Abstract
The aim of this work was to compare the antimicrobial activity against Paenibacillus larvae and the antioxidant capacity of two Laurus nobilis L. extracts obtained by different extraction methods. The hydroalcoholic extract was moreover added as supplementary diet to bees in field conditions to test behavioural effects and colony strength. Both laurel extracts were subjected to different phytochemical analysis to identify their bioactive compounds. Antimicrobial activity was analyzed by the minimal inhibitory concentration (MIC) determination by means the agar dilution method. The hydroalcoholic extract (HE) was able to inhibit the bacterial growth of all P. larvae strains, with 580 µg/mL mean value. This better antibacterial activity in relation to the essential oil (EO) could be explained by the presence of some phenolic compounds, such as flavonoids, evidenced by characteristic bands resulting from the Fourier Transform Infrared Spectroscopy (FTIR) analysis. Antioxidant activities of the extracts were evaluated by 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical-scavenging ability and ferric reducing antioxidant power (FRAP) assays. The HE showed the highest antioxidant activity as measured by DPPH, with IC50 values of 257 ± 12 μg/mL. The FRAP assay method showed that the HE was 3-fold more effective reducing agent than the EO. When the bee colonies were supplied with laurel HE in sugar paste an improvement in their general condition was noticed, although neither the hygienic behavior nor the proportions of the breeding cells varied statistically due to the treatment. In conclusion, the inhibition power against P. larvae attributable to the phenolic compounds, the antioxidant capacity of the HE, and the non-lethal effects on adult honey bees on field trials suggest the HE of laurel as a promising substance for control American foulbrood disease.
Keywords: Laurus nobilis, Paenibacillus larvae, Apis mellifera, Colony strength, Antimicrobial activity, Antioxidant capacity, Hygienic behavior
1. Introduction
Apis mellifera colonies are threatened by numerous pathogens, including viruses, fungi, bacteria, and protozoa. One of the most contagious and destructive diseases is American foulbrood (AFB), caused by the Gram-positive, spore-forming bacterium Paenibacillus larvae (Genersch et al., 2006). The AFB affects the larval stage of the honey bees. Because of the resilience and long life of the pathogen spores (Lindström, 2008) is difficult to prevent. The burn of the affected combs is used in most of the European Community countries as an extreme alternative to control this pest. However, a common strategy employed in some other countries is the use of antibiotics (Alippi, 1996). Oxytetracycline hydrochloride has been used for decades to prevent and treat AFB. Nevertheless, tetracycline resistant strains have been identified in USA, Canada, and Argentina (Alippi, 1996, Miyagi et al., 2000, Evans, 2003, Reynaldi et al., 2008). Moreover, its residues can persist in honey affecting its quality for human consumption, reducing the honey bee lifespan, and increasing the risk of emergence of new resistant strains (Thompson et al., 2005, Johnson and Jadon, 2010). Thus, the development of new strategies to treat the AFB infected colonies become necessary.
Some natural alternatives including essential oils (Gende et al., 2008, Gende, 2009, Ansari et al., 2016), plant extracts (Flesar et al., 2010, González and Marioli, 2010, Damiani et al., 2014), propolis extracts (Bastos et al., 2008) and bee venom (Fernández et al., 2014) as well as their purified individual components (Gende et al., 2008, Gende et al., 2014, Bilikova et al., 2013) have proved to inhibit the P. larvae growth. Most of them only have been in vitro tested. Much of components of a plant extract, such as flavonoids, are known to have also a high antioxidant capacity (Škerget et al., 2005, Kaurinovic et al., 2010). Farjan et al. (2012) showed favorable responses as higher contents of protein and enzymes and less loss of bees during the winter.in honey bees fed supplemented with antioxidants. Johnson et al. (2013) reported an increased bee mortality associated with an oxidative stress increment resulting of interactions between fungicides and acaricides. This phenomenon would occur by the production of reactive oxygen species (ROS) that lead to damage of molecules by lipid peroxidation (Hsieh and Hsu, 2013).
Bay laurel (Laurus nobilis L., Lauraceae) is an aromatic herb used extensively to add a distinctive aroma and flavor to food. Their leaves can be used as a spice, to treat a variety of complaints, neuralgia, and intestinal cramps and for its beneficial effect upon the digestive system (Chaudhry and Tariq, 2006). Its antimicrobial activity against both gram-positive and gram-negative bacterial species has been also demonstrated (El Malti and Amarouch, 2009). From the laurel leaves can be obtained a complex mixture of phytochemicals according to the extraction process that was used. The essential oil is the hydrophobic and volatile fraction recovered by distillation and their chemical composition commonly included mono- and sesquiterpenes (Di Leo Lira et al., 2009, Ortiz et al., 2009); while by alcoholic or hydroalcoholic extraction, polar compounds such as flavonoids, saponins, alkaloids, and polyphenols are usually obtained (Škerget et al., 2005).
Thereby, in the framework of an integrated pest management, the aim of this work was to compare the antimicrobial activity against P. larvae and the antioxidant capacity of two extracts from L. nobilis obtained by different extraction methods. Moreover, L. nobilis hydroalcoholic extract was added as supplementary diet to bees in field conditions to test behavioral effects and colony strength.
2. Materials and methods
2.1. Plant material and extracts of Laurus nobilis
Laurel leaves were collected in Henderson (36°10′S; 61°20′W), Buenos Aires province, Argentina. The plant material was identified by J. Ringuelet, and a voucher sample was deposited in the collection of the Herbarium of the National University of La Plata (collection name: Argentina, Buenos Aires province, Henderson, 2007, Ringuelet s.n., LPAG) (Di Leo Lira et al. 2009). The essential oil (EO) was obtained by steam distillation of dried leaves and was provided by Esencias Nuestras, Planned Industrial Zone of Henderson, Buenos Aires province, Argentina (E. Tkacik Company).
The hydroalcoholic extract (HE) was obtained mixing 100 g of laurel leaves (dried at 20–27 °C and 20% RH) with 500 ml of 80% (v/v) ethanol (extraction fluid). The mixture was kept for 5 days in tightly sealed vessels at room temperature, protected from sunlight, and shaken several times daily. This blend was vacuum filtered and further extractions of the leaf residues were repeated until a clear supernatant was obtained. The extracted liquid was subjected to evaporation in a stove (40 °C) to remove the ethanol until getting a semisolid extract (Porrini et al., 2011). Both extracts were stored in screw-capped dark glass vials at 4 °C until further testing.
2.2. Spectroscopic characterization
2.2.1. Fourier transform infrared spectroscopy (FTIR)
FTIR spectra were obtained in transmission mode using a Nicolet 6700 spectrophotometer (Thermo Scientific), in the mid infrared region from 4000 to 600 cm−1, with a resolution of 4 cm−1 and 32 scans. The EO was analyzed as a thin film between two NaCl windows while the HE was ground with KBr powder and pressed into transparent disks.
2.2.2. UV–visible spectroscopy
The EO and HE were subjected to ultraviolet–visible (UV–Vis) spectroscopy at a concentration of 200 µg/mL in ethanol, using a Shimadzu UV-2101PC scanning spectrophotometer.
2.3. Total phenolic content
2.3.1. Essential oil
For the essential oil it was considered the phenol content previously reported by chromatographic analysis by Di Leo Lira (2009).
2.3.2. Hydroalcoholic extract
The amount of total phenolics was determined by the procedure of Folin–Ciocalteu described by Amerine and Ough (1980). The HE suspended in 0.5 mL 96% (v/v) ethanol was mixed with 30 mL of water and 2.5 mL of Folin–Ciocalteu’s reagent (Merck 9001, Darmstadt, Germany). After 30 s, 7.5 mL of 20% w/v sodium carbonate solution were added and the solution was mixed and diluted with water to a final volume of 50 mL. After 2 h in the dark at 20 °C, the absorbance of the samples was measured at 765 nm using a Shimadzu UV-1200 spectrophotometer (Shimadzu, Kyoto, Japan). The phenolic content was expressed in mg of gallic acid (GA) per gram of sample. The standard curve (50–750 mg L−1) was based on analytical grade GA (Sigma-Aldrich, Steinheim, Germany).
2.4. Antioxidant capacity
2.4.1. DPPH radical scavenging activity
Radical scavenging activity was determined according to the method of Yen and Hsieh (1995) with slight modifications. Four hundred µL of each EO or HE solution in methanol (0–4000 µg/mL) were mixed with 2 mL of 0.06 mM solution of DPPH in methanol. Mixtures were vigorously shaken and then, were allowed to stand for 30 min at room temperature in the dark. The reduction of DPPH radical was measured at 517 nm using an UV–Visible spectrophotometer (Agilent 8453, China). DPPH radical scavenging activity (RSA) was calculated as follows:
Where A517 sample and A517 control are the sample and control absorbance, respectively. For control, ethanol was used instead of the sample. A lower absorbance of the reaction mixture indicate a higher DPPH radical-scavenging activity. To analyze the capability to capture free radicals, the IC50 was estimated as the concentration of antioxidant required to decrease the initial DPPH concentration at half. All tests were performed in triplicate.
2.4.2. Ferric reducing antioxidant power (FRAP) assay
Antioxidant activity of the extracts was determined by the method described by Oyaizu (1986). The EO and HE were dissolved in methanol (0–4000 µg/mL). One mL of each solution was mixed with 2.5 mL of phosphate buffer (0.2 mol/L, pH 6.6) and 2.5 mL of potassium hexacyanoferrate III (K3Fe (CN)6, 1% w/v). Each mixture was incubated at 50 °C for 20 min. Afterwards, 2.5 mL of 10% (w/v) trichloroacetic acid solution was added, and then, centrifuged at 5000 rpm for 10 min (Sartorius type 4–15, Germany). An aliquot of 2.5 mL of the upper layer was mixed with 2.5 mL of deionized water and 0.5 mL of 0.1% (w/v) FeCl3 solution, and the absorbance was measured at 700 nm in a UV–visible spectrophotometer (Agilent 8453, China). Increased absorbance of the reaction mixture indicates increased of the reducing power. Ascorbic acid (AA) was used as positive control and the final results were expressed as µg/mL of AA equivalents (AAE). All tests were performed in triplicate.
2.4.3. Data values obtained from both experiments were statistically analyzed by one-way analysis of variance (ANOVA)
Differences between pairs of means were assessed on the basis of confidence intervals using the Tukey test. The level of significance was p < 0.05.
2.5. Antimicrobial activity
2.5.1. Microorganisms and culture media
Bacterial strains of P. larvae were isolated from brood combs of beehives with AFB clinical symptoms corresponding to five localities in Argentina (Mechongué, La Plata, Vidal, Estafeta, Sierra de los Padres). Isolations were achieved on Mueller-Hinton broth, yeast extract, glucose, and sodium pyruvate (MYPGP) (Digman and Stahly, 1983) agar supplemented with 9 µg/mL of nalidixic acid to inhibit Paenibacillus alvei growth, and incubated under microaerobic conditions (5–10% of CO2). All strains were genotypically identified using PL2-Fw and PL2-Rv primers (Martínez et al., 2010), and characterized like ERIC I with ERIC1R and ERIC2 primers (Versalovic et al., 1994). Pure strains were maintained on MYPGP agar with 15% (v/v) glycerol until used. Two references strains were used from OIE Reference Laboratories for American foulbrood acquired in UB‐CIDEFI.
2.5.2. Determination of minimal inhibitory concentration by agar dilution method
Vegetative cells of P. larvae previously cultivated on MYPGP agar for 48 h at 35–37 °C were suspended in double distilled sterile water. The suspension was standardized according to FDA method (1998) and adjusted to 0.5 McFarland scale.
An aliquot of the EO or the HE, v/v or w/v, respectively, in 8% v/v propylene glycol (as an emulsifier) was mixed in MYPGP agar into Petri dishes until to achieve dilutions from 1000 to 125 µg/mL of each antimicrobial agent in the culture medium. As control, 8% v/v propylene glycol was mixed in MYPGP agar into Petri dishes. Then, 100 µL of the microbial suspension was added and spread on the surface of each plate. Incubation was at 35–37 °C for 48 h. Antimicrobial activity was tested in triplicate for each antimicrobial agent and strain. The lowest concentration of the antimicrobial substance that showed inhibition was considered as the minimal inhibitory concentration (MIC) (Lennette et al., 1987).
2.6. Apiary trial
The laurel HE effects were evaluated in field trials on A. mellifera colonies. These experiments were carried out from April to July 2013 in an experimental apiary from the Centro de Investigación en Abejas Sociales at the Universidad Nacional de Mar del Plata (Buenos Aires, Argentina) placed on route 11 km 32 (38°10′06″S, 57°38′10″W).
Twelve small healthy colonies (22 × 14 × 25 cm) of homogeneous bee population were selected. Each colony consists of four brood combs (18 × 10 cm) plus one feeder. It was not allowed that the brood/food (nectar and pollen) ratio exceed the 4:1 proportion. Thus, a colony had approximately 1000 brood cells with mixed development breeding, and 2000 worker bees (OECD, 2007). To minimize the genetic variability, young sister queens from the same progeny mated in the same place were used. Two groups with six mini-hives each one were randomly established. The first group received weekly one oral treatment with candy paste (10 g paste of glucose + sucrose) plus laurel HE at a concentration of 2000 μg/mL for three weeks. This concentration was selected according to previous data obtained in Gende, 2009, Porrini et al., 2011. The second group received only 10 g sugar paste once a week for three weeks as controls. Thus, the total assay comprised a complete development cycle of workers.
2.6.1. Colony strength estimation
The colony strength was weekly assessed during all the trial. On both treatment groups were recorded: adult bee population (Liebefeld estimation method), egg laying quality, and ratio between areas with food (pollen and honey), and with uncapped and capped brood cells. These ratio assessments were performed by estimating 4 sub-areas for all combs (both sides) into each hive (Burgett and Burikam, 1985).
2.6.2. Hygienic behavior
Once a week, a 2 × 2 cm area on the central comb was marked from each colony. This fragment contained about 10 ± 2 capped brood cells. Each cell from this area was centrally pierced using a needle until killing the inside pupa, and then the comb was returned to their original place. After 24 h, the number of pierced cells that were Opened and Cleaned (OC), Opened but Not Cleaned (ONC), and Not Opened (NO) was counted.
2.6.3. Statistical analysis
Generalized linear mixed models (GLMMs) were used in order to include “hives” as a random effect. These models have a negative binomial distribution. Model selection and parameter estimation were performed using the Akaike information criterion for small samples (AICc) (Crawley, 2005, Symonds and Moussalli, 2011). To quantify the plausibility of each model with the data and the set of models, the AICc was calculated. Thus, the models that best describe the data are those with a lower AICc. Since the treatments were evaluated in different hives, this variable was added as random. These statistical analyses were performed with R software (R Development Core Team, 2012).
3. Results and discussion
3.1. Plant material and extracts of Laurus nobilis
The yield of the EO (green colored) obtained by steam distillation was 0.9 ± 0.5% v/w while the HE (dark semisolid) was 20% w/w.
3.2. Spectroscopic characterization
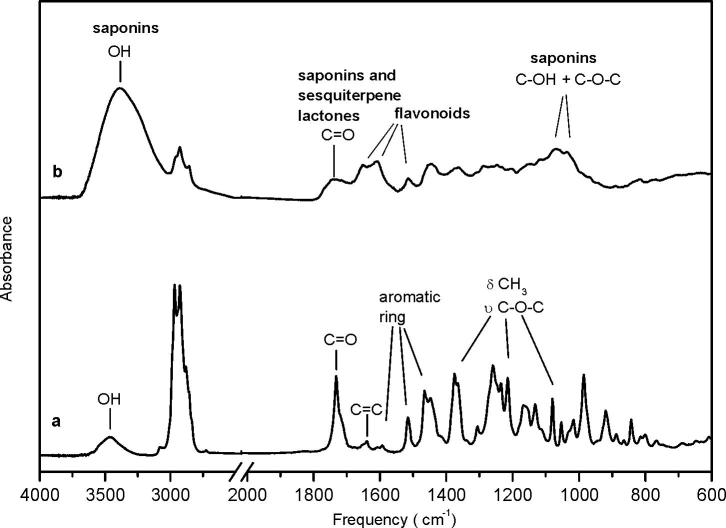
The infrared spectroscopy allowed identifying signals that could be assigned to the main constituents of the EO and HE (Fig. 1), as they have functional groups that absorb IR light at characteristic frequencies. The EO spectrum showed a broad band with a maximum at 3463 cm−1, characteristic of OH stretching from linalool and terpineol; a sharp and intense peak at 1724 cm−1 assigned to carbonyl stretching from terpinyl acetate; and a smaller one but broad at 1639 cm−1 related to C C stretching from terpene compounds such as β-caryophyllene, linalool, α- and β-pinene, sabinene, and myrcene (Schulz and Baranska, 2007). Bands observed at 1600, 1515 and 1465 cm−1 can be associated to the aromatic ring from methyl eugenol (Wang and Sung, 2011). Peaks at 1375, 1214 and 1079 cm−1 evidenced the presence of 1,8-cineol, assigned to methyl bending and C—O—C asymmetric and symmetric stretching of this compound (Baranska et al., 2005). In summary, the infrared spectroscopy results suggest that the main constituents of the laurel EO, already reported by Di Leo Lira et al., (2009) may be present.
Fig. 1.
Infrared spectra of: (a) the essential oil and (b) the hydroalcoholic extract of Laurus nobilis.
On the other hand, from the IR analysis it can be inferred that the HE contains sesquiterpene lactones, flavonoids, and, possibly, saponins (Fig. 1). A broad band with a maximum at 1724 cm−1, can be assigned to carbonyl stretching in sesquiterpene lactones (Dall’Acqua et al., 2006, Julianti et al., 2012) but also in saponins, and the peak at 1615 cm−1 with a shoulder at 1646 cm−1, together with another one at 1515 cm−1 suggest the presence of flavonoids (Heneczkowski et al., 2001). An peak intensity at 3387 cm−1 due to OH stretching and additional bands in the 1070–1030 cm−1 region, associated to C—OH and C—O—C linkages in oligosaccharides (Kareru et al., 2008) let us infer that this extract also contains saponins.
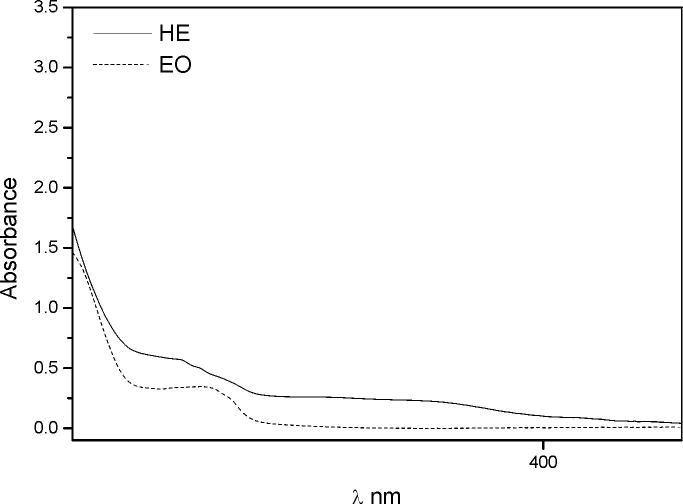
The UV spectroscopy detects chromophore groups responsible for absorbing the UV light. The UV absorption curves of both extracts were different. The EO showed a peak with a maximum at 270 nm while the UV–Vis spectra from the HE included two absorbance peaks in the 316–349 nm range (Fig. 2). Peaks observed for the HE could be assigned to flavones and flavonols (Martínez, 2005) while the absorption peak for the EO could be due to its main compound, the 1,8-cineol. Thus, as it was expected, the spectroscopic analysis confirmed that the EO is rich in terpenoids while the HE contains more polar compounds as flavonoids, sesquiterpene lactones, and saponins.
Fig. 2.
UV spectra of: (EO) the essential oil and (HE) the hydroalcoholic extract of Laurus nobilis.
3.3. Antioxidant capacity
In the iron (III)-reduction assay, the general ability of an extract to donate electrons is tested whereas, in the DPPH assay, hydrogen atoms are involved as well. DPPH is a stable nitrogen-centered free radical, whose colour change from violet to yellow upon reduction by either the process of hydrogen -or electron- donation. Substances which are able to perform this reaction can be considered as antioxidant and therefore radical scavengers (Brand-Williams et al., 1995).
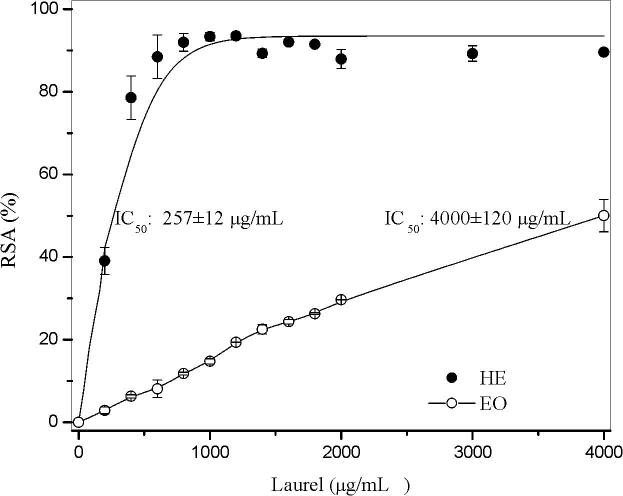
The DPPH radical scavenging of both extracts is shown in Fig. 3. In the case of laurel EO, the DPPH radical scavenging activity was significantly lower than the HE one (IC50 4000 ± 120 µg/ml vs 257 ± 12 µg/mL, respectively), in concordance with the lower amount of phenolic compounds present in the essential oil (Goudjil et al., 2015). Hinneburg et al., (2006) showed an IC50 of 490 µg/mL for a hydrodistilled laurel extract. The differences with our results could be associated with the extraction method and the essential oil chemotypes (Chizzola et al., 2008). The phenolic chemotypes express a higher antioxidant capacity than those non-phenolic ones (Goudjil et al., 2015).
Fig. 3.
Radical scavenging activity (RSA%) and Index concentration (IC50) values of essential oil (EO) and hydroalcoholic extract (HE) of Laurus nobilis.
For the HE, the maximum RSA was achieved around 1000 µg/mL and the IC50 values obtained was 257 ± 12 µg/mL. Kaurinovic and co-workers (2010) analyzed antioxidant activities of different extracts from laurel leaves; they found IC50 values ranged from 81 to 182 µg/mL, depending on the solvent used for the extraction. These authors analyzed the extraction with four solvents: ether, chloroform, ethyl acetate, and n-butanol. Prior to the DPPH analyzes they dissolve the extracts in ethanol. The n-butanol extract exhibited the weakest inhibitory effect (IC50 182 µg/mL) due to the lowest flavonoids content. In our work, the results obtained are in agreement with those reported by the authors mentioned before.
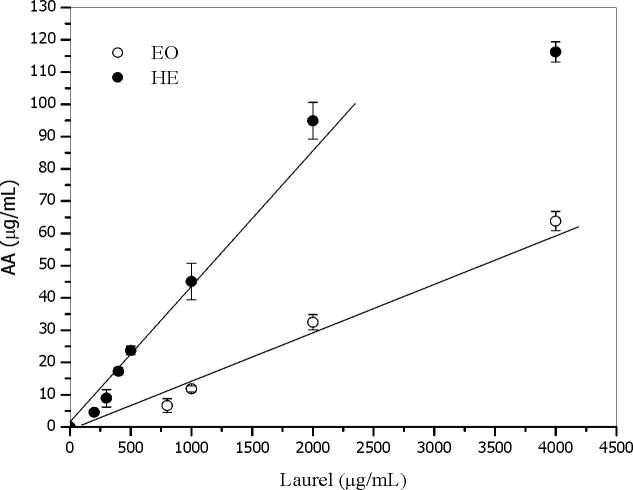
Fe (III) reduction is often used as an indicator of electron-donating activity, which is an important mechanism of phenolic antioxidant action (Yildirim et al., 2001). The iron (III) to iron (II)-reducing activity is expressed as AA equivalents (µg/mL AA). Some authors demonstrated that the iron (III)-reducing activity is correlated with the content of total phenols (Hinneburg et al., 2006, Ortiz et al., 2009, Kaurinovic et al., 2010). The reducing power of the EO and the HE determined by FRAP assay is shown in Fig. 4. The IC50 value for AA (90 ± 10 µg/mL) was significantly minor than those for both extracts. The FRAP method showed that the laurel HE is a 3-fold more effective reducing agent than the EO, according to the relationship between the slopes (Fig. 4). As in the DPPH method, a decrease in the concentration caused a reduction in the reducing power for all samples. Clearly, the HE showed the highest reducing power probably associated with their phenolic content (110.43 mg gallic acid/g extract) in contrast with the low content of phenols of the EO (0.4–1% v/w of eugenol and 0.05% v/w of carvacrol) (Di Leo Lira et al., 2009).
Fig. 4.
Iron(III) to iron(II)-reducing activity (FRAP assay) of essential oil (EO) and hydroalcoholic extract (HE) of Laurus nobilis. Ascorbic acid (AA).
However, the HEs from other plant species have registered a higher antioxidant activity (i.e. IC50 Emex spinosus 20.73 and Thymus vulgaris 147.02 µg/mL) than the laurel HE (IC50 257 µg/mL) possibly due to a greater amount of flavonoids in their composition (Emam et al., 2010). The low antioxidant activity of the EO (50.0 ± 3.9% DPPH at 4000 µg/mL, Fig. 3) is due to its composition rich in terpenoids (Di Leo Lira et al., 2009); however, other authors have reported essential oils with more antioxidant power than the laurel EO e.i. the EOs of Lavandula stoechas and Mentha pulegium scavenged 63.05 ± 2.5% and 30.38 ± 0.8% at 10 μL/mL of the free radical DPPH, respectively, while Satureja calamintha EO showed the lowest ability of DPPH scavenging (22.01 ± 3.13% at 10 μL/mL) (Cherrat et al., 2014).
An efficient antioxidative system is of particular importance for insects with high metabolic rate, which naturally generates huge amounts of free radicals (Candy et al., 1997). Due to the extreme sensitivity of honey bees to oxidative stress, the application of antioxidant substances into colonies is promising. Farjan and co-workers (2012) showed that the supplementation of the honey bee diet with vitamin C like antioxidant increased their lifespan. In addition, Strachecka et al., (2014) showed that food supplementation with CoQ10 increased the survival of winter bees, suggesting that it could enhance their vitality. Some components of natural substances like essential oils have been defined as Generally Recognised As Safe (GRAS) food substances, and, thereby, it could be added as antioxidant dietary supplements to honey bee colonies without leaving residues in honey (Bogdanov et al., 2002).
3.4. Antibacterial activity
The MIC values for both extracts against the different strains are shown in Table 1. The HE was capable of the bacterial growth inhibiting. The MIC values for the different P. larvae strains exposed to the HE were according with the values reported by some authors (Gende et al., 2008, González and Marioli, 2010) and higher than other plant extracts (Flesar et al., 2010). The better antibacterial activity of the HE could be explained by the presence of some phenolic compounds, like flavonoids evidenced by characteristic bands from FTIR and UV analysis. It has been reported that phenolic compounds inhibit the peptidoglycan synthesis (Ogunlana et al., 1987), damage the microbial membrane structure (Cox et al., 2000), modify the bacterial membrane surface hydrophobicity (Türi et al., 1997), and modulate the quorum sensing (Gao and Teplitski, 2003). Ultee et al. (2002) hypothesized that the presence of a system of delocalized electrons is responsible for the antimicrobial activity of phenolic compounds.
Table 1.
Minimal inhibitory concentration (µg/mL) of the hydroalcoholic extract and the essential oil of Laurus nobilis against five Paenibacillus larvae isolated strains two references were used from OIE Reference Laboratories for American foulbrood acquired in (UB‐CIDEFI).
| P. larvae strains | Hydroalcoholic extract | Essential oil |
|---|---|---|
| Mechongué | 400 | >1000 |
| La Plata | 800 | >1000 |
| Vidal | 400 | >1000 |
| Estafeta | 500 | >1000 |
| Sierra | 800 | >1000 |
| PL15 | 500 | >1000 |
| PL33 | 800 | >1000 |
The MIC values obtained for the EO were higher than those reported by other authors. Damiani and co-workers (2014) obtained MIC values between 600–1200 µg/mL for the same laurel EO and Ansari and co-workers (2016) obtained MIC value of 1287.9 µg/mL for laurel EO, both using the microdilution method. The differences could be attributed to the use of different techniques for the MIC determination. The EO has volatile compounds which make that the antimicrobial activity depends on a combination of direct effects of volatilization and indirect effects by the culture medium (Moleyar and Narasimtram, 1986). In this work, it was not possible to determine MIC values below 1000 µg/mL. This could be due to the evaporation of volatile compounds by heating of the culture medium for the agar dilution method. Concentrations among 50–250 μg/mL of EOs from other plants have been able to inhibit the P. larvae growth (Alippi et al., 1996, Albo et al., 2001, Albo et al., 2003, Gende, 2009).
3.5. Apiary trial: Colony strength and hygienic behavior
Considering the results of the antimicrobial activity and the no deleterious or toxic effects of both laurel extracts during topical or oral administration on adult worker bees (Damiani et al., 2014, Porrini, 2013), a group of healthy bee colonies were provided with the laurel HE added to sugar paste, in order to study the effect of this differential feeding on the hive dynamics. The analysis of data about the variations in cell proportion (capped, uncapped, honey, pollen, and empty cells) due to the treatment, did not allow the setting to any statistical model for the treatment performed. This let us to infer that the variables analyzed were not affected by the treatment. The data registered for each cell state come from different hives, for that, the hive was regarded as a random effect. In most of the cases, the random effect was responsible for the variations (Table 2). It should be noted that while there were no differences between the hives fed with candy paste + extract and control food, a remarkable strength and health state of hives supplemented with the laurel extract was evidenced (data not shown). This additional benefit could be attributed to an antioxidant effect related to the phenolic compounds present in the HE.
Table 2.
Possible generalized linear mixed (GLM) models with binomial distribution explaining the variations of cell ratio: uncapped (U), capped (C), honey (H), bee bread (B) and empty (E) cells.
| Models | AIC (AIC null) |
|---|---|
| U ∼ treatment(Ex/Ctrl) + (1|Hive) | 27.49 (26.27) |
| C ∼ treatment(Ex/Ctrl) + (1|Hive) | 17.59 (16.91) |
| H ∼ treatment(Ex/Ctrl) + (1|Hive) | 11.42 (9.436) |
| B ∼ treatment(Ex/Ctrl) + (1|Hive) | 6.114 (4.121) |
| E ∼ treatment(Ex/Ctrl) + (1|Hive) | 28.95 (28.6) |
Random variable, expressed as (1|Hive). AICc, Akaike information criterion for small samples. In brackets: AICc of the corresponding null model for each variable evaluated. Ex: Laurel extract treatment; Ctrl: Control treatment.
At least one compound isolated from leaves, the 3α-acetoxyeudesma-1, 4(15), 11(13)-trien-12,6α-olide (AETO) has proven a potent neuroprotective and inhibitor of ROS formation in human cells (Koo et al., 2011) and could play a role in the antioxidant system of bees. Several bee behaviors that help to confer colony-level resistance against parasites and pathogens have been characterized, including the hygienic behavior (HB) (Spivak and Gilliam, 1993). It involves the detection of dead or diseased bees in brood cells, cell uncapping, and the removal of affected larvae or pupae by the nurse bees (Spivak and Reuter, 2001). Thus, the HB is considered a mechanism that enhances the resistance of colonies to common brood diseases such as AFB (Palacio et al., 2000). The GLMM analysis showed that any of the variables analyzed affected the HB in the mini-hives (AIC null lower than AICs of the models; Table 3). The random effect (1|Hive) explained the 100% of the variation in the data of hygienic behavior while the laurel extract addition and/or the time had no effect in the analysis. The mean percent values of removal were 75.36 ± 25.47% and 85.39 ± 20.01% for control and HE treatment, respectively. While significant differences between these values were not found, it could be observed a slight trend of increasing HB in the colonies fed with the HE. These discrete differences could be due to the use of concentrations and doses in the food that fail to produce significant changes in the dynamics of the colonies. More assays could be done supplying bee colonies a more concentrated laurel HE sugar paste or for a more prolonged time. Increased hygienic behavior is a desirable effect because it is a natural way to manage honey bee pathologies, especially those affecting brood.
Table 3.
Possible generalized linear mixed (GLM) models with binomial distribution explaining the variations of the cleaned cell ratio.
| MODELS | AIC | AICNULL |
|---|---|---|
| NC ∼ Treat + T + (1|Hive) | 44.69 | 39.98 |
| NC ∼ T + (1|Hive) | 43.9 |
Random variable, expressed as (1 | Hive). AICc: Akaike information criterion for small samples; AICc null of the corresponding null model.. NC: number of cleaned cells; Treat: control and Laurel extract treatments; T: time.
4. Conclusion
A better inhibition power against P. larvae, attributable to the presence of phenolic compounds, plus the antioxidant capacity of the laurel HE allow us to think in different forms of administration of this botanical extract in honey bee colonies for control purposes of American foulbrood in a safe way.
Acknowledgements
The authors would like to thank Enrique Tkacik for plant material and essential oil. This work was supported by UNMdP-Argentina, CONICET and PICT 1625 to LG.
Footnotes
Peer review under responsibility of King Saud University.
References
- Albo G.N., Cerimene E., Re M.S., De Giusti M.R., Alippi A.M. Loque americana. Ensayos de campo para evaluar la efectividad de algunos aceites esenciales. Vida Apícola. 2001;108:41–46. [Google Scholar]
- Albo G.N., Henning C., Ringuelet J., Reynaldi F.J., De Giusti M.R., Alippi A.M. Evaluation of some essential oils for the control and prevention of American Foulbrood disease in honey bees. Apidologie. 2003;34:1–11. [Google Scholar]
- Alippi A.M. Caracterización de aislamientos de Paenibacillus larvae mediante tipo bioquímico y resistencia a oxitetraciclina. Rev. Arg. Microbiol. 1996;28:197–203. [PubMed] [Google Scholar]
- Alippi A.M., Ringuelet J.A., Cerimele E.L., Re M.S., Henning C.P. Antimicrobial activity of some essential oils against Paenibacillus larvae, the causal agent of American Foulbrood Disease. J. Herbs. Spices Med. Plants. 1996;4(2):9–16. [Google Scholar]
- Amerine M.A., Ough C.S. Methods for analysis of must and wines. Wiley-Interscience Publication; New York: 1980. pp. 181–184. [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Usmani S., Al-Waili N., Nuru A., Sharma D., Khan K.A., Kaur M., Omer M. In vitro evaluation of the effects of some plant essential oils on Paenibacillus larvae, the causative agent of American foulbrood. Biotechnol. Biotechnol. Equip. 2016;30(1):49–55. [Google Scholar]
- Baranska M., Schulz H., Uhlemann U., Strehle M.A., Krüger H., Quilitzsch R., Foley W., Popp J. Vibrational spectroscopic studies to acquire a quality control method of eucalyptus essential oils. Biopolymers. 2005;78:237–248. doi: 10.1002/bip.20284. [DOI] [PubMed] [Google Scholar]
- Bastos E.M., Simone M., Jorge D.M., Soares A.E., Spivak M. In vitro study of the antimicrobial activity of Brazilian propolis against Paenibacillus larvae. J. Invertebr. Pathol. 2008;97(3):273–281. doi: 10.1016/j.jip.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Bilikova K., Popova M., Trusheva B., Bankova V. New anti-Paenibacillus larvae substances purified from propolis. Apidologie. 2013;44(3):278–285. [Google Scholar]
- Bogdanov S., Charrière J.D., Imdorf A., Kilchenmann V., Fluri P. Determination of residues in honey after treatments with formic and oxalic acid under field conditions. Apidologie. 2002;33:399–409. [Google Scholar]
- Brand-Williams W., Cuvelier M., Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995;28:25–30. [Google Scholar]
- Burgett M., Burikam I. Number of adult honey bees (Hymenoptera: Apidae) occupying a comb: a standard for estimating colony populations. J. Econ. Entomol. 1985;78:1154–1156. [Google Scholar]
- Candy D.J., Becker A., Wegener B. Coordination and integration of metabolism in insect flight. Comp. Biochem. Physiol. 1997;117B:497–512. [Google Scholar]
- Chaudhry N.M., Tariq P. Bactericidal activity of black pepper, bay leaf, aniseed and coriander against oral isolates. Pak. J. Pharm. Sci. 2006;19(3):214–218. [PubMed] [Google Scholar]
- Cherrat L., Espina L., Bakkali M., Pagán R., Laglaoui A. Chemical composition, antioxidant and antimicrobial properties of Mentha pulegium, Lavandula stoechas and Satureja calamintha Scheele essential oils and an evaluation of their bactericidal effect in combined processes. Innov. Food Sci. Emerg. Technol. 2014;22:221–229. [Google Scholar]
- Chizzola R., Michitsch H., Franz C. Antioxidative Properties of Thymus vulgaris Leaves: Comparison of Different Extracts and Essential Oil Chemotypes. J. Agric. Food Chem. 2008;56:6897–6904. doi: 10.1021/jf800617g. [DOI] [PubMed] [Google Scholar]
- Cox S.D., Mann J.L., Markham H.C., Bell J.E., Gustafson J.R., Warmington S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil) J. Appl. Microbiol. 2000;88:170–175. doi: 10.1046/j.1365-2672.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- Crawley M.J. Wiley; Chichester: 2005. Statistics: An Introduction using R; p. 342. [Google Scholar]
- Dall’Acqua, S., Viola, G., Giorgetti, M., Loi, M.C., Innocenti, G., 2006. Two new sesquiterpene lactones from the leaves of Laurus nobilis. Chem. Pharm. Bull. 54(8), 1187–1189. [DOI] [PubMed]
- Damiani N., Fernández N.J., Porrini M.P., Gende L.B., Álvarez E., Buffa F., Brasesco C., Maggi M.D., Marcangeli J.A., Eguaras M.J. Laurel leaf extracts for honey bee pest and disease management: antimicrobial, microsporicidal, and acaricidal activity. Parasitol. Res. 2014;113(2):701–709. doi: 10.1007/s00436-013-3698-3. [DOI] [PubMed] [Google Scholar]
- Development Core Team R. R foundation for Statistical Computing; Vienna, Austria: 2012. R: A language and environment for statistical computing. [Google Scholar]
- Di Leo Lira P., Retta D., Tkacik E., Ringuelet J., Coussio J.D., van Baren C., Bandoni A.L. Essential oil and by-products of distillation of bay leaves (Laurus nobilis L.) from Argentina. Ind. Crop. Prod. 2009;30:259–264. [Google Scholar]
- Dingman D.W., Stahly D.P. Medium promoting sporulation of Bacillus larvae and metabolism of medium components. Appl. Environ. Microbiol. 1983;46:860–869. doi: 10.1128/aem.46.4.860-869.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Malti J., Amarouch H. Antibacterial effect, histological impact and oxidative stress studies from Laurus nobilis extract. J. Food Quality. 2009;32:190–208. [Google Scholar]
- Emam A.M., Mohamed M.A., Diab Y.M., Megally N.Y. Isolation and structure elucidation of antioxidant compounds from leaves of Laurus nobilis and Emex spinosus. Drug Discov. Ther. 2010;4(3):202–207. [PubMed] [Google Scholar]
- Evans E. Diverse origins of tetracycline resistance in the honey bee bacterial pathogen Paenibacillus larvae. J. Invertebr. Pathol. 2003;83 doi: 10.1016/s0022-2011(03)00039-9. 56 50. [DOI] [PubMed] [Google Scholar]
- Farjan M., Dmitryjuk M., Lipiński Z., Biernat-Łopieńska E., Żółtowska K. Supplementation of the honey bee diet with vitamin C: The effect on the antioxidative system of Apis mellifera carnica brood at different stages. J. Apicult. Res. 2012;51(3):263–270. [Google Scholar]
- Food and Drug Administration (FDA), 1998. App.3.73. In: Bacteriological Analytical Manual, 8th Ed. pp 581, AOAC International, Gaithersburg, MD.
- Fernández N.J., Porrini M.P., Podaza E.A., Damiani N., Gende L.B., Eguaras M.J. A scientific note on the first report of honeybeehoney bee venom inhibiting Paenibacillus larvae growth. Apidologie. 2014;45:719–721. [Google Scholar]
- Flesar J., Havlik J., Kloucek P., Rada V., Titera D., Bednar M., Stropnicky M., Kokoska L. In vitro growth-inhibitory effect of plant-derived extracts and compounds against Paenibacillus larvae and their acute oral toxicity to adult honey bees. Vet. Microbiol. 2010;145(2010):129–133. doi: 10.1016/j.vetmic.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Gao M., Teplitski J.B. Production of substances by Medicago truncatula that affect bacterial quorum sensing. Mol. Plant-Microbe In. 2003;16:827–834. doi: 10.1094/MPMI.2003.16.9.827. [DOI] [PubMed] [Google Scholar]
- Gende L.B. Facultad de Farmacia y Bioquímica. Universidad de Buenos Aires; Tesis Doctoral: 2009. Principales componentes de aceites esenciales relacionados con actividad antimicrobiana frente a Paenibacillus larvae; p. 232. [Google Scholar]
- Gende, L., Mendiara, S., Fernández, N.J., Van Baren, C., Di Leo Lira, A., Bandoni, A., Fritz, R., Floris, I., Eguaras, M.J., 2014. Essentials oils of some Mentha spp. and their relation with antimicrobial activity against Paenibacillus larvae, the causative agent of American foulbrood in honey bees, by using the bioautography technique. B. Insectol. 67 (1):13–20.
- Gende L.B., Principal J., Maggi M.D., Palacios S.M., Fritz R., Eguaras M.J. Extracto de Melia azedarach y aceites esenciales de Cinnamomun zeylanicum, Mentha piperita y Lavandula officinalis como control de Paenibacillus larvae. Zootecnia Trop. 2008;26(2):151–156. [Google Scholar]
- Genersch E., Forsgren E., Pentikainen J., Ashiralieva A., Rauch S., Kilwinski J., Fries I. Reclassification of Paenibacillus larvae subsp. Pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation. Int. J Syst. Evolut. Microbiol. 2006;56:501–511. doi: 10.1099/ijs.0.63928-0. [DOI] [PubMed] [Google Scholar]
- González M.J., Marioli J.M. Antibacterial activity of water extracts and essential oils of various aromatic plants against Paenibacillus larvae, the causative agent of American Foulbrood. J. Invertebr. Pathol. 2010;104:209–213. doi: 10.1016/j.jip.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Goudjil M.B., Ladjel S., Bencheikh S.E., Zighmi S., Hamada D. Study of the chemical composition, antibacterial and antioxidant activities of the essential oil extracted from the leaves of Algerian Laurus nobilis Lauraceae. J. Chem. Pharm. Res. 2015;7(1):379–385. [Google Scholar]
- Henczkowski M., Kopacz M., Nowak D., Kuzniar A. Infrared spectrum analysis of some flavonoids. Acta Pol. Pharm. 2001;58(6):415–420. [PubMed] [Google Scholar]
- Hinneburg I., Dorman H.J., Hiltunen R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006;97:122–129. [Google Scholar]
- Hsieh Y.S., Hsu C.Y. Oxidative stress and anti-oxidant enzyme activities in the trophocytes and fat cells of queen honeybee (Apis mellifera) Rejuvenation Res. 2013;16(4):295–303. doi: 10.1089/rej.2013.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, S., Jadon, N., 2010. Antibiotic residues in honey. Centre for science and environment, Tughlakabad Institutional Area, New Delhi, 48pp.
- Johnson R.M., Dahlgren L., Siegfried B.D., Ellis M.D. Acaricide, fungicide and drug interactions in honey bees (Apis mellifera) PLOS ONE. 2013;8(1) doi: 10.1371/journal.pone.0054092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julianti E., Jang K.H., Lee S., Lee D., Mar W., Oh K., Shin J. Sesquiterpenes from the leaves of Laurus nobilis L. Phytochem. 2012;80:70–76. doi: 10.1016/j.phytochem.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Kareru P.G., Keriko J.M., Gachanja A.N., Kenji G.M. Direct detection of triterpenoid saponins in medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2008;5(1):56–60. doi: 10.4314/ajtcam.v5i1.31257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaurinovic B., Popovic M., Vlaisavljevic S. In vitro and in vivo effects of Laurus nobilis L. leaf extracts. Molecules. 2010;15(5):3378–3390. doi: 10.3390/molecules15053378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo U., Nam K.W., Ham A., Lyu D., Kim B., Lee S.J., Kim K.H., Oh K.B., Mar W., Shin J. Neuroprotective Effects of 3α-Acetoxyeudesma-1,4(15),11(13)- trien-12,6α-olide Against Dopamine-Induced Apoptosis in the Human Neuroblastoma SH-SY5Y Cell Line. Neurochem. Res. 2011;36:1991–2001. doi: 10.1007/s11064-011-0523-1. [DOI] [PubMed] [Google Scholar]
- Lennette S., Balows R., Hansler L., Shadony E. Clínica. Ed. Panamericana; Buenos Aires, Argentina: 1987. Manual de Microbiología. [Google Scholar]
- Lindström A. Distribution of Paenibacillus larvae spores among adult honey bees (Apis mellifera) and the relationship with clinical symptoms of American Foulbrood. Microb. Ecol. 2008;56:253–259. doi: 10.1007/s00248-007-9342-y. [DOI] [PubMed] [Google Scholar]
- Martínez A.M. Universidad de Antioquia, Medellin; Facultad de Química Farmacéutica: 2005. Flavonoides. [Google Scholar]
- Martínez J., Simon V., Gonzalez B., Conget P. A real-time PCR-based strategy for the detection of Paenibacillus larvae vegetative cells and spores to improve the diagnosis and the screening of American foulbrood. Lett. Appl. Microbiol. 2010;50:603–610. doi: 10.1111/j.1472-765X.2010.02840.x. [DOI] [PubMed] [Google Scholar]
- Miyagi T., Peng C.Y.S., Chuang R.Y., Mussen E.C., Spivak M.S., Doi R.H. Verification of oxytetracycline-resistant American Foulbrood pathogen Paenibacillus larvae in the United States. J. Invertebr. Pathol. 2000;75:95–96. doi: 10.1006/jipa.1999.4888. [DOI] [PubMed] [Google Scholar]
- Moleyar V., Narasimtram P. Antifungal activity of some essential oil components. Food Microbiol. 1986;3:331–336. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). 2007 Guidance Document On The Honey Bee (Apis Mellifera L.) Brood Test Under Semi-Field Conditions.
- Ogunlana E.O., Hoeglund S., Onawunmi G., Skoeld O. Effects of lemongrass oil on the morphological characteristics and peptidoglycan synthesis of Escherichia coli cells. Microbios. 1987;50:43–59. [PubMed] [Google Scholar]
- Ortiz H.F., Sánchez W.F., Méndez J.A., Murillo E.P. Potencial antioxidante de hojas y corteza de Bauhinia kalbreyeri Harms: Contribución de sus flavonoides en esta actividad. Rev. Acad. Colomb. Cienc. 2009;33(127):183–191. [Google Scholar]
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Japanese J. Nutrition. 1986;44:307–331. [Google Scholar]
- Palacio M.A., Figini E.E., Ruffinengo S.R., Rodriguez E.M., Hoyo M.L. Changes in population of Apis mellifera L. selected for hygienic behaviour and its relation to brood disease tolerance. Apidologie. 2000;31(4):471–478. [Google Scholar]
- Porrini M., Fernández N., Garrido P., Gende L., Medici S., Eguaras M. In vivo evaluation of antiparasitic activity of plant extracts on Nosema ceranae (Microsporidia) Apidologie. 2011;42(6):700–707. [Google Scholar]
- Porrini, M.P., 2013. Formulaciones bacterianas y sustancias alternativas para el control de la parasitosis causada por Nosema spp. (Microspora, Nosematidae) en colonias de Apis mellifera (Hymenoptera, Apidae). Doctoral Thesis. Universidad Nacional de Mar del Plata; pp.193.
- Reynaldi F.J., Albo G.N., Alippi A.M. Effectiveness of tilmicosin against Paenibacillus larvae, the causal agent of American Foulbrood disease of honeybeehoney bees. Vet. Microbiol. 2008;132:119–128. doi: 10.1016/j.vetmic.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Schulz H., Baranska M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007;43(1):13–25. [Google Scholar]
- Škerget M., Kotnik P., Hadolin M., Hraš A., Simonič M., Knez Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191–198. [Google Scholar]
- Spivak M.S., Gilliam M. Facultative expression of hygienic behaviour of honey bees in relation to disease resistance. J. Apicultural Res. 1993;32(3):147–157. [Google Scholar]
- Spivak M.S., Reuter G.S. Resistance to American fouldbrood disease by honey bee colonies, Apis mellifera, bred for hygienic behavior. Apidologie. 2001;32:555–565. [Google Scholar]
- Strachecka A., Olszewski K., Paleolog J., Borsuk G., Bajda M. Coenzyme Q10 treatments influence the lifespan and key biochemical resistance systems in the honeybee Apis mellifera. Arch. Insect. Biochem. Physiol. 2014;86(3):165–179. doi: 10.1002/arch.21159. [DOI] [PubMed] [Google Scholar]
- Symonds M.R.E., Moussalli A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using akaike’s infromation criterion. Behav. Ecol. Sociobiol. 2011;65:13–21. [Google Scholar]
- Thompson H.M., Waite R.J., Wilkins S., Brown M.A., Bigwood T., Shaw M., Ridgway C., Sharman M. Effects of European foulbrood treatment regime on oxytetracycline levels in honey extracted from treated honeybee (Apis mellifera) colonies and toxicity to brood. Food Addit. Contam. 2005;22:573–578. doi: 10.1080/02652030500089986. [DOI] [PubMed] [Google Scholar]
- Türi M., Türi E., Kõljalg S., Mikelsaar M. Influence of aqueous extracts of medicinal plants on surface hydrophobicity of Escherichia coli strains of different origin. APMIS. 1997;105:956–962. [PubMed] [Google Scholar]
- Ultee A., Bennik M.H., Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002;68:1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versalovic J., Schneider M., de Bruijn F.J., Lupski J.R. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell. Biol. 1994;5:25–40. [Google Scholar]
- Wang L., Sung W. Rapid evaluation and quantitative analysis of eugenol derivatives in essential oils and cosmetic formulations on human skin using attenuated total reflectance-infrared spectroscopy. J. Spectrosc. 2011;26:43–52. [Google Scholar]
- Yen G.C., Hsieh P.P. Antioxidative activity and scavenging effects on active oxygen of xylose-lysine Maillard reaction products. J. Scie. Food Agricult. 1995;67:415–420. [Google Scholar]
- Yildirim A., Mavi A., Kara A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agricult. Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]