Signal transducer and activator of transcription (STAT) 3 is a member of the STAT family, and plays a major role in various immunological mechanisms.1 Mutations in STAT3 are associated with a broad spectrum of manifestations, including immunodeficiency, autoimmunity, and malignancy.2 In particular, heterozygous germline loss-of-function (LOF) mutations cause Hyper-IgE syndrome (HIES),3–5 while heterozygous germline gain-of-function (GOF) mutations have recently been associated to multi-organ autoimmune manifestations (i.e. type 1 diabetes, enteropathy, cytopenia, interstitial lung disease, hypothyroidism), lymphoproliferation, short stature, and recurrent infections (OMIM #615952).6–8
We report a 7-year-old boy who presented with early-onset severe enteropathy, and diffuse eczematous dermatitis since birth. During the first weeks of life, Hirschsprung disease was also suspected and surgically treated. Gastrointestinal and cutaneous manifestations were first ascribed to food allergy with quite a good response to amino acid-based formula. In the following months, the patient failed to thrive, and developed respiratory tract infections. At two years, the patient presented with progressive interstitial lung disease characterized by lymphocytic interstitial infiltration leading to pulmonary hypertension, tricuspid insufficiency, and right ventricular heart failure with hepatomegaly. Because of the increased risk of infections, he received intravenous (IV) immunoglobulin infusions (400 mg/kg), prophylaxis with cotrimoxazole and fluconazole. Methylprednisolone at 0.3 mg/kg/day was also given to treat autoimmune manifestations.
Later, growth hormone (GH) deficiency, hypothyroidism and type 1 diabetes were diagnosed.
Informed consent was obtained from the patient’s parents. Ethical approval was obtained by the Institutional Review Board of Spedali Civili of Brescia. Genomic DNA was extracted from peripheral whole blood, whole exome sequencing (WES) was performed while the identified mutation was validated by Sanger sequencing.5 STAT3 phosphorylation was analyzed by flow cytometry on whole blood after stimulation with interferon (IFN)-α (40,000 U/mL) or interleukin (IL)-27 (100 ng/mL) for 30 minutes (min) according to Becton Dickinson protocol: cells were acquired using FACSCalibur (BD Bioscence) and analyzed by FlowJo version 7.5 Software (TreeStar). Epstein-Barr virus (EBV)-immortalized B cells western blot assay was performed after overnight starvation, as previously described.5
Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) was performed with specific probes (Applied Biosystems) on CFX96 RT System (BIO-RAD). EBV cells were stimulated with 200 ng/mL of IL-6 or IL-10 for 1 hour (h) with/without overnight starvation. Results are shown as mean normalized expression (MNE) units after GAPDH normalization.
Chromatin immunoprecipitation (ChIP) assay was performed on 5×106 EBV-transformed cell lines stimulated with IL-10 or IL-6 for 45 min and executed as described.9 To establish the background levels of ChIP experiments, STAT3 was quantified also at the promoter of prolactin (PRL) since it is completely silent in leukocytes.
Because of the association of severe early-onset enteropathy and autoimmune manifestations, immunod-eficiency polyendocrinopathy enteropathy X-linked (IPEX) syndrome or other IPEX-like inherited disorders were suspected. But the search for mutations in FOXP3, CD25 and STAT5b resulted negative.10,11 Subsequently, WES was performed and a de novo heterozygous mis-sense STAT3 mutation was identified that clustered in the DNA-binding domain and caused methionine-to-arginine substitution (c.1986T>G) at position 329 (p.M329R). Lymphocyte subsets analysis revealed normal levels of regulatory (CD4+ CD25hi FOXP3+) and effector (CD45RA−CCR7−) CD3+T lymphocytes.
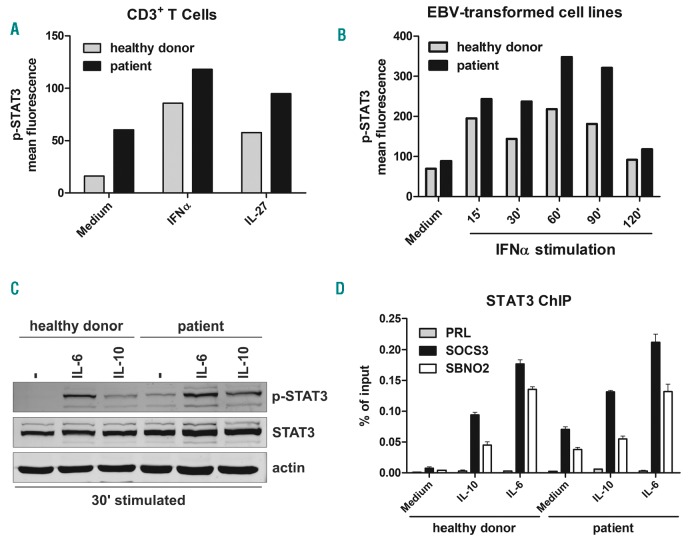
STAT3 activation in response to cytokines such as IL-6, IL-10, IL-21, IL-23 and IFN-α is characterized by phosphorylation of tyrosine 705 (pY705) by associated Janus activating kinases (JAK) and subsequent STAT–STAT dimerization and nucleus translocation.12 We comparatively evaluated by ELISA assay plasmatic cytokines con-centration in the patient and in age-matched healthy donors. Detectable levels of IL-6 (72.04 pg/mL), IL-10 (32.5 pg/mL), and IL-17(55.82 pg/mL) were observed in the patient, but not in the healthy donors (data not shown). The flow cytometry analysis of CD3+ lymphocytes showed detectable levels of STAT3 phosphorylation (pSTAT3) in the patient, but not in healthy donors. Increased pSTAT3 levels were observed both in unstimulated conditions and after IFN-α or IL-27 stimulation (Figure 1A).
Figure 1.
STAT3 phosphorylation and chromatin immunoprecipitation assay in the STAT3 gain-of-function (GOF) patient. (A) STAT3 phosphorylation in T cells (CD3+ cells) in response to interferon-α (IFNα) and IL-27 stimulation or medium alone. Cells from whole blood samples were lysed, fixed and permeabilized, then stained with anti-p-STAT3 and anti-CD3 antibodies. Extent of p-STAT3 is shown on y-axis as mean fluorescent intensity. (B) Time course of STAT3 phosphorylation of Epstein-Barr virus (EBV)-transformed B-cell lines obtained from a control subject or from the STAT3 GOF patient. Cells were kept in culture with serum free medium overnight and stimulated with IFNα for 15, 30, 60, 90 or 120 minutes. Then, cells were permeabilized and stained with anti-p-STAT3. Extent of p-STAT3 is shown on y-axis as mean fluorescent intensity. (C) Western blot analysis of STAT3 tyrosine phosphorylation in EBV-transformed B-cell lines from a control subject or from the STAT3 GOF patient. Cells were kept in culture with serum free medium overnight and then either untreated or stimulated with IL-6 (200 ng/mL) and IL-10 (200 ng/mL) for 30 minutes. (D) Evaluation, by chromatin immunoprecipitation analysis, of STAT3 recruitment to the SOCS3, SBNO2, IL-10 and PRL promoters of EBV-transformed B cells cultured in the presence or absence of IL-6 (200 ng/mL) and IL-10 (200 ng/mL) for up to 1 hour. Co-immunoprecipitated DNA was expressed as percent of the total input. Error bars represent standard errors calculated from triplicate quantitative polymerase chain reactions.
Next, we analyzed STAT3 phosphorylation in unstimulated cells or after 15, 30, 60, 90 and 120 min after stimulation of EBV-transformed cell lines with IFN-α (20,000 U/mL). The patient showed a delayed dephosphorylation of STAT3, especially 90 min after stimulation (Figure 1B).
Finally, we investigated the levels of pSTAT3 by western blot assay in unstimulated cells, and following IL-6 or IL-10 stimulation in EBV-transformed cell lines. Analysis of cells derived from the patient with GOF mutation showed detectable levels of pSTAT3 also in unstimulated conditions, which increased after IL-6 (200 ng/mL) or IL-10 (200 ng/mL) stimulation. This was associated with increased levels of STAT3 protein as compared to the healthy subjects (Figure 1C).
In order to evaluate STAT3 binding capacity to specific DNA sequences involved in the regulation of inflammation and immune response (e.g. SOCS3 and SNBO2), we performed a ChIP assay in EBV-transformed cells from the patient with STAT3 GOF mutation and from a healthy subject. We observed that unstimulated cells from the patient showed enhanced STAT3 binding to SOCS3 and SBNO2 promoters. But after stimulation with IL-6 or IL-10, STAT3 binding was weakly increased as compared to cells from healthy controls (Figure 1D).
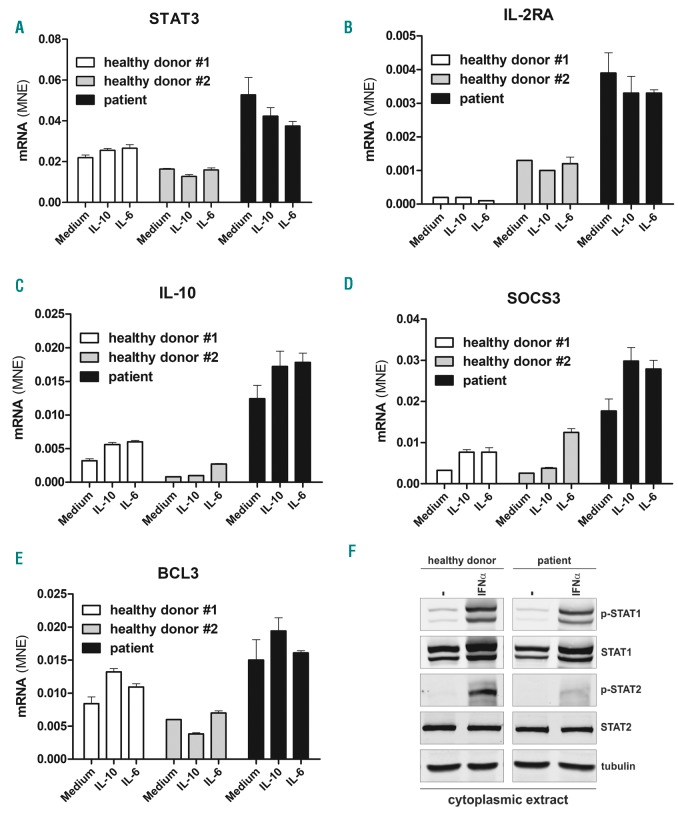
Moreover, we evaluated the expression of STAT3, IL-10, BCL-3, IL2RA and SOCS3 mRNAs by -RT-qPCR. Analysis of mRNAs in unstimulated cells from the patient showed high-level expression of STAT3, IL-10, BCL-3, IL2RA, and SOCS3 at baseline, and further increase of SOCS3 and IL10 mRNAs after stimulation with IL-6 or IL-10, while cell starvation did not influence the extent of gene expression in the cells of the patient (Figure 2A-E).
Figure 2.
Analysis of STAT3, IL10, BCL3, IL2RA, and SOCS3 mRNA expression in Epstein-Barr virus (EBV)-transformed B-cell lines. (A-E) EBV-transformed B cell lines from two healthy donors or the STAT3 gain-of-function (GOF) patient were kept overnight in serum-free medium and then either untreated or stimulated for 1 hour with IL-6 (200 ng/mL) and IL-10 (200 ng/mL) to investigate STAT3, IL10, BCL3, IL2RA, and SOCS3 mRNAs by RT-qPCR. (F) Western blot analysis of STAT1 and STAT2 levels in cytoplasmic extracts from EBV-transformed B cells from a control subject or the STAT3 GOF patient. Cells were cultured overnight without serum and then stimulated with IFNα (1000 U/mL) for 30 minutes. Levels of p-STAT1, p-STAT2, total STAT1 and STAT2 in the cytoplasmic fractions were evaluated. Samples were also immunoblotted for tubulin, a cytoplasmic marker.
Patients with GOF-STAT3 mutation share similar phenotypic manifestations (i.e. eczema, autoimmunity) with STAT5b deficient patients,13 suggesting that STAT proteins phosphorylation and expression can be negatively regulated by STAT3-mediated induction of SOCS3.14 Therefore we investigated cytokine-induced phosphorylation of both STAT1 and STAT2, and the expression of the total amount of STAT1 protein in the EBV-transformed cell line from the patient with STAT3 GOF mutation and from a healthy subject.
After preparation of cytoplasmic extracts, we observed that STAT2 phosphorylation levels were lower in STAT3-mutated cells after IFNα stimulation, as compared to healthy control. In contrast, the level of phosphorylated and total STAT1 was similar in both control and patient EBV-transformed cell lines (Figure 2F). These results suggest that the STAT3 GOF mutation p.M329R affects not only STAT3 but also STAT2-mediated response.
Immunological studies in this patient revealed normal T-cell subsets but severe depletion of both plasmacytoid (BDCA2+/CD123+/CD4+) and myeloid dendritic (CD1c+/CD4+/CD19−/CD14−) cells: pDC 0.06%, mDC 0.05% of total peripheral blood mononuclear cells (PBMC) as compared to healthy subjects (pDC n.v. 0.16-0.7%, mDC n.v. 0.18-0.92%). This is in contrast with two other cases that showed increased T-effector cell counts and a reduction of T-regulatory cells,1,15 but these differences might be related to inter-individual variations. Moreover, high plasmatic concentrations of IL-6, IL-10 and IL-17 in this patient were also observed, suggesting that these cytokines might be involved in the mechanism of the autoimmune diseases. All these atypical immunological features are probably related to the novel STAT3 mutation that was associated with increased phosphorylation of STAT3, while most of patients with STAT3 GOF mutations display normal STAT3 phosphorylation, but abnormal gene regulation.7
Current therapies for multiple organ autoimmune diseases in patients with STAT3 GOF mutations include immunosuppressive drugs and hematopoietic stem cell transplantation in selected patients.16 Because tyrosine kinases inhibitors, such as ruxolitinib, have been extensively used in conditions characterized by abnormal STAT activation, their use could be favorably extended to immunological diseases associated with increased STAT3 phosphorylation, such as observed in this case.17
Supplementary Material
Footnotes
Funding: this work was supported in part by Ministero della Salute (grant n. NET-2013-02355002) to RB.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Villarino AV, Kanno Y, Ferdinand JR, O’Shea JJ. Mechanisms of Jak/STAT Signaling in Immunity and Disease. J Immunol. 2015; 194(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forbes LR, Milner J, Haddad E. Signal transducer and activator of transcription 3: A year in review. Curr Opin Hematol. 2016;23(1):23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland SM, DeLeo FR, Elloumi HZ, et al. STAT3 Mutations in the Hyper-IgE Syndrome. N Engl J Med. 2007;357(16):1608–1619. [DOI] [PubMed] [Google Scholar]
- 4.Minegishi Y, Saito M, Tsuchiya S, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448(7157):1058–1062. [DOI] [PubMed] [Google Scholar]
- 5.Giacomelli M, Tamassia N, Moratto D, et al. SH2-domain mutations in STAT3 in hyper-IgE syndrome patients result in impairment of IL-10 function. Eur J Immunol. 2011;41(10):3075–3084. [DOI] [PubMed] [Google Scholar]
- 6.Haapaniemi EM, Kaustio M, Rajala HLM, et al. Autoimmunity, hypogammaglobulinemia, lymphoproliferation, and mycobacterial disease in patients with activating mutations in STAT3. Blood. 2015; 125(4):639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milner JD, Vogel TP, Forbes L, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125(4):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flanagan SE, Haapaniemi E, Russell MA, et al. Activating germline mutations in STAT3 cause early-onset multi- organ autoimmune disease. Nat Genet. 2014;46(8):812–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamassia N, Castellucci M, Rossato M, et al. Uncovering an IL-10-dependent NF-κB recruitment to the IL-1ra promoter that is impaired in STAT3 functionally defective patients. FASEB J. 2010;24(5):1365–1375. [DOI] [PubMed] [Google Scholar]
- 10.Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: A paradigm of immunodeficiency with autoimmunity. Front Immunol. 2012; 3(July):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr. 2013;25(6):708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenzini T, Dotta L, Giacomelli M, Vairo D, Badolato R. STAT mutations as program switchers: turning primary immunodeficiencies into autoimmune diseases. J Leukoc Biol. 2017;101(1):29–38. [DOI] [PubMed] [Google Scholar]
- 13.Kanai T, Jenks J, Nadeau KC. The STAT5b Pathway Defect and Autoimmunity. Front Immunol. 2012;3:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer DC, Restifo NP. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 2009;30(12):592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Consonni F, Dotta L, Todaro F, Vairo D. Signal transducer and activator of transcription gain-of-function primary immunodeficiency/ immunodysregulation disorders. Curr Opin Pediatr. 2017;29(6):711–717. [DOI] [PubMed] [Google Scholar]
- 16.Vogel TP, Milner JD, Cooper MA. The Ying and Yang of STAT3 in Human Disease. J Clin Immunol. 2015;35(7):615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miklossy G, Hilliard TS, Turkson J. Therapeutic modulators of STAT signalling for human diseases. Nat Rev Drug Discov. 2013;12(8):611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.