Abstract
Background
Diabetic cardiomyopathy (DC) is defined as a ventricular diastolic and/or systolic dysfunction, which is directly related to diabetes mellitus (DM) in the absence of coronary artery disease, valvular, congenital or hypertensive heart disease, and alcoholism. In this report, we present an unusual case of a patient with DC and reversible, acute left ventricular systolic dysfunction due to cardiotoxicity of hyperosmolar hyperglycaemic state (HHS).
Case summary
A 20-year-old male patient presented with weakness and polyuria. Physical examination and electrocardiogram were normal. Laboratory results and arterial blood gas analysis were consistent with HHS. Baseline echocardiography showed global left ventricular hypokinesis with an ejection fraction (EF) of 36%. The patient’s clinical condition improved after blood glucose level normalization and echocardiography revealed progressive improvement in the left ventricular systolic function with an EF of 54% at the 5-day follow-up and an EF of 69% at the 15-day follow-up.
Discussion
Uncontrolled DM and hyperglycaemic crisis may result in cardiotoxicity, acute left ventricular systolic dysfunction, and DC. The pathophysiological mechanism of this phenomenon is still unclear. Blood glucose control is the most important strategy for the prevention of DC.
Keywords: Cardiotoxicity, Case report, Diabetic cardiomyopathy, Hyperglycaemic crisis, Systolic dysfunction
Learning points
Diabetic cardiomyopathy (DC) is defined as a ventricular diastolic and/or systolic dysfunction, which is directly related to diabetes mellitus (DM) in the absence of coronary artery disease, valvular, congenital or hypertensive heart disease, and alcoholism.
Uncontrolled DM and hyperglycaemic crisis such as diabetic ketoacidosis or hyperglycaemic hyperosmolar state may result in cardiotoxicity, acute left ventricular systolic dysfunction, and DC.
Introduction
Diabetes mellitus (DM) is a major public health problem whose rates have risen dramatically.1 High blood glucose levels can lead to serious life-threatening complications such as coronary artery disease (CAD), cerebrovascular accident, heart failure, and nephropathy.2 Diabetic cardiomyopathy (DC) is characterized by myocardial systolic and/or diastolic dysfunction without CAD, valvular or hypertensive heart disease, which was firstly defined by Rubler in 1972.3 The mechanisms of DC and cardiotoxicity due to hyperglycaemia has not been fully understood yet and it is an under-recognized phenomenon in cardiology. In this report, we present a case of a patient with acute and reversible left ventricular systolic dysfunction due to cardiotoxicity of hyperosmolar hyperglycaemic state (HHS) and DC.
Timeline
| Day 1 | A 20-year-old male patient presented with weakness and polyuria. Laboratory results revealed hyperglycaemia, hypertriglyceridaemia, and hyperosmolar hyperglycaemic state. Transthoracic echocardiography revealed normal valvular functions and global left ventricular hypokinesis with an ejection fraction (EF) of 36%. |
| Day 2 | The patient’s clinical condition and laboratory results improved after intravenous fluid and insulin infusion therapy and blood glucose level normalization. |
| Day 5 | Transthoracic echocardiography revealed progressive improvement in the left ventricular systolic function with an EF of 54% at the 5-day follow-up. |
| Day 15 | Transthoracic echocardiography revealed progressive improvement in the left ventricular systolic function with an EF of 69% at the 15-day follow-up and the patient was discharged with intensive insulin, metoprolol, ramipril, and fenofibrate therapy. |
Case presentation
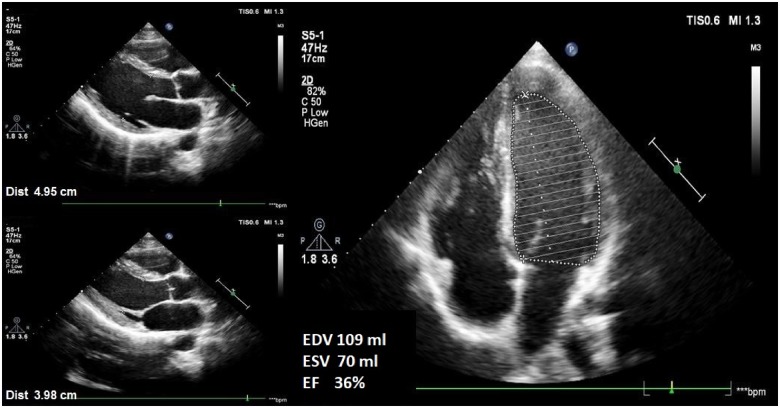
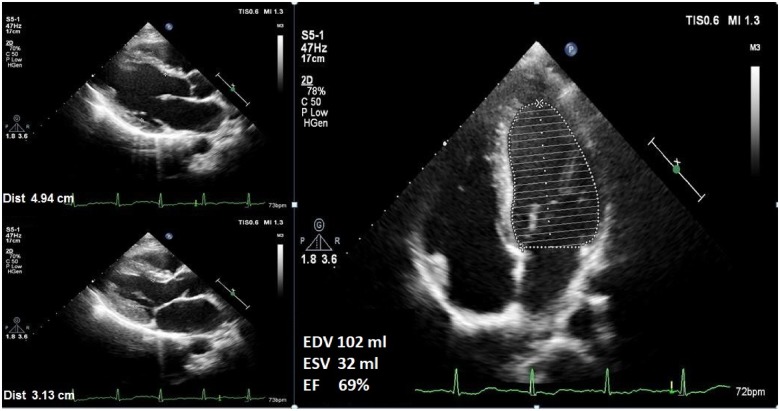
A 20-year-old male patient presented to internal medicine clinic with weakness and polyuria. His past medical history was unremarkable. On initial assessment, the patient had no chest pain or shortness of breath, and he was afebrile, with blood pressure of 107/69 mmHg, and heart rate of 62 b.p.m. Cardiac and pulmonary auscultation were normal and further physical examination showed no other abnormalities. The electrocardiogram (ECG) showed a normal sinus rhythm and a heart rate of 59 b.p.m. without ischaemic changes. Laboratory results revealed hyperglycaemia (glucose 1104 mg/dL, reference range: 74–106 mg/dL), hyponatraemia (Na 121 mmol/L, corrected Na 145 mmol/L, reference range: 135–145 mmol/L), hypertriglyceridaemia (triglyceride 1306 mg/dL, reference range: 0–150 mg/dL), haemoglobin A1c 17.7% (reference range: 4.5–6.0%), negative high-sensitive troponin (reference range <1.5 ng/L), N-terminal pro-brain natriuretic peptide 1220 pg/mL (reference range: 0–100 pg/mL), C-reactive protein 0.82 mg/dL (reference range: 0–0.5 mg/dL), and effective osmolality 361 mOsm/L. Arterial blood gas analysis revealed pH 7.44 (reference range: 7.35–7.45), pO2 78 mmHg (reference range: 80–100 mmHg), pCO2 37.4 mmHg (reference range: 35–45 mmHg), and bicarbonate 25.2 mmol/L (reference range: 22–26 mmol/L) (Table 1). The patient was admitted to intensive care unit (ICU) with HHS as a first presentation of unknown DM. After hospitalization, intravenous fluid and insulin infusion therapy were started. Baseline transthoracic echocardiography (TTE) revealed normal valvular functions and global left ventricular hypokinesis with an ejection fraction (EF) of 36% (left ventricular end-diastolic and end-systolic volumes were 109 and 70 mL, respectively and left ventricular end-diastolic and end-systolic diameters were 50 and 40 mm, respectively), which was determined by the Simpson’s method (Figure 1, Supplementary material online, Movie S1–S3). After TTE, metoprolol 25 mg/day, ramipril 2.5 mg/day, and fenofibrate 267 mg/day were also started. The patient’s clinical condition improved after blood glucose level normalization. During ICU follow-up, troponin levels were within normal reference range and ECG showed normal sinus rhythm without ischaemic changes. Transthoracic echocardiography revealed progressive improvement in the left ventricular systolic function with an EF of 54% at the 5-day follow-up and an EF of 69% (left ventricular end-diastolic and end-systolic volumes were 102 and 32 mL, respectively and left ventricular end-diastolic and end-systolic diameters were 49 and 31 mm, respectively) at the 15-day follow-up after blood glucose control (Figure 2, Supplementary material online, Movie S1–S3). The patient was discharged with intensive insulin, metoprolol 50 mg/day, ramipril 5 mg/day, and fenofibrate 267 mg/day therapy and a programme of intensive follow-up for blood glucose regulation.
Table 1.
Laboratory analysis on admission
| Laboratory test | Result | Reference range |
|---|---|---|
| Blood glucose (mg/dL) | 1104 | 74–106 |
| Creatinine (mg/dL) | 1.3 | 0.7–1.3 |
| Na (mmol/L)/corrected Naa | 121/145 | 135–145 |
| K (mmol/L) | 5 | 3.5–5.0 |
| Alanine amino transferase (U/L) | 44 | 5–49 |
| hs-troponin I (ng/L) | <1.5 | <1.5 |
| NT-proBNP (pg/mL) | 1220 | 0–100 |
| C-reactive protein (mg/dL) | 0.82 | 0–0.5 |
| Triglyceride (mg/dL) | 1306 | 0–150 |
| Hba1c (%) | 17.7 | 4.5–6.0 |
| Haemoglobin (g/dL) | 17.1 | 12–16 |
| Effective serum osmolality (mOsm/L)b | 361 | 280–295 |
| Arterial blood gas analysis | ||
| pH | 7.44 | 7.35–7.45 |
| pO2 (mmHg) | 78 | 80–100 |
| pCO2 (mmHg) | 37 | 35–45 |
| Bicarbonate (mmol/L) | 25 | 22–26 |
Corrected sodium (Hillier, 1999) = measured sodium + 0.024 * (Serum glucose − 100).
Effective serum osmolality (Eosm) = 2 [Na (meq/L) + K (meq/L)] + [Serum glucose (mg/dL)/18].
Figure 1.
Baseline transthoracic echocardiography showing left ventricular hypokinesis with an ejection fraction of 36% (left ventricular end-diastolic and end-systolic volumes were 109 and 70 mL, respectively and left ventricular end-diastolic and end-systolic diameters were 50 and 40 mm, respectively).
Figure 2.
Follow-up echocardiography at the 15th day demonstrating progressive improvement in the left ventricular systolic function with an ejection fraction of 69% (left ventricular end-diastolic and end-systolic volumes were 102 and 32 mL, respectively and left ventricular end-diastolic and end-systolic diameters were 49 and 31 mm, respectively).
Discussion
Diabetic cardiomyopathy is defined as a ventricular diastolic and/or systolic dysfunction, which is directly related to DM in the absence of CAD, valvular, congenital or hypertensive heart disease, and alcoholism.3,4
The pathogenesis of DC has been poorly understood, includes complex and multifactorial mechanisms such as hyperglycaemia, hyperinsulinaemia, insulin resistance, increased free fatty acids (FFA), microvascular damage and inflammatory cytokines change cellular metabolic pathways in cardiomyocytes, and impair cardiac function.5 The non-diabetic healthy heart obtains 60–90% of its energy from FFA oxidation, with the balance from lactate and glucose.6 In patients with uncontrolled DM, glucose uptake significantly decreases, FFA uptake increase and metabolic balance shifts to lipid oxidation. Increased FFA oxidation is complicated with high level of triglycerides synthesis that causes lipotoxicity and myocyte apoptosis. Additionally, in diabetic heart, increased lipid oxidation boosts mitochondrial uncoupling and oxidative stress that may eventuate in reduced myocardial energy production and myocardial contractile dysfunction.7–9 On the other hand, hyperglycaemia is an another important component in the mechanism of DC. In DC, glucotoxicity give rise to cardiac dysfunction by induction of oxidative stress and generation of advanced glycation end-products.10 In addition, hyperglycaemia activates renin–angiotensin–aldosterone system (RAAS), which may result in increased cell necrosis and fibrosis.11 Inflammation is another key factor in the pathogenesis of diabetes and its complications.10 Expression of inflammatory cytokines such as tissue necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) are increased in the myocardium, which are associated with myocardial contractile dysfunction.12 Stentz et al.13 reported that acute hyperglycaemic crisis such as diabetic ketoacidosis (DKA) and HHS are associated with inflammatory state and independently induce changes in proinflammatory cytokines, oxidative stress, and cardiovascular markers. The authors point out the importance of insulin therapy and its anti-inflammatory effects.
Some important clinical studies demonstrated subclinical left ventricular systolic dysfunction in adolescents and young adults with Type 1 DM who have normal left ventricular EF.14,15 The mechanisms of ventricular systolic dysfunction in young adults with Type 1 DM are mainly related to hyperglycaemia and impaired FFA metabolism rather than hyperinsulinaemia and insulin resistance.14 Another study showed significant reduction in global longitudinal strain in diabetic patients with normal coronary arteries and normal left ventricular EF, which is independently correlated with glycosylated haemoglobin and fasting blood sugar levels.16 Aboukhoudir et al.17 demonstrated dobutamine infusion during dobutamine stress echocardiography can induce a significant deterioration in left ventricular EF in the absence of CAD or vasospasm in patients with uncontrolled DM and normal left ventricular EF at rest. This condition largely reversed by appropriate blood glucose control and RAAS inhibition after 60 days.
To conclude, uncontrolled DM and hyperglycaemic crisis such as DKA and HHS may result in cardiotoxicity, acute left ventricular systolic dysfunction and DC. The pathophysiological mechanism of this phenomenon is still unclear and there is no specific treatment for DC. Blood glucose control is the most important strategy for the prevention of DC.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
Footnotes
† Presented at 33rd Turkish Cardiology Congress 2017, Antalya, Turkey, as an oral presentation.
References
- 1. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB.. Executive summary: heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 2012;125:188–197. [DOI] [PubMed] [Google Scholar]
- 2. Low Wang CC, Hess CN, Hiatt WR, Goldfine AB.. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus—mechanisms, management, and clinical considerations. Circulation 2016;133:2459–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A.. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 1972;30:595–602. [DOI] [PubMed] [Google Scholar]
- 4. Seferović PM, Paulus WJ.. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J 2015;36:1718–1727. [DOI] [PubMed] [Google Scholar]
- 5. Bugger H, Abel ED.. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014;57:660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stanley WC, Chandler MP.. Energy metabolism in the normal and failing heart: potential for therapeutic interventions. Heart Fail Rev 2002;7:115–130. [DOI] [PubMed] [Google Scholar]
- 7. Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME.. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med 2008;121:748–757. [DOI] [PubMed] [Google Scholar]
- 8. Rodrigues B, Cam MC, McNeill JH.. Metabolic disturbances in diabeticcardiomyopathy. Mol Cell Biochem 1998;180:53–57. [PubMed] [Google Scholar]
- 9. Boudina S, Abel ED.. Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda) 2006;21:250–258. [DOI] [PubMed] [Google Scholar]
- 10. Schilling JD, Mann DL.. Diabetic cardiomyopathy: bench to bedside. Heart Fail Clin 2012;8:619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P.. Myocardial cell death in human diabetes. Circ Res 2000;87:1123–1132. [DOI] [PubMed] [Google Scholar]
- 12. Westermann D, Van Linthout S, Dhayat S, Dhayat N, Schmidt A, Noutsias M, Song XY, Spillmann F, Riad A, Schultheiss HP, Tschöpe C.. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol 2007;102:500–507. [DOI] [PubMed] [Google Scholar]
- 13. Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE.. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes 2004;53:2079–2086. [DOI] [PubMed] [Google Scholar]
- 14. Jędrzejewska I, Król W, Światowiec A, Wilczewska A, Grzywanowska-Łaniewska I, Dłużniewski M, Braksator W.. Left and right ventricular systolic function impairment in type 1 diabetic young adults assessed by 2D speckle tracking echocardiography. Eur Heart J Cardiovasc Imaging 2016;17:438–446. [DOI] [PubMed] [Google Scholar]
- 15. Altun G, Babaoğlu K, Binnetoğlu K, Özsu E, Yeşiltepe Mutlu RG, Hatun Ş.. Subclinical left ventricular longitudinal and radial systolic dysfunction in children and adolescents with type 1 diabetes mellitus. Echocardiography 2016;33:1032–1039. [DOI] [PubMed] [Google Scholar]
- 16. Zoroufian A, Razmi T, Taghavi-Shavazi M, Lotfi-Tokaldany M, Jalali A.. Evaluation of subclinical left ventricular dysfunction in diabetic patients: longitudinal strain velocities and left ventricular dyssynchrony by two-dimensional speckle tracking echocardiography study. Echocardiography 2014;31:456–463. [DOI] [PubMed] [Google Scholar]
- 17. Aboukhoudir F, Rekik S.. Left ventricular systolic function deterioration during dobutamine stres echocardiography as an early manifestation of diabetic cardiomyopathy and reversal by optimized therapeutic approach. Int J Cardiovasc Imaging 2012;28:1329–1339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.