Abstract
Tea is one of the top beverages used around the world every day, which contains a high amount of polyphenols and antioxidants. The main aim of this research is to quantify some marketed black tea (Rabea, Lipton, Alkbous, Green gold and Haritham) for phenolic contents and antioxidant potential evaluation by ultrasound solvent extraction and was compared with conventional extraction. Ultrasonic extraction was optimized by considering frequencies (26 kHz, 40 kHz), temperature (30, 40 and 50 °C), and power (30, 40 and 50%) at a fixed time of 30 min. In both the ultrasonic frequencies, 40 °C temperature and 40% power combination exhibited highest cumulative yield (mg/100 g DW), total phenolic content (mg gallic acid/g DW), flavonoids (mg/g DW) and DPPH radical scavenging activity (%) in all branded tea. Within each brand of tea, at any temperature-power combination at particular frequency results were not significantly different. But, at a similar condition of temperature power results were found significantly different between two frequencies. Furthermore, ultrasonic extraction process was analyzed thermodynamically by selecting some basic parameters. Thermodynamics results showed the extraction process was feasible, spontaneous and irreversible. Also, 26 kHz ultrasonic probe is more appropriate for the extraction purpose and thermodynamically more acceptable as compared to 40 kHz ultrasonic bath. Moreover, Haritham was selected as the best tea brand due to its high polyphenol contents and antioxidant potential.
Keywords: Ultrasound extraction, Branded tea, Polyphenol, Antioxidant, Thermodynamic study
1. Introduction
Tea (Camellia sinensis, Theaceae) is the only beverage in the world which is consumed by all age group and it is just after water as a consumable substance. There is an increasing importance in the field of nutrition and food industry of plant-derived products (Food and agriculture organization of the united nation). There is a dramatic growth rate from 6% to 15% higher predicted of plant-based food and pharmaceutical (Martin, 2012). Treatment of plant materials for the extraction of valuable components are the top priority steps in any food/ beverage processing. Preclinical and epidemiological studies suggest the value of tea and its health benefits (Banerjee and Chatterjee, 2015). Tea extract and tea biomolecules have to gain demand in pharmaceutical and food industries as a natural antioxidant and for other purposes. Depending on the processing technique tea is classified as Black tea, Green tea, White tea, Yellow tea, Dark tea, etc. Each variety of tea is known for the amount of bioactive/antioxidant components present in it (Banerjee and Chatterjee, 2015). Tea biomolecules preferentially contained non-protein amino acid (theanine), free sugars (Unachukwu et al., 2010), methylxanthine, alkaloid like caffeine, theobromine, theophylline, phenolic acids like gallic acid and different catechins (Unachukwu et al., 2010, Fukino et al., 2005, Seeley et al., 2005). Since major bioactive of tea are polyphenols and methylxanthines and different extraction procedure and solvent largely affect its concentration in extracts. Various researchers used different extraction techniques and solvents for the evaluation of potential bioactive (Jun et al., 2009, Lin et al., 2008) such as supercritical carbon dioxide (Chang et al., 2000), Microwave extraction (Xuejun et al., 2003, Nikhil et al., 2009), high hydrostatic pressure (Jun et al., 2009, Jun et al., 2010) and ultrasound (Mason and Zhao, 1994, Tao et al., 2006, Simon et al., 2014).
Ultrasound technology was explored to enhance the food quality by reducing the process time, energy and increasing shelf life (Altemimi et al., 2015). It has been reported that ultrasound energy can be used to increase extraction yield by disrupting cell tissues (Miracea, 2001). There are different ways to utilize ultrasonic frequency for the extraction of value-added substances. Water bath shaker, Ultrasonic bath & probe were used as extraction techniques at different temperatures and adopting different solvents in order to optimize the methods and solvents (Banerjee and Chatterjee, 2015, Altemimi et al., 2015). A mechanical and thermal effect of ultrasound is used for the extraction of plant materials (Tao et al., 2006, Simon et al., 2014). Thermal effects were observed for the extraction, as a result of sonic waves converted to heat which was absorbed by plant tissues, while mechanical effects generate acoustic cavitation which leads to cell disruption to enhance extraction (Miracea, 2001, Horžić et al., 2012). Extensive studies have been carried out in the present research using the ultrasonic treatment on different branded tea in order to improve the yield of polyphenol contents as compared to conventional method. The main aim of present study is to compare important components such as yield of total phenol, total flavonoids and antioxidant activity, extracted from the ultrasonic probe (26 kHz), ultrasonic bath (40 kHz) by the treatments of various temperature-power combinations for five branded tea. Additionally, the data obtained as a result of ultrasonic treatment were used to establish the thermodynamics of the extraction process.
2. Materials and methods
2.1. Chemicals and reagents
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical, Folin-Ciocalteu reagent, butylated hydroxytoluene (BHT) and gallic acid were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Rest of the chemicals and reagents (analytical grade) used were also purchased from Sigma Aldrich unless stated otherwise.
2.2. Sample preparation
Five different brands (sample) of black tea (Camellia sinensis) were purchased from local market of Al-Kharj (South Riyadh), Saudi Arabia.
2.2.1. Conventional extraction
In conventional extraction, the tea samples were extracted using 80% aqueous methanol. Tea samples (10 g) were mixed with 200 mL of aqueous methanolic solvent and stirred with boiling at 80 °C up to 30 min. The extracts obtained were freed of excess solvents under reduced pressure. All the extractions were performed in triplicate. The extracts produced were evaluated for yield, total phenolic contents, total flavonoids and DPPH scavenging activity calorimetrically.
2.2.2. Ultrasonic extraction (probe)
Tea samples (10 g) for ultrasonic (26 kHz) extraction were placed in 500 mL beaker with same the amount of solvent (80% aqueous methanol) as discussed in conventional method using similar time of frame (30 min) with power ultrasound, low frequency, and high intensity, 26 kHz probe (Ultrasonic processor-200Ht, power 200 W, made in Germany). The probe used for extraction has vibrating horn diameter of 14 mm which is directly immersed in the solvent containing sample which is irradiated with ultrasonic waves generated from the tip of the probe. Amplitude applied for this experiment was 30%, 40% and 50% with a corresponding temperature 30 °C, 40 °C and 50 °C. Each experiment was repeated three times.
2.2.3. Ultrasonic extraction (Bath)
Digital ultrasonic bath of Branson (Model, 5800) was used for the extraction purpose of the present study with the frequency of 40 kHz. Ultrasonic bath dimension was length16, width 15.5 and depth 14.5 in. respectively. During the extraction, the processed amplitude was set at 30%, 40% and 50% with the corresponding temperature was 30 °C, 40 °C, and 50 °C. Before the start of the experiment, the bath was filled with 3 L of distilled water. Rest of the procedure were followed same as ultrasonic Probe.
2.3. Estimation of polyphenol and antioxidant potential
Effect of ultrasonic extraction and conventional extraction performance was estimated in terms of extract yield, total phenols, total flavonoids and antioxidant potential (DPPH assay).
2.3.1. Estimation of extract yield
Extract yield was calculated as a reported method (Altemimi et al., 2015) as follows
| (1) |
2.3.2. Estimation of total phenol
Total phenolics in branded tea extracts was evaluated by a reported method (Hussain et al., 2008) spectrophotometrically using Folin-Ciocalteu's reagent. Briefly, In a test tube, 1 g of the tea extract, 10 mL of methanol, 7.5 mL distilled water, 0.5 mL Folin-Ciocalteu's reagent and 1.5 mL sodium carbonate (20%) were taken. This mixture incubated for 30 min at room temperature and then reading was measured at 755 nm on spectrophotometer against blank. Results are expressed as mg/liter of gallic acid equivalents (GAE).
2.3.3. Estimation of total flavonoids
The total amount of flavanoid was estimated calorimetrically, with the support of previously published work (Altemimi et al., 2015). Nearly, 0.3 mL of extracts was taken in a test tube with the further addition of 6 mL of cinnamaldehyde with manual shaking for 30 min at room temp. Absorbance was determined at 640 nm. The standard calibration curve was established using a known amount of catechin. Total flavonoid was calculated with reference to the standard calibration curve of catechin, expressed as mg CE/g of dry matter.
2.3.4. Estimation of DPPH radical scavenging
The antioxidant activity of all the branded tea samples was also assessed by neutralizing purple colored 2,2′-diphenyl-1-picrylhydrazyl free radicals (DPPH°). This assay was performed by the method as described earlier (Hussain et al., 2008). Approximately, 10 µg/mL methanolic extract solution of branded tea was allowed to mix with 1 mL of freshly prepared DPPH solution (90 μM). This mixture was made up to 4 mL with 95% MeOH. After vigorous shaking, the reaction mixture was kept under ambient conditions in the darkness for 30 min for any reaction to accomplish. The absorbance of the incubated final mixture and the blank (control) was noted at 515 nm using UV/VIS spectrophotometer (V-630, JASCO International Co. Ltd., Hachioji, Tokyo, Japan). A well-known equation for calculating percent inhibition of oxidation is as follows:
2.4. Thermodynamic analysis
In present research ultrasonically extraction of branded tea samples was thermodynamically analyzed to determine some basic thermodynamic quantities such as Gibbs free energy (ΔG°), Enthalpy change (ΔH°), Entropy change (ΔS°), activation energy (Ea) to investigate spontaneity of extraction, heat of reaction, reversibility and excitation energy for the extraction process respectively. Following equations are used to generate these important parameters-
| (2) |
where ke is the reaction rate constant for the extraction; A is the Arrhenius constant; Ea is the activation energy; R is the universal gas constant and T is absolute temperature.
Slope resulted from the plot of lnk vs 1/T gives the values of activation energy (Ea).
Determination of phenolic contents in both ultrasonic frequencies (26 kHz and 40 kHz) assisted extraction and Gibbs free energy (ΔG°), Enthalpy change (ΔH°), Entropy change (ΔS°) was calculated using following equations:
| (3) |
| (4) |
| (5) |
where Ct is the concentration of total phenolic contents in the methanolic extracts at a given temperature and Cmax is the concentration in the saturated form of extract.
The values of Gibbs free energy change (ΔG°) (kJ/mol) gives an idea of the spontaneity of the extraction process. A negative and positive value of ΔG° is an indicator of spontaneous and non-spontaneous extraction process (Meziane and Kadi, 2008). Similarly, enthalpy change (ΔH°) (kJ/mol) postulates whether the reaction is exothermic or endothermic having value negative and positive respectively. Disorder or randomness of an extraction process was calculated from values of ΔS°. A reaction of values of ΔS° higher than zero is indicative of irreversible reaction (Meziane and Kadi, 2008).
2.5. Statistical analysis
Variation in the amount of polyphenol content and antioxidant activity mainly depends on frequency, temperature and power as independent variables, which were analyzed by repeated measures ANOVA within the samples of tea to test overall differences between means after repeated interaction of temperature and power. When the effect of the main comparison was found significant, simple comparison effects were analyzed for differences in both frequencies by Tukey-Kramer multiple comparison tests, when p < 0.05. Graph pad Instat version 3 was used for all statistical analysis.
3. Results and discussion
In the present research, we have selected five brands of black tea and extraction was performed conventionally (control) and using an ultrasonic bath (40 kHz) and probe (26 kHz) in order to calculate and compare the extract yield and antioxidant potential of samples. Further, the yield of samples and major antioxidant components such as total phenolics, flavonoids contents with DPPH radical scavenging potential were evaluated and accessed for both ultrasonic treatments (bath, probe). To optimize the two ultrasonic techniques different temperature-power combination were used and results were analyzed to identify the superiority of two ultrasonic techniques in terms of percent yield and antioxidant components results. Additionally, some thermodynamic parameters were also calculated to judge the merits of both ultrasonic techniques.
3.1. Impact of extraction parameters on the yield of branded tea samples using ultrasound technology
Extraction yield largely depended on the different variables such as frequency, temperature, and power with an exposure time of 30 min are statistically significant (P < 0.0001).
Effect of each temperature power pairing showed significant results among the selected treatment for 26 kHz and 40 kHz frequencies. In both of the chosen frequencies, temperature 40 °C and power 40% resulted in higher extraction yield as compared to the rest of the temperature-power groupings (Table 5). The yield of polyphenols (mg/100 g DW) at this temperature power pairing was found to be 18.61 ± 0.27, 15.65 ± 0.10, 22.43 ± 0.12, 19.36 ± 0.13, 32.68 ± 0.14 for Rabea, Lipton, Alkbous, Green gold and Haritham brands of tea respectively at 26 kHz (Table 1, Table 5).
Table 5.
Effect of ultrasonic treatment at each temperature power combination to extract yield and antioxidant potential at frequency 26 kHz.
| Sample (Brand) | Temperature –power | Extract yield* (mg/100 g DW) | Total Phenolics* GAE mg gallic/g DW | Flavanoids* (mg/g DW) | DPPH radical scavenging* (%) |
|---|---|---|---|---|---|
| Rabea | 30 °C-30% | 9.65ad | 18.61acd | 15.71acd | 37.95ad |
| 30 °C-40% | 10.78abd | 19.57ad | 17.15abd | 38.19ad | |
| 30 °C-50% | 11.68 ad | 23.29cd | 21.81ad | 39.22abd | |
| 40 °C-30% | 15.58 abd | 26.59acd | 24.56abcd | 43.87abcd | |
| 40 °C-40% | 18.61ad | 33.76ac | 27.77acd | 51.22ab | |
| 40 °C-50% | 16.88acd | 27.17acd | 21.43abd | 48.71abc | |
| 50 °C-30% | 15.98abcd | 24.88acd | 18.96ad | 46.48abcd | |
| 50 °C-40% | 14.89abcd | 25.77acd | 19.89ad | 42.66ad | |
| 50 °C-50% | 13.71abcd | 22.61acd | 16.12abcd | 41.18abd | |
| Lipton | 30 °C-30% | 9.56ad | 20.92acd | 14.78acd | 36.13ad |
| 30 °C-40% | 10.19abd | 21.89ad | 15.71abd | 38.82ad | |
| 30 °C-50% | 11.62ad | 25.29cd | 18.98ad | 40.80abd | |
| 40 °C-30% | 13.42abd | 28.82acd | 21.18abcd | 44.52abcd | |
| 40 °C-40% | 15.65ad | 38.13ac | 24.00acd | 53.78ab | |
| 40 °C-50% | 14.42acd | 27.97acd | 22.92abd | 49.82abc | |
| 50 °C-30% | 12.37abcd | 24.12acd | 18.75acd | 44.76abcd | |
| 50 °C-40% | 12.86acd | 25.91acd | 19.08ad | 42.85ad | |
| 50 °C-50% | 11.66abd | 21.78acd | 16.45abcd | 40.98abd | |
| Alkbous | 30 °C-30% | 16.31ad | 14.98acd | 8.24acd | 34.45ad |
| 30 °C-40% | 17.33abd | 15.66ad | 9.09abd | 37.28ad | |
| 30 °C-50% | 18.17ad | 17.49cd | 11.39ad | 40.16abd | |
| 40 °C-30% | 19.22abd | 20.92acd | 14.69abcd | 42.56abcd | |
| 40 °C-40% | 22.43ad | 24.71ac | 17.50acd | 56.38ab | |
| 40 °C-50% | 21.76acd | 19.87acd | 13.90abd | 49.89abc | |
| 50 °C-30% | 17.88abcd | 16.14acd | 9.88acd | 46.08abcd | |
| 50 °C-40% | 18.21ad | 15.68acd | 10.13ad | 42.81ad | |
| 50 °C-50% | 13.51abcd | 13.42acd | 8.98abcd | 40.61abd | |
| 30 °C-30% | 11.98ad | 23.87acd | 16.05acd | 46.17ad | |
| Green Gold | 30 °C-40% | 12.20abd | 24.43ad | 17.33abd | 47.49ad |
| 30 °C-50% | 13.24ad | 27.40cd | 19.81ad | 44.98abd | |
| 40 °C-30% | 15.57abd | 29.77acd | 22.39abcd | 55.39abcd | |
| 40 °C-40% | 19.36ad | 32.73ac | 25.11acd | 65.30ab | |
| 40 °C-50% | 18.33acd | 26.90acd | 21.29abd | 57.86abc | |
| 50 °C-30% | 15.35abcd | 24.45acd | 18.61acd | 53.41abcd | |
| 50 °C-40% | 16.88acd | 26.20acd | 17.93ad | 51.85ad | |
| 50 °C-50% | 14.29abcd | 22.65acd | 15.89abcd | 48.94abd | |
| Haritham | 30 °C-30% | 22.11ad | 48.76acd | 39.59acd | 56.96ad |
| 30 °C-40% | 23.34ab | 51.61ad | 41.84abd | 59.22ad | |
| 30 °C-50% | 25.42ad | 55.92cd | 42.18ad | 61.56abd | |
| 40 °C-30% | 27.49abd | 59.97acd | 46.08abcd | 64.82abcd | |
| 40 °C-40% | 32.68ad | 71.13ac | 58.25acd | 71.29ab | |
| 40 °C-50% | 29.72acd | 63.12acd | 55.69abd | 65.91abc | |
| 50 °C-30% | 26.44abcd | 54.58acd | 51.38acd | 60.11abcd | |
| 50 °C-40% | 27.78acd | 56.88acd | 48.78ad | 58.79ad | |
| 50 °C-50% | 24.45abcd | 49.30acd | 47.23abcd | 56.88abd | |
DW = Dry weight.
Means with different superscript letters (with the same column) indicate significantly different.
Table 1.
Extract yield (mg/100 g DW) from different brands of tea using conventional and ultrasonic treatment.
| Sample (Brand) | Extract Yield (mg/100 g DW) |
||
|---|---|---|---|
| Conventional | Ultrasonic (26 kHz) | Ultrasonic (40 kHz) | |
| Rabea | 12.35 ± 0.15 | 18.61 ± 0.27 | 15.71 ± 0.10 |
| Lipton | 7.46 ± 0.19 | 15.65 ± 0.10 | 8.98 ± 0.08 |
| Alkbous | 12.83 ± 0.14 | 22.43 ± 0.12 | 14.79 ± 0.04 |
| Green Gold | 11.71 ± 0.11 | 19.36 ± 0.13 | 11.95 ± 0.07 |
| Haritham | 17.68 ± 0.14 | 32.68 ± 0.14 | 21.93 ± 0.04 |
Using 40 kHz ultrasonic frequency, it was 15.71 ± 0.10, 8.98 ± 0.08, 14.79 ± 0.04, 11.95 ± 0.07, 21.93 ± 0.04 (Table 1). Combination of the temperature of 30 °C and power at 30% were found lowest extract yield in both ultrasonic frequencies. The frequency, temperature, and power of ultrasound influence the efficiency of extraction. In these results, lower operating frequency of 26 kHz was more effective than 40 kHz with regard to producing extract yield. This was supported by the almost similar outcomes of few reported work (Altemimi et al., 2015, Zhou, 2010).
At 40% power combination with temperature in both frequencies, the results in terms of extract yield were in similar fashion (Table 5, Table 6, and Table 7). Considering 40% powers setting in both the frequencies the results were not significantly differenced at any temperature (p < 0.001). It was also evident that the yield was increased almost 1.5 to 2-fold from 30 °C to 40 °C in all temperature-power coupling but fall at 5 °C.
Table 6.
Effect of ultrasonic treatment at each temperature power combination to extract yield and antioxidant potential at frequency 40 kHz.
| Sample (Brand) | Temperature power combination | Extract yield* (mg/100 g DW) | Total Phenolics* GAE mg gallic/g DW | Flavanoids* (mg/g DW) | DPPH radical scavenging* (%) |
|---|---|---|---|---|---|
| Rabea | 30 °C-30% | 6.58abd | 11.66abd | 5.91acd | 21.78ad |
| 30 °C-40% | 7.78acd | 12.12ad | 6.37ad | 22.16abd | |
| 30 °C-50% | 9.93acd | 14.29abd | 8.84acd | 24.79abd | |
| 40 °C-30% | 11.12bcd | 16.98abd | 10.06abcd | 29.44abcd | |
| 40 °C-40% | 15.71abd | 21.29ab | 14.85ad | 36.74abd | |
| 40 °C-50% | 12.17acd | 19.17abd | 12.13abd | 32.93acd | |
| 50 °C-30% | 11.44bcd | 17.70ad | 9.84abd | 29.15abd | |
| 50 °C-40% | 14.64bd | 15.51abd | 8.11ad | 27.07acd | |
| 50 °C-50% | 13.56ad | 13.54ad | 6.98abd | 24.88abcd | |
| Lipton | 30 °C-30% | 3.66abd | 16.54abd | 9.73acd | 21.06ad |
| 30 °C-40% | 4.10acd | 17.78ad | 10.33ad | 22.58abd | |
| 30 °C-50% | 5.89acd | 19.90abd | 12.42acd | 24.09abd | |
| 40 °C-30% | 6.75bcd | 21.91ad | 14.08abcd | 27.11abcd | |
| 40 °C-40% | 8.98abd | 24.71ab | 17.82ad | 39.12abd | |
| 40 °C-50% | 7.59acd | 21.72abd | 16.09abd | 35.76acd | |
| 50 °C-30% | 6.77bcd | 19.66abd | 15.02abd | 32.19abd | |
| 50 °C-40% | 7.91bd | 20.14abd | 14.21ad | 30.17abcd | |
| 50 °C-50% | 6.28ad | 17.95ad | 12.24abd | 29.49abcd | |
| Alkbous | 30 °C-30% | 7.73abd | 6.82abd | 3.44acd | 24.95ad |
| 30 °C-40% | 8.64acd | 7.88ad | 4.95ad | 25.11abd | |
| 30 °C-50% | 9.80acd | 9.39abd | 5.59acd | 27.66abd | |
| 40 °C-30% | 11.91bcd | 11.71abd | 8.22abcd | 29.49abcd | |
| 40 °C-40% | 14.79abd | 14.34a | 11.19ad | 36.38abd | |
| 40 °C-50% | 13.51acd | 12.17abd | 10.39abd | 34.89acd | |
| 50 °C-30% | 9.86bcd | 10.10abd | 9.15abd | 32.60abd | |
| 50 °C-40% | 7.27bd | 9.99abd | 7.04ad | 30.20abcd | |
| 50 °C-50% | 5.57ad | 9.12ad | 6.08abd | 35.75abcd | |
| Green Gold | 30 °C-30% | 4.92abd | 12.49abd | 7.77acd | 34.50ad |
| 30 °C-40% | 4.89acd | 13.68ad | 8.80ad | 35.13abd | |
| 30 °C-50% | 5.96acd | 14.54abd | 9.13acd | 37.19abd | |
| 40 °C-30% | 7.97bcd | 16.89abd | 12.83abcd | 39.04abcd | |
| 40 °C-40% | 11.95abd | 20.48ab | 17.86ad | 45.23abd | |
| 40 °C-50% | 9.17acd | 18.83abd | 15.31abd | 42.78acd | |
| 50 °C-30% | 8.41bcd | 17.49abd | 13.06abd | 39.09abd | |
| 50 °C-40% | 7.54bd | 16.05abd | 11.16ad | 37.81abcd | |
| 50 °C-50% | 6.32ad | 14.81ad | 9.01abd | 36.72abcd | |
| Haritham | 30 °C-30% | 11.91abd | 29.91abd | 23.56acd | 30.64ad |
| 30 °C-40% | 12.76acd | 30.78ad | 24.19ad | 33.42abd | |
| 30 °C-50% | 13.33acd | 31.99abd | 26.09acd | 37.16abd | |
| 40 °C-30% | 16.19bcd | 36.17abd | 28.68abcd | 42.79abcd | |
| 40 °C-40% | 21.93abd | 40.88ab | 34.14ad | 50.86abd | |
| 40 °C-50% | 18.79acd | 38.14abd | 32.96abd | 47.18acd | |
| 50 °C-30% | 17.18bcd | 36.72abd | 31.18abd | 44.13abd | |
| 50 °C-40% | 16.12bd | 35.89abd | 30.04ad | 42.69abcd | |
| 50 °C-50% | 15.66ad | 31.59ad | 27.36abd | 39.14abcd | |
DW = Dry weight.
Means with different superscript letters (with the same column) indicate significantly different.
Table 7.
Effect of ultrasonic treatment measured by temperature at 40% power for each frequency.
| Sample (Brand) | Temperature (°C) | Extract yield* (mg/100 g DW) | Total Phenolics* GAE mg gallic/g DW | Flavanoids* (mg/g DW) | DPPH radical Scavenging* (%) |
|---|---|---|---|---|---|
| Rabea | Frequency = | ||||
| 26 kHz | |||||
| 30 | 10.78c | 19.57c | 17.15c | 38.19c | |
| 40 | 18.61ab | 33.76ab | 27.77ab | 51.22ab | |
| 50 | 14.89b | 25.77b | 19.89b | 42.66b | |
| Frequency = | |||||
| 40 kHz | |||||
| 30 | 7.78bd | 12.12bd | 6.37bd | 22.16bd | |
| 40 | 15.71bc | 21.29bc | 14.85bc | 36.74bc | |
| 50 | 14.64cd | 15.51cd | 8.11cd | 27.07cd | |
| Frequency = | |||||
| 26 kHz | |||||
| 30 | 10.19bd | 21.89bd | 15.71bd | 38.82bd | |
| 40 | 15.65bc | 38.13bc | 24.00bc | 53.78bc | |
| 50 | 12.86cd | 25.91cd | 19.08cd | 42.85cd | |
| Lipton | Frequency = | ||||
| 40 kHz | |||||
| 30 | 4.10bd | 17.78bd | 10.33bd | 22.58bd | |
| 40 | 8.98bd | 24.71bd | 17.82bd | 39.12bd | |
| 50 | 7.91d | 20.14d | 14.21d | 30.17d | |
| Frequency = | |||||
| 26 kHz | |||||
| 30 | 17.33bd | 15.66bd | 9.09bd | 37.28bd | |
| 40 | 22.43bc | 24.71bc | 17.50bc | 56.38bc | |
| 50 | 18.21cd | 15.68cd | 10.13cd | 42.81cd | |
| Alkbous | Frequency = | ||||
| 40 kHz | |||||
| 30 | 8.64bd | 7.88bd | 4.95bd | 25.11bd | |
| 40 | 14.79b | 14.34b | 11.19b | 36.38b | |
| 50 | 7.27bd | 9.99bd | 7.04bd | 30.20bd | |
| Frequency = | |||||
| 26 kHz | |||||
| 30 | 12.20bd | 24.43bd | 17.33bd | 47.49bd | |
| 40 | 19.36bc | 32.73bc | 25.11bc | 65.30bc | |
| 26 | |||||
| 50 | 16.88cd | 26.20cd | 17.93cd | 51.85cd | |
| Green gold | Frequency = | ||||
| 40 kHz | |||||
| 30 | 4.89bd | 13.68bd | 8.80bd | 35.13bd | |
| 40 | 11.95bc | 20.48bc | 17.86bc | 45.23bc | |
| 50 | 7.54cd | 16.05cd | 11.16cd | 37.81cd | |
| Frequency = | |||||
| 26 kHz | |||||
| 30 | 23.34ad | 51.61ad | 41.84ad | 59.22ad | |
| 40 | 32.68ab | 71.13ab | 58.25ab | 71.29ab | |
| 50 | 27.78bd | 56.88bd | 48.78bd | 58.79bd | |
| Haritham | Frequency = | ||||
| 40 kHz | |||||
| 30 | 12.76ac | 30.78ac | 24.19ac | 33.42a | |
| 40 | 21.93ac | 40.88ac | 34.14ac | 50.86ac | |
| 50 | 16.12c | 35.89c | 30.04c | 42.69c | |
DW = Dry weight
Means with different superscript letters (with the same column) indicate significantly different.
At 40 °C, with chosen power there is no significant difference (p < 0.001) were observed in both frequencies. With a 40% power setting and 40 °C was the highest extract yield and are significant, but no variation at 30% and 50% power in all samples at both frequencies (Table 8). The optimal temperature in the ultrasonic extraction of tea was discussed by some researcher (Milica et al., 2015).
Table 8.
Effect of ultrasonic treatment measured by power setting at 40 °C temperature for each frequency.
| Sample (Brand) | Power setting (%) (mg/100 g DW) | Extract yield | Total Phenolics GAE mg gallic/g DW | Flavanoids (mg/g DW) | DPPH radical scavenging (%) |
|---|---|---|---|---|---|
| Rabea | Frequency = 26 kHz | ||||
| 30 | 15.58cd | 26.95cd | 24.56cd | 43.87cd | |
| 40 | 18.61cd | 33.76cd | 27.77cd | 51.22cd | |
| 50 | 16.88d | 27.17d | 21.43d | 48.71d | |
| Frequency = 40 kHz | |||||
| 30 | 11.12a | 16.98a | 10.06a | 29.44a | |
| 40 | 15.71abc | 21.29abc | 14.85abc | 36.74abc | |
| 50 | 12.17bc | 19.17bc | 12.13bc | 32.93bc | |
| Lipton | Frequency = 26 kHz | ||||
| 30 | 13.42d | 28.82d | 21.18d | 44.52d | |
| 40 | 15.65d | 38.13d | 24.00d | 53.78d | |
| 50 | 14.42d | 25.91d | 22.92d | 49.82d | |
| Frequency = 40 kHz | |||||
| 30 | 6.75d | 21.91d | 14.08d | 27.11d | |
| 40 | 8.98d | 24.71d | 17.82d | 39.12d | |
| 50 | 7.59d | 21.72d | 16.09d | 35.76d | |
| Frequency = 26 kHz | |||||
| 30 | 19.22d | 20.92d | 14.69d | 42.56d | |
| 40 | 22.43d | 24.71d | 17.50d | 56.38d | |
| 50 | 21.76d | 19.87d | 13.90d | 49.89d | |
| Alkbous | Frequency = 40 kHz | ||||
| 30 | 11.91cd | 11.71cd | 8.22cd | 29.49cd | |
| 40 | 14.79cd | 14.34cd | 11.19cd | 36.38cd | |
| 50 | 13.51d | 12.17d | 10.39d | 34.89d | |
| Frequency = | |||||
| 26 kHz | |||||
| 30 | 15.57cd | 29.77cd | 22.39cd | 55.39cd | |
| 40 | 19.36c | 32.73c | 25.11c | 65.30c | |
| 50 | 18.33d | 26.90d | 21.29d | 57.86d | |
| Green Gnold | Frequency = 40 kHz | ||||
| 30 | 7.97ab | 16.89ab | 12.83ab | 39.04ab | |
| 40 | 11.95ab | 20.48ab | 17.86ab | 45.23ab | |
| 50 | 9.17b | 18.83b | 15.31b | 42.78b | |
| Frequency = 26 kHz | |||||
| 30 | 27.49bd | 59.97bd | 46.08bd | 64.82bd | |
| 40 | 32.68bd | 71.13bd | 58.25bd | 71.29bd | |
| 50 | 29.72d | 63.12d | 55.69d | 65.91d | |
| Haritham | Frequency = 40 kHz | ||||
| 30 | 16.19ab | 36.17ab | 28.68ab | 42.79ab | |
| 40 | 21.93a | 40.88a | 34.14a | 50.86a | |
| 50 | 18.79bc | 38.14bc | 32.96bc | 47.18bc | |
DW = dry weight.
*Means with different superscript letters (with the same column) indicate significantly different.
Moreover, the temperature at 40 °C and 40% power setting was results in good extraction in both the ultrasonic frequencies. But results were found better in case of ultrasonic probe of frequency 26 kHz than ultrasonic bath of frequency 40 kHz which might be due to cavitational growth, collapse was better in lower frequency (Ashley, 1998) and thus good rarefaction time to grow bubble to sufficient size leads to disruption of cells resulting in increased in extraction yield.
3.2. Impact of extraction parameters on total phenol (GAE mg gallic/g DW) of branded tea samples using ultrasound technology
Results of total phenol content of different brands of tea sample were obtained quite significant (p < 0.0001). There was also found significant results among all parameters in both frequencies (Table 2). Considering the temperature-power combination there was a significant difference between the total phenol content of each brand of tea, but there is no difference were found within respective samples for a particular temperature power combination for 26 kHz and 40 kHz frequencies. Maximum total phenol content observed at 40 °C and 40% power as compared to other alliances was 33.76 ± 0.12, 38.13 ± 0.12, 24.71 ± 0.14, 32.76 ± 0.10& 71.13 ± 0.13 at 26 kHz and 22.29 ± 0.08, 24.71 ± 0.14, 14.34 ± 0.12, 20.48 ± 0.13 & 40.88 + 0.14 at 40 kHz for Rabea, Lipton, Alkbous, Green gold and Haritham brands of tea respectively (Tables 5 and 6).
Table 2.
Total Phenolics content of different brands of tea using conventional and ultrasonic treatment.
| Sample (Brand) | GAE mg gallic/g DW |
||
|---|---|---|---|
| Conventional | Ultrasonic (26 kHz) | Ultrasonic (40 kHz) | |
| Rabea | 20.26 ± 0.10 | 33.76 ± 0.12 | 21.29 ± 0.08 |
| Lipton | 22.78 ± 0.11 | 38.13 ± 0.12 | 24.71 ± 0.14 |
| Alkbous | 13.58 ± 0.11 | 24.71 ± 0.14 | 14.34 ± 0.12 |
| Green Gold | 19.73 ± 0.12 | 32.73 ± 0.10 | 20.48 ± 0.13 |
| Haritham | 36.23 ± 0.12 | 71.13 ± 0.13 | 40.88 + 0.14 |
Thus, this study showed that an optimal temperature-power (40 °C-40%) was needed to obtain good results in terms of total phenol content (TPC) in either of the utilized ultrasound frequencies.
The results of TPC also indicated that at low temperature-power (30 °C-30%) and high temperature-power (50 °C-50%) combination was quite similar (Tables 5 and 6) with regard to obtained results. Consequently, the extraction of total phenol depends on both variable factors i.e temperature and power. Ignorance of any single factor might be unsatisfactory in relation to results of total phenol content. Hence, our study and some previous reports pointed out similar facts that combined effects of temperature-power variables was more valuable in the extraction of total phenolic content (TPC) (Horžić et al., 2012).
At a power setting of 40%, there was a significant difference (p < 0.0001) between temperatures (Table 7). There were also significant differences were found between 30 °C and 50 °C, but the highest total phenol was achieved at 40 °C for 26 kHz and 40 kHz frequencies. TPC of studied tea samples increased significantly with increasing temperature, attained maximum at 40 °C then declined. It has been reported that to the extraction of TPC from soybean temperature affects the mass transfer, solubility and in turn stability of phenolics (Lien et al., 2015).
For two frequencies (26 kHz and 40 kHz) all chosen power settings at 40 °C, total phenol content was significant (p < 0.0001) (Table 8). Power of 40% at 40 °C temperature exhibited a maximum total phenol outcome. Statistical analysis also revealed that no differences were found at power treatments of 30% and 50% at a constant temperature of 40 °C for both targeted frequencies. But results of TPC among different brands of tea using the same treatments of power temperature were found differently. In the present series, Haritham brand of tea was found maximum TPC at 40 °C temperature and 40% power was 71.13 and 40.88 mg equivalent to gallic/g DW respectively for 26 and 40 kHz. Likewise, extraction of multi bioactive compounds of Hypericum perforatum L. was different with similar ultrasonic conditions (Zou et al., 2004).
3.3. Impact of extraction parameters on total flavonoids (mg/g DW) of branded tea samples using ultrasound technology
Results of total flavonoid content of branded samples of tea were found significant (p < 0.0001) utilizing frequency, temperature, and power as independent variables. There were significant results were observed for both 26 and 40 kHz frequencies within each optimizing condition (Tables 3, 5 and 6). Total flavonoid concentration was maximum at both the treated frequencies at 40 °C and power setting 40% compared to the rest of the temperature power amalgamation. A close examination revealed that almost similar results were observed at other temperature power combination of all samples except Haritham, where the flavanoid content observed 2–3 folds higher in concentration (Tables 5 and 6).
Table 3.
Total flavonoids content of different brands of tea using conventional and ultrasonic treatment.
| Sample (Brand) | Conventional | (mg/g DW) | |
|---|---|---|---|
| Ultrasonic (26 kHz) | Ultrasonic (40 kHz) | ||
| Rabea | 8.12 ± 0.30 | 27.77 ± 0.01 | 14.85 ± 0.45 |
| Lipton | 9.0 ± 0.33 | 24.00 ± 0.01 | 17.82 ± 0.30 |
| Alkbous | 6.97 ± 0.37 | 17.50 ± 0.28 | 11.19 ± 0.27 |
| Green Gold | 12.02 ± 0.32 | 25.11 ± 0.24 | 17.86 ± 0.45 |
| Haritham | 39.87 ± 0.48 | 58.25 ± 0.41 | 34.14 ± 0.33 |
For 40% power, observed a significant difference (p < 0.0001) for 26 and 40 kHz frequencies between treated temperatures. A fair combination of 40% power with 40 °C temperature achieved the highest total flavonoid content (TFC) at both the frequencies and each tea samples. In addition, there were different TFC results were found at 30 °C and 50 °C temperatures at both frequencies (Table 7). Similar findings were reported in the ultrasonic extraction of polysaccharides of Boletus edulis mycelia and concluded that an optimal temperature (below 50 °C) and power (below 50%) were needed to increase antioxidant potential (Chen et al., 2011).
At 40 °C treatment paired with different power (30, 40, 50%), flavanoid content results were not much significant difference within each frequency of 26 kHz and 40 kHz, but it was significantly different (p < 0.0001) at two different frequencies. At similar power, with a temperature of 40°, C TFC results were observed in similar fashion (Table 8). Highest cumulative results of TFC was 58.25 ± 0.41 and 34.14 ± 0.33 mg/g at 40 °C temperature paired with 40% power of Haritham brand tea for 26 kHz and 40 kHz respectively. A quantitative variation of flavanoid content of two frequencies was might have been being due to slow mass transfer and cavitational performance in case of the ultrasonic bath (40 kHz) in comparison to ultrasonic probe (26 kHz) (Wang et al., 2013).
3.4. Impact of extraction parameters on DPPH radical scavenging (%) activity of branded tea samples using ultrasound technology
The effect of optimization parameters (temperature and power) on the rate of DPPH radical scavenging activity of all the samples of tea at two ultrasonic frequencies (26 kHz and ‘40 kHz) was significant (p < 0.0001).
DPPH radical scavenging capacity of all the tea sample with each treatment of temperature-power was significantly (p < 0.0001) difference between ultrasonic frequencies. In another way, adopting similar conditions of optimization it was not significantly (p < 0.0001) difference within each frequency. The antioxidant potential of each brand of tea for both the frequencies was highest at 40 °C-40% temperature-power combination. The maximum mean results of DPPH radical scavenging was 71.29 ± 0.11 and 50.86 ± 0.10 for Haritham brand of tea at 26 kHz and 40 kHz ultrasonic frequencies respectively (Table 4). Rest of the temperature-power grouping showed almost similar DPPH radical scavenging within each sample at both the frequencies. Results also pointed out that as the operating frequency increased from 26 kHz to 40 kHz the rate of DPPH radical scavenging was decreased which was in agreement with some parallel research reports (Altemimi et al., 2015, Wang et al., 2013).
Table 4.
DPPH radical scavenging (%) activity from different brands of tea using conventional and ultrasonic treatment.
| Sample (Brand) | DPPH radical scavenging (%) |
||
|---|---|---|---|
| Conventional | Ultrasonic (26 kHz) | Ultrasonic (40 kHz) | |
| Rabea | 24.49 ± 0.46 | 51.22 ± 0.10 | 36.74 ± 0.13 |
| Lipton | 29.21 ± 0.65 | 53.78 ± 0.90 | 39.12 ± 0.01 |
| Alkbous | 36.79 ± 0.90 | 56.38 ± 0.42 | 36.38 ± 0.90 |
| Green Gold | 41.95 ± 0.92 | 65.30 ± 0.80 | 45.23 ± 0.39 |
| Haritham | 46.61 ± 0.87 | 71.29 ± 0.11 | 50.86 ± 0.10 |
Considering 40% power, there was a significant difference (p < 0.0001) between temperatures for 40 kHz frequency, but no difference was obtained for 26 kHz. Maximum DPPH radical scavenging was exhibited at 40 °C for both the operating frequencies (Table 7). Also, for both operating frequencies, the DPPH radical rate was suddenly dropping at 50 °C, possibly due to some heat-sensitive bioactive present in the samples.
Statistical analysis revealed that at 40 °C, no difference in DPPH scavenging was observed at 30% and 50% power when exposed to ultrasonic frequency of 40 kHz. But at similar operating temperature and power with 26 kHz frequency, results were differing around 5–10 digits in a row for each brand of tea sample (Table 8). In the case of 26 kHz ultrasonic frequency, direct probe immersed in the reaction medium, therefore results might have been changes even alteration in power 5–10%. On the other hand, in case of the ultrasonic bath (40 kHz), no any significant changes was observed as it was not in direct contact with the medium and hence slight tuning of temperature or power wouldn’t affect results.
3.5. Appraisal of ultrasonic methods for yield, total phenols, total flavonoids and DPPH radical scavenging activities with conventional (control) extraction of branded tea samples
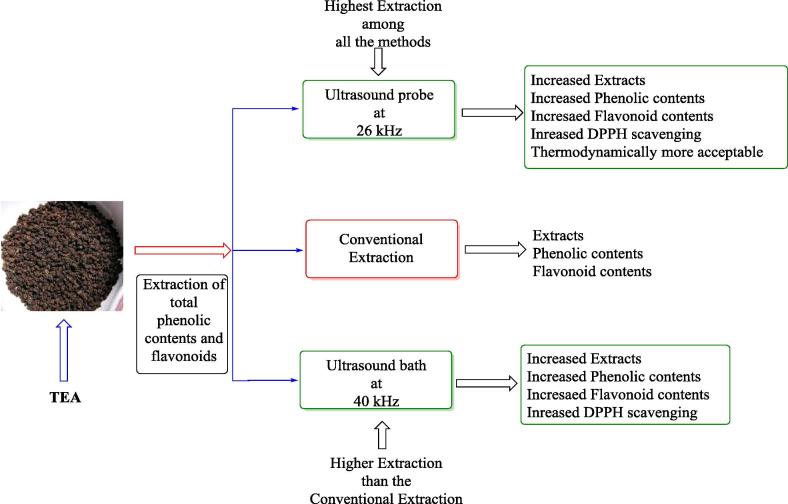
Performance evaluation of two ultrasonic techniques i.e. ultrasonic probe (26 kHz) and ultrasonic bath (40 kHz) in terms of extract percent yield and antioxidant potential was compared with experiments conducted by a conventional method (Fig. 1).
Fig. 1.
Comparison of ultrasonic extraction with conventional (control) in terms of polyphenol content and antioxidant potential.
All the branded tea samples went through extraction, conducted at 40 °C temperature and 40% power for 30 min by both ultrasonic frequencies. A parallel experiment was also run by a conventional method at 40 °C temperature for 30 min. significant results were obtained in both ultrasonic set-ups. The polyphenolic extract was significantly (p < 0.0001) higher for 26 kHz frequency compared to 40 kHz and conventional (control) method (Table 1). The highest yield was 32.68 ± 0.14, 21.93 ± 0.04 and 17.68 ± 0.14 exhibited by Haritham brand of tea for 26 kHz, 40 kHz, and control respectively. Total phenol content (TPC) (Table 2) and total flavonoid content (TFC) (Table 3) produce significant (p < 0.0001) results in the order ultrasonic (26 kHz) > ultrasonic (40 kHz) > control. Many satisfied results of percent DPPH radical scavenging were also observed by the ultrasonic probe (26 kHz) and bath (40 kHz) with respect to control (Table 4). Furthermore, results of presents study were in agreements with various recent work reported on tea extraction, mentioned the efficiency of ultrasonication to the enhancement of polyphenolic compounds extract yield compared to conventional technique at low-temperature power decreasing the threats of degradation of valuable compounds (Horžić et al., 2012, Pasrija and Krishnan, 2015).
3.6. Impact of the thermodynamic parameter on the extraction of polyphenolic compounds using ultrasound technology
In the present study, a process of extraction for a branded tea samples was analyzed thermodynamically under the influence of two ultrasound frequencies i.e ultrasound probe (26 kHz) and ultrasound bath (40 kHz). All the basic thermodynamic parameters were calculated from Eqs. (2), (3), (4), (5) in material method section and results are summarized in Tables 9 and 10.
Table 9.
Thermodynamic parameters at ultrasonic frequency 26 kHz.
| Sample (Brand) | Temp, K | ΔG0, kJmol−1 | Ea, kJmol−1 | ΔH0, kJ | ΔS0, Jmol−1 |
|---|---|---|---|---|---|
| Rabea | 303.15 | −0.72 | |||
| 313.15 | −0.78 | 3.64 | 1.04 | 5.83 | |
| 323.15 | −0.84 | ||||
| Lipton | 303.15 | −0.60 | |||
| 313.15 | −0.61 | 2.42 | −0.18 | −1.31 | |
| 323.15 | −0.62 | ||||
| Alkbous | 303.15 | −0.53 | |||
| 313.15 | −0.58 | 3.44 | 0.84 | 4.55 | |
| 323.15 | −1.29 | ||||
| Green Gold | 303.15 | −1.00 | 4.01 | 1.41 | 7.98 |
| 313.15 | −1.08 | ||||
| 323.15 | −1.16 | ||||
| Haritham | 303.15 | −1.52 | 4.75 | 2.15 | 12.12 |
| 313.15 | −1.64 | ||||
| 323.15 | −1.76 |
Table 10.
Thermodynamic parameters at ultrasonic frequency 40 kHz.
| Sample (Brand) | Temp, K | ΔG0, kJmol−1 | Ea, kJmol−1 | ΔH0, kJ | ΔS0, Jmol−1 |
|---|---|---|---|---|---|
| Rabea | 303.15 | −0.92 | |||
| 313.15 | −0.99 | 3.96 | 1.36 | 7.53 | |
| 323.15 | −1.06 | ||||
| Lipton | 303.15 | −2.96 | |||
| 313.15 | −3.19 | 11.43 | 4.12 | 23.36 | |
| 323.15 | −3.42 | ||||
| Alkbous | 303.15 | −2.07 | |||
| 313.15 | −2.24 | 5.57 | 2.97 | 16.65 | |
| 323.15 | −2.41 | ||||
| Green Gold | 303.15 | −3.50 | 7.48 | 4.88 | 27.67 |
| 313.15 | −3.78 | ||||
| 323.15 | −4.06 | ||||
| Haritham | 303.15 | −3.62 | 7.64 | 5.04 | 28.59 |
| 313.15 | −3.91 | ||||
| 323.15 | −4.19 |
The values of ke for all brands of samples were found decreased as the temperature increased during the extraction in both ultrasonic frequencies (Tables 9 and 10) but at temperature 303.15 K it was lowest. Total phenolic contents (TPC) also found maximum at this temperature. 303.15 K (40 °C) temperature yielded the highest TPC in all brand of tea under the influence of both ultrasound frequencies. The values of Gibbs free energy (ΔG°) were found negative which confirmed the extraction process was spontaneous and feasible in both utilized ultrasound frequencies (Tables 9 and 10). Also, the degree of spontaneity was proportionally increased with increased in extraction temperature (Kim and Kim, 2016).
The heat of reaction (ΔH°) was obtained positive values in all brands of tea under the influence of both ultrasound technologies except Lipton, which exhibited the negative value (ΔH° = −0.18) using 26 kHz ultrasound (Tables 9 and 10). Therefore, the negative values indicating, that the extraction of tea samples requires energy and process were endothermic. The heat of reaction (ΔH°) during the extraction of Rabea, Lipton, Alkbous, Green gold and Haritham were found 1.04, −0.18, 0.84, 1.41 and 2.15 utilizing 26 kHz, whereas, 1.36, 4.12, 2.97, 4.88 and 5.04 kJ/mol with 40 kHz frequency. Interestingly, heats of reaction in our results were far less than some recent reports. The value of ΔH° = 8.040 kJ/ mol was recorded by the extraction of paclitaxel from biomass (Kim and Kim, 2016). Extraction of gossypol from defatted cottonseed meal (Saxena et al., 2012), extraction of oil from sunflower seeds (Topallar and Geçgel, 2000) and the extraction of oil from caster cake (Amarante et al., 2014) were found ΔH° = 27.749–28.093, 11.200 and 12.270 kJ/mol which is more than that of our present results as shown in Tables 9 and 10. Moreover, comparing values of heat of reaction (ΔH0) of each brand and under the influence of two frequencies was quite variable. Amount of polyphenolic contents using 26 kHz ultrasonic probe were found more with the expense of less energy but in case of 40 kHz ultrasonic bath, comparatively less amount of polyphenolic contents received with more energy dissipation considering any brand of tea sample (Tables 9 and 10). In both ultrasonic frequencies, Haritham brand of tea used maximum energy, ΔH° = 2.15 and ΔH° = 5.04 kJ/mol with 26 kHz and 40 kHz respectively yielded highest polyphenolic contents.
Further, the value of activation energy (Ea) should be more than their corresponding heat of reaction (ΔH°) in an endothermic reaction (Kim and Kim, 2016) and thus in our results indicated that all Ea values in either of the frequency were higher than ΔH°. Utilizing 26 kHz and 40 kHz frequencies, Ea values range from 2.42 to 4.75 and 3.96 to 11.43 kJ/mol respectively (Tables 9 and 10). These values also pointed out that relatively less activation energy (Ea) needed when 26 kHz ultrasound technique was employed compared to 40 kHz for the extraction process. Also, the Ea values in all brand of tea were less than that of most of the reported results. During the extraction of flavanols from green tea Ea was used 30–50 kJ/mol (Price and Spitzer, 1994). In another research, Ea = 8.56 kJ/mol was utilized for the oil extraction from the olive cake (Meziane and Kadi, 2008). All the positive value of ΔS° in both ultrasound extraction methods was irreversible.
4. Conclusion
In conclusion, ultrasound-assisted extraction was found to be the most reliable method for the extraction of polyphenol compounds from branded tea. Results of ultrasonic treatment were found statistically significant at temperature of 40 °C and power setting of 40% for both utilized frequencies. Total yield, total phenolic content, total flavonoid content and antioxidant potential (%DPPH) were found highest when used 26 kHz ultrasound probe as an extraction technique than 40 kHz bath. Thermodynamic analysis of branded tea sample for both ultrasonic treatments showed that the extraction process was spontaneous, endothermic and irreversible in the ranges of temperature employed. Under similar thermodynamic set up 26 kHz ultrasonic probe was found superior over 40 kHz ultrasonic bath. The futuristic study could be employed for the different food and beverages under the effect of ultrasound by varying parameters to access antioxidant potential as a techno-economical application.
Acknowledgments
Authors wish to thanks, Prince Sattam Bin Abdulaziz University for providing research facilities for the accomplishment of this work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Altemimi A., Choudhary R., Watson D.G., Lighfoot D.A. Effects of ultrasonic treatments on the polyphenol and antioxidant content of spinach extracts. Ultrason. Sonochem. 2015;24:247–255. doi: 10.1016/j.ultsonch.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Amarante R.C.A., Oliveira P.M., Schwantes F.K., Moron-villarreyes J.A. Oil extraction from castor cake using ethanol: kinetics and thermodynamics. Ind. Eng. Chem. Res. 2014;53:6824–6829. [Google Scholar]
- Ashley K. Ultrasonic extraction of heavy metals from environmental and industrial hygiene samples for their subsequent determination. Trends. Analyt. Chem. 1998;17:366–372. [Google Scholar]
- Banerjee S., Chatterjee J. Efficient extraction strategies of tea (Camellia sinensis) biomolecules. Food. Bioprocess. Tech. 2015;52:3158–3168. doi: 10.1007/s13197-014-1487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.J., Chiu K.L., Chen Y.L., Chang C.Y. Separation of catechins from green tea using carbon dioxide extraction. Food. Chem. 2000;68:109–113. [Google Scholar]
- Chen W., Wang W., Zhang H., Huang Q. Optimization of ultrasonic-assisted extraction of water-soluble polysaccharides from Boletus edulis mycelia using response surface methodology. Carbohyd. Polym. 2011;87:614–619. doi: 10.1016/j.carbpol.2011.08.029. [DOI] [PubMed] [Google Scholar]
- Fukino Y., Shimbo M., Aoki N., Okubo T., Iso H. Randomized controlled trial for an effect of green tea consumption on insulin resistance and inflammation markers. J. Nut. Sci. Vitaminnol. 2005;51:335–342. doi: 10.3177/jnsv.51.335. [DOI] [PubMed] [Google Scholar]
- Horžic D., Jambrak A.R., B-Cvitanović A., Komes D., Lelas V. Comparison of conventional and ultrasound assisted extraction techniques of yellow tea and bioactive composition of obtained extracts. Food. Bioproc. Tech. 2012;5:2858–2870. [Google Scholar]
- Hussain A.I., Anwar F., Sherazi S.T.H., Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food. Chem. 2008;108:986–995. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Jun X., Deji S., Shou Z., Bingbing L., Ye L., Rui Z. Characterization of polyphenols from green tea leaves using a high hydrostatic pressure extraction. Int. J. Pharm. 2009;382:139–143. doi: 10.1016/j.ijpharm.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Jun X., Shuo Z., Bingbing L., Rui Z., Ye L., Deji S. Separation of major catechins from green tea by ultrahigh pressure extraction. Int. J. Pharmaceutics. 2010;386:229–231. doi: 10.1016/j.ijpharm.2009.10.035. [DOI] [PubMed] [Google Scholar]
- Kim W.K., Kim J.H. Kinetics, and thermodynamics of paclitaxel extraction from cell culture, Korean. J. Chem. Eng. 2016;33:3175–3183. [Google Scholar]
- Lien D.T.P., Tram P.T.B., Toan H.T. Effects of extraction process on phenolic content and antioxidant activity of soybean. J. Food. Nut. Sci. 2015;3:33–38. [Google Scholar]
- Lin S.D., Liu E.H., Mau J.L. Effect of different brewing methods on antioxidant properties of steaming green tea. LWT-Food. Sci. Tech. 2008;41:1616–1623. [Google Scholar]
- Martin T. Plant extraction: Key technology for the sustained use of bio-resources. Chem. Ingenieur. Tech. 2012;84:880–882. [Google Scholar]
- Mason T.J., Zhao Y.Y. Enhanced extraction of tea solids using ultrasound. Ultrason. Sonochem. 1994;32:375–377. [Google Scholar]
- Meziane S., Kadi H. Kinetics and thermodynamics of oil extraction from olive cake. J. Am. Oil. Chem. Soc. 2008;85:391–396. [Google Scholar]
- Milica R., Senka V., Zoran Z., Jelena V., Aleksandra C., Branimir P. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochem. 2015;23:360–368. doi: 10.1016/j.ultsonch.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Miracea V. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001;8:303–313. doi: 10.1016/s1350-4177(01)00071-2. [DOI] [PubMed] [Google Scholar]
- Nikhil E., Tomao V., El Hajji H., El Boustani E.S., Chemat F., Dangles O. Microwave-assisted water extraction of green tea polyphenols. Phytochem. Anal. 2009;20:408–415. doi: 10.1002/pca.1141. [DOI] [PubMed] [Google Scholar]
- Pasrija D., Krishnan C.A.H. Techniques for extraction of green tea polyphenols: a review. Food. Bioproc. Technol. 2015;8:935–950. [Google Scholar]
- Price W.E., Spitzer J.C. The kinetics of extraction of individual flavanols and caffeine from a Japanese green tea (Sen Cha Uji Tsuyu) as a function of temperature. Food. Chem. 1994;50:19–23. [Google Scholar]
- Saxena D., Sharma S., Sambi S. Kinetics and thermodynamics of gossypol extraction from defatted cottonseed meal by ethanol. Pol. J. Chem. Tech. 2012;14:29–34. [Google Scholar]
- Seeley D., Mills E.J., Wu P., Verma S., Guyatt G.H. The effects of green tea consumption on incidence of breast cancer and recurrence of breast cancer: a systematic review and meta-analysis. Inga. Canc. Therapies. 2005;4:144–155. doi: 10.1177/1534735405276420. [DOI] [PubMed] [Google Scholar]
- Simon B., Farid C., Jochen S. Extraction of polyphenols from black tea – conventional and ultrasound assisted extraction. Ultrason. Sonochem. 2014;21:1030–1034. doi: 10.1016/j.ultsonch.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Tao X., Siquan S., Xiaochun W. Impact of ultrasonic-assisted extraction on the chemical and sensory quality of tea infusion. J. Food. Eng. 2006;74:557–560. [Google Scholar]
- Topallar H., Geçgel U. Kinetics and thermodynamics of oil extraction from sun flower seeds in the presence of aqueous acidic. Turk. J. Chem. 2000;24:247–254. [Google Scholar]
- Unachukwu U.J., Ahmed S., Kavalier A., Lyles J.T., Kennelly E.J. White and green teas (Camellia sinensis varsinensis) variation in phenolic methylxanthine and antioxidant profiles. J. Food. Sci. 2010;75:C541–C548. doi: 10.1111/j.1750-3841.2010.01705.x. [DOI] [PubMed] [Google Scholar]
- Wang X.S., Wu Y.F., Chen G.Y., Yue W., Liang Q.L., Wu Q.N. Optimization of ultrasound-assisted extraction of phenolic compounds from Sparganii rhizoma with response surface methodology. Ultrason. Sonochem. 2013;20:846–854. doi: 10.1016/j.ultsonch.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Xuejun P., Guoguang N., Huizhou L. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process. 2003;42:129–133. [Google Scholar]
- Zhou B. UIUC; Urbana, Illinois: 2010. Investigation on factors influencing ultrasound-assisted surface decontamination of fresh and fresh-cut vegetables (Dissertation) [Google Scholar]
- Zou Y., Lu Y., Wei D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food. Chem. 2004;52:5032–5039. doi: 10.1021/jf049571r. [DOI] [PubMed] [Google Scholar]