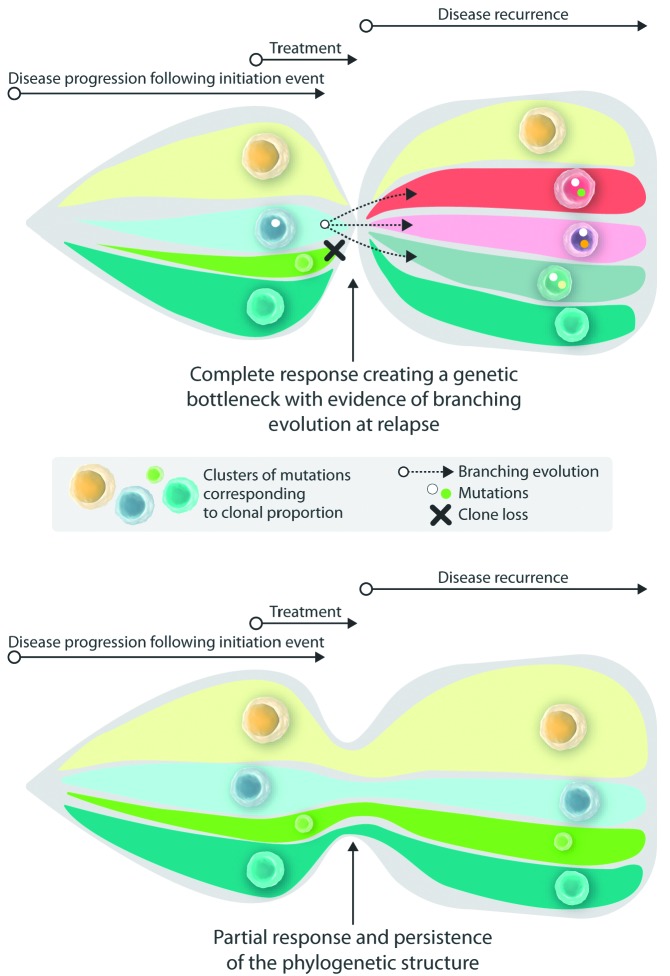
Over the last twenty years, a wave of new therapies has made a profound impact on the treatment and prognosis of multiple myeloma. The advent of proteasome inhibitors and thalidomide and its successors have increased the response rates to first-line therapy in myeloma to more than 90%, with a median time to progression of over 45 months.1 This development continues, with further improvements expected through up and coming agents, such as second- and third-generation proteasome inhibitors and immunomodulatory drugs (IMID), novel antibodies, and targeted immunotherapeutics.2 Furthermore, new approaches to measuring minimal residual disease (MRD) have demonstrated a clear connection between progression-free survival (PFS)/overall survival (OS) and the achievement of MRD negativity as determined by high sensitivity flow cytometry or next-generation sequencing (NGS),3,4 opening up new approaches for prognostic and therapeutic stratification. Interestingly, the impact of MRD negativity also holds true for high-risk myeloma patients, who show an improved prognosis if they reach MRD negative status, implying that treatment resistance leading to incomplete clearance of clonal plasma cells represents the basis of early relapse and worse outcome. Previous studies analyzing the clonal evolution of myeloma during therapy have demonstrated different types of clonal changes, which are categorized as stable, linear, and branching evolution of myeloma clones.5–7 Whereas clonally stable myeloma contains roughly the same mutational spectrum over the course of disease, linear evolution is characterized by gains of additional genetic aberrations on top of existing mutations, in contrast to a branching evolution, where clones with a sometimes completely new set of genetic aberrations appear over time. Still, the impact of the depth of remission on the clonal substructure, and the clonal behavior during relapse after maintenance therapy has not so far been studied in great detail. In this issue of the Journal, Jones et al. report their study in which they performed whole exome sequencing (WES) on diagnosis/relapse samples from a subset of 30 myeloma patients receiving lenalidomide maintenance and 26 patients without maintenance from a large myeloma treatment study (Myeloma XI trial), with a focus on patients with high-risk disease and early relapse.8,9 Strikingly, the authors observed a change in the mutational spectrum and an increase in the mutation load in the majority of relapse samples, whereas only a subset of patients showed a stable mutational landscape. Thus, patients achieving a good response to the initial therapy [either a very good partial remission (vgPR) or a complete response (CR)] seemed to go through a clonal bottleneck leading to a branched clonal evolution upon relapse, whereas most patients with incomplete responses showed a linear evolution or stable clonal patterns (Figure 1). The authors did not identify specific mutations associated with relapse; for this type of analysis, higher patient numbers will be required. As has been described before, structural aberrations involving a gain of chromosome 1q or translocations targeting the Myc oncogene on 8q were increased at relapse,10 with additional evidence of bi-allelic inactivation of common tumor suppressor genes like RB1, TRAF3, and TP53 in a subset of relapse patients.
Figure 1.
Clonal evolution during therapy (adapted from Jones et al.8). Myeloma patients achieving a complete or very good partial remission are generally characterized by a branching evolution of the clonal architecture upon relapse (top), with frequent loss of clones present at diagnosis and concomitant gain of clones carrying additional new mutations. In contrast, myeloma patients with a partial response to the initial therapy mostly showed stable clonal patterns without evidence of significant clonal selection (bottom).
Interestingly, maintenance therapy did not significantly impact the clonal composition predetermined by the response category. Similar to patients without maintenance, patients on lenalidomide therapy who achieved a CR presented with branching clonal architecture at relapse, whereas patients with a less profound response maintained more or less the clones already present at diagnosis. The finding that maintenance therapy did not seem to impact clonal patterns, while clearly having a clinical effect on disease free survival,9 may hint at an immune-dependent, non-selective mode of action of lenamidomide, which leaves the clonal composition relatively unchanged. A more non-specific, indirect mode of action of lenalidomide in these patients would also be supported by the observation that only a few mutations in the Cereblon/IRF4 pathway were detected in the relapsed patients under analysis. Alternatively (or additionally), maintenance therapy may not have had a sufficient effect on the overall clonal population such as to create an evolutionary bottleneck. The study by Jones et al. centered on early relapse patients from the Myeloma XI trial who received 10 mg lenalidomide as maintenance therapy. Thus, the maintenance lenalidomide dose and the more aggressive biology of the myloma may have contributed to a reduced clonal selection pressure. Future studies will need to look further into the effects of maintenance lenalidomide on clonal evolution to confirm these findings, and to expand the analysis to patients with less aggressive disease. Reassuringly, lenalidomide maintenance therapy did not seem to increase the number of new genetic aberrations or the overall mutational load compared to the non-treated patients.
Overall, the study by Jones et al.8 demonstrates a clear connection between the depth of response and the pattern of clonal evolution in myeloma patients, with dormant new clones contributing to relapse in high-risk patients who initially responded well to therapy. These findings raise the hope that further in-depth analysis of the clonal composition of relapsed myeloma, including the application of novel techniques such as single cell sequencing,11 may shed light on the mechanisms of resistance leading to therapy failure, and help to guide subsequent salvage therapy. An in-depth analysis of the residual clonal substructure after intensive treatment may help to refine myeloma therapy in such a way as to ultimately prevent clonal escape, thus making an important contribution to the still elusive goal of achieving a cure for myloma patients.
Supplementary Material
References
- 1.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunacheewa C, Orlowski RZ. New Drugs in Multiple Myeloma. Annu Rev Med. 2019;70:521–547. [DOI] [PubMed] [Google Scholar]
- 3.Paiva B, Chandia M, Puig N, et al. The prognostic value of multiparameter flow cytometry minimal residual disease assessment in relapsed multiple myeloma. Haematologica. 2015;100(2):e53–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrot A, Lauwers-Cances V, Corre J, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132(23):2456–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keats JJ, Chesi M, Egan JB, et al. Clonal competition with alternating dominance in multiple myeloma. Blood. 2012;120(5):1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egan JB, Shi CX, Tembe W, et al. Whole-genome sequencing of multiple myeloma from diagnosis to plasma cell leukemia reveals genomic initiating events, evolution, and clonal tides. Blood. 2012;120(5):1060–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker BA, Wardell CP, Melchor L, et al. Intraclonal heterogeneity and distinct molecular mechanisms characterize the development of t(4;14) and t(11;14) myeloma. Blood. 2012;120(5):1077–1086. [DOI] [PubMed] [Google Scholar]
- 8.Jones JR, Weinhold N, Ashby C, et al. Clonal evolution in myeloma: the impact of maintenance lenalidomide and depth of response on the genetics and sub-clonal structure of relapsed disease in uniformly treated newly diagnosed patients. Haematologica. 2019;104(7):1440–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20(1):57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker BA, Wardell CP, Brioli A, et al. Translocations at 8q24 juxtapose MYC with genes that harbor superenhancers resulting in overexpression and poor prognosis in myeloma patients. Blood Cancer J. 2014;4:e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledergor G, Weiner A, Zada M, et al. Single cell dissection of plasma cell heterogeneity in symptomatic and asymptomatic myeloma. Nat Med. 2018;24(12):1867–1876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.