Abstract
Background
Burkholderia pseudomallei is a Gram negative, soil-water saprophytic bacterium endemic in South-East Asia and Northern Australia. Melioidosis is being increasingly diagnosed in other regions like India, China, and Sri Lanka during recent years. The clinical presentation of melioidosis is extremely variable.
Case summary
We present a case of melioidosis presenting as native valve infective endocarditis with concomitant hepatic and splenic abscesses. This is the second case of melioidosis with infective endocarditis reported from India.
Discussion
Melioidosis can present with pneumonia, pleural effusion, subcutaneous abscesses, visceral abscesses, osteomyelitis, and septicaemia, but cardiac involvement is rare. Endocarditis due to melioidosis is rare (∼1%) and is rarely reported in literature. This case highlights the unusual presentation of this emerging disease.
Keywords: Melioidosis, Infective endocarditis, Disseminated melioidosis, Case report
Learning points
Burkholderia pseudomallei infection should be suspected, when there is multiple organ involvement, in patients with underlying risk factors like diabetes mellitus, alcohol intake, chronic liver disease, chronic kidney disease, immune-compromised status, malignancies, and trauma.
Cardiac involvement though rare, should always be ruled out in patients with melioidosis.
Introduction
Melioidosis is an infection caused by a Gram negative soil-water saprophytic bacterium, Burkholderia pseudomallei. It is endemic in South-East Asia and Northern Australia. During recent years, melioidosis is being increasingly diagnosed in other regions like India, China, and Sri Lanka. The clinical presentation of melioidosis is extremely variable, varying from acute fulminant septicaemia to subclinical and chronic forms. Varied presentations include pneumonia, pleural effusion, subcutaneous abscesses, visceral abscesses, osteomyelitis, septicaemia, etc. Melioidosis can involve any part of the body; however, cardiac involvement is very rare (∼1%).1
Timeline
| Day 0 | Presentation in emergency room with fever of 1 month duration and abdominal pain. |
| Day 0 | Ultrasound abdomen revealed possible abscess in liver. |
| Day 1 | Computed tomography (CT) abdomen with contrast—multiple liver and splenic abscesses. |
| CT chest—right lower lobe consolidation. | |
| Day 2 | Transoesophageal echo—multiple vegetations on anterior mitral leaflet. |
| Day 2 | Blood cultures—grew Burkholderia pseudomallei. Started on intravenous ceftazidime, oral doxycycline, and oral trimethoprim-sulfamethoxazole. |
| Day 14 | Discharged in stable afebrile state. |
| 4 weeks | On review patient afebrile and asymptomatic. Repeat transthoracic echo showed no vegetation. |
Case presentation
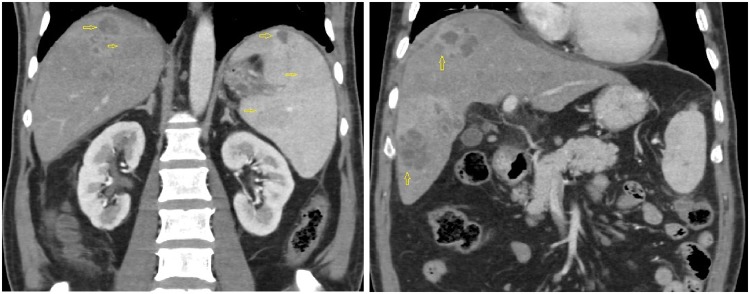
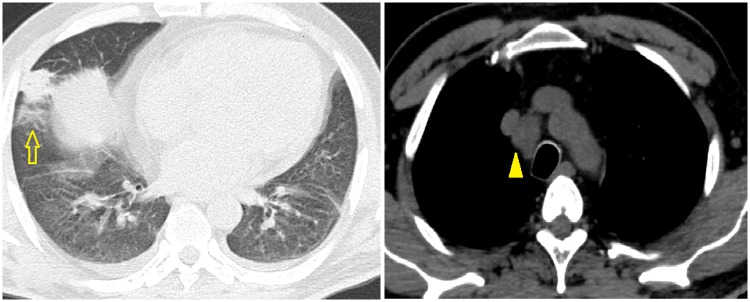
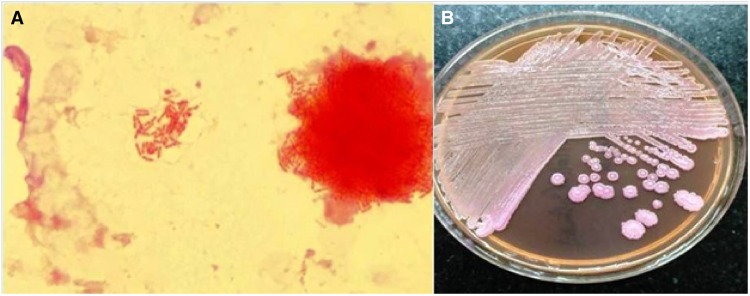
A 60-year-old diabetic male presented to our hospital with high-grade fever (38.8°C), cough with mucoid expectoration, and generalized abdominal pain of 1 month duration. He had previously served in the army and was currently a farmer by occupation. He was an ex-smoker and consumed alcohol daily. The physical examination revealed pallor, tachypnoea, crepitations over the right mammary, axillary and interscapular areas, and tender hepatomegaly. His jugular venous pulse was normal and there was no pedal oedema. He was tachycardiac with a heart rate of 110 b.p.m. His respiratory rate was 20 per minute. His blood pressure and oxygen saturation were normal. The cardiac auscultation was also normal. The baseline investigations revealed mild anaemia with haemoglobin of 11.4 g/dL (normal range: 13–16 gm/dL), leucocytosis 15 830 cells/mL (normal range: 4 000–11 000 cells/mL) with neutrophilia 82% (normal value: 40–60%), and erythrocyte sedimentation rate of 64 mm/h (normal range: 0–22 mm/h). Liver function tests revealed elevated alkaline phosphatase 206 IU/L (normal range: 0—40 IU/L) and gamma glutamyl transpeptidase 185 IU/L (normal range: 0–35 IU/L), while the renal parameters were normal. The test for HIV ELISA was negative. The chest X-ray did not reveal any obvious abnormalities. The ultrasound of the abdomen revealed multiple, ill defined, hypoechoic areas in the liver and mild hepatosplenomegaly. The contrast enhanced computed tomography (CT) scan of the abdomen revealed multiple hypodense lesions in the liver and spleen (Figure 1). The CT chest revealed consolidation in the right lower lobe and mediastinal lymphadenopathy along with mild bilateral pleural effusions (Figure 2). In view of disseminated infection with multiple liver and splenic abscesses, possible infective endocarditis was suspected. The transthoracic echocardiogram was suboptimal due to poor echo windows, but there was a suspicion of vegetation on the anterior mitral leaflet. The transoesophageal echocardiogram revealed multiple vegetations on the anterior mitral leaflet (Figure 3). There was trivial mitral regurgitation and the other valves were normal. There was normal biventricular function, with no pericardial effusion. The blood cultures grew B. pseudomallei (identified on Vitek 2 Compact—bioMerieux) (Figure 4). The sensitivity testing and interpretation was done by disk diffusion as per Clinical and Laboratory Standards Institute recommendations and found to be sensitive to cotrimoxazole, ceftazidime, aminoglycosides, ciprofloxacin, trimethoprim-sulfamethoxazole, and carbapenems. The patient was treated with intravenous ceftazidime. Two weeks into admission, the patient became afebrile; the cough subsided and hence was discharged on intravenous ceftazidime, oral trimethoprim-sulfamethoxazole, and doxycycline. Upon follow-up after 4 weeks, the patient’s symptoms had completely subsided. A repeat transthoracic echocardiogram was done, which showed no evidence of vegetation. He was continued on oral medications for 3 months with doxycycline and trimethoprim-sulfamethoxazole.
Figure 1.
Multiple hypodense lesions in the liver and spleen are seen (arrows), in contrast enhanced computed tomography scan of abdomen.
Figure 2.
Computed tomography chest revealed consolidation of the right lower lobe (arrow) and mediastinal lymphadenopathy (arrowhead) along with mild bilateral pleural effusions.
Figure 3.
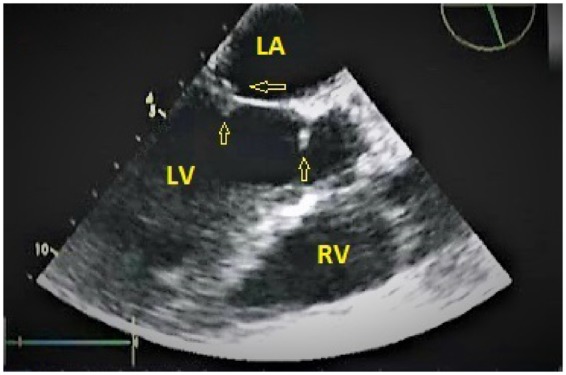
Transoesophageal echocardiogram showing vegetations attached (arrows) to tip and base of the anterior mitral leaflet. LA, left atrium; LV, left ventricle; RV, right ventricle.
Figure 4.
(A) Gram stain of positive blood culture broth, showing Gram-negative bacilli with bipolar staining (‘safety pin’ appearance). (B) MacConkey agar with non-fermenting, wrinkled colonies of Burkholderia pseudomallei with metallic sheen.
Discussion
Burkholderia pseudomallei is a Gram negative, motile, non-spore forming bacillus with bipolar staining, and safety pin appearance. Stanton and Fletcher in 1932 termed melioidosis from the Greek words melis (distemper of asses) and eidos (resemblance). Melioidosis is endemic in South-Eastern Asia and Northern Australia.1 The recognized risk factors for melioidosis are diabetes mellitus, alcohol intake, chronic liver disease, chronic kidney disease, immune-compromised status, malignancies, and trauma. The common modes of acquisition of infection are inhalation, ingestion, and inoculation.2
The clinical spectrum of melioidosis is extremely diverse, consisting of the acute fulminant septicaemic form, sub-acute illness, chronic infection, and subclinical disease. In the acute fulminant form, death can occur in 24–48 h from onset of symptoms.3 Most commonly it presents as community acquired pneumonia, with rapid progression of severity.4,5 The sub-acute form is characterized by prolonged febrile illness, with multi organ involvement, systemic abscess formation, and bacteraemia. The clinical manifestations can last for weeks to months or even years.6,7 Chronic melioidosis is the presentation in about 10% of the disease. It generally remains undiagnosed until activated by a traumatic event or found only at the time of postmortem examination of the tissues.8 Subclinical melioidosis is an infection with no symptoms, resulting in a chronic carrier state, which only becomes clinically apparent in immunocompromised patients.2
Clinically it can cause pneumonia and pleural effusion; skin and soft tissue infections like skin ulcers, subcutaneous abscess, cellulitis, and muscle abscess; genitourinary system infections like cystitis, pyelonephritis, prostatitis and prostatic abscess; and musculoskeletal system infections like septic arthritis and osteomyelitis. Abscess formation is common in organs like the spleen, liver, pancreas, kidney, prostate, genital organs, brain, parotid, and adrenal glands.2
Cardiac involvement is rare in melioidosis. Pericarditis is probably the most common of the cardiac manifestations, followed rarely by myocardial abscess9 and endocarditis.10 Pericardial effusion occurs in 1–3% of patients with melioidosis, mostly confined to the endemic regions.1,11 In the Darwin prospective melioidosis study from Australia, only 4 out of 540 (<1%) documented cases had pericarditis.12 The incidence of pericardial effusion was similar in other large studies.10,13 Apart from these, very few case reports of pericardial effusion have been reported worldwide.14–19 In most of the cases of pericarditis, the presentation was sub-acute to chronic, mimicking tuberculous pericardial effusion.20
Endocarditis in melioidosis is extremely rare. In one case report from India, a 60-year-old gentleman was admitted with complaints of upper abdominal pain and vomiting of 1 month duration. The contrast enhancing CT of the abdomen showed multiple hepatic, splenic, and pancreatic non-enhancing cystic lesions. The culture of CT-guided aspirate from the lesion involving the liver showed growth of B. pseudomallei. A few days after the admission, the patient developed massive infarct involving the middle cerebral artery. An echocardiogram was done, which revealed a vegetation on the aortic valve.21
Another case of melioid endocarditis was reported from Sri Lanka in a 73-year-old gentleman. He was a known chronic obstructive pulmonary disease patient, who presented clinically as community acquired pneumonia. On evaluation, he had multiple vegetations on the mitral and aortic valves, with blood cultures flagging B. Pseudomallei.22
Conclusion
Melioidosis causing the endocardial involvement is extremely rare. Usually the cardiac involvement manifests as pericarditis and pericardial effusion (1–3%). We report a case of native valve endocarditis with evidence of haematogenous dissemination in the form of multiple hepatic and splenic abscesses. We hope our case might remind the readers about such unusual presentation of this emerging disease.
Lead author biography

Hariharan Subramony is a senior consultant and is presently head of the Department of Internal Medicine, Apollo Hospitals, Greams Road, Chennai. He has been a practicing as a consultant for the last 25 years and is actively involved in the undergraduate and postgraduate clinical teaching.
Supplementary Material
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Cheng AC, Currie BJ.. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 2005;18:383–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee SH, Nathan S.. Burkholderia pseudomallei: an update on disease, virulence and host interaction. Malays Appl Biol 2013;42:1–14. [Google Scholar]
- 3. Ip M, Osterberg LG, Chau PY, Raffin TA.. Pulmonary melioidosis. Chest 1995;108:1420–1424. [DOI] [PubMed] [Google Scholar]
- 4. Nachiangmai N, Patamasucon P, Tipayamonthein B, Kongpon and Nakaviroj AS.. Pseudomonas pseudomallei in southern Thailand. Southeast Asian J Trop Med Public Health 1985;16:83–87. [PubMed] [Google Scholar]
- 5. Brett PJ, Woods DE.. Pathogenesis of and immunity to melioidosis. Acta Trop 2000;74:201–210. [DOI] [PubMed] [Google Scholar]
- 6. Leelarasamee A, Bovornkitti S.. Melioidosis: review and update. Rev Infect Dis 1989;11:413–425. [DOI] [PubMed] [Google Scholar]
- 7. Smith CJ, Allen JC, Embi MN, Othman O, Razak N, Ismail G.. Human melioidosis: an emerging medical problem. MIRCEN J 1987;3:343–366. [Google Scholar]
- 8. Weinberg AN, Heller HM.. Infectious Diseases of the Lung. New York, NY: Thieme-Stratton; 1997. p2413–2430. [Google Scholar]
- 9. Baumann BB, Morita ET.. Systemic melioidosis presenting as myocardial infarct. Ann Intern Med 1967;67:836–842. [DOI] [PubMed] [Google Scholar]
- 10. Punyagupta S. Melioidosis. Review of 686 cases and presentation of a new clinical classification. In: Punyagupta S, Sirisanthana T, Stapatayavong B, eds. Melioidosis. Bangkok: Bangkok Medical Publisher; 1989. p217–229. [Google Scholar]
- 11. Schultze D, Müller B, Bruderer T, Dollenmaier G, Riehm JM, Boggian K.. A traveller presenting with severe melioidosis complicated by a pericardial effusion: a case report. BMC Infect Dis 2012;12:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Currie BJ, Ward L, Cheng AC.. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis 2010;4:e900.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Puthucheary SD, Parasakthi N, Lee MK.. Septicaemic melioidosis: a review of 50 cases from Malaysia. Trans R Soc Trop Med Hyg 1992;86:683–685. [DOI] [PubMed] [Google Scholar]
- 14. Ruff MJ, Lamkin N Jr, Braun J, Barnwell P.. Melioidosis complicated by pericarditis. Chest 1976;69:227–229. [DOI] [PubMed] [Google Scholar]
- 15. Majid AA. Successful surgical management of a case of pulmonary and pericardial melioidosis. Aust N Z J Surg 1990;60:139–141. [PubMed] [Google Scholar]
- 16. Lim KB, Oh HM.. Melioidosis complicated by pericarditis. Scand J Infect Dis 2007;39:357–359. [DOI] [PubMed] [Google Scholar]
- 17. Vidyalakshmi K, Chakrapani M, Shrikala B, Damodar S, Lipika S, Vishal S.. Tuberculosis mimicked by melioidosis. Int J Tuberc Lung Dis 2008;12:1209–1215. [PubMed] [Google Scholar]
- 18. Chung H-C, Liang S-H, Lai C-H, Lin J-N, Lee C-T, Lin H-H, Huang C-K.. Non-septicemic melioidosis presenting as cardiac tamponade. Am J Trop Med Hyg 2008;79:455–457. [PubMed] [Google Scholar]
- 19. De Keulenaer BL, Van Hooland SR, Damodaran PR, Currie BJ, Powell BP, Jenkins IR.. Burkholderia pseudomallei sepsis presenting with pericardial effusion and tamponade. Crit Care Resusc 2008;10:137–139. [PubMed] [Google Scholar]
- 20. Chetchotisakd P, Anunnatsiri S, Kiatchoosakun S, Kularbkaew C.. Melioidosis pericarditis mimicking tuberculous pericarditis. Clin Infect Dis 2010;51:46–49. [DOI] [PubMed] [Google Scholar]
- 21. Abdulla MC, Alungal J.. Melioidosis with endocarditis and massive cerebral infarct. Ital J Med 2015;10:55–57. [Google Scholar]
- 22. Piyasiri LB, Wickramasinghe SA, Lekamvasam VC, Corea EM, Gunarathne R, Priyadarshana U.. Endocarditis in melioidosis. Ceylon Med J 2016;61:192–193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.