Although allogeneic hematopoietic stem cell transplantation (HCT) is still the only documented curative treatment option for patients with high-risk chronic lymphocytic leukemia (CLL),1 the advent of pathway inhibitors has substantially altered its role in the treatment landscape of CLL..2 In 2014, the European Research Initiative on CLL (ERIC) and the European Society for Blood and Marrow Transplantation (EBMT) proposed a transplant algorithm for patients with high-risk CLL taking into account use of the B-cell receptor inhibitors ibrutinib and idelalisib as well as the BCL-2 inhibitor venetoclax.3 Patients can enter the ERIC/EBMT algorithm at two different levels. Level 1 (L1) includes patients with CLL harboring TP53 abnormalities and/or del(11q) who have relapsed after or are refractory to chemoimmunotherapy but are responding to treatment with a first pathway inhibitor. At this level allogeneic HCT is only suggested as an option for those patients who have a low transplant risk, i.e. availability of a well-matched donor and absence of comorbidity. Level 2 (L2) is defined by disease that has relapsed after or is refractory to both chemoimmunotherapy and the first pathway inhibitor. Patients on L2 are considered to have a higher disease-specific risk, justifying allogeneic HCT even with mismatched donors or comorbidity. A recent refinement of the ERIC/EBMT algorithm eliminated del(11q) as a high-risk criterion but retained the two-level risk grading.4
The purpose of the present study was to explore whether the ERIC/EBMT algorithm is generally feasible and whether allogeneic HCT can still be performed successfully at L2. Patients with CLL who were referred to our institution between January 2014 and October 2017 and matched the criteria for inclusion in the algorithm, thus triggering a donor search, were eligible for entry into this study. Survival times were calculated on an intent-to-treat basis from entry into the algorithm unless indicated otherwise. The analysis was performed according to the Declaration of Helsinki and upon approval by the institutional ethics review committee.
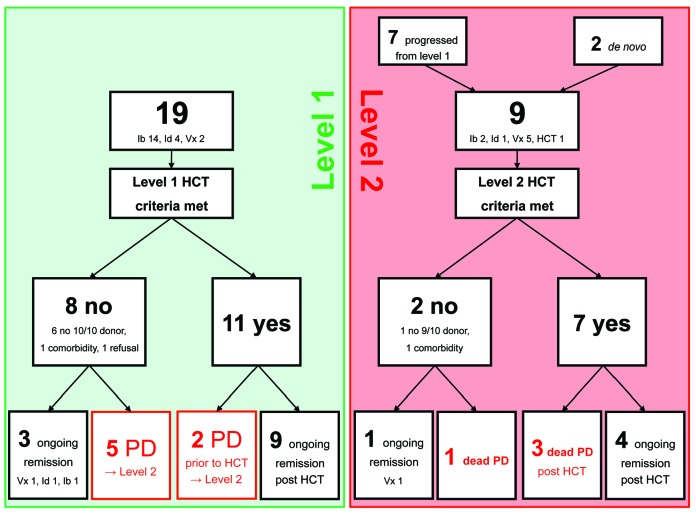
In total, 21 patients were included. Nineteen patients entered the algorithm at L1 and two patients at L2. Of the 19 patients entering at L1, 11 patients met the eligibility criteria for allogeneic HCT, whereas eight patients did not (6 did not have a 10/10 donor, 1 had comorbidity, 1 refused allogeneic HCT) (Figure 1). These 19 L1 patients (13 males, 6 females) had a median age of 58 years (range, 38-63 years). TP53 lesions were present in 14 patients and del(11q) without TP53 lesions in five. All patients were on their first pathway inhibitor, which was ibrutinib in 13 patients, idelalisib in four, and venetoclax in two. Prior to pathway inhibitor treatment, patients had received a median of three (range, 1-8) treatment lines. There were no significant difference in baseline characteristics between the 11 patients meeting transplant eligibility criteria and the eight patients who did not.
Figure 1.
Flow of patients entering the EBMT/ERIC transplant algorithm for high-risk chronic lymphocytic leukemia. Ib: ibrutinib; Id: idelalisib; Vx: venetoclax; HCT: hematopoietic stem cell transplantation; PD: progressive disease.
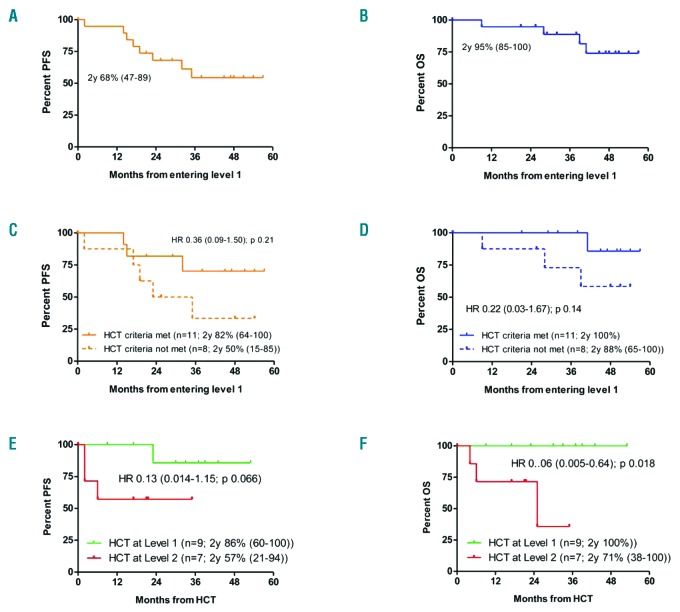
At a median follow-up of 48 months (range, 21-57 months) after algorithm entry, disease progression had occurred in L1 in seven patients (Figure 1). In the absence of any non-relapse mortality, this translated into 2-year progression-free and overall survival rates from entering L1 of 68% (range, 47-89%) and 95% (range, 85-100%), respectively (Figure 2A,B). The 11 patients meeting the transplant eligibility criteria had no outcome disadvantage compared to the eight patients who did not (hazard ratio for progression-free survival = 0.36, 95% confidence interval: 0.09-1.50, P=0.21; hazard ratio for overall survival = 0.22, 95% confidence interval: 0.03-1.67, P=0.14) (Figure 2C,D).
Figure 2.
Survival of patients entering the EBMT/ERIC transplant algorithm for high-risk chronic lymphocytic leukemia. (A) Progression-free survival (orange line) for patients entering the algorithm at level 1. (B) Overall survival (blue line) for patients entering the algorithm at level 1. (C) Progression-free survival for patients entering the algorithm at level 1 (orange solid line = meeting HCT criteria; orange dashed line = not meeting HCT criteria). (D) Overall survival for patients entering the algorithm at level 1 (blue solid line = meeting HCT criteria; blue dashed line = not meeting HCT criteria). (E) Progression-free survival for patients undergoing allogeneic transplantation at level 1 (green line) and at level 2 (red line). (F) Overall survival for patients undergoing allogeneic transplantation at level 1 (green line) and at level 2 (red line). PFS: progression-free survival; OS: overall survival; HCT: hematopoietic stem cell transplantation; y: year; HR: hazard ratio.
Per definition, the seven patients with progressive disease while on first pathway inhibitor therapy proceeded to L2. Together with two patients entering L2 directly because of failure of pathway inhibitor treatment without prior L1-defining risk, a total of nine patients progressed to L2. Rescue treatments at L2 were a second pathway inhibitor in eight patients (venetoclax 5, ibrutinib 2, idelalisib 1), and direct allogeneic HCT in the remaining patient. Two patients could not be transplanted because of lack of a 9/10 donor or comorbidity, so seven L2 patients underwent allogeneic HCT. With four cases of progression and no non-relapse deaths, the progression-free and overall survival rates were 56% (range, 23-88%) and 65% (range, 42-87%), respectively, 2 years after entering L2.
Considering all 16 transplanted patients, the disease progressed in four (in 3 as rapidly fatal transformation in patients with refractory disease at the time of allogeneic HCT, 1 as untransformed CLL in a patient who was still responding to ibrutinib at the time of allogeneic HCT) but no non-relapse mortality was observed. With a median post-transplant follow-up of survivors of 30 months (range, 9-53), the 2-year progression-free and overall survival rates after allogeneic HCT were 71% (range, 46-91%) and 88% (range, 71-100%), respectively. Although patients transplanted at L2 had an outcome disadvantage compared to those transplanted at L1, which was statistically significant for overall survival (Figure 2E,F), it should be noted that none of the L2 patients who proceeded to allogeneic HCT while responding to a second pathway inhibitor had an event after transplantation. All surviving patients except two were negative for minimal residual disease tested by flow cytometry5 at their most recent visit.
In conclusion, this series shows for the first time that the algorithm proposed in the 2014 ERIC/EBMT position paper is feasible. Of note, our L1 progression-free and overall survival rates were comparable to those in the PCYC 1102/1103 trial of ibrutinib as first pathway inhibitor in relapsed/refractory CLL, although the proportion of patients with TP53 abnormalities in the latter trial appeared much smaller.6 Moreover, the survival outcomes in our series compared well with those observed in the M13-982 trial studying venetoclax in patients with relapsed/refractory CLL harboring del(17p).7 Most importantly, it seems that early transplantation in patients with chemoimmunotherapy-resistant, genetically poor-risk CLL responding to a first pathway inhibitor might have the potential to ameliorate outcome compared to continuing pathway inhibitors (which was mainly ibrutinib in our study). Larger numbers of patients are necessary to prove that this approach does indeed improve the prognosis of this population which was designated as high-risk-I in a recent consensus paper.4
Furthermore, our analysis provides evidence, for the first time, that allogeneic HCT can be successfully performed in patients in whom treatment with a first pathway inhibitor (which was ibrutinib in 7 cases and idelalisib in the remainder) has failed. The overall good outcome of the transplanted patients was partly due to the low non-relapse mortality. The reason for this could just be chance because of the small sample size, but it is in line with a recent EBMT analysis demonstrating 12-month non-relapse mortality rates of 5% and 10% in ibrutinib-pretreated patients with mantle cell lymphoma and CLL, respectively.8 In contrast to the findings of the EBMT study, the post-transplant relapse risk was also quite low in our series with only four events, three of which occurred in patients with refractory disease prior to transplantation. It remains to be shown whether our transplant strategy (bridging with pathway inhibitor therapy until maximum response, intensive conditioning with 8 Gy total body irradiation plus fludarabine in 11 patients, treosulfan 30 g plus fludarabine in 5 patients; pathway inhibitor discontinuation at day -1) contributed to this, or whether other factors played a role.
Although still preliminary, the collective results of this study suggest that the risk-adapted move to cellular therapy pursued in the ERIC/EBMT algorithm is feasible and might improve the prognosis of patients with relapsed/refractory CLL and is worth being explored further. Moreover, these results provide the first evidence that allogeneic HCT is feasible and can be effective after failure of therapy with a first pathway inhibitor.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Krämer I, Stilgenbauer S, Dietrich S, et al. Allogeneic hematopoietic cell transplantation for high-risk CLL: 10-year follow-up of the GCLLSG CLL3X trial. Blood. 2017;21;130(12):1477–1480. [DOI] [PubMed] [Google Scholar]
- 2.Passweg JR, Baldomero H, Bader P, et al. Impact of drug development on the use of stem cell transplantation: a report by the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2017;52(2):191–196. [DOI] [PubMed] [Google Scholar]
- 3.Dreger P, Schetelig J, Andersen N, et al. Managing high-risk CLL during transition to a new treatment era: stem cell transplantation or novel agents? Blood. 2014;124(26):3841–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dreger P, Ghia P, Schetelig J, et al. High-risk chronic lymphocytic leukemia in the era of pathway inhibitors: integrating molecular and cellular therapies. Blood. 2018;132(9):892–902. [DOI] [PubMed] [Google Scholar]
- 5.Böttcher S, Stilgenbauer S, Busch, et al. Standardized MRD flow and ASO IGH RQ-PCR for MRD quantification in CLL patients after rituximab-containing immunochemotherapy: a comparative analysis. Leukemia. 2009;23(11):2007–2017. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien S, Furman RR, Coutre S, et al. Single-agent ibrutinib in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: results from the full population of a phase II pivotal trial. J Clin Oncol. 2018; 36(19):1973–1980. [DOI] [PubMed] [Google Scholar]
- 8.Dreger P, Michallet M, Bosman P, et al. Ibrutinib for bridging to allogeneic hematopoietic cell transplantation in patients with chronic lymphocytic leukemia or mantle cell lymphoma: a study by the EBMT Chronic Malignancies and Lymphoma Working Parties. Bone Marrow Transplant. 2019;54(1):44–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.