Abstract
Background
The aim of the present study was to evaluate the neuroprotective effect of allantoin in cisplatin‐induced toxicity in rats.
Methods
Adult male Wistar rats weighing 160‐200 g were used. Neuropathy was induced by injecting cisplatin (2 mg/kg, ip, twice a week for 6 weeks) and the rats were concurrently treated with allantoin (200 and 400 mg/kg, po) for 8 weeks. At the end of the study, body weight and hemogram were measured. Behavioural tests were performed, including tests for cold and hot hyperalgesia, motor co‐ordination, locomotor activity, mechano‐tactile allodynia and mechanical hyperalgesia. The rats were then sacrificed and sciatic nerve conduction velocity was determined. The antioxidant enzyme and nitric oxide levels in sciatic nerve homogenates were measured.
Results
In this study, allantoin restored the motor nerve conduction velocity deficits induced by cisplatin, and the allantoin‐treated rats showed improvement in cold and thermal hyperalgesia, mechano‐tactile allodynia, and mechanical hyperalgesia. Allantoin treatment also improved the rats’ hematological status, increasing haemoglobin, platelet and RBC counts compared to the cisplatin‐treated group. Allantoin treatment also mitigated the functional abnormalities seen in the cisplatin neuropathy group, protecting neurons from the neurotoxic effects of cisplatin.
Conclusion
Allantoin shows promise for use as an adjuvant drug in cancer treatment to protect against cisplatin‐induced neuropathy.
Keywords: allantoin, cisplatin, neurotoxicity, rats
1. INTRODUCTION
Chemotherapy‐induced peripheral neuropathy (CIPN) remains one of the major limitations in oncology clinics due to the increasing number of cancer patients, the lack of effective treatment strategies and relapse of the disease. Around 30%‐40% of patients undergoing chemotherapy develop peripheral neuropathy and experience symptoms of pain and sensory disturbances.1 According to the National Cancer Institute (NCI), CIPN is one of the major reasons given for cessation of treatment, and hence decreased chemotherapeutic efficacy and a higher number of relapses.2 Symptoms of peripheral nerve damage range from sensorimotor deficits (tingling sensations, burning pain in the arms, allodynia and hyperalgesia) to various functional deficits (impaired axonal transmission and reduced nutritive blood flow to nerves). The most common agents causing CIPN are platinum compounds (eg cisplatin), taxane derivatives, vinca alkaloids, epothilones, thalidomide, and bortezomib, which adversely affect the peripheral nervous system through dissimilar mechanisms. Although the molecular patho‐mechanism and severity may vary with the inducing agent, physical damage to the neurons by chemotherapeutic agents is a common mechanism underlying the disease pathology. Physical damage by chemotherapeutic drugs leads to functional impairment in neurons through oxidative stress, inflammation, apoptosis, and electrophysiological disturbances.
Most chemotherapeutic drugs penetrate the blood–brain barrier (BBB) poorly, but readily penetrate the blood–nerve barrier (BNB) and bind to the dorsal root ganglia (DRG) and peripheral axons. Experimental studies reveal that chemotherapeutic drugs preferentially accumulate and bind in the DRG and peripheral nerves. The blood–nerve barrier is less efficient than the BBB, specifically in the areas of the DRG and nerve terminals, which allows easier access for potential neurotoxins into the periphery.3 Additionally, the endoneural compartment lacks a lymphatic system to remove toxins. These factors increase peripheral nerve vulnerability to potentially toxic medications compared to the central nervous system. Although various CIPN mechanisms based on in vitro and in vivo experiments have been proposed, the pathogenesis of CIPN has not been fully elucidated and level neurotoxic chemotherapeutic agents damage microtubules and interfere with microtubule‐based axonal transport, interrupt mitochondrial function, or directly target DNA, and subsequently lead to peripheral nerve degeneration or small fiber neuropathy. Interestingly, nerve biopsies from experimental animals and patients treated with paclitaxel, oxaliplatin, or vincristine show similar morphological changes even though these compounds have different neurotoxic targets. Peripheral nerve degeneration or small fiber neuropathy is thus generally accepted as the underlying mechanism in the development of CIPN.2
This study investigated the agent allantoin, which is a metabolic intermediate of purine catabolism that often accumulates in stressed plants, for its protective effects against neuropathy induced by the platinum compound cisplatin. A previous study used arabidopsis knockout mutants (aln) of allantoinase to show that this purine metabolite activates abscisic acid (ABA) production, thereby stimulating stress‐related gene expression and enhancing seedling tolerance to abiotic stress. Allantoin is reported to activate the jasmonate signaling in a MYC2.4 In this study, it was hypothesized that, because of its ability to cross the blood–nerve barrier and reach the areas of dorsal root ganglions and nerve terminals affected by cisplatin, allantoin has an anti‐oxidant activity, reducing the potential toxic effect of cisplatin and thus reducing degeneration of the small nerve fibers. In a literature survey, allantoin has been reported to possess anti‐diabetic,5 anti‐hypertensive,6 and analgesic activity.7
2. MATERIALS AND METHODS
2.1. Chemicals
Cisplatin, allantoin and other chemicals were purchased from commercial supplies (Cipla, Himedia, Sigma etc). All reagents and chemicals were of analytical reagent grade.
2.2. Experimental animals
Adult male Wistar rats weighing 160‐200 g were used in the study. Female rats were not included to rule out the influence of female sex hormones. The use of animals in these experiments was authorized by the Institutional Animal Ethical Committee. Throughout the experiment, experimental rats were handled in accordance with the CPCSEA guidelines. The animals were housed in groups of four in polypropylene cages. The lids were made of strong steel mesh, and the cages were designed to contain a feed hopper and a bracket to hold drinking water bottles. Animals were maintained at a controlled temperature of 20 ± 2℃ and a relative humidity of 50%‐60%, with an alternating 12 hour light/dark cycle (light on 6:00‐18:00 hours) and water provided ad libitum. UV sterilized clean paddy husk was used for bedding. Bedding was changed every alternate day by trained personnel to maintain the animals in approved hygienic conditions. The animals are fed with commercially available standard pellet chow (Amrut Feeds, Bangalore) and filtered tap water. All animals were free from diseases.
2.3. Methodology
2.3.1. Animal groupings
The animals were grouped into four groups, each group containing six rats (n = 6) as follows. Group I: normal control (distilled water po for 8 weeks). Group II: cisplatin control (2 mg/kg ip for 6 weeks). Group III: cisplatin (2 mg/kg ip for 6 weeks) + allantoin (200 mg/kg po for 8 weeks). Group IV: cisplatin (2 mg/kg ip for 6 weeks) + allantoin (400 mg/kg po for 8 weeks).
2.3.2. Study protocol
Cisplatin (2 mg/kg) was administered to the rats twice a week ip for 6 weeks to induce neuropathy.8 The dose of cisplatin was standardized in our laboratory. The protective drug allantoin (200 & 400 mg/kg) was given orally daily from day 0 to 8 weeks using an oral feeding needle (size 16G × 1.1/2) to groups 3 and 4. The dose of allantoin was decided based on LD50 studies9 reporting an LD50 (oral) in rats of >5000 mg/kg. After 8 weeks of treatment, blood was withdrawn from the retro‐orbital sinus under light anesthesia for measurement of biochemical parameters. Behavioral studies to ascertain the preventive effect of allantoin after stopping cisplatin administration were carried out before the start of the drug regime on day 0 and after completion of the drug regime at 8 weeks. Rats were sacrificed by an overdose of anesthesia and the sciatic nerves were isolated for nerve conduction velocity tests and for checking antioxidant activity in tissue homogenates.
2.3.3. Body weight measurement
Body weights were measured using a digital weighing scale (Advanturer OHAUS) before and after treatment.
2.4. Behavioral studies
2.4.1. Tail immersion test
Cold and thermal hyperalgesia was assessed by tail immersion test. The terminal part of the tail (1 cm) was immersed in water maintained at either 46 or 4℃. The time to tail withdrawal reflex or signs of struggle were recorded as a response to hot and cold sensations and a cut‐off time of 20 seconds was maintained. Rats were habituated to the testing procedure and handling by the investigator during the week before the experiment. All the animals were held in a restrainer to maintain uniform stress for all the groups.10
2.4.2. Locomotor activity test
Spontaneous locomotor activity was monitored using a digital actophotometer (Techno Electronics, India) equipped with infrared sensitive photo cells. The apparatus was placed in a darkened, light‐ and sound‐attenuated, and ventilated testing room. Each interruption of the beam on the x or y axis generated an electric impulse, which was recorded by a digital counter. Activity was measured as counts per 5 minutes.1
2.4.3. Mechanical allodynia test
Mechanical allodynia was defined as a significant decrease in withdrawal thresholds in response to electronic von‐Frey testing (in‐house fabricated electronic von Frey instrument). Rats were placed individually on an elevated mesh platform (1 cm2 perforations) in a clear plastic cage and adapted to the testing environment for at least 15 minutes. To assess the withdrawal threshold of the rat hind paw, a rigid polypropylene tip mounted on the cone of the probe was used. All measurements were performed in a quiet, temperature‐controlled room.
Paw withdrawal threshold measurements were standardized by using 3 out of 5 values and Positive responses were noted if the paw was robustly and immediately withdrawn.11
2.4.4. Mechanical hyperalgesia test
The nociceptive flexion reflex was quantified using an in‐house fabricated electronic Randall‐Selitto paw pressure device which applies a linearly increasing mechanical force (in grams) to the dorsum of the rat's hindpaw. The nociceptive threshold, expressed in grams, was assessed by increasing pressure to the hind paw until a squeak (vocalisation threshold) was elicited. As this test involves animal handling, the rat was habituated to being handled as follows. Three days before the experiment rats were handled continuously for 20 seconds 2 or 3 times depending on their capacity to remain quiet. On the day of the experiment, rats were again handled 2‐3 times for 20 seconds. Rats showing an aversive reaction during handling were excluded from the experiment. Then the paw of the rat was placed under the probe tip, and progressive pressure was applied until the rat vocalised. The vocalisation threshold was measured 3 or 4 times in order to obtain two consecutive values that differed no more than 10%, with an interval of at least 10 minutes between each measurement, and the measurements were averaged.12
2.5. Measurement of sciatic nerve conduction velocity
A sciatic nerve conduction velocity test (SNCV) was conducted using a PowerLab data acquisition system (ADInstruments, Australia). This test that determines the strength of the conduction of the nerve impulse as it courses down a nerve and is used to detect signs of nerve injury. This test followed a non‐survival procedure: rats were anesthetized with ketamine and xylazine (dose 80:5 mg/kg ip) hind limbs were used for the experiment, the left sciatic nerve was dissected rapidly and place in an insulate box and stimulated at the distal end. The nerve was stimulated with a biphasic pulse (duration 0.1 second, intensity 200 mV). The average of 10 potential traces was calculated and the nerve length between stimulant and recording electrode was recorded. The time interval between the stimulus artifact and the start of the first negative deflection was taken as the latency.13
2.6. Measurement of haematological parameters
At the end of the experimental period, approximately 0.5 ml of blood was drawn from the retro‐orbital plexus of the rats under light anaesthesia. Each blood sample was collected in a clean, dry, labelled micro‐centrifuge tube containing 10% EDTA solution (0.5 ml blood). The blood samples were analyzed within 6 hours using standard procedures for the estimation of haemoglobin, and WBC, RBC and platelet counts.14
2.7. Measurement of biochemical parameters
All the experimental animals were killed by an overdose of anesthesia after the experimental period. For measurement of biochemical parameters, sciatic nerve obtained from right hind limbs was rinsed and homogenized (10% w/v) in 0.1 M phosphate buffer (pH 7.0) and centrifuged for 10 minutes, and the resulting supernatant was used for measurement of lipid peroxide (malondialdehyde, MDA),15 catalase activity16 and superoxide dismutase (SOD).17 The remaining supernatant was centifuged at 3200 rpm for 20 minutes and used for measurement of reduced glutathione (GSH)18 and nitric oxide.19
2.8. Statistical analysis
All data were expressed as means ± SEM. One‐way analysis of variance between the groups followed by Dunnett's multiple comparison test was used to assess differences between the control, cisplatin‐treated and cisplatin + allantoin‐treated groups. A probability value of *P < 0.05 was considered as significant, **P < 0.01 was considered as highly significant and ***P < 0.001 was considered very highly significant.
3. RESULTS
3.1. Effect on behavioural studies
3.1.1. Cold and thermal hyperalgesia
The tail withdrawal latency of control cisplatin‐treated (group 2) rats was significantly lower than that of normal rats (group 1), indicating development of hyperalgesia. Treatment with allantoin (200 mg/kg) significantly increased the tail withdrawal latency compared to the cisplatin control group. At a higher dose (400 mg/kg), allantoin did not exhibit a significant effect (Figures 1 and 2).
Figure 1.
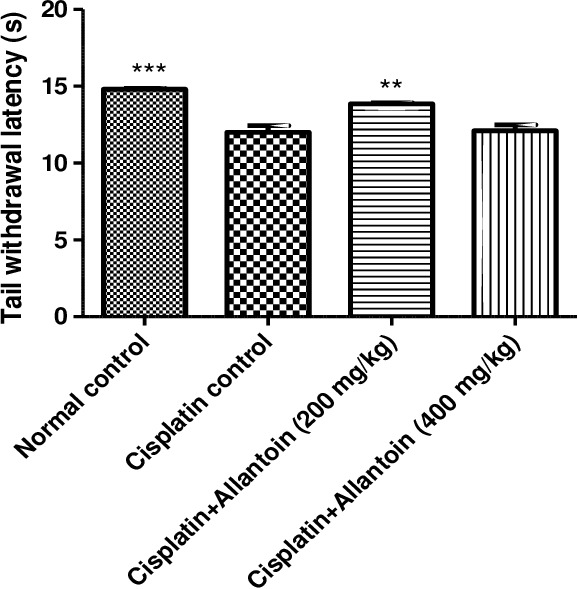
Effect of oral administration of allantoin on cold hyperalgesia in cisplatin‐treated rats
Figure 2.
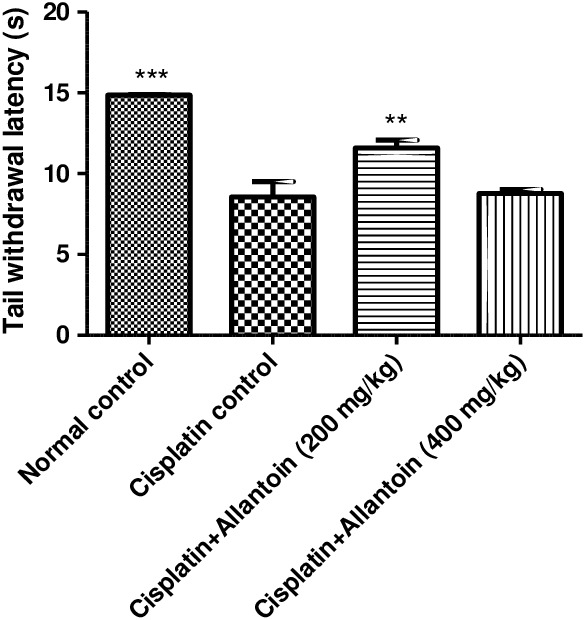
Effect of oral administration of allantoin on thermal hyperalgesia in cisplatin‐treated rats
3.1.2. Locomotor activity
Activity measured as the number of counts/10 minutes of cisplatin control rats was significantly lower than in normal rats. Allantoin treatment at the high dose (400 mg/kg) resulted in a significant increase in activity. At the lower dose (200 mg/kg), allantoin did not exhibit any significant change compared to the cisplatin control (Figure 3).
Figure 3.
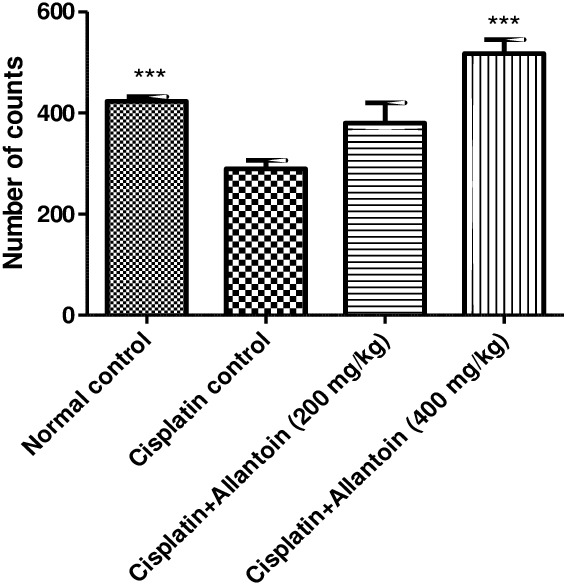
Effect of oral administration of allantoin on locomotor activity in cisplatin‐treated rats
3.1.3. Mechano‐tactile allodynia
The paw withdrawal threshold of cisplatin control rats was significantly lower than in normal rats. Treatment with allantoin (200 mg/kg) significantly increased the paw withdrawal threshold compared to cisplatin control rats. At the higher dose (400 mg/kg), allantoin did not exhibit a significant effect compared to the cisplatin control (Figure 4).
Figure 4.
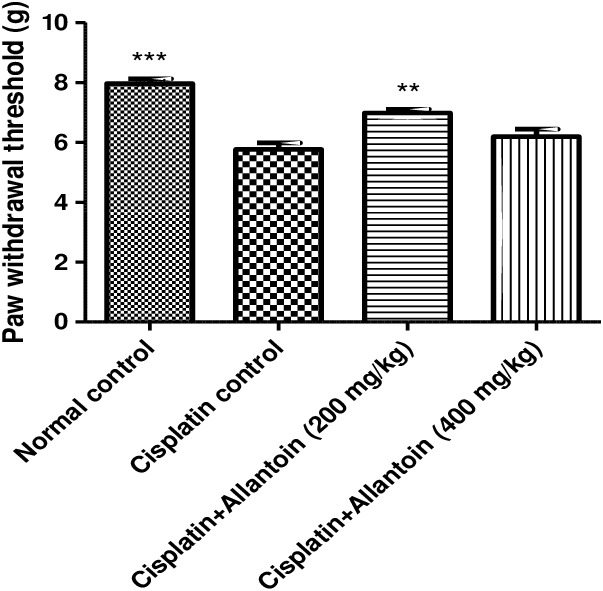
Effect of oral administration of allantoin on mechano‐tactile allodynia in cisplatin‐treated rats
3.1.4. Mechanical hyperalgesia
The mechanical paw pressure threshold of cisplatin control rats was significantly lower than in normal rats. Treatment with allantoin (200 mg/kg) significantly increased the mechanical threshold compared to the cisplatin control. At the higher dose (400 mg/kg), allantoin did not exhibit any significant effect (Figure 5).
Figure 5.
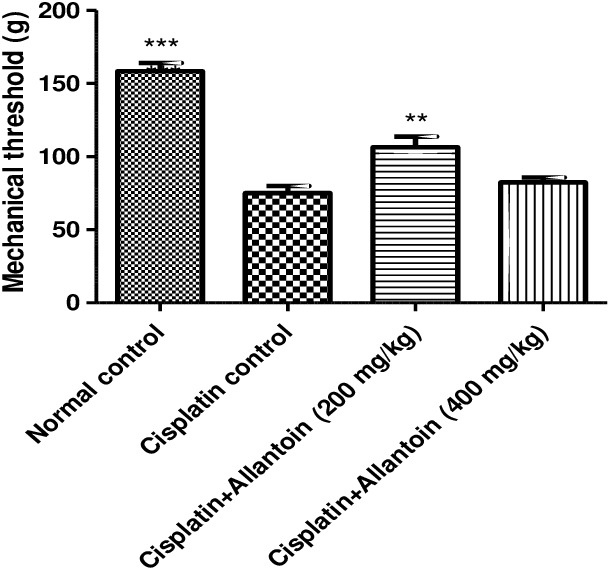
Effect of oral administration of allantoin on mechanical hyperalgesia in cisplatin‐treated rats
3.2. Effect on sciatic nerve conduction velocity
The sciatic nerve conduction velocity of cisplatin control rats was significantly lower than that of normal rats. The sciatic nerve conduction velocity in rats treated with allantoin (200 and 400 mg/kg oral) was significantly higher than in the cisplatin‐treated rats (Figure 6).
Figure 6.
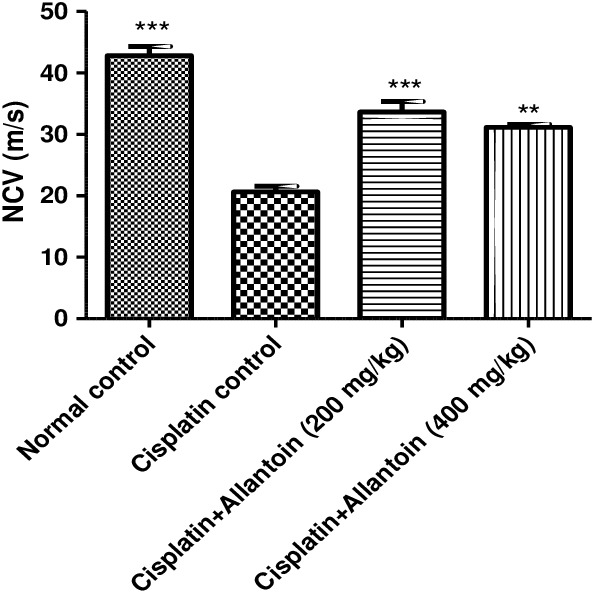
Effect of oral administration of allantoin on sciatic nerve conduction velocity (NVC) in cisplatin‐treated rats
3.3. Effect on haematological parameters
Haematological parameters in cisplatin‐treated animals were found to be significantly altered compared to those of the normal control group (group 1). The WBC count in rats treated with allantoin (200 and 400 mg/kg oral) showed no significant changes compared to the cisplatin‐treated rats. RBC counts, haemoglobin content and platelet counts in rats treated with allantoin (200 and 400 mg/kg oral) were significantly higher than in the cisplatin‐treated rats (Table 1).
Table 1.
Effect of allantoin on body weight and haematological parameters in cisplatin‐treated rats
| Parameters | Group 1 Normal control | Group 2 Cisplatin (2 mg/kg ip) | Group 3 Cisplatin (2 mg/kg ip) + allantoin (200 mg/kg po) | Group 4 Cisplatin (2 mg/kg ip) + allantoin (400 mg/kg po) |
|---|---|---|---|---|
| Body weight (g) | 269.166 ± 11.432 | 212.833 ± 15.413 | 217.833 ± 7.516 | 219.333 ± 18.957 |
| WBC count (×103/µl) | 6.733 ± 0.313** | 5.133 ± 0.301 | 5.483 ± 0.291 | 4.916 ± 0.257 |
| RBC count (×106/µl) | 6.836 ± 0.111*** | 4.285 ± 0.371 | 6.008 ± 0.363*** | 5.936 ± 0.292** |
| Haemoglobin (g/dL) | 13.083 ± 0.327*** | 8.216 ± 0.703 | 10.317 ± 0.241* | 10.117 ± 0.509* |
| Platelet count (×103/µl) | 538.833 ± 20.956*** | 403.333 ± 25.489 | 500.000 ± 6.875* | 541.666 ± 36.137*** |
Values are expressed as means ± SEM (n = 6). The data were statistically analyzed by one‐way.
ANOVA followed by Dunnet's multiple comparison test.
*P < 0.05 was considered as significant, **P < 0.01 was considered as highly significant and ***P < 0.001 was considered as very highly significant when compared with the cisplatin control group.
3.4. Effect on biochemical parameters
The catalase activity, superoxide dismutase activity and reduced glutathione levels of cisplatin control rats were significantly lower than in normal control rats. In rats treated with allantoin (200 mg/kg oral), the catalase activity, superoxide dismutase activity and reduced glutathione levels were significantly higher than in the cisplatin‐treated rats. The malonaldehyde levels and nitric oxide concentration of cisplatin‐treated rats were higher than in normal rats, while in rats treated with allantoin (200 and 400 mg/kg orally) they were significantly lower than in the cisplatin‐treated rats (Table 2).
Table 2.
Effect of allantoin on biochemical parameters in cisplatin‐treated rats
| Parameters | Group 1 Normal control | Group 2 Cisplatin (2 mg/kg ip) | Group 3 Cisplatin (2 mg/kg ip) + allantoin (200 mg/kg po) | Group 4 Cisplatin (2 mg/kg ip) + allantoin (400 mg/kg po) |
|---|---|---|---|---|
| Catalase activity(µmol/min/mg of protein) | 68.757 ± 2.888*** | 42.111 ± 1.509 | 50.442 ± 2.505* | 44.414 ± 2.491 |
| SOD (units/mg protein) | 14.656 ± 0.669*** | 6.804 ± 0.472 | 10.412 ± 0.526*** | 7.924 ± 0.451 |
| GSH (µg/mg protein) | 41.192 ± 4.986*** | 15.466 ± 1.638 | 37.916 ± 3.924** | 24.397 ± 2.244 |
| MDA (nmol/mg protein) | 3.596 ± 0.287*** | 7.618 ± 0.684 | 5.679 ± 0.283** | 7.700 ± 0.360 |
| NO (µg/ml) | 34.972 ± 0.481*** | 75.466 ± 0.234 | 54.138 ± 0.651** | 66.935 ± 0.220* |
Values are expressed as means ± SEM (n = 6). The data were statistically analyzed by one‐way ANOVA followed by Dunnet's multiple comparison test.
*P < 0.05 was considered as significant, **P < 0.01 was considered as highly significant and ***P < 0.001 was considered as very highly significant when compared with the cisplatin control group.
4. DISCUSSION
Cisplatin is an important component of chemotherapy used against various tumour types such as pancreatic cancer, breast cancer, osteosarcoma and metastatic melanoma. However, its clinical use is severely restricted by dose‐limiting neurotoxicity. As well as affecting the nervous system, cisplatin has also been reported to cause ROS generation and lipid peroxidation. Studies suggest that cisplatin reduces peripheral nerve blood supply through a potent angiogenic effect which may result in nerve damage.20
In this study rats administered cisplatin (2 mg/kg ip weekly twice for 6 weeks) exhibited decreased bodyweight, thermal hyperalgesia, decreased motor co‐ordination, mechanical allodynia, mechanical hyperalgesia, nerve conduction velocity deficits and decreased antioxidant levels, all of which are symptoms frequently occurring in patients with peripheral neuropathy. Our data show that peripheral neuropathy was successfully induced in the rats and this model can be used for screening neuroprotective drugs for the treatment of cisplatin‐induced peripheral neuropathy. Our data also show that treatment with allantoin (200 mg/kg, po) significantly alleviated cisplatin‐induced peripheral neuropathy in rats. Rats treated with allantoin exhibited reduced cold and thermal hyperalgesia, improved nerve conduction velocity, improved mechano‐tactile sensitivity and mechanical hyperalgesia, and higher levels of antioxidant enzymes when compared with cisplatin‐treated rats. Rats treated with allantoin at a higher dose (400 mg/kg po) exhibited improved locomotor activity and nerve velocity but the treatment had no significant effect on cold and thermal hyperalgesia, mechano‐tactile allodynia and mechanical hyperlgesia. This may be because of the peripheral antinociceptive activity of allantoin, which has been reported to involve activation of opioid receptor‐mediated ATP‐sensitive channels.21 Thus at a high dose (400 mg/kg) allantoin may exhibit analgesic activity. Allantoin has been shown to have significant anti‐inflammatory and wound healing properties. Reports suggest that allantoin exerts antidiabetic activity in streptozotocin‐induced diabetic rats by decreasing the levels of fasting blood glucose and HbAlc, while conversely increasing the levels of insulin, GLP‐1, and C‐peptide. In recent reports allantoin treatment increased the serum antioxidant activities of tGSH, GSH, and SOD, but decreased the levels of MDA and GSSG.5 These findings suggested that allantoin treatment may be able to ameliorate the effects of chronic oxidative stress in β‐cells and other bodily organs.
Allantoin has also been shown to exert anti‐inflammatory effects. IL‐6, a multifunctional pro‐inflammatory cytokine, affects the secretion of GLP‐1 by intestinal L cells. The levels of circulating GLP‐1 have been found to correlate with the concentration of systemic IL‐6 and also with the concentrations of other markers of inflammation, suggesting that the regulation of GLP‐1 is inflammation dependent. It is thus worth noting that treatment with allantoin may promote the release of GLP‐1 and improve the function of β‐cells, thereby maintaining insulin and glucose levels.21
The effects of allantoin in this study could be due to its anti‐inflammatory and antioxidant activity, which may have restored motor nerve conduction velocity deficits and improved cold and thermal hyperalgesia, mechano‐tactile allodynia, and mechanical hyperalgesia compared to cisplatin‐treated rats. Reports suggest that neurotoxic chemotherapy agents can damage sympathetic nerves in the bone marrow and compromise hematopoietic stem cell mobilization and hematopoietic regeneration. This study provides evidence that allantoin improves the haematological status by showing that haemoglobin, platelet and RBC levels were significantly increased in allantoin‐treated rats compared to cisplatin‐treated rats, while the WBC count in treated groups remained normal.16
5. CONCLUSION
Our findings suggest favourable effects of allantoin (200 mg/kg) on functional abnormalities in cisplatin neuropathy. We have shown that allantoin can ameliorate cisplatin‐induced neurotoxic insults to neurons. It therefore shows promise as an adjuvant drug in cancer treatment.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
MA performed the experimental work, and RR and VNS were involved in experimental and intellectual work on the paper. All authors have read and approved the manuscript.
ACKNOWLEDGEMENTS
The authors are grateful to the administration of Al‐Ameen College of Pharmacy, Bangalore, India, for financial assistance and facilities provided.
N S V, Mohamad A, Razdan R. Allantoin attenuates deficits of behavioural and motor nerve conduction in an animal model of cisplatin‐induced neurotoxicity in rats. Animal Model Exp Med. 2019;2:114–120. 10.1002/ame2.12070
REFERENCES
- 1. Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy‐induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507‐1515. [DOI] [PubMed] [Google Scholar]
- 2. Argyriou A, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy‐induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82:51‐77. [DOI] [PubMed] [Google Scholar]
- 3. Wang XM, Lehky TJ, Brell JM, Dorsey SG. Discovering cytokines as targets for chemotherapyinduced painful peripheral neuropathy. Cytokine. 2012;59:3‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takagi H, Ishiga Y, Watanabe S, et al. Allantoin, a stress‐ related purine metabolite, can activate jasmonate signaling in a MYC2‐regulated and abscisic acid‐dependent manner. J Exp Bot. 2016;67:2519‐2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Go H‐K, Rahman MD, Kim G‐B, et al. Antidiabetic effects of yam (Dioscorea batatas) and its active constituent, allantoin, in a rat model of streptozotocin‐induced diabetes. Nutrients. 2015;7:8532‐8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen M‐F, Tsai J‐T, Chen L‐J, et al. Antihypertensive action of allantoin in animals. Biomed Res Int. 2014;2014:690135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang HZ, Wang CJ. Isolation, characterization and analgesic activity of natural allantoin from portulaca oleracea seed. Mod Chem Appl. 2018;8:114‐3. [Google Scholar]
- 8. Bianchi R, Brines M, Lauria G, et al. Protective effect of erythropoietin and its carbamylated derivative in experimental cisplatin peripheral neurotoxicity. Clin Cancer Res. 2006;12:2607‐2612. [DOI] [PubMed] [Google Scholar]
- 9. EMEA . Committee for veterinary medicinal products: Allantoin. 2001. http://www.ema.europa.eu
- 10. Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp Neurol. 2003;182:12‐20. [DOI] [PubMed] [Google Scholar]
- 11. Martinov T, Mack M, Sykes A, Chatterjea D. Measuring changes in tactile sensitivity in the hind paw of mice using an electronic von frey apparatus. J Vis Exp. 2013;e51212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santos‐Nogueira E, Redondo Castro E, Mancuso R, Navarro X. Randall‐Selitto test: a new approach for the detection of neuropathic pain after spinal cord injury. J Neurotrauma. 2012;29:898‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bhadri N, Sanji T, Madakasira Guggilla H, Razdan R. Amelioration of behavioural, biochemical, and neurophysiological deficits by combination of monosodium glutamate with resveratrol/alpha‐lipoic acid/coenzyme Q10 in rat model of cisplatin‐induced peripheral neuropathy. Sci World J. 2013;2013:565813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohan H. Pathology Practical Book. New Delhi: Jaypee Brothers Medical Publishers; 2005. [Google Scholar]
- 15. Saadé NE, Jabbur SJ. Nociceptive behavior in animal models for peripheral neuropathy: spinal and supraspinal mechanisms. Prog Neurogibol. 2008;86:22‐47. [DOI] [PubMed] [Google Scholar]
- 16. Reddy DS, Kulkarni SK. Possible role of nitric oxide in the nootropic and antiammestic effects of neurosteroids on aging and dizocilpine induced learning impairement. Brain Res. 1998;2:215‐229. [DOI] [PubMed] [Google Scholar]
- 17. Awasthi S, Kakkar P, Viswanathan PN, Bharadwaj R. Effects of anesthetic ether on lipid peroxidation and superoxide dimulatase isoenzymes of young and adult rat brain. Indian J Exp Biol. 1984;27:647‐649. [PubMed] [Google Scholar]
- 18. Tiwari V, Kuhad A, Chopra K. Tocotrienol ameliorates behavioral and biochemical alterations in the rat model of alcoholic neuropathy. Pain. 2009;145:129‐135. [DOI] [PubMed] [Google Scholar]
- 19. Mittal R, Gonzalez‐Gomez I, Goth K, Prasadarao N. Inhibition of inducible nitric oxide controls pathogen load and brain damage by enhancing phagocytosis of Escherichia coli K1 in neonatal meningitis. Am J Pathol. 2010;176:1292‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy‐induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15:3081‐3094. [DOI] [PubMed] [Google Scholar]
- 21. Florentino IF, Silva D, Galdino PM, et al. Antiniciceptive and anti‐inflammatory effects of Memora nodosa and allantoin in mice. J Ethnopharmacol. 2016;186:298‐304. [DOI] [PubMed] [Google Scholar]


