JAK2-V617F is present in nearly 95% of patients with polycythemia vera and 50-60% of those with essential thrombocythemia or primary myelofibrosis.1 In addition to erythrocytosis, thrombocytosis and bone marrow fibrosis (as in polycythemia vera, essential thrombocythemia, and primary myelofibrosis, respectively), the clinical course of patients with myeloproliferative neoplasia (MPN) is characterized by increased risks of thrombosis, splenomegaly and an inflammatory response syndrome.2,3 Clinical studies of JAK kinase inhibitors have shown that these agents can produce considerable improvements of splenomegaly, constitutive symptoms, and overall survival in MPN patients.3 However, the therapeutic response is often limited and short-lived. In addition, transformation to acute leukemia remains a major problem. It is, therefore, essential to identify novel nodes of constitutive JAK2-V617F signaling and to develop better approaches to the therapy of these neoplasias.4 Downstream of JAK2-V617F, NFκB signaling (p65) is constitutively active and regulates expression of CXCL10 in MPN.5 In bortezomib-resistant multiple myeloma cells6 and in acute myeloid leukemia cells with MLL-AF9 rearrangements,7 constitutively active NFκB drives the expression of Bruton tyrosine kinase (BTK). Of note, it was found that BTK interacted with erythropoietin receptor-JAK2 and was tyrosine phosphorylated in response to treatment with erythropoietin.8 These observations led us to investigate whether JAK2-V617F kinase also induces BTK expression (via p65) and activation and to characterize its physiological relevance in JAK2-V617F-positive cells.
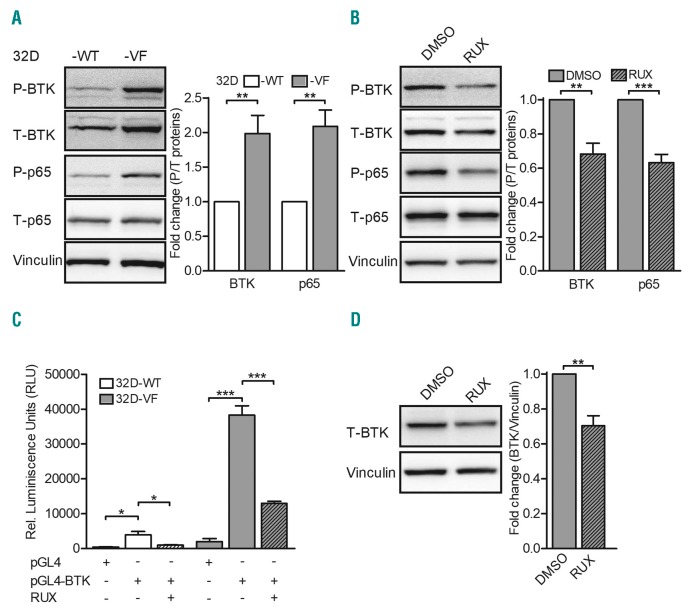
To investigate whether BTK is activated by JAK2-V617F, we used 32D myeloid progenitor cells ectopically expressing human erythropoietin receptor and JAK2-wildtype (32D JAK2-WT) or JAK2-V617F (32D JAK2-V617F).9 Tyrosine phosphorylation of BTK was indeed elevated in whole cell lysates of 32D JAK2-V617F cells compared to the level in 32D JAK2-WT cells (Figure 1A). Consistent with this finding, erythropoietin additionally induced BTK phosphorylation in 32D JAK2-WT cells (Online Supplementary Figure S1A). Of note, BTK was overexpressed in 32D JAK2-V617F cells. In line with our previous observations,5 32D JAK2-V617F whole cell lysates demonstrated constitutive activation of p65 (Figure 1A). Emphasizing the contribution of the constitutively active JAK2-V617F kinase, the JAK1/2 inhibitor ruxolitinib inhibited phosphorylation of both BTK and p65 in 32D JAK2-V617F cells (Figure 1B). These findings were confirmed in human cell lines with the JAK2-V617F mutation (Online Supplementary Figure S1B). HEL and SET2 (with the JAK2-V617F mutation) demonstrated strong expression and activation of BTK when compared to OCI AML3 (without the JAK2-V617F mutation). MOLM13 cells (with the FLT3-internal tandem duplication) served as a positive control for BTK expression.7 The contribution of activated p65 in BTK expression,6,7 together with the observations presented in Figure 1A (p65 activation and BTK overexpression), led us to hypothesize a role for activated p65 in BTK expression. Luciferase-based BTK promoter assays were performed by electroporating 32D JAK2-WT and JAK2-V617F cells with an empty construct (pGL4) or test construct (pGL4-BTK promoter).6 The BTK promoter region in the test construct harbors two p65 binding sites.10 In comparison to JAK2-WT cells, 32D JAK2-V617F cells demonstrated elevated luciferase activity, indicating enhanced transcriptional activity of p65 on the BTK promoter (Figure 1C). Confirming a role for JAK2-V617F kinase in p65 activity, ruxolitinib treatment negatively affected BTK promoter activity (Figure 1C) and protein expression (Figure 1D). Thus, downstream of JAK2-V617F signaling, our data suggest a novel role for activated p65 in the regulation of BTK expression.
Figure 1.
Bruton tyrosine kinase is overexpressed and constitutively active in 32D myeloid progenitor cells ectopically expressing erythropoietin receptor and JAK2-V617F kinase. (A) 32D JAK2-wildtype (WT) and JAK2-V617F (VF) cells were starved of serum for 4 h and the whole cell lysates were analyzed for expression of phosphorylated (P) Bruton tyrosine kinase (BTK) (pY223) or p65 (pS536) and total (T) proteins. Densitometric analysis of P/T-BTK and -p65 is shown (n=5). (B) 32D VF cells were cultured in the presence of dimethyl sulfoxide (DMSO) or 0.5 μM ruxolitinib (RUX) overnight and the whole cell lysates were analyzed for expression of P-BTK or -p65 and T proteins. Densitometric analysis of P/T-BTK and -p65 is shown (n=4). (C) 32D WT and VF cells were electroporated with control (pGL4) or BTK promoter construct (pGL4-BTK) and treated with DMSO or 0.5 mM RUX overnight and promoter assays were performed as described previously5 (n=3). (D) 32D VF cells were cultured in the presence of DMSO or 0.5 mM RUX overnight and whole cell lysates were analyzed for expression of BTK. Densitometric analysis of T-BTK relative to vinculin is shown (n=4). Vinculin served as a loading control in all the experiments. Columns represent mean ± standard error of mean from independent experiments. Statistical significance between different conditions was calculated using the Student t-test; *P<0.05, **P<0.01 and ***P<0.001.
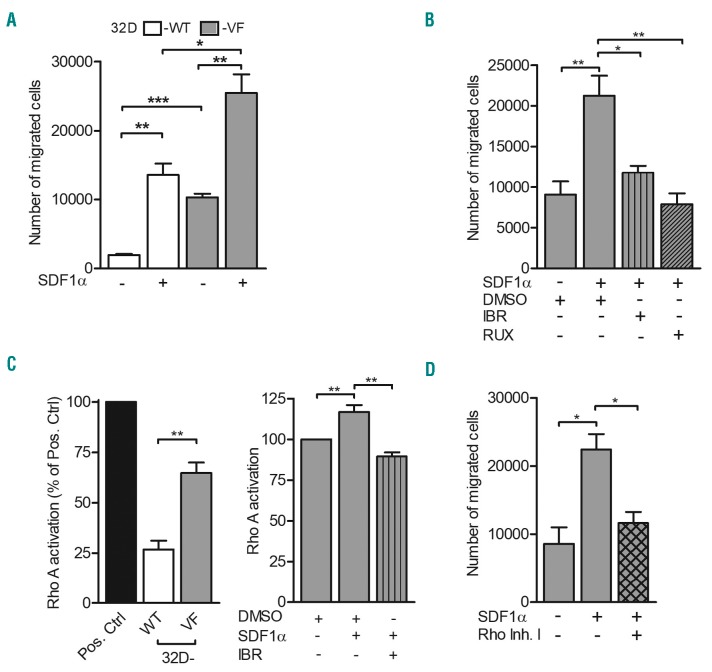
Growing evidence supports an important contribution of BTK in cell migration via the stromal cell-derived factor 1 alpha (SDF1α)-CXCR4 axis.11,12 BTK mediates activation of phospholipase C gamma (PLCγ) 2 and regulates SDF1α-induced migration and homing of B cells to the spleen and bone marrow.11 We showed earlier that erythropoietin receptor-JAK2-dependent PLCγ1 activation is essential in erythroid progenitor differentiation.9 Recently it was demonstrated that MPL-W515L or cytokine-dependent JAK2 activation cooperates with SDF1α-CXCR4 signaling to enhance chemotactic response via phosphoinotiside 3 kinase (PI3K) signaling.13 These observations led us to investigate whether PLCγ1 and PI3K signaling are activated downstream of the JAK2-V617F-BTK signaling cascade and whether they contribute to cell migration. 32D JAK2-V617F cells were treated with a BTK inhibitor (ibrutinib) or ruxolitinib and were analyzed for AKT and PLCγ1 phosphorylation. Positioning BTK upstream of PI3K-AKT and PLCγ1, ibrutinib treatment inhibited AKT and PLCγ1 phosphorylation (Online Supplementary Figure S2A,B). Plasma and splenic SDF1α levels are elevated in patients with poly-cythemiavera and primary myelofibrosis.14 Consequently, we hypothesized that SDF1α-dependent cell migration occurs via BTK and its downstream signaling nodes PI3K-AKT and PLCγ1 in JAK2-V617F cells. Basal migration of 32D JAK2-V617F cells was significantly higher than that of 32D JAK2-WT cells (Figure 2A). Supporting this observation, eyrthropoietin-induced JAK2 activation enhanced chemotaxis in 32D JAK2-WT cells (Online Supplementary Figure S3A). Erythropoietin in combination with the chemokine of interest, SDF1α, failed to promote further chemotaxis in 32D JAK2-V617F cells (Online Supplementary Figure S3B). Interestingly, our data also demonstrate a collaboration between JAK2 and SDF1α signaling in enhancing chemotaxis of both 32D JAK2-WT and JAK2-V617F cells (Figure 2A). Underscoring the contribution of activated BTK, ibrutinib blocked basal cell migration in 32D JAK2-V617F cells (Online Supplementary Figure S3C). Furthermore, ruxolitinib and ibrutinib attenuated SDF1α-induced chemotaxis in these cells (Figure 2B). This observation could be recapitulated in BaF3 JAK2-V617F cells (Online Supplementary Figure S3D). Demonstrating an important role for activated PLCγ1 and PI3K-AKT signaling down stream of JAK2-V617F and activated BTK (Online Supplementary Figure S2A,B), PI3K-AKT and PLCγ1 inhibitors (LY294002 and U73122, respectively) suppressed SDF1α-induced chemotaxis. Ibrutinib in combination with LY294002 or U73122 additionally inhibited SDF1α-induced chemotaxis (Online Supplementary Figure S3E). Importantly, ibrutinib treatment failed to affect either basal or SDF1α-induced cell migration in JAK2-V617F-negative control cells (primary T-lymphocytes and 32D parental cells) (Online Supplementary Figure S4A,B). In contrast, ruxolitinib blocked SDF1α-induced cell migration in these cells. Since p65 transcriptionally regulated BTK expression, we investigated whether p65 inhibition could affect cell migration. NFκB inhibitors (BAY11-7082 and IKK inhibitor VII) did indeed block basal and SDF1α-induced cell migration in 32D and BaF3 JAK2-V617F cells (Online Supplementary Figure S4C,D). Since BTK is activated downstream of the JAK2-V617F mutation and ibrutinib has been reported to have a cytotoxic effect,7,15 we examined the therapeutic potential of this drug (Online Supplementary Figure S4E). 32D JAK2-V617F cells did not respond to ibrutinib even after 48 h, suggesting a cell survival-independent function of BTK activation in these cells.
Figure 2.
Bruton tyrosine kinase mediates stromal cell-derived factor 1α-induced chemotaxis via activation of the small GTPase RhoA. (A, B) 32D JAK2-wild-type (WT) and JAK2-V617F (VF) cells were starved of serum for 2 h and left alone (n=3) or treated with dimethyl sulfoxide (DMSO) and inhibitors against Bruton tyrosine kinase (BTK) (2 mM of ibrutinib: IBR) and JAK2 (1 mM of ruxolitinib: RUX) as indicated for an additional 2 h (n=4) and chemotaxis assays were performed using transwells (pore size: 5 mm). Starvation medium (0.5% fetal bovine serum) containing stromal cell-derived factor 1 alpha (SDF1α: 100 ng/mL) served as the chemoattractant. (C) Active RhoA was determined by the G-LISA RhoA activation kit (Cytoskeleton #BK124) following the manufacturer’s instructions. 32D WT and VF cells were starved of serum for 4 h and left alone (left panel) (n=4) or treated with DMSO and 1 mM IBR as indicated (right panel) (n=4). After 1 h, the cells were stimulated with SDF1α (100 ng/mL) for an additional 2 h and whole cell lysates were analyzed for RhoA activation. (D) 32D VF cells were treated with 2 mM of Rho inhibitor (Rho Inh.I) for 4 h in serum starvation medium as indicated and chemotaxis assays were performed using transwells (pore size: 5 μm) (n=3). Starvation medium (0.5% fetal bovine serum) containing SDF1α (100 ng/mL) served as the chemoattractant. Columns represent mean ± standard error of mean from independent experiments. Statistical significance between different conditions was calculated using the Student t-test; *P<0.05, **P<0.01 and ***P<0.001.
To exclude potential off-target effects associated with the PLCγ inhibitor U73122, we evaluated cell migration in 32D JAK2-V617F cells upon PLCγ1 knockdown (Online Supplementary Figure S5A). Both the short hairpin RNA (#14, #133) tested successfully knocked down PLCγ1 (left panel) and this resulted in suppression of SDF1α-induced chemotactic migration (right panel). Importantly, SDF1α significantly induced chemotaxis in 32D JAK2-V617F cells but not in cells with PLCγ1 knockdown. This observation intrigued us and led us to investigate whether SDF1α induced chemotaxis via PLCγ1 phosphorylation. In support of this possibility, SDF1α significantly enhanced phosphorylation on PLCγ1 (Online Supplementary Figure S5B). Knockdown of PLCγ1 in con trol cells (32D and BaF3 parental cells) did not affect basal migration but negatively affected SDF1α-induced cell migration (Online Supplementary Figure S5C,D). The data thus indicate an important role for PLCγ1 activation downstream of JAK2-V617F kinase or SDF1α signaling. Since SDF1α induced significant chemotaxis in 32D JAK2-WT and JAK2-V617F cells, we were intrigued to evaluate its receptor CXCR4 expression. 32D JAK2-WT and JAK2-V617F cells demonstrated the highest and lowest surface expression of CXCR4, respectively (Online Supplementary Figure S6A). Erythropoietin resulted in a dose-dependent decrease of surface CXCR4 expression in 32D JAK2-WT cells. Hence, JAK2 activation regulates chemotaxis potentially via inducing changes in CXCR4 turnover on the cell surface. Although ibrutinib inhibited cell migration, it failed to affect CXCR4 expression (Online Supplementary Figure S6B). Ibrutinib thus affects cell migration by blocking intracellular signaling in JAK2-V617F cells.
We next investigated the signaling molecules influencing SDF1α-controlled migration downstream of BTK. Ras homolog gene family, member A (RhoA) activation is important in the regulation of cytoskeletal dynamics and cell migration in diverse cell types. In Jurkat cells, SDF1α-induced activation of RhoA and RhoC plays a pivotal role in the regulation of chemotaxis.16 Furthermore, a role for the BTK family of kinases in the regulation of Rho GTPases has been described.17 Quantitative mass spectrometry-based investigations of the granulocyte proteome revealed an underexpression of Rho-GDP dissociation inhibitors (Rho-GDI 1 and 2) in JAK2-V617F-positive MPN.18 Since Rho-GDI negatively regulate Rho GTPases, we hypothesized an increased RhoA activation downstream of JAK2-V617F kinase. RhoA GTPase activation assays, which specifically recognize active GTP-bound forms, were performed in 32D JAK2-WT and JAK-V617F cells. RhoA activation was indeed elevated in 32D JAK2-V617F cells in comparison to that in 32D JAK2-WT cells (Figure 2C, left panel). Underscoring the contribution of activated JAK2, erythropoietin induced RhoA activation in 32D JAK2-WT cells (Online Supplementary Figure S7A). SDF1α enhanced basal RhoA activation in 32D JAK2-V617F cells and pretreatment of the cells with ibrutinib significantly attenuated SDF1α-induced RhoA activation (Figure 2C, right panel). RhoA activation was validated in granulocytes of MPN patients with JAK2-V617F mutation (Online Supplementary Figure S7B). In response to external stimuli, RhoA regulates cell migration via actin remodeling.19 Establishing a role for RhoA activation in cell migration, 32D JAK2-V617F cells demonstrated increased actin polymerization (F-actin) (Online Supplementary Figure S8A). SDF1α further augmented F-actin staining in these cells. Granulocytes from JAK2-V617F MPN patients demonstrated higher actin polymerization than those from healthy donors (Online Supplementary Figure S8B). Confirming an essential role for RhoA activation, Rho inhibitor treatment blocked SDF1α-induced cell migration in 32D JAK2-V617F cells (Figure 2D). Collectively our observations highlight RhoA GTPase as one of the essential downstream targets of JAK2-V617F-BTK signaling in regulating cell migration.
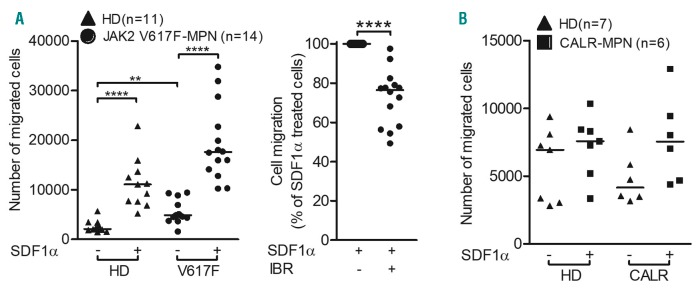
Finally, we sought to validate our cell migration results in primary granulocytes isolated from the peripheral blood of healthy donors, JAK2-V617F-positive and CALR-mutated MPN patients. Supporting our cell migration data using 32D and BaF3 JAK2-V617F cells, granulocytes isolated from JAK2-V617F MPN patients demonstrated increased basal migration (2.1-fold higher) as compared to granulocytes from healthy donors (Figure 3A, left panel). SDF1α treatment further enhanced migration in granulocytes from patients with MPN in comparison to those from healthy donors. Clinically achievable concentrations of ibrutinib (0.5 mM)7 significantly reduced SDF1α-stimulated migration in JAK2-V617F MPN granulocytes (Figure 3A, right panel). Of note, in comparison to granulocytes from healthy donors, granulocytes isolated from CALR-mutated MPN patients demonstrated no detectable changes with respect to basal or SDF1α-induced transmigration (Figure 3B).
Figure 3.
Stromal cell-derived factor 1 alpha-induced chemotaxis of primary granulocytes isolated from peripheral blood of JAK2-V617F-positive patients with myeloproliferative neoplasms could be impeded by a clinically relevant dose of ibrutinib. Granulocytes were isolated (Ficoll-paque density gradient centrifugation- erythrocyte lysis based method) from peripheral blood of healthy donors (▲: HD), and patients with untreated JAK2-V617F-positive (●: JAK2 V617F-MPN) (A) or CALR-mutated (■: CALR-MPN) (B) myeloproliferative neoplasia (MPN). The protocol was approved by the local ethics committee (protocol n. MD115/08), and all patients signed informed consent. The cells were rested for 30 min in starvation medium (0.5% fetal bovine serum) and left alone (A, left panel and B) or treated with dimethylsulfoxide (DMSO) or 0.5 mM of ibrutinib (IBR) (A, right panel) for an additional 1 h and chemotaxis assays were performed (pore size: 3 mm). Starvation medium (0.5% fetal bovine serum) containing stromal cell-derived factor 1 alpha (SDF1α: 100 ng/mL) served as the chemoattractant. Chemotaxis of IBR-treated cells was normalized to that of DMSO-treated cells. The horizontal lines indicate medians and the statistical significance of differences between control and treated samples was calculated by the Mann-Whitney test; *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001.
Transmigration of leukocytes and of progenitor cells is crucial to the process of extramedullary hematopoiesis. This process is regulated by chemokines such as SDF1α and is mediated by the integrins LFA1 and VLA4. Recently, we showed that JAK2-V617F but not mutated CALR stimulates integrin signaling via activation of the small GTPase Rap1, resulting in increased binding of granulocytes to ICAM-1 and VCAM-1 (abundantly expressed in spleen).20 Thus, it is tempting to speculate that differences in chemotaxis in concert with differential integrin binding of JAK2-V617F versus CALR mutated leukocytes might contribute to extramedullary hematopoiesis. This hypothesis is in line with recent data showing that in primary myelofibrosis, the risk of splenomegaly is less pronounced in CALR-mutated patients than in JAK2-V617F-positive individuals.21
In conclusion, our data demonstrate that JAK2-V617F kinase, via its signaling intermediates BTK, PI3K/AKT, PLCγ1, and RhoA, collaborates with chemokine SDF1α and regulates cell migration. These findings expand our current understanding of the physiological role of activated JAK2-V617F signaling. The data further provide a rationale for investigating the contribution of these downstream molecules in abnormal cell motility of JAK2-V617F-positive myeloid progenitors and stem cells migrating from bone marrow to peripheral blood and to extramedullary organs. The findings may also be useful to the clinical exploration of ruxolitinibibrutinib combinations to inhibit abnormal migration and homing of the JAK2-V617F-positive clone in MPN. Future studies are warranted to clarify the molecular mechanisms and clinical potential of these targets.
Supplementary Material
Acknowledgments
The authors would like to thank: Prof. Kristian Bowles and Dr. Lyubov Zaitseva (Norwich Medical School, UK) for their generous gift of pGL4-empty and pGL4-BTK-promoter constructs and Corinna Fahldieck, Uta Schönborn and Anja Sammt for their excellent technical support.
Footnotes
Funding: this project was funded by grants from DFG (SFB854, project A20 to TF and SFB1335, project P3 to UK) and BMBF (e:Bio JAK-Sys to TF).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Cross NCP. Genetic and epigenetic complexity in myeloproliferative neoplasms. Hematology Am Soc Hematol Educ Program. 2011;2011:208–214. [DOI] [PubMed] [Google Scholar]
- 2.Hermouet S, Bigot-Corbel E, Gardie B. Pathogenesis of myeloproliferative neoplasms: role and mechanisms of chronic Inflammation. Mediators Inflamm. 2015;145293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. New Engl J Med. 2010;363(12):1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan M, Siddiqi R, Gangat N. Therapeutic options for leukemic transformation in patients with myeloproliferative neoplasms. Leuk Res. 2017;63:78–84. [DOI] [PubMed] [Google Scholar]
- 5.Schnoder TM, Eberhardt J, Koehler M, et al. Cell autonomous expression of CXCL-10 in JAK2V617F-mutated MPN. J Cancer Res Clin. 2017; 143(5):807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray MY, Zaitseva L, Auger MJ, et al. Ibrutinib inhibits BTK-driven NF-kappa B p65 activity to overcome bortezomib-resistance in multiple myeloma. Cell Cycle. 2015;14(14):2367–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nimmagadda SC, Frey S, Edelmann B, et al. Bruton’s tyrosine kinase and RAC1 promote cell survival in MLL-rearranged acute myeloid leukemia. Leukemia. 2018;32(3):846–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt U, van den Akker E, Parrenvan Amelsvoort M, et al. Btk is required for an efficient response to erythropoietin and for SCF-controlled protection against TRAIL in erythroid progenitors. J Exp Med. 2004;199(6):785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnoder TM, Arreba-Tutusaus P, Griehl I, et al. Epoinduced erythroid maturation is dependent on Plc gamma 1 signaling. Cell Death Differ. 2015;22(6):974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L, Mohamed AJ, Simonson OE, et al. Proteasome-dependent autoregulation of Bruton tyrosine kinase (Btk) promoter via NF-kappaB. Blood. 2008;111(9):4617–4626. [DOI] [PubMed] [Google Scholar]
- 11.de Gorter DJJ, Beuling EA, Kersseboom R, et al. Bruton’s tyrosine kinase and phospholipase C gamma 2 mediate chemokine-controlled B cell migration and homing. Immunity. 2007;26(1):93–104. [DOI] [PubMed] [Google Scholar]
- 12.Zaitseva L, Murray MY, Shafat MS, et al. Ibrutinib inhibits SDF1/CXCR4 mediated migration in AML. Oncotarget. 2014; 5(20):9930–9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdelouahab H, Zhang YY, Wittner M, et al. CXCL12/CXCR4 pathway is activated by oncogenic JAK2 in a PI3K-dependent manner. Oncotarget. 2017;8(33):54082–54095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang XL, Cho SY, Hu CS, et al. C-X-C motif chemokine 12 influences the development of extramedullary hematopoiesis in the spleens of myelofibrosis patients. Exp Hematol. 2015;43(2):100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rushworth SA, Murray MY, Zaitseva L, et al. Identification of Bruton’s tyrosine kinase as a therapeutic target in acute myeloid leukemia. Blood. 2014;123(8):1229–1238. [DOI] [PubMed] [Google Scholar]
- 16.Luo JX, Li DY, Wei D, et al. RhoA and RhoC are involved in stromal cell-derived factor-1-induced cell migration by regulating F-actin redistribution and assembly. Mol Cell Biochem. 2017;436(1-2):13–21. [DOI] [PubMed] [Google Scholar]
- 17.Mao JH, Xie W, Yuan HD, et al. Tec/Bmx non-receptor tyrosine kinases are involved in regulation of Rho and serum response factor by G alpha 12/13. Embo J. 1998;17(19):5638–5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Socoro-Yuste N, Cokic VP, Mondet J, et al. Quantitative proteome heterogeneity in myeloproliferative neoplasm subtypes and association with JAK2 mutation status. Mol Cancer Res. 2017;15(7):852–861. [DOI] [PubMed] [Google Scholar]
- 19.Sit ST, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci. 2011;124(Pt 5):679–683. [DOI] [PubMed] [Google Scholar]
- 20.Edelmann B, Gupta N, Schnoeder TM, et al. JAK2-V617F promotes venous thrombosis through beta1/beta2 integrin activation. J Clin Invest. 2018;128(10):4359–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pei YQ, Wu Y, Wang F, et al. Prognostic value of CALR vs. JAK2V617F mutations on splenomegaly, leukemic transformation, thrombosis, and overall survival in patients with primary fibrosis: a meta-analysis. Ann Hematol. 2016;95(9):1391–1398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.