing of CMV vaccination strategies using this model could spur progress toward the decades-long goal of controlling the spread of CMV.
Materials and Methods
Experimental Animals.
This study was approved in advance by the University of California, Davis (UC Davis) Institutional Animal Care and Use Committee (IACUC) and was performed at the California National Primate Research Center (CNPRC). Housing, medical care, and all procedures were performed according to UC Davis IACUC-established policies. UC Davis has an Animal Welfare Assurance on file with the NIH Office of Laboratory Animal Welfare and is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Animals were administered 10 mg/kg body weight ketamine-HCl intramuscularly when necessary for immobilization. Analgesics were administered at the discretion of the CNPRC veterinary staff in an effort to minimize pain and discomfort.
Twenty-four weanling rhesus macaques (Macaca mulatta) were selected from the conventional colony of the CNPRC. They were moved to RhCMV-free housing in pairs until confirmation of RhCMV-negative serostatus by enzyme-linked immunosorbent assay (ELISA) and PCR screening. They were then assembled in a group housing enclosure. At the start of the study, 22 of the macaques were juveniles and ranged in age from 11 to 17 mo. The remaining two macaques were used to establish social order within the group and were 4 and 10 y old. PB and saliva samples were collected and stored at −80 °C as previously described (20).
Immunogens.
Two vIL-10 immunogens (rhcmvIL-10M1 and rhcmvIL-10M2) that lack IL-10 biological activity were used for vIL-10 vaccination (SI Appendix). Previous studies demonstrated that vaccination with a mixture of RhcmvIL-10M1 and M2 proteins elicits vIL-10 binding and neutralizing antibodies (18, 20). Recombinant RhCMV gB protein for vaccination, containing an alteration in the furin site, was produced as described in SI Appendix.
Vaccinations and Virus.
Vaccinations consisted of recombinant protein delivered with Addavax (Invivogen) as an adjuvant via intramuscular delivery. Macaques vaccinated with gB received 50 μg of protein. Macaques vaccinated with vIL-10 received 50 μg of both rhcmvIL-10M1 and rhcmvIL-10M2 proteins. The four inoculated macaques were infected intramuscularly in the back with 105 plaque-forming units of RhCMV UCD52, an epithelial- and endothelial-tropic RhCMV isolate (21).
ELISA.
ELISAs were performed on plasma samples (1:100 dilution) as previously described for the vaccine strain, RhCMV 68–1 (21), and pp65 (31). Total UCD52 binding antibody responses were measured following the same procedure described for RhCMV 68–1 ELISA, except that lysate generated from telomerized rhesus fibroblasts cells infected with UCD52 was used as the coating antigen.
Neutralizing Antibody Assays.
RhCMV-neutralizing antibody responses were measured in plasma using RhCMV 68–1 expressing green fluorescent protein according to previously published protocols (16). vIL-10–neutralizing antibody responses were measured in plasma (1:1,000 dilution) according to previously published protocols (18).
qPCR.
DNA was extracted from plasma and oral swab samples using a QIAsymphony DNA extraction system (Qiagen) according to the manufacturer’s protocols. Extracted samples were stored at −80 °C until PCR analysis. RhCMV DNA was quantified in extracted samples using real-time PCR for UL132 according to previously published protocols (21, 39).
Statistics.
Statistical analyses were performed using GraphPad Prism version 8.0.1 for MacOS, GraphPad Software (https://www.graphpad.com/). Multiple-group comparisons were performed using one-way ANOVA. Comparisons between two groups were performed using unpaired Student’s t tests, except for the comparison between peak and terminal ELISA responses (Fig. 4C), which utilized paired t tests. Cox survival analysis (Fig. 3A) was performed in the R programming environment.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants AI097629 (to M.R.W. and P.A.B.), AI049342 (to M.R.W. and P.A.B.), AI143554 (to M.R.W., P.A.B., and D.J.H.-O.), AI131568 (to P.A.B. and D.J.H.-O.), and OD P51 OD011107 (to the CNPRC) and the Margaret Deterding Infectious Disease Research Support Fund (P.A.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1903317116/-/DCSupplemental.
References
- 1.Cannon M. J., Hyde T. B., Schmid D. S., Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev. Med. Virol. 21, 240–255 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hertel L., Human cytomegalovirus tropism for mucosal myeloid dendritic cells. Rev. Med. Virol. 24, 379–395 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvin A. M., Fast P., Myers M., Plotkin S., Rabinovich R.; National Vaccine Advisory Committee , Vaccine development to prevent cytomegalovirus disease: Report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 39, 233–239 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Crough T., Khanna R., Immunobiology of human cytomegalovirus: From bench to bedside. Clin. Microbiol. Rev. 22, 76–98 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffiths P. D., Burden of disease associated with human cytomegalovirus and prospects for elimination by universal immunisation. Lancet Infect. Dis. 12, 790–798 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Hanshaw J. B., Congenital cytomegalovirus infection: A fifteen year perspective. J. Infect. Dis. 123, 555–561 (1971). [DOI] [PubMed] [Google Scholar]
- 7.Britt W. J., Maternal immunity and the natural history of congenital human cytomegalovirus infection. Viruses 10, E405 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pass R. F., et al. , Vaccine prevention of maternal cytomegalovirus infection. N. Engl. J. Med. 360, 1191–1199 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths P. D., et al. , Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: A phase 2 randomised placebo-controlled trial. Lancet 377, 1256–1263 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Britt W. J., Mach M., Human cytomegalovirus glycoproteins. Intervirology 39, 401–412 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Wille P. T., Wisner T. W., Ryckman B., Johnson D. C., Human cytomegalovirus (HCMV) glycoprotein gB promotes virus entry in trans acting as the viral fusion protein rather than as a receptor-binding protein. MBio 4, e00332-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D., Shenk T., Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl. Acad. Sci. U.S.A. 102, 18153–18158 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wussow F., et al. , A vaccine based on the rhesus cytomegalovirus UL128 complex induces broadly neutralizing antibodies in rhesus macaques. J. Virol. 87, 1322–1332 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deere J. D., Barry P. A., Using the nonhuman primate model of HCMV to guide vaccine development. Viruses 6, 1483–1501 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abel K., et al. , A heterologous DNA prime/protein boost immunization strategy for rhesus cytomegalovirus. Vaccine 26, 6013–6025 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abel K., et al. , Vaccine-induced control of viral shedding following rhesus cytomegalovirus challenge in rhesus macaques. J. Virol. 85, 2878–2890 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockridge K. M., et al. , Primate cytomegaloviruses encode and express an IL-10-like protein. Virology 268, 272–280 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Logsdon N. J., Eberhardt M. K., Allen C. E., Barry P. A., Walter M. R., Design and analysis of rhesus cytomegalovirus IL-10 mutants as a model for novel vaccines against human cytomegalovirus. PLoS One 6, e28127 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eberhardt M. K., et al. , Host immune responses to a viral immune modulating protein: Immunogenicity of viral interleukin-10 in rhesus cytomegalovirus-infected rhesus macaques. PLoS One 7, e37931 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eberhardt M. K., et al. , Vaccination against a virus-encoded cytokine significantly restricts viral challenge. J. Virol. 87, 11323–11331 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oxford K. L., et al. , Open reading frames carried on UL/b′ are implicated in shedding and horizontal transmission of rhesus cytomegalovirus in rhesus monkeys. J. Virol. 85, 5105–5114 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang W. L., Tarantal A. F., Zhou S. S., Borowsky A. D., Barry P. A., A recombinant rhesus cytomegalovirus expressing enhanced green fluorescent protein retains the wild-type phenotype and pathogenicity in fetal macaques. J. Virol. 76, 9493–9504 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson C. S., et al. , HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions. Proc. Natl. Acad. Sci. U.S.A. 115, 6267–6272 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baraniak I., et al. , Protection from cytomegalovirus viremia following glycoprotein B vaccination is not dependent on neutralizing antibodies. Proc. Natl. Acad. Sci. U.S.A. 115, 6273–6278 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkins A. L., et al. , AS03- and MF59-adjuvanted influenza vaccines in children. Front. Immunol. 8, 1760 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips B., et al. , Adjuvant-dependent enhancement of HIV env-specific antibody responses in infant rhesus macaques. J. Virol. 92, e01051-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang W. L., Barry P. A., Attenuation of innate immunity by cytomegalovirus IL-10 establishes a long-term deficit of adaptive antiviral immunity. Proc. Natl. Acad. Sci. U.S.A. 107, 22647–22652 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ejrnaes M., et al. , Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 203, 2461–2472 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks D. G., Lee A. M., Elsaesser H., McGavern D. B., Oldstone M. B., IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J. Exp. Med. 205, 533–541 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson E. B., Brooks D. G., The role of IL-10 in regulating immunity to persistent viral infections. Curr. Top. Microbiol. Immunol. 350, 39–65 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yue Y., Kaur A., Zhou S. S., Barry P. A., Characterization and immunological analysis of the rhesus cytomegalovirus homologue (Rh112) of the human cytomegalovirus UL83 lower matrix phosphoprotein (pp65). J. Gen. Virol. 87, 777–787 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Young V. P., et al. , Modulation of the host environment by human cytomegalovirus with viral interleukin 10 in peripheral blood. J. Infect. Dis. 215, 874–882 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannino M. H., et al. , The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 367, 103–107 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Rousset F., et al. , Long-term cultured CD40-activated B lymphocytes differentiate into plasma cells in response to IL-10 but not IL-4. Int. Immunol. 7, 1243–1253 (1995). [DOI] [PubMed] [Google Scholar]
- 35.Rousset F., et al. , Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 89, 1890–1893 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy Y., Brouet J. C., Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J. Clin. Invest. 93, 424–428 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brière F., Servet-Delprat C., Bridon J. M., Saint-Remy J. M., Banchereau J., Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J. Exp. Med. 179, 757–762 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen S. G., et al. , Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15, 293–299 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Assaf B. T., et al. , Limited dissemination and shedding of the UL128 complex-intact, UL/b′-defective rhesus cytomegalovirus strain 180.92. J. Virol. 88, 9310–9320 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.