Abstract
This study was conducted at the apiary of the Beekeeping Research Section at the Sakha Agricultural Research Station, ARC, Kafrelsheikh, and other apiaries in Kafrelsheikh province, during two successive years 2015 and 2016. The study aimed to survey nectar and pollen floral resources in Kafrelsheikh province. Ninty seven plant species belonging to 33 families were recorded as nectar sources, and 82 plant species belonging to 36 families were recorded as pollen sources during the whole year. The largest amount of monthly trapped pollen was obtained during May followed by August. It can be concluded that, beekeepers in Kafrelsheikh province can harvest good honey yield at the end of blooming seasons of citrus (Citrus spp.) during March and April, Egyptian clover (Trifolium alexandrinum L.) during May and June, loofah (Luffa aegyptiaca Mill.) during June to October, cotton (Gossypium spp.) during July and August, and banana (Musa spp.) during August and September. They also, could be trapping pollen loads collected from faba bean (Vicia faba L.) and flax (Linum usitatissimum L.) during January to March, date palm (Phoenix dactylifera L.) during March and April, Egyptian clover during May and June, summer seed watermelon (Citrullus lanatus var. colothynthoides L.) during June and July, loofah and maize (Zea mays L.) during June to November.
Keywords: Flora, Honeybee, Kafrelshiekh, Nectar, Pollen
1. Introduction
The main honey flow seasons in Egypt are citrus during March and April, Egyptian clover during April to June, and cotton during July and August (Taha, 2005). In addition, two secondary honey flow seasons: loofah during June to October (Taha et al., 2006), and banana during August and September (Taha, 2007) were recorded in Kafrelsheikh province. Long gaps in the availability of bee floral resources between the flow seasons affect the growth and productivity of bee colonies (Taha, 2000, Taha, 2005). During such floral dearth periods, particularly when pollen is not available, colonies gradually use up stored resources within the combs in their nests, while the queens stop laying eggs and the colonies become weak (Manning, 2008, Taha and AL-Kahtani, 2013). Such colonies use up a major part of nectar and pollen collected directly after the dearth for buildup of colony population. For beekeepers, this process is destructive for their business. Honeybee colonies should to be populous in order to store surplus nectar, which is then harvested (Taha and Al-Kahtani, 2013). Beekeepers usually provide their colonies with artificial feeding including sugar syrup and pollen substitutes/supplements during dearth periods (Taha, 2015c). However, no artificial feeds have been found equivalent to nectar and pollen (Mohanna, 1989). The knowledge on the major floral resources lead beekeepers to maintain colony strength, economize the cost of feeding, and harvest good honey yield (Carol, 1999, Taha, 2005, Taha, 2015a).
In tropical and sub-tropical areas, bee floral resources are available around the year, and the activity of honeybee colonies in gathering nectar and/or pollen is continued throughout the year (Neupane and Thapa, 2005). However, the foraging activities of honeybees for pollen are greatly influenced by weather factors and availability of pollen (Taha and AL-Kahtani, 2013, Taha, 2014). It is very useful for beekeepers to have a knowledge on nectar and/or pollen floral resources in their areas, that's could help them to plan for managing their colonies, and they may be decided to move it to another area rich with nectar and pollen floral resources during certain periods to have good honey yield, producing bee swarms, or queen production (Taha, 2005, Taha, 2015a).
Studies on nectar and/or pollen plants for honeybees were conducted in Brazil (Luz and Barth, 2012); in Costa Rica (Freitas, 1994); in Bulgaria (Atanassova and Lazarova, 2010); in Egypt (Taha, 2000, Helal et al., 2003, Fathy, 2008, Ismail et al., 2013, Abou-Shaara, 2015, Esmael et al., 2016); in Germany (Köppler et al., 2007, Beil et al., 2008); in India (Singh, 2003); in Iran (Mossadegh, 1990); in Italy (Fortunato et al., 2006); in Mexico (Villanueva, 1989); in Nibal (Paudayal and Gautam, 2011); in Nigeria (Dukku, 2003); in Palestine (Reyahi, 1999); in Philippines (Payawal et al., 1991); in Poland (Wróblewska et al., 2010); in Saudi Arabia (Taha, 2013, Taha, 2015a, Taha, 2015b, Taha et al., 2016, Adgaba et al., 2017, Al-Kahtani et al., 2017, Taha et al., 2019); in Spain (Seijo et al., 1994); in Turkey (Bilisik et al. 2008); in USA (Baum et al., 2011).
The present study aimed to survey major and minor sources of nectar and/or pollen for honeybees in Kaferelsheikh province, northern Egypt throughout the year. The second goal was to throw the light on the periods in which beekeepers can harvesting honey yield, and/or trapping pollen loads collected from the major sources.
2. Materials and method
The present study was carried out at the apiary of the Beekeeping Research Section at Sakha Agricultural Research Station, ARC, Kafrelsheikh, and other apiaries in Kafrelsheikh province, northern Egypt throughout two successive years, from January 2015 to December 2016. Kafrelsheikh lies at latitude 31°06′42″N and longitude 30°56′45″E at an altitude of 17 m above sea level. It comprises 10 cities: Metoubes, Sidi Salem, Fuwwah, Desouk, Qallin, Kafrelsheikh, El-Reyad, Beila, El-Hamool, and Baltim.
Fresh flowers were collected from the plants as buds before anthesis, and reference slides were prepared according to Louveaux et al. (1978). The anthers were washed out 3 times in a watch glass filled with ether and then were dried. The pollen grains were transferred to a slide and spread out. A drop of fructose solution (20 g fructose + 0.5 g crystallized phenol in 100 ml of distilled water) was added to facilitate the transferring, and accelerate the swelling of the pollen grains. The preparation was dried on a warming plate at 40 °C and mounted with glycerine gelatine. This procedure was done for the pollen grains of each plant species. The prepared reference slides were stored in a refrigerator.
Five colonies (each 10 combs) of hybrid Carniolan (Apis mellifera carnica Pollmann) honeybees were selected in each city to study the fluctuation of collected bee-pollen throughout the year in Kaferelsheikh province. All colonies were approximately in the same strength (brood combs, stored food, and adult bee population). Pollen traps with efficiency of 25% were fitted on the entrance of the hives in each city. Pollen loads were collected from pollen traps every two days. Pollen loads were dried at room temperature in a shady place for two hrs to make the separation easy. The pollen loads were hand-sorted according to their color and appearance, then referred to their sources, and were weighted using an electrical balance. Microscopical examinations were conducted to identify the floral origin of pollen grains according to their shape and size by comparing with the previously prepared reference pollen slide.
Recording of all plant species which were observed to be visited by honeybee workers was done throughout the year in the ten regions represented Kaferelsheikh province. Unidentified plant species were collected, transferred to the laboratory, then were identified and recorded. Each source was identified by its scientific name and botanical family. Average date of the blooming period of each plant and the value for bees as a source of nectar and/or pollen were recorded.
Data were statistically analyzed by the analysis of variance (ANOVA) using PROC GLM ver. 9.1.3 SAS® software computer program (SAS Institute, 2003). Means of monthly collected bee-pollen were compared using Least Significant Difference (LSD) tests (α: 0.05).
3. Results
Data listed in Table 1 showed that 110 plant species belonging to 39 plant families were recorded as pollen and/or nectar floral resources in Kafrelshiekh province throughout 2015 and 2016. Ninty seven plant species belonging to 33 families were recorded as nectar sources and 82 plant species belonging to 36 families were recorded as pollen sources during the whole year. The most important species for honeybees in Kafrelshiekh province were faba bean (Vicia faba L.), flax (Linum usitatissimum L.), peach (Prunus persica L.) citrus (Citrus spp.), date palm (Phoenix dactylifera L.), Egyptian clover (Trifolium alexandrinum L.), guava (Psidium guajava L.), summer seed watermelon (Citrullus lanatus var. colothynthoides L.), loofah (Luffa aegyptiaca Mill.), maize (Zea mays L.), cotton (Gossypium spp.), sunflower (Helianthus annuus L.), banana (Musa spp.). The highest numbers (64 spp.) of blooming species were recorded during March, while the lowest numbers (7 spp.) were recorded during January.
Table 1.
Nectar and pollen floral resources in Kafrelsheikh province during 2015 and 2016 years.
| Latin name | Common name | Family | Flowering period | Source |
||
|---|---|---|---|---|---|---|
| Nectar | Pollen | |||||
| 1 | Vicia faba L. | Faba bean | Fabaceae | January–March | ++ | ++ |
| 2 | Lupinus albus L. | White lupin | Fabaceae | January–March | + | + |
| 3 | Trigonella foenum-graecum L. | Fenugreek | Fabaceae | January–March | + | + |
| 4 | Linum usitatissimum L. | Flax | Linaceae | January–March | ++ | ++ |
| 5 | Ocimum spp. | Basil | Lamiaceae | January–Decmber | + | + |
| 6 | Prunus persica L. | Peach | Rosaceae | Febraury–March | ++ | ++ |
| 7 | Prunus armeniaca L. | Apricot | Rosaceae | Febraury–March | + | + |
| 8 | Anagallis arvensis L. | Scarlet pimpernel | Myrsinaceae | Febraury–March | + | + |
| 9 | Citrus limon (L.) Osbeck | Eureka lemon | Rotaceae | Febraury–March | + | + |
| 10 | Citrus aurantiifolia Swingle | Key lime | Rotaceae | Febraury–March | + | + |
| 11 | Citrus latifolia (Yu.Tanaka) Tanaka | Persian Lime | Rotaceae | Febraury–March | + | + |
| 12 | Pisum sativum L. | Pea | Fabaceae | February–March | + | + |
| 13 | Avena fatua L. | Wild oat. | Poaceae | February–March | − | + |
| 14 | Setaria viridis (L.) P.Beauv | Green foxtail | Poaceae | February–March | − | + |
| 15 | Sisumbrium irio L. | Thumble mustard | Brassicaceae | February–April | + | + |
| 16 | Raphanus sativus L. | Radish | Brassicaceae | February–April | + | + |
| 17 | Eruca sativa Mill. | Rocket | Brassicaceae | February–April | + | + |
| 18 | Melilotus siculus All. | Yellow sweet clover | Fabaceae | February–April | − | + |
| 19 | Melilotus indicus All. | Sour clover | Fabaceae | February–April | − | + |
| 20 | Medicago hispida Gaertn. | Bur clover | Fabaceae | February–April | − | + |
| 21 | Malus domestica Borkh. nom. illeg. | Apple | Rosaceae | February–April | + | + |
| 22 | Salex safsaf Forssk | Egyptian willow | Salicaceae | February–April | − | + |
| 23 | Casuarina equisetifolia L. | Casuarina | Casuarinaceae | February–April | − | + |
| 24 | Persea americana Mill. | Avocado | Lauraceae | February–April | + | + |
| 25 | Anethum graveolens L. | Dill | Asteraceae | February–May | + | + |
| 26 | Petroselenum crispum Mill. | Parsley | Asteraceae | February–May | + | + |
| 27 | Senecio vulgaris L. | Groundsel | Asteraceae | February–May | + | + |
| 28 | Malva sylvestris L. | High mallow | Malvaceae | February–May | + | + |
| 29 | Ricinus communis L. | Castor bean | Euphorbiaceae | February–May | + | + |
| 30 | Coriandrum sativum L. | Coriander | Apiaceae | February–October | + | + |
| 31 | Barasica oleracea var.capitata L. | Cabbage | Brassicaceae | February–April | + | + |
| 32 | Barasica rapa L. | Turnip | Brassicaceae | February–April | + | + |
| 33 | Barasica napus L. | Rape | Brassicaceae | February–April | + | + |
| 34 | Barasica nigra Koch | Wild mustard | Brassicaceae | March–May | + | + |
| 35 | Pyrus malus L. | Apple | Rosaceae | March–April | + | + |
| 36 | Pyrus communis L. | Pear | Rosaceae | March–April | + | + |
| 37 | Citrus sinensis Osbeck | Orange | Rotaceae | March–April | ++ | + |
| 38 | Citrus reticulata Blanco | Mandarin orange | Rotaceae | March–April | ++ | + |
| 39 | Citrus aurantium Linn | Sour orange | Rotaceae | March–April | + | + |
| 40 | Phoenix dactylifera L. | Date palm | Arecaceae | March–April | − | ++ |
| 41 | Mangifera indica L. | Mango | Anacardiaceae | March–April | + | + |
| 42 | Punica granatum L. | Pomegranate | Lythraceae | March–April | + | + |
| 43 | Papaver rhoeas L. | Common poppy | Papaveraceae | March–April | + | + |
| 44 | Borago officinalis L. | Borage | Boraginaceae | March–April | + | + |
| 45 | Matricaria chamomilla L. | Chamomile | Asteraceae | March–April | + | + |
| 46 | Rosmarinus officinalis L. | Rosemary | Lamiaceae | March–April | + | + |
| 47 | Emex spinosus L. | Devil's thorn | Polygonaceae | March–April | + | + |
| 48 | Oxalis griffithii Edgew. & Hook. | Pink doubl flower | Oxalidaceae | March–April | + | + |
| 49 | Hordeum murinum L. | Wall barley | Poaceae | March–April | − | + |
| 50 | Urtica urens L. | Nettle | Urticaceae | March–April | + | + |
| 51 | Lolium multiflorum Lam. | Ryegrass | Poaceae | March–April | − | + |
| 52 | Nerium oleander L. | Nerium | Apocynaceae | March–April | + | + |
| 53 | Silybum marianum L. | Star-thistle | Asteraceae | March–May | + | + |
| 54 | Morus alba L. | White mulberry | Moraceae | March–May | + | + |
| 55 | Morus nigra L. | Mulberry | Moraceae | March–May | + | + |
| 56 | Alcea rosea L. | Hollyhock | Malvaceae | March–September | + | − |
| 57 | Convolvulus arvensis L. | Field Bindweed | Convolvulaceae | March–October | + | + |
| 58 | Libbia nodiflora L. | Lebya | Verbenaceae | March–October | + | + |
| 59 | Petunia spp. | Petunia | Solanaceae | March–October | + | + |
| 60 | Solanum lycopersicum L. | Tomato | Solanaceae | March–December | + | + |
| 61 | Solanum melongena L. | Eggplant | Solanaceae | March–December | + | + |
| 62 | Capsicum annuum L. | Chili pepper | Solanaceae | March–December | + | + |
| 63 | Capsicum annuum var. glabriusculum Dunal | Chiltepin | Solanaceae | March–December | + | + |
| 64 | Zantedeschia aethiopica (L.) Spreng. | Arum lily | Araceae | April–May | + | + |
| 65 | Albizia julibrissin Durazz | Pink siris | Fabaceae | April–May | + | + |
| 66 | Melaleuca viminalis (Sol. ex Gaertn.) Byrnes | Weeping bottlebrush | Myrtaceae | April–May | + | + |
| 67 | Acacia saligna (Labill.) H.L. Wendl | Coojong | Fabaceae | April–May | + | + |
| 68 | Tanacetum parthenium (L.) Sch. Bip. | Feverfew | Asteraceae | April–May | + | + |
| 69 | Olea europaea L. | Olive | Oleaceae | April–May | + | + |
| 70 | Euphorbia milii Des Moul | Christ thorn | Euphorbiaciae | April–May | + | + |
| 71 | Opuntia ficus-indica (L.) Mill. | Barbary fig | Cactaceae | April–May | + | + |
| 72 | Cucumis melo var. cantalupensis Naudin | Cantaloupe | Cucurbitaceae | April–May | + | + |
| 73 | Citrullus lanatus var. lanatus (Thunb.) Matsum. & Nakai | Watermelon | Cucurbitaceae | April–May | ++ | ++ |
| 74 | Ammi visnaga L. | Tooth pick | Asteraceae | April–May | + | + |
| 75 | Cichorium intybus L. | Chicory | Asteraceae | April–May | + | + |
| 76 | Trifolium alexandrinum L. | Egyptian clover | Fabaceae | April–June | ++ | ++ |
| 77 | Cucurbita pepo Thunb | Squash | Cucurbitaceae | April–September | + | + |
| 78 | Portulaca oleracea L. | Purslane | Portulacaceae | April–October | − | + |
| 79 | Pluchea dioscoridis (L.) DC. | Camphorweeds | Asteraceae | April–October | + | + |
| 80 | Hibiscus rosa-sinensis L. | Chinese hibiscus | Malvaceae | May–June | + | − |
| 81 | Ammi spp. | Celery | Apiaceae | May–June | + | + |
| 82 | Malvaviscus arboreus Cav. | Sleeping hibiscus | Malvaceae | May–June | + | − |
| 83 | Carduus acanthoides L. | Welted thistle | Asteraceae | May–June | + | + |
| 84 | Pelargonium zonale (L.) L'Hér. ex Aiton | Horse-shoe pelargonium | Geraniaceae | May–June | + | + |
| 85 | Delonix regia (Boj. ex Hook.) Raf | Royal poinciana | Fabaceae | May–June | + | + |
| 86 | Aptenia cordifolia (L.f.) N.E.Br | Baby sun rose | Aizoaceae | May–June | + | + |
| 87 | Leucaena leucocephala (Lam.) de Wit | White leadtree | Fabaceae | May–June | + | + |
| 88 | Cichorium pumilum Gacq | Willd chicory | Asteraceae | May–June | + | + |
| 89 | Psidium guajava L. | Guava | Myrtaceae | May–July | + | ++ |
| 90 | Ipomoea carnea Jace. | Pink morning glory | Convolvulaceae | May–December | + | − |
| 91 | Citrullus lanatus var. colothynthoides L. | Summer seed watermelon | Cucurbitaceae | June–July | + | ++ |
| 92 | Duranta spp. | Duranta | Verbenaceae | June–August | + | + |
| 93 | Abelmoschus esculentus (L.) Moench | Okra | Malvaceae | June–September | + | − |
| 94 | Cucumis sativus L. | Cucmber | Cucurbutaceae | June–October | + | + |
| 95 | Luffa aegyptiaca Mill. | Loofah | Cucurbitaceae | June–November | ++ | ++ |
| 96 | Zea mays L. | Maize or corn | Poaceae | June–November | − | ++ |
| 97 | Gossypium spp. | Cotton | Malvaceae | July–August | ++ | − |
| 98 | Origanum majorana L. | Marjoram | Lamiaceae | July–August | + | + |
| 99 | Hibiscus canabinus L. | Kenaf | Malvaceae | July–August | + | − |
| 100 | Helianthus annuus L. | Sunflower | Asteraceae | July–August | ++ | ++ |
| 101 | Sesamum indicum L. | Sesame | Pedaliaceae | July–August | ++ | ++ |
| 102 | Vigna unguiculata (L.) Walp. | Cowpea | Fabaceae | July–August | + | + |
| 103 | Musa spp. | Bananas | Musaceae | August–September | ++ | − |
| 104 | Oryza sativa L. | Rice | Poaceae | August–September | − | + |
| 105 | Schinus terebinthifolius Radd. | Brazilian pepper | Anacardiaceae | September–October | + | + |
| 106 | Phaseolus vulgaris L. | Bean | Fabaceae | September–October | + | + |
| 107 | Pisum sativum L. | Pea | Fabaceae | October–January | + | + |
| 108 | Eucalyptus globulus Labill | Eucalyptus | Myrtaceae | October–April | + | + |
| 109 | Eriobotrya japonica (Thunb.) Lindl. | Loquate | Rosaceae | November–December | + | + |
| 110 | Tecoma stans (L.) Juss.ex Kunth | Trumpet flower | Bignoniaceae | November–December | + | + |
++ Major source. + Minor source. − Not source.
As shown in Table 2 faba bean, Egyptian clover, summer seed watermelon, and cotton were recorded as major nectar floral resources in all regions of Kafrelshiekh province. Faba bean, Egyptian clover, summer seed watermelon, and maize were recorded as major pollen floral resources in all regions of Kafrelshiekh province. Citrus was recorded as a major nectar, and minor pollen floral resource in Metoubes, Fuwwah, and Desouk regions. Banana was recorded as a honey plant in Fuwwah, and Desouk regions. Loofah was recorded as a major floral resource of nectar and pollen in Metoubes region. In Metoubes and Baltim regions, guava was recorded as a major nectar and pollen floral resource, while date palm was recorded as a major pollen floral resource. Sunflower was recorded as a major source of nectar and pollen in Sidi Salem, Qallin and Kafrelsheikh regions, and as a minor source in the others regions.
Table 2.
Major nectar and/or pollen floral resources in Kafrelsheikh province in 2015 and 2016.
| Floral resources | Metoubes | Sidi Salem | Fuwwah | Desouk | Qallin | Kafrelsheikh | El-Reyad | Beila | El-Hamool | Baltim |
|---|---|---|---|---|---|---|---|---|---|---|
| Citrus | ++ | − | ++ | ++ | − | − | − | − | − | ++ |
| Egyptian clover | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Loofah | ++ | + | + | + | + | + | + | + | + | + |
| CottonN | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + |
| BananaN | + | + | ++ | ++ | + | + | + | + | + | + |
| Faba bean | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Flax | + | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ |
| Peach | ++ | − | ++ | ++ | − | − | − | − | − | − |
| Date palmP | ++ | + | + | + | + | + | + | + | + | ++ |
| Guava | ++ | + | + | + | + | + | + | + | + | ++ |
| Summer seed watermelon | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ |
| Sunflower | + | ++ | + | + | ++ | ++ | + | + | + | + |
| MaizeP | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Watermelon | + | + | + | + | + | + | + | + | + | ++ |
++ Major source. + Minor source. − Not source. N Source for nectar not for pollen, P Source for pollen not for nectar.
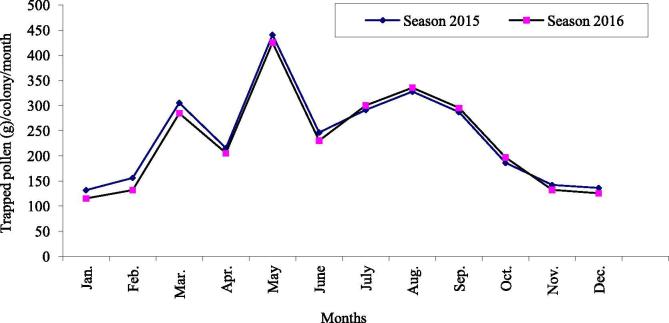
The average yearly amounts of collected bee-pollen were 2.866 and 2.780 kg/colony in 2015 and 2016, respectively. Significant (P < .01) variations were found among amounts of trapped pollen loads during months throughout the year. The largest amounts of collected bee-pollen were obtained during May (440.77 and 425.33 g/colony), followed by August (327.73 and 335.62 g/colony), then March (305.33 and 284.80 g/colony), while the lowest amounts of trapped pollen loads were obtained during January (131.92 and 115.66 g/colony), followed by December (136.36 and 125.65 g/colony) in 2015 and 2016, respectively (Fig. 1).
Fig. 1.
Seasonal fluctuation of collected bee-pollen in Kafrelsheikh province during 2015 and 2016.
4. Discussion
The survey of bee plants in Kafrelshiekh province showed that 110 plant species were visited by honeybees (A. mellifera L.); worker bees can collect nectar from 97 species, and can collect pollen from 82 floral resources during the whole year. Previous studies in Egypt showed that, there were 39 bee forages belonging to 15 families in Kafrelshiekh region (Taha, 2005), 26 pollen species in 15 families in Dakahlia (Fathy, 2008), 24 pollen sources belonging to 16 families at Fayoum (Ismail et al., 2013), and 65 bee plants belonging to 25 plant families in Alexandria and El-Beheira provinces (Esmael et al., 2016). In India, more than 60 plant species were visited by A. cerana, five species were potential sources of nectar and five species were sources for pollen (Nehru et al., 1988). In Iran, 173 bee plants belonging to 32 families were recorded; 89 plants produce surplus honey and 28 species as major pollen sources (Mossadegh, 1990). In Mexico, 102 plant species were visited by the bees (Villanueva, 1989). In Saudi Arabia, 79 bee plant species belonging to 24 botanical families were recorded as nectar and/or pollen sources in Al-Ahsa province (Taha, 2015a), meanwhile 182 species from 49 plant families were identified as bee forages in Al-Baha region (Adgaba et al., 2017).
Of the total 110 species were recorded, 76 (69.10%) were herbs, 29 (26.36%) trees, and 5 (4.54%) shrubs. The main 5 sources of honey were classified as 3 herbs (T. alexdrinum, L. aegyptiaca, and Gossypium spp.) and two trees (Citrus spp., and Musa spp.). Meanwhile, all major pollen floral resources were classified as herbs except for date palm, guava and peach were trees. All major floral resources of nectar and/or pollen were cultivated plants. Similar results were reported by Taha, 2000, Taha, 2005, Fathy, 2008, Ismail et al., 2013, Esmael et al., 2016 in Egypt, and Taha (2015a) in the Al-Ahsa oasis of eastern Saudi Arabia. Kafrelsheikh is an Agricultural province, that’s could explains why all of major floral resources of nectar and/or pollen in this province were classified as cultivated plants. There were some wild plants visited by bees, but non of them is major source either of nectar or pollen.
Of the total 110 species were recorded as bee plants only five species (Citrus spp., T. alexdrinum, L. aegyptiaca, Gossypium spp., and Musa spp.) represented 4.55% of the identified plants produced surplus honey in Kafrelsheikh province. This percentage is higher than that (3.30%) for bee plants in Al-Baha region (Adgaba et al., 2017), and that (3.80%) in Al-Ahsa province (Taha, 2015a) in Saudi Arabia, and that (1.6%) of world bee plants (Crane, 1990), while our percentage is too low compared with that (51.44%) of Villanueva (1989) in Iran. Beekeepers in Kafrelsheikh province usually harvest honey yield during the blooming seasons of Citrus spp., T. alexdrinum and Gossypium spp. (Taha, 2000, Taha, 2005), L. aegyptiaca (Taha et al., 2006) and Musa spp. (Taha, 2007). In Saudi Arabia, Medicago sativa L., Ziziphus spp. and Citrus spp. were the dominant sources of nectar, while Cucurbita pepo Thunb, Ph. dactylifera L., Helianthus annuus L., M. sativa L. and Brassica napus L. were the main sources of pollen in Al-Ahsa province (Taha, 2015a,b). Besides, the major sources of nectar in Al-Baha region were Z. spina-christi L., Acacia tortilis (Forssk) Hayne, A. asak, Lavandula dentata L., and Hypoestes forskaolii (Adgaba et al., 2017). In USA, 7 bee plants non-Lamiaceae and nine Lamiaceae species were recorded as nectar-producing plants (Widrlechner and Senechal, 1992).
Faba bean and flax were bloomed during the period from January to March in most parts of Kafrelshiekh province. Their flowers were produced much pollen and nectar. The extra-floral nectaries of faba bean were produced nectar before flowering starts and this continues through and to the end of flowering (Kirk, 2004). Peach was bloomed during early spring, and honeybees were collected nectar and pollen from flowers. These plants did not produce surplus honey, but they are very important for building up the colonies and swarms production. Date palm was flowered during March and April, male trees produced more pollen. It considered an important source of pollen in Metoubes (Taha, 2005) and Baltim regions. Summer seed watermelon was bloomed during a dearth period between Egyptian clover and cotton flow seasons so, it was served on maintaining the colony strength and economizing the cost of feeding during this period (Taha and Bayomi, 2009). Guava was bloomed from May to July and considered good source of nectar and pollen in Metoubes and Baltim regions. Sunflower and sesame were bloomed during July and August. They were good sources of nctare and pollen during this period. These results are in lin with the findings of Hussein et al. (1992) in Assiut, Taha, 2000, Taha, 2005 in Kafrelshiekh, Fathy (2008) in Dakahlia, Ismail et al. (2013) in Fayoum, Esmael et al. (2016) in Alexandria and El-Beheira provinces.
The number of available bee plants throughout the months of the year could be arranged in a descending order: March and April > May > February > June > July and August > September > October > November > December > January. Seven nectar and pollen floral resources were bloomed during January; faba bean and flax were the most important plants for honeybees. During February, 34 pollen and 27 nectar floral resources were recorded; faba bean, flax, and peach were the most beneficial plants for honeybees in this period. Sixty-four bee forage plants were recorded during March and April. The most important sources during March were citrus, faba bean, date palm, peach, and flax. Similar results were recorded by Hussein et al. (1992) in Assiut province, and by Taha (2005) in Kafrelshiekh province. Unfortunately, most of the bloomed plants in April were dried before mid-April. Citrus was the predominant bee flora in April. These results confirmed the findings of Taha (2005). During May, 46 nectar and pollen floral resources were recorded; Egyptian clover was recorded as a major nectar and pollen floral resource. These results are in agreement with those obtained by Hussein et al. (1992) in Assiut, Esmael et al. (2016) in El-Beheira, and Taha (2005) in Kafrelshiekh province. During June, 33 pollen and/or nectar floral resources were recorded. The most abundant species were Egyptian clover, guava, and loofah. Twenty-nine pollen and/or nectar floral resources were recorded throughout July and August; the most abundant species were watermelon, guava, loofah, cotton, banana, sunflower, and maize. These results are in harmony with those obtained by Hussein et al. (1992) in Assiut, and Taha (2005) in Kafrelshiekh province. Twenty-two bee forage plants were recorded during September; loofah, and maize were the most important species. Nineteen taxa were bloomed in October; loofah, and maize were the major sources in this period. Although, 12 plant species flowered in November, and 10 plants in December, it considered dearth period because all of these plants are minor sources of nectar and/or pollen.
The most represented families were Fabaceae (15 spp.), Asteraceae (12 spp.), Brassicaceae and Malvaceae (7 spp.), Cucurbitaceae, Poaceae, Rosaceae and Rutaceae (6 spp.), and solanaceae (5 spp.). They contributed by 63.64% of total bee flora in Kafrelshiekh province. These results are in agreement with those obtained by Zoratti et al. (1995) in Italy, Taha, 2005, Esmael et al., 2016 in Egypt, and Taha, 2015a, Adgaba et al., 2017 in Saudi Arabia, they reported that the most represented families were Asteraceae, Brassicaceae, Cucurbitaceae, Fabaceae, Rosaceae. Besids, the most abundant species in Nigerian honey samples were from Asteraceae and Arecaceae (Adekanmbi and Ogundipe, 2009). Apiaceae, Lamiaceae, and Myrtaceae were represented by 3 spp. The following families: Anacardiaceae, Convolvulaceae, Euphorbiaceae, Moraceae, and Verbenaceae each was represented by two plant species. Moreover, 22 families (Arecaceae, Aizoaceae, Apocynaceae, Araceae, Bignoniaceae, Boraginaceae, Cactaceae, Casuarinaceae, Geraniaceae, Lauraceae, Linaceae, Lythraceae, Musaceae, Myrsinaceae, Papaveraceae, Pedaliaceae, Polygonaceae, Portulacaceae, Oleaceae, Oxalidaceae, Salicaceae, and Urticaceae) were represented by one nectar and/or pollen floral resource.
The monthly weight of trapped pollen loads reflects the activity of honeybee colonies in gathering pollen. Pollen collection is a continuous prosses throughout the year. The colonies started their activities in pollen collection during January, then significant (P < .01) increasing occurred during February and reached the first peak during March. Decreasing in pollen collection occurred during April, then increased significantly (P < .01) and formed the second and the major peak during May coincieded with the flowering period of Egyptian clover, then significant (P < .01) decrease occurred during June. Gradually increase occurred during July, and formed the third peak in August, then gradually and significantly (P < .01) decrease occurred from September to December. These results are confirmed by the findings of Shawer, 1987, Fathy, 1996, Shawer et al., 2003, Taha, 2005 in Egypt, Taha and Al-Kahtani (2019) in Saudi Arabia, and Al-Humyarie et al. (1999) in Yemen who recorded the maximum area of stored pollen during May. Besides, Sattigi and Lingappa (1993) found the maximum area of stored pollen during March in India. Three peaks of pollen collection were recorded during March, May and August in the Al-Ahsa oasis of eastern Saudi Arabia (Taha and AL-Kahtani, 2013, Taha and Al-Kahtani, 2019, Taha, 2014, Taha, 2015a). On contrary, the maximum area of stored pollen was recorded during September in the Island of Hawaii (Arita and Fujii, 1992). April considered a dearth period in Kafrelsheikh province because of the shortage of pollen and nectar floral resources, as a result of dried of most flowering plants after a short time in April. The decline in pollen collecting during April was recorded in Saudi Arabia (Taha, 2014, Taha and Al-Kahtani, 2019) due to the presence of bee-eater (Merops spp.) birds in the apiary area (Ali and Taha, 2012). The lowest amount of collected bee-pollen was trapped during January, this may be related to the low number of flowering plants during this period. Similar results were obtained by Khanbash and Bin Ghodel (1994) in Yemen, and Taha, 2014, Taha, 2015a in the Al-Ahsa oasis of eastern Saudi Arabia.
5. Conclusion
Based on the present data, it can be concluded that beekeepers at Kafrelsheikh province can obtain high honey yield, during the blooming seasons of citrus (March and April), Egyptian clover (April to June), loofah (June to October), cotton (July and August), and banana (August and September). They can trap pollen loads during the flowering periods of faba bean (January to March), Egyptian clover (April to June), summer seed watermelon (June and July), loofah (June to October), and maize (June to November).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abou-Shaara H.F. Potential honeybee plants of Egypt. Cercetări Agronomice în Moldova. 2015;48:99–108. [Google Scholar]
- Adekanmbi O., Ogundipe O. Nectar sources for the honeybee (Apis mellifera adansonii) revealed by pollen content. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2009;37:211–217. [Google Scholar]
- Adgaba N., Alghamdi A., Sammoud R., Shenkute A., Tadesse Y., Ansari M.J., Sharma D., Hepburn C. Determining spatio-temporal distribution of bee forage species of Al-Baha region based on ground inventorying supported with GIS applications and Remote Sensed Satellite Image analysis. Saudi J. Biol. Sci. 2017;24:1048–1055. doi: 10.1016/j.sjbs.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Humyari A.A., El-Sherif M.E., Naser Kh.S.A. Brood rearing, food storage and worker longevity of Yemeni bee colonies and their Carniolan hybrid. J. Agric. Sci. Mansoura Univ. 1999;24:1345–1358. [Google Scholar]
- Ali M.A., Taha E.A. Bee-eating birds (Coraciiformes: Meropidae) reduce virgin honeybee queen survival during mating flights and foraging activity of honeybees (Apis mellifera L.) Inter. J. Sci. Eng. Res. 2012;3:1–8. [Google Scholar]
- Al-Kahtani S.N., Taha E.A., Al-Abdulsalam M. Alfalfa (Medicago sativa L.) seed yield in relation to phosphorus fertilization and honeybee pollination. Saudi J. Biol. Sci. 2017;24:1051–1055. doi: 10.1016/j.sjbs.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita L.H., Fujii J.K. Quantity and seasonal variation of pollen types collected by honeybees at two localities on the island of Hawaii. Proc. Hawaiian Entomol. Soc. 1992;31:119–123. [Google Scholar]
- Atanassova J., Lazarova M. Pollen analysis of bee pollen loads from the region of town Shumen (NE Bulgaria) Comptes Rendus de l'Académie Bulgare des Sciences. 2010;63:369–374. [Google Scholar]
- Baum K.A., Rubink W.L., Coulson R.N., Bryant J.R., Vaughn M. Diurnal patterns of pollen collection by feral honeybee colonies in Texas, USA. Palynology. 2011;35:85–93. [Google Scholar]
- Beil M., Horn H., Schwabe A. Analysis of pollen loads in a wild bee community (Hymenoptera: Apidae)- a method for elucidating habitat use and foraging distances. Apidologie. 2008;39:456–467. [Google Scholar]
- Bilisik A., Cakmak I., Bicakci A., Malyer H. Seasonal variation of collected pollen loads of honeybees (Apismellifera L.anatoliaca) Grana. 2008;47:70–77. [Google Scholar]
- Crane E. Oxford Heinemann Newnes; 1990. Bees and beekeeping: Science, practice and world resources. [Google Scholar]
- Carol G. Wind pollination and reproductive assurance in Linanthus parviflorus (Polemoniaceae) Amer. J. Biol. 1999;86:948–954. [PubMed] [Google Scholar]
- Dukku U.H. Acacia ataxacantha: a nectar plant for honeybees between two dearth periods in the sudan savanna of northern Nigeria. Bee World. 2003;84:32–33. [Google Scholar]
- Esmael M.E., Salem M.H., Mahgoub M.S., El-Barbary N.S. Photographer guide of pollen grains collected from apiaries in Alexandria and El-Beheira Governorates (West Nile Delta, Egypt) Alex. J. Agric. Sci. 2016;61:267–290. [Google Scholar]
- Fathy D.M. Fac. Agric., Mansoura Univ.; Egypt: 2008. Types and quantities of pollen grains collected by honeybee Apis mellifera L. with reference to brood rearing activity. Unpublished M.Sc. Thesis. [Google Scholar]
- Fathy H.M. Honeybee colony population in relation to brood rearing and stored pollen. Apiacta. 1996;31:36–44. [Google Scholar]
- Fortunato L., Gazziola F., Barbattini R. A study on the pollen sources for honeybees in Udine province (northern Italy) Bull. Insectol. 2006;59:39–43. [Google Scholar]
- Freitas B.M. Proc. 5th Inter. Conf. Apic. Tropical Climates, Trinidad and Tobago, 7–12 Sep. 1994. Pollen identification of pollen and nectar loads collected by Africanized honeybees in the state of Ceara, Brazil. pp. 73–79. [Google Scholar]
- Helal R.M., El-Dakhakhni T.N., Shawer M.B., Taha E.A. Effect of moving the apiaries on activity of honeybee colonies. 2-Flight activity, gathering of nectar and sugar concentration contents and honey. J. Agric. Res. Tanta Univ. 2003;29:268–282. [Google Scholar]
- Hussein M.H., Mannaa S.H., Omar M.O., Mostafa A.M. Proc. 4th Nat. Conf. Pests Dis. Veg. & Fruits in Egypt. 1992. Species composition of collected pollen loads by honeybee (Apis mellifera L.), pollen flora and floral calender of Assiut region; pp. 177–195. [Google Scholar]
- Ismail A.M., Owayss A.A., Mohanny K.M., Salem R.A. Evaluation of pollen collected by honeybee, Apis mellifera L. colonies at Fayoum Governorate, Egypt. Part 1: Botanical origin. J. Saudi Soc. Agric. Sci. 2013;12:129–135. [Google Scholar]
- Kirk W. Faba bean (Vicia faba) Bee World. 2004;85:60–62. [Google Scholar]
- Khanbash M.S., Bin Ghodel A.Y. Seasonal collection and storage of pollen in honeybee colonies with different sizes. J. Yemeni Agric. Res. Aden Univ. 1994;5:1–14. [Google Scholar]
- Köppler K., Vorwohl G., Koeiger N. Comparison of pollen spectra collected by four different subspecies of the honeybee Apis mellifera. Apidologie. 2007;38:341–353. [Google Scholar]
- Louveaux J., Maurizio A., Vorwohl G. Methods of melissopalynology. Bee World. 1978;59:139–157. [Google Scholar]
- Luz C.F., Barth O.M. Pollen analysis of honey and beebread derived from Brazilian mangroves. Brazil. J. Botany. 2012;35:79–85. [Google Scholar]
- Manning, R., 2008. The effect of high and low fat pollens on honeybee longevity. RIRDC Publication No. 08/031. Dept. Agric., Western Australia.
- Mohanna N.E. An important source of nectar and pollen during the dearth period in Egypt. Alex. J. Agric. Res. 1989;34:173–182. [Google Scholar]
- Mossadegh M.S. Honey and pollen sources in Lorestan. Iran. Bee World. 1990;71:25–32. [Google Scholar]
- Nehru C.R., Thankamani S., Jayarathnam K., Levijospph P.M. Nectar and pollen plants for extending the flow period in Rubber-growing areas of India. Bee World. 1988;60:118–119. [Google Scholar]
- Neupane K.R., Thapa R.B. Pollen collection and brood production by honeybees (Apis mellifera L.) under Chitwan condition of Nepal. J. Inst. Agric. Anim. Sci. 2005;26:143–148. [Google Scholar]
- Paudayal K.N., Gautam I. Scanning Electron Microscopic studies on surface pattern of the pollen loads from Apis cerana in Jajarkot district. Nepal J. Sci. Technol. 2011;12:340–349. [Google Scholar]
- Payawal P.C., Tilde A.C., Manimtim A.L. Year round pollen sources of Italian honeybees (Apismellifera L.) in the Philippines III. Selected areas. Philippine Agric. 1991;74:503–509. [Google Scholar]
- Reyahi B.A. Proc. 36th Apimondia Cong. Vancouver, Canada, 12–17 Sep. 1999. Melliferous flora of Palestine: some important species with potenial for introduction to other regions of the world; p. 269. [Google Scholar]
- SAS Institute, 2003. SAS/STAT User’s Guide release 9.1. SAS Institute Inc, Cary, NC 27513.
- Sattigi H.N., Lingappa S. Foraging activities of Indian honeybee Apis Cerana Fabr. Under Dhrwad Conditions Karnataka. J. Agric. Sci. 1993;6:352–354. [Google Scholar]
- Seijo M.C., Aira M.J., Lglesias M.I., Jato M.V. Foraging activity of the honeybee on Actinidia deliciosa Chev. as shown by pollen analysis. Grana. 1994;33:286–291. [Google Scholar]
- Shawer M.B. Major pollen sources in Kafrelsheikh, Egypt and the effect of pollen supply on brood area and honey yield. J. Apic. Res. 1987;26:43–46. [Google Scholar]
- Shawer M.B., El-Dakhakhni N.M., Helal R.M., Taha E.A. Effect of moving the apiaries on activity of honeybee colonies. 1-Gathering and storing pollen, brood rearing and wax secretion. J. Agric. Res. Tanta Univ. 2003;29:250–267. [Google Scholar]
- Singh R.K. Studies on pollen and nectar sources to honeybees at Dehradun, Uttaranchal. India. Asian Bee J. 2003;5:129–138. [Google Scholar]
- Taha E.A. Fac. Agric. Tanta Univ; Egypt: 2000. Effect of transferring the apiaries on activity of honeybee colonies. Unpublished M.Sc. Thesis. 117 pp. [Google Scholar]
- Taha E.A. Fac. Agric. Tanta Univ.; Egypt: 2005. Studies on honeybee (Apis mellifera L.) Unpublished Ph.D. Thesis. 151 pp. [Google Scholar]
- Taha E.A. Importance of banana Musa sp. (Musaceae) for honeybee Apis mellifera L. (Hymenoptera: Apidae) in Egypt. Bull. Ent. Soc. Egypt. 2007;II:125–133. [Google Scholar]
- Taha, E.A., 2013. Survey of nectar and pollen sources in Al-Ahssa district, Saudi Arabia. In: Proc. 43rd Inter. Apic. Cong. 29 Sep.–4 Oct. Kyiv, Ukrania, P 247.
- Taha E.A. Seasonal variation of foraging activity, pollen collection and growth of honeybee colonies in Al-Ahsa. Saudi Arabia. Bull. Ent. Soc. Egypt. 2014;91:163–175. [Google Scholar]
- Taha E.A. A study on nectar and pollen sources for honeybee Apis mellifera L. in Al-Ahsa, Saudi Arabia. J. Entomol.d ZoolSt. 2015;3:272–277. [Google Scholar]
- Taha E.A. Chemical composition and amounts of mineral elements in honeybee-collected pollen in relation to botanic origin. J. Apic. Sci. 2015;59:75–81. [Google Scholar]
- Taha E.A. The impact of feeding certain pollen substitutes on maintaining the strength and productivity of honeybee colonies (Apis mellifera L.) Bull. Ent. Soc. Egypt, Econ. Ser. 2015;41:63–74. [Google Scholar]
- Taha E.A., Bayoumi Y.A. The value of honeybee (Apis mellifera L.) as pollinator of summer seed watermelon (Citrullus lanatuscolothynthoides L.: Cucurbitaceae) in Egypt. Acta Biol. Szeg. 2009;53:33–37. [Google Scholar]
- Taha E.A., AL-Kahtani S.N. Relationship between population size and productivity of honeybee colonies. J. Entomol. 2013;10:163–169. [Google Scholar]
- Taha E.A., Al-Kahtani S.N. Comparison of the activity and productivity of Carniolan (Apis mellifera carnica Pollmann) and Yemeni (Apis mellifera jemenitica Ruttner) subspecies under environmental conditions of the Al-Ahsa oasis of eastern Saudi Arabia. Saudi J. Biol. Sci. 2019;26(4):681–687. doi: 10.1016/j.sjbs.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha E.A., Nour M.E., Shawer M.B. Loofah, Luffa aegyptiaca Mill. (Cucurbitaceae), a source of nectar and pollen for honeybee Apis mellifera L. (Hymenoptera: Apidae) in Egypt. Bull. Ent. Soc. Egypt. 2006;83:337–345. [Google Scholar]
- Taha E.A., Al-Abdulsalam M., Al-Kahtani S.N. Insect pollinators and foraging behavior of honeybees on alfalfa (Medicago sativa L.) in Saudi Arabia. J. Kansas Entomol. Soc. 2016;89:92–99. [Google Scholar]
- Taha E.A., Al-Kahtani S.N., Taha R. Protein content and amino acids composition of bee-pollens from major floral sources in Al-Ahsa, eastern Saudi Arabia. Saudi J. Biol. Sci. 2019;26(2):232–237. doi: 10.1016/j.sjbs.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva R.G. Proc. 4th Inter. Conf. Apic. Tropical Climates, Cairo, Egypt. 1989. Important plant species for Apiculture in Ejido plan Del Rio, Veracrus, Mexico; pp. 138–145. [Google Scholar]
- Widrlechner M.P., Senechal N.P. Relationships between nectar production and honeybee preference. Bee World. 1992;73:119–127. [Google Scholar]
- Wróblewska A., Warakomska Z., Kaminska M. The pollen spectrum of beebread from the Lublin region (Poland) J. Apic. Sci. 2010;54:81–89. [Google Scholar]
- Zoratti M.L., Barbattini R., Frill F. Bee flora in the Codroipo area (Italy) Ape Nostra Amica. 1995;17:5–14. [Google Scholar]