The basis of regulatory approval of generic imatinib (GI) has been acceptable bioequivalence, i.e. bioavailability within 80-125% of brand-name imatinib (BI).1 Clinical data on the use of GI in patients with chronic myeloid leukemia in chronic phase (CP-CML) have been conflicting and lack parallel-group comparisons. To address current concerns about the similarity of GI to BI,2 we designed a prospective cohort study comparing medication persistence with GI and BI in Canadian patients with CP-CML. Medication persistence, defined as the duration of time from treatment initiation to cessation,3 may be a valuable metric to evaluate the tolerability of anticancer medications. We show that persistence is lower with GI, and that this is driven mainly by excessive adverse events with GI use.
This study used a prospective registry managed by the Groupe Québécois de Recherche en Leucémie Myéloïde Chronique et Néoplasies Myéloprolifératives (GQR LMC-NMP), which includes demographic and clinical information on more than 80% of CML patients in the province of Québec, Canada. All patients provided informed consent to the use of their data and ethics approval was obtained through a Quebec multicenter ethics review process. The source population comprised patients over 18 years of age who initiated frontline BI for CP-CML starting from 1 September, 2001. We excluded patients who were diagnosed with accelerated or blast phase CML, and those who received non-BI frontline therapy excepting hydroxyurea. Patients who had switched to a non-imatinib tyrosine kinase inhibitor (TKI) before, or had no follow-up after 1 January, 2013, were not eligible for selection into the study cohorts.
To each BI user, we matched a GI user based on use at the same calendar date, nearest duration of prior BI use, and closest age on a 1:1 ratio. Further details on the matching process are provided in Appendix 1 in the Online Supplementary Material. Patients were followed from GI use until a switch or discontinuation of the original TKI, death, or end of the study period (31 December, 2016).
The primary outcome was non-persistence, defined as a switch to an alternate TKI within 45 days of stopping the TKI used at entry into the cohort. Switching between different types of GI within 45 days of stopping the original type was considered continuous GI use. BI users were censored upon switching to GI, because this switch was required by insurers and thus did not represent non-persistence. The secondary outcome was TKI discontinuation, defined as a 45-day gap in TKI therapy.
Baseline covariates and clinical definitions of reasons for switching and discontinuation are detailed in Appendix 2 in the Online Supplementary Material. We used the Kaplan-Meier estimator to assess persistence and treatment without discontinuation, and the log-rank test to assess the differences between GI and BI use. In Cox models, we determined the hazard ratios (HR) and corresponding 95% confidence intervals (CI) for the association between GI use and switching, compared with BI use, and adjusted for baseline covariates. In a secondary analysis, we determined the adjusted HR for discontinuation with GI versus BI use.
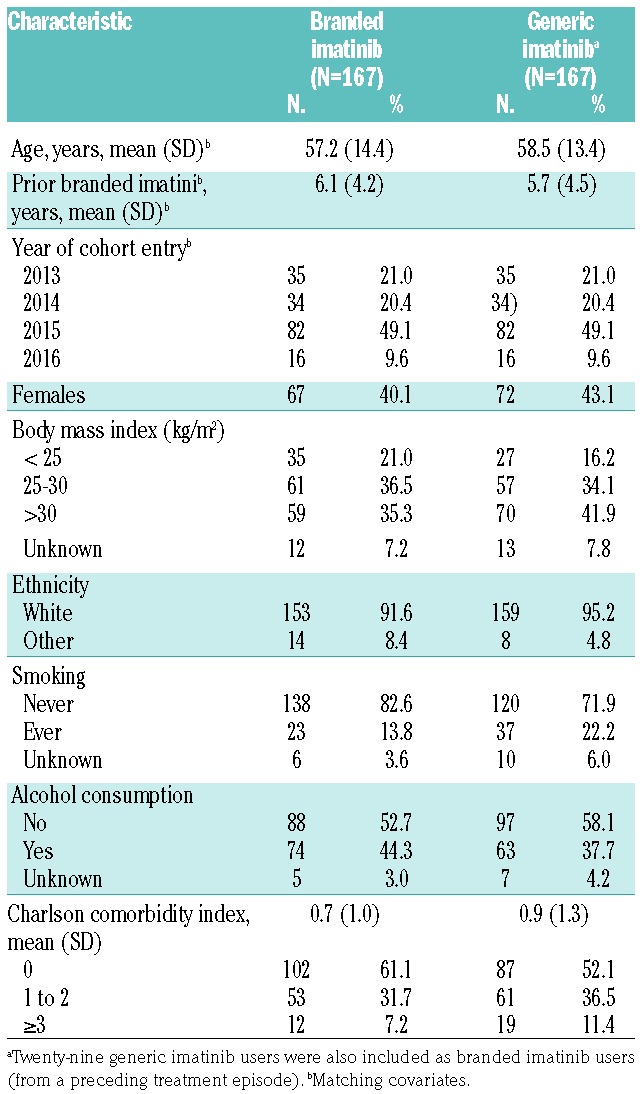
The matched cohorts included 167 patients each (Online Supplementary Figure S1). The mean (standard deviation) follow up was 15.8 (11.7) and 19.6 (11.8) months for BI and GI users, respectively. Age and prior use of BI were overall well balanced between GI and BI users (Table 1).
Table 1.
Baseline characteristics of the matched cohorts.
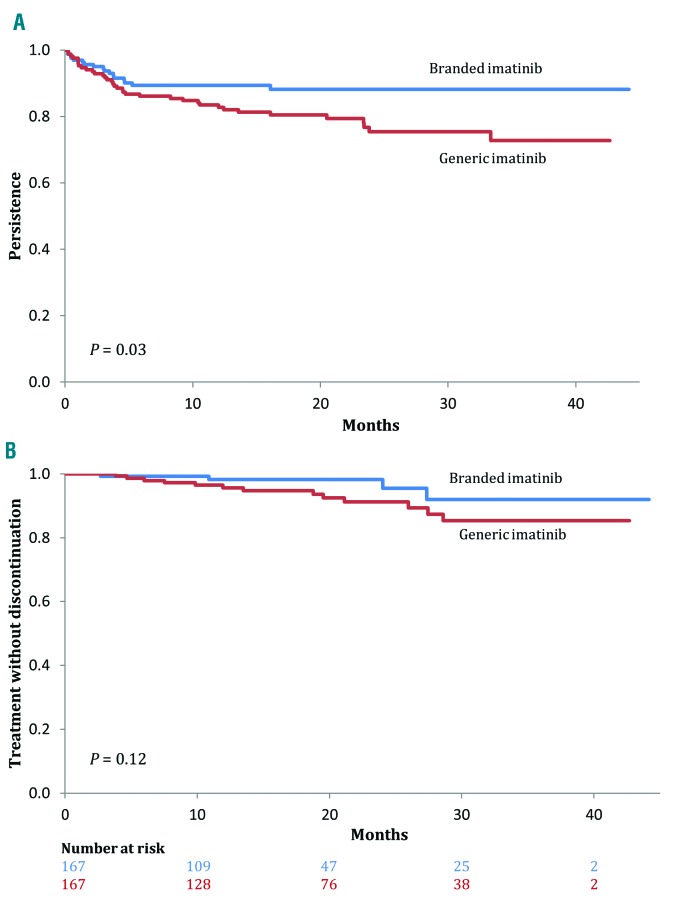
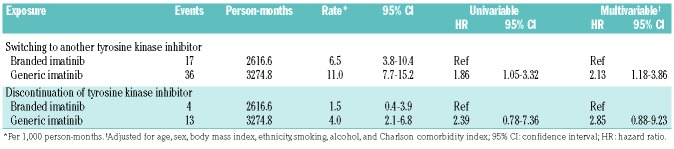
At 3 years, the rate of persistence with GI use was 72.8% (95% CI: 63.9%-81.6%), whereas with BI use it was 88.2% (95% CI: 82.8%-93.6%, P=0.03) (Figure 1A). Most of the switches occurred early: 23 (63.9%) and 16 (94.1%) of switches from GI and BI, respectively, were in the first 6 months from cohort entry. The probability of switching to another TKI at any time was more than 2-fold higher among GI users than among BI users (HR, 2.13; 95% CI: 1.18-3.86) (Table 2).
Figure 1.
Primary and secondary outcomes of tyrosine kinase inhibitor therapy. (A) Probability of persistence with branded (blue) and generic (red) imatinib. (B) Probability of treatment without discontinuation with branded (blue) and generic (red) imatinib.
Table 2.
Use of generic imatinib and non-persistence outcomes.
At 3 years, the rate of treatment without discontinuation among GI users was 85.3% (95% CI: 77.2%-93.5%), whereas that among BI users was 92.0% (95% CI: 83.1%-100.0%, P=0.12) (Figure 1B). The probability of discontinuing TKI at any time was suggested to be higher with GI use than with BI use (HR, 2.85; 95% CI: 0.88-9.23) (Table 2).
Among 36 switchers from GI, intolerance was recorded in 25 (69.5%), while resistance was noted in 12 (33.3%) (Online Supplementary Table S2). Most adverse events recorded in GI users were grade 2 or lower; there were ten grade 3 adverse events in seven patients, all non-hematologic. The TKI to which GI users switched was BI in 23 (63.9%) cases and dasatinib in ten (27.8%). In switchers from BI, intolerance and resistance were recorded in nine (52.9%) patients each. There were five grade 3 adverse events in four BI users, all non-hematologic. While 60% of grade 3 adverse events with GI were gastrointestinal, no such grade 3 adverse events were recorded with BI use. Dasatinib was the main target of switching from BI (76.5%).
All 13 discontinuations of GI were in patients with a molecular response of 0.01% or deeper. Following discontinuation, nine patients remained in treatment-free remissions of varying duration, three were re-treated and the other patient lost the major molecular response without resumption of TKI therapy. Among four BI users who discontinued their TKI, two remain in treatment-free remissions of 35 and 15 months, one patient is being retreated, and the other was lost to follow-up.
The probability of switching TKI was twice as high among GI users than among BI users, which was a significant difference. Our finding that adverse effects were the main cited reason for stopping GI reiterates the observations from a growing number of quantitative and qualitative studies indicating that toxicity is a central determinant of TKI-taking behavior.4,5 However, our results also point to differential tolerability of GI and BI, which has not thus far been addressed in comparative studies.
Observations from previous studies range from a deleterious effect of switching to GI6–8 to comparable effectiveness when examined against that of BI in historical controls.9–11 None of the published studies provided a contemporaneous comparison of GI to BI use, which may have introduced bias from historical controls.12 Furthermore, the time-varying nature of external constraints on patients from health insurance providers to switch from BI to GI means parallel-group analysis is easier to interpret. Our design was akin to having two CP-CML patients seen at the clinic for follow up on the same day (calendar date) and, at coin toss, one is assigned to be switched to GI while the other to continue taking BI, as they would in a parallel-group clinical trial.
A further difficulty stems from incomplete and inconsistent reporting. A switch due to intolerance was reported in only four out of 12 published cohort studies. As with effectiveness outcomes, this outcome varied among studies, and was reported predominantly among initiators of GI (accounting for only 8.4% of our study population). Nevertheless, the 15% switch rate we report approximates that reported for GI initiators in a study conducted in Poland.11
The clinical applicability of our findings pertains to ensuring treatment continuity with GI use. As intolerance was the main reason for switching early with GI use, awareness of possible adverse events and their prompt management may be beneficial. We found that among severe adverse events, diarrhea, nausea and vomiting were the most common, and this was in keeping with previous trials in imatinib users.13
This study has several limitations. First, GI users in our study could switch to BI (the preferred target of switch) and have BI reimbursed by their insurance provider, as long as they had enough cause to stop GI. While it could be argued that some patients had a pre-determined notion to stop taking GI and therefore reported intolerance, the switching from GI still reflects real-world choices made by patients. However, small numbers prevented us from drawing conclusions about GI initiators, who may not hold the same bias. Second, the sample size in our study did not allow for stratification of GI use by specific generic versions or duration of use. In addition, a low number of discontinuation events decreased the precision around this secondary analysis. Lastly, we could not account for CML severity by comparing prognostic scores such as Sokal14 or EUTOS,15 but these scores were developed to predict response to TKI and overall survival, and their association with TKI persistence is unclear. Furthermore, patients in this study had relatively stable CML, having taken BI only and for an average of 6 years and thus it would be unlikely that disease severity at baseline biased our results. Notwithstanding, the applicability of our findings to patients with poor medication adherence who consequently switch is unknown.
Apart from study design, factors associated with polypharmacy and medication-taking behavior, such as age (matching and adjustment) and comorbidity (adjustment), were accounted for in our study, as were lifestyle factors such as smoking and alcohol consumption, thus reducing the possibility of residual confounding.
In conclusion, this matched cohort study of GI and BI users suggests that CP-CML patients stay on GI for a shorter time than on BI and that intolerance is the main reason for this higher non-persistence. If replicated, these findings should alert prescribing physicians and CP-CML patients alike to manage early adverse effects with GI use and prevent interruptions of TKI therapy. They also suggest that further comparative work on GI tolerability is warranted.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Yang YT, Nagai S, Chen BK, et al. Generic oncology drugs: are they all safe? Lancet Oncol. 2016;17(11):e493–e501. [DOI] [PubMed] [Google Scholar]
- 2.Geissler J, Sharf G, Cugurovic J, et al. Chronic myeloid leukemia patients call for quality and consistency when generics are introduced to treat their cancer. Leukemia. 2016;30(12):2396–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. [DOI] [PubMed] [Google Scholar]
- 4.Eliasson L, Clifford S, Barber N, Marin D. Exploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leuk Res. 2011;35(5):626–630. [DOI] [PubMed] [Google Scholar]
- 5.Verbrugghe M, Duprez V, Beeckman D, et al. Factors influencing adherence in cancer patients taking oral tyrosine kinase inhibitors: a qualitative study. Cancer Nurs. 2016;39(2):153–162. [DOI] [PubMed] [Google Scholar]
- 6.Alwan AF, Matti BF, Naji AS, Muhammed AH, Abdulsahib MA. Prospective single-center study of chronic myeloid leukemia in chronic phase: switching from branded imatinib to a copy drug and back. Leuk Lymphoma. 2014;55(12):2830–2834. [DOI] [PubMed] [Google Scholar]
- 7.Islamagic E, Hasic A, Kurtovic S, et al. The efficacy of generic imatinib as first- and second-line therapy: 3-year follow-up of patients with chronic myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2017; 17(4):238–240. [DOI] [PubMed] [Google Scholar]
- 8.Saavedra D, Vizcarra F. Deleterious effects of non-branded versions of imatinib used for the treatment of patients with chronic myeloid leukemia in chronic phase: a case series on an escalating issue impacting patient safety. Leuk Lymphoma. 2014;55(12):2813–2816. [DOI] [PubMed] [Google Scholar]
- 9.Entasoltan B, Bekadja MA, Touhami H, et al. Outcome of frontline treatment with “generic” imatinib in adult patients with chronic myeloid leukemia in Algerian population: a multicenter study. Mediterr J Hematol Infect Dis. 2017;9(1):e2017062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eskazan AE, Ayer M, Kantarcioglu B, et al. First line treatment of chronic phase chronic myeloid leukaemia patients with the generic formulations of imatinib mesylate. Br J Haematol. 2014;167(1):139–141. [DOI] [PubMed] [Google Scholar]
- 11.Sacha T, Gora-Tybor J, Szarejko M, et al. A multicenter prospective study on efficacy and safety of imatinib generics: a report from Polish Adult Leukemia Group imatinib generics registry. Am J Hematol. 2017; 92(7):E125–e128. [DOI] [PubMed] [Google Scholar]
- 12.Papageorgiou SN, Koretsi V, Jager A. Bias from historical control groups used in orthodontic research: a meta-epidemiological study. Eur J Orthod. 2017;39(1):98–105. [DOI] [PubMed] [Google Scholar]
- 13.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. [DOI] [PubMed] [Google Scholar]
- 14.Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63(4):789–799. [PubMed] [Google Scholar]
- 15.Hasford J, Baccarani M, Hoffmann V, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118(3):686–692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.