Abstract
Background
Type 1 myotonic dystrophy (DM1) is associated with a variety of cardiac conduction abnormalities and the frequent need for permanent pacing. However, the role of ventricular tachycardia (VT) and the implied risk of sudden cardiac death (SCD) is poorly understood.
Case summary
This study examined a 56-patient DM1 cohort of men and women, and identified five patients (two females and three males) with ventricular arrhythmias (8.9%). Patients were reviewed on a case-by-case basis, with their clinical presentation and management of VT and the associated cardiomyopathy indicated. Patient cardiac function was determined by 12-lead electrocardiogram, 48-h Holter monitor, and transthoracic echocardiography. These patients were therefore suitable candidates for implantable cardioverter-defibrillator implantation and received these devices; four of the five patients also received cardiac resynchronization therapy. Medical therapies included angiotensin converting enzyme inhibition, mineralocorticoid receptor antagonist, and following device implantation, beta-blocker therapy was initiated.
Discussion
Our case series demonstrates the prevalence of VT in patients with DM1 highlighting the associated risks of SCD in this patient population. The burden of ventricular arrhythmias, advanced conduction disease, and cardiomyopathy are best treated with a combination of device and medical therapies.
Keywords: Myotonic dystrophy, Cardiomyopathy, Conduction disease, Arrhythmia, Ventricular tachycardia , Sudden cardiac death, Case series
Learning points
Ventricular tachycardia is an important arrhythmia in patients with type 1 myotonic dystrophy.
Ventricular arrhythmias can account for the increased risk of sudden cardiac death in these patients.
Appropriate use of device therapy coupled with effective pharmacological therapies is important interventions.
Introduction
Myotonic dystrophy is the most common form of muscular dystrophy in adults and is characterized by cardiac conduction abnormalities with various other comorbidities.1–3 Type 1 myotonic dystrophy (DM1), also known as Steinert’s disease, is inherited through an autosomal dominant pattern, presenting with myotonia and distal muscle weakening. DM1 is associated with conduction abnormalities and left ventricular (LV) dysfunction at a greater frequency and prevalence than type 2 myotonic dystrophy.4 We describe the presentation and outcome of malignant ventricular arrhythmias in a cohort of patients with DM1 as a basis for the increased risk of sudden cardiac death (SCD) in this vulnerable patient population.5
Timeline
| Initial clinical presentation |
|
| Presentation of ventricular tachycardia (VT) |
|
| Interventions |
|
Case presentation
Case 1
A 44-year-old female DM1 patient, with a mild dilated cardiomyopathy (DCM), a left bundle branch block (LBBB), and associated LV systolic dysfunction had a dual-chamber pacemaker implanted for secondary prophylaxis (Table 1). This patient had an episode of syncope witnessed by her husband who immediately performed cardiopulmonary resuscitation; she regained consciousness during chest compressions. Subsequent device interrogation demonstrated sustained, rapid, and polymorphic ventricular tachycardia (VT) at the time of her event (Figure 1A). Physical examination showed a blood pressure of 101/54 mmHg, and a heart rate of 67 b.p.m. She had normal heart sounds, with no S3 or S4, or murmurs; jugular venous pressure (JVP) was not elevated. The patient was independently ambulatory, though reported occasional falls and chronic fatigue. She complained of myotonia in the hands and feet as well as frequent leg cramps. She had distal muscle weakness with myotonia. An echocardiogram indicated global hypokinesis, mild ventricular dilation, and a mild reduction in LV systolic function (Table 1). Her device was upgraded to a cardiac resynchronization therapy-defibrillator (CRT-D) device and medical therapy was expanded to include metoprolol 25 mg twice-daily (b.i.d.) and spironolactone 25 mg once-daily (q.d.), in addition to her previous medical therapy: mexiletine 200 mg three times daily and perindopril 2 mg b.i.d. In her follow-up visits, 1 week and 11 months following her device upgrade, her condition was stable and device interrogation showed no recurrent VT.
Table 1.
Clinical characteristics of patients with type 1 myotonic dystrophy presenting with ventricular tachycardia
| Patient | Age (years)/ gender | HR (b.p.m.) | ECG findings (ms) | Echocardiographic parameters | VT detection | Presentation | Medications |
|---|---|---|---|---|---|---|---|
| 1 | 44/F | 82 |
|
|
CRT-P | Cardiac Arrest |
|
| 2 | 36/F | 69 |
|
|
DC-PPM | Asymptomatic |
|
| 3 | 49/M | 86 |
|
|
Tele-monitoring | Syncope |
|
| 4 | 61/M | 64 |
|
|
DC-PPM | Palpitations |
|
| 5 | 40/M | 71 |
|
|
CRT-D | ICD shock |
|
Refer to Methods section for how values were obtained or calculated.
ASA, acetylsalicylic acid; CRT-D, cardiac resynchronization therapy defibrillator; CRT-P, cardiac resynchronization therapy pacemaker; DC-PPM, dual-chamber permanent pacemaker; ECG, electrocardiogram; LBBB, left bundle branch block; LV, left ventricle; LVEF, left ventricular ejection fraction; LVIDd, left ventricle internal diameter end diastole; LVIDs, left ventricle internal diameter end systole; VT, ventricular tachycardia.
Figure 1.
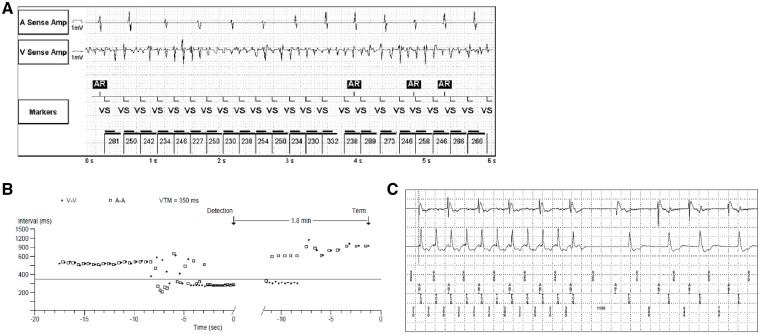
Rapid polymorphic ventricular tachycardia resulting in cardiac arrest as illustrated by the electrocardiogram (A), as described for Patient 1. R-R Interval histogram (B) and electrocardiogram (C) illustrating monomorphic ventricular tachycardia detected during device interrogation of a dual-chamber permanent pacemaker as described for Patient 4.
Case 2
A 36-year-old female DMI patient with a dual-chamber pacemaker for primary prophylaxis was diagnosed with sustained VT upon device interrogation during routine follow-up. The VT recorded was at an average rate of 147 b.p.m. and spontaneously terminated after 32 s. Previous device interrogations had shown five episodes of non-sustained VT up to 26 s in total. The patient was asymptomatic for all recorded episodes. Physical examination showed a blood pressure of 119/55 mmHg, and a heart rate of 60 b.p.m. She had normal heart sounds, with no S3 or S4, or murmurs; JVP was not elevated. The patient was reliant on a cane and walker for ambulation. She reported fatigue, myotonia in her hands, and difficulty swallowing. Her 12-lead electrocardiogram (ECG) demonstrated an atrial-paced rhythm and LBBB (Table 1). An echocardiogram showed mild LV systolic dysfunction (Table 1). Her device was upgraded to a CRT-D device. Her medical therapy, which included ramipril 5 mg q.d., furosemide 40 mg q.d., and spironolactone 25 mg q.d., was expanded to include metoprolol 12.5 mg b.i.d. At her 1-month follow-up visit, she reported that she stopped taking metoprolol. Device interrogation showed the delivery of two shocks for rapid atrial fibrillation with rapid ventricular response. The patient was encouraged to continue taking metoprolol. In her follow-up visits, 2 weeks and 4 months following her device upgrade, she has been compliant with her medications. Her condition was stable and device interrogation showed no recurrent VT.
Case 3
A 49-year-old male DM1 patient was admitted to the cardiology ward following two episodes of syncope. Electrocardiographic tele-monitoring detected asymptomatic episodes of sustained VT. Physical examination showed a blood pressure of 112/74 mmHg, and a heart rate of 82 b.p.m. He had normal heart sounds, with no S3 or S4, or murmurs; JVP was not elevated. The patient was independently ambulatory. He reported fatigue, weakness in the hands, and dysphagia. He had distal weakness and facial myotonia. The patient received an echocardiogram which showed global hypokinesis of the LV with an LVEF of 45% (Table 1). This patient also received a cardiac magnetic resonance imaging (MRI) study with gadolinium contrast showing normal biventricular volumes and atrial sizes, no hypertrophy, and a LVEF of 45%. The absence of late gadolinium enhancement (LGE) indicates that there was no myocardial scarring. Following VT detection, the patient received an MRI compatible dual-chamber implantable cardiac defibrillator (ICD). He was also newly diagnosed with type 1 diabetes. The patient was prescribed acetylsalicylic acid 81 mg q.d., ramipril 2.5 mg q.d., metoprolol 25 mg b.i.d., rosuvastatin 10 mg q.d., and metformin and insulin. He has had subsequent appropriate ICD shocks and his ICD therapy was adjusted to optimize-anti-tachycardic pacing (ATP)-termination and to minimize ICD shocks, and his dose of metoprolol, was increased to 50 mg b.i.d. At his 6-month follow-up visit, device interrogation showed 45 incidents of VT or ventricular fibrillation at rates of 170–200 b.p.m. He received 53 episodes of ATP and 3 ICD shocks were delivered. In his most recent follow-up visit, at 11 months, there were seven incidents which required two to three ATP and four shocks had to be delivered for four separate VT events detected at 200 b.p.m. each. Given his recurrent VT, metoprolol was uptitrated to 75 mg b.i.d.
Case 4
A 61-year-old male DM1 patient with a history of symptomatic bradycardia, and a dual-chamber pacemaker for secondary prophylaxis, was diagnosed with recurrent asymptomatic VT. ECG findings for this patient included first-degree atrioventricular (AV) block and LBBB (Table 1). A 48-h Holter monitor showed an atrial-paced rhythm and a 1% burden of premature ventricular contractions. During his regular pacemaker clinic visit, device interrogation revealed one episode of rapid VT at an average rate of 186 b.p.m., lasting 1 min and 49 s (Figure 1B). Physical examination showed a blood pressure of 107/69 mmHg, and a heart rate of 85 b.p.m. He had normal heart sounds, with no S3 or S4, or murmurs; JVP was not elevated. The patient was wheelchair-dependent. He showed distal myotonia, weakness, and muscle wasting. His echocardiogram showed mild global hypokinesis of the LV with an LVEF of 45%. A cardiac MRI study with gadolinium contrast showed normal biventricular volumes and atrial sizes, no hypertrophy, and a LVEF of 40%. The absence of LGE indicates that there was no myocardial scarring. His device was upgraded to a CRT-D device. Prior to the event, his medical therapy included candesartan 12 mg q.d., atorvastatin 10 mg q.d., and spironolactone 25 mg b.i.d. Bisoprolol 2.5 mg q.d. was initiated prior to hospital discharge. In his follow-up visits, 1 week and 2 months following his device upgrade, his condition was stable and device interrogation showed no recurrent VT.
Case 5
A 40-year old male diagnosed with DM1, permanent atrial fibrillation, and a DCM with an ejection fraction of 30–35% on echocardiography, received a single-chamber defibrillator for primary prophylaxis. Two years later, as a result of progressive AV block and predominantly RV pacing, he received a CRT-D device. In the following year, he had two separate episodes of VT, one of which resulted in an ICD shock. Physical examination showed a blood pressure of 109/70 mmHg, and a heart rate of 63 b.p.m. He had normal heart sounds, with no S3 or S4. A soft systolic murmur was detected at the apex. JVP was not elevated and he had mild peripheral oedema. The patient was independently ambulatory. He reported progressive difficulty in maintaining balance as well as dysphagia. He showed distal muscle weakness and mild myotonia. An echocardiogram showed moderate LV chamber dilation and global hypokinesis of the LV with a LVEF of 38% (Table 1). His initial medical therapy included warfarin 2 mg q.d., ramipril 2.5 mg q.d., and digoxin 0.125 mg q.d.; perindopril 2 mg q.d. and bisoprolol 7.5 mg q.d. were added prior to discharge. Given recurrent ventricular arrhythmias in this patient, he was initiated on amiodarone therapy at a dose of 200 mg q.d. In his most recent follow-up visit, 3.5 years following initiation of amiodarone therapy, device interrogation showed no recurrent VT.
Discussion
Our cohort is comprised of patients with a high burden of arrhythmias and conduction abnormalities including AV block, left anterior fascicular block, and LBBB; with a high prevalence of cardiomyopathy, and all five patients having reduced ejection fraction. However, four of the five patients in this study had mild cardiomyopathies with ejection fractions well above 35%, the recommended level for primary prophylaxis ICD insertion.6 These results demonstrate that in patients with DM1, adverse cardiac electrical remodelling occurs prior to the progression to advanced cardiomyopathy. LV systolic dysfunction is likely primarily attributed to the LBBB and the associated electromechanical dysynchrony (Table 1).7,8 Cardiac MRI is useful to define the biventricular remodelling and dysfunction, and the location and degree of myocardial fibrosis in this patient population. Two of our patients who received cardiac MRI with gadolinium contrast did not show myocardial scarring (absence of LGE). The overall cohort of 56 DM1 patients had relatively preserved ejection fractions with a much lower prevalence of conduction abnormalities (Supplementary material online, Table S1).
Pacemaker device use in four of our five patients, prior to the detection of VT, were inserted in response to conduction disease and bradycardia associated with DM1, in accordance with clinical practice guidelines.6 The development of VT is of considerable concern in DM1 patients, as demonstrated by our case series. One of the five patients in this study experienced cardiac arrest requiring resuscitation. Implantable cardioverter-defibrillator and appropriate medical therapy are crucial to prevent SCD in these patients. The implantation of pacemakers or ICD is particularly challenging in patients with myotonic dystrophy given the low skeletal muscle mass and respiratory involvement. Four of our five patients had a successful upgrade of their pacemakers to a CRT-D device without complications. A recent study tracking 1388 patients over a 12-year period reported an incidence of 2.3% (26 of 1388 patients) of sustained VT in patients with DM1.3 The much higher prevalence of VT in our case series, at an incidence of 8.9% over four years (5 out of 56 patients) suggest that our cohort of contemporary DM1 patients had more advanced heart disease. As such, while it is important that we recognize the severe conduction abnormality, the potential of VT leading to SCD must be appropriately investigated and managed in patients with DM1. An electrophysiological study may be helpful to evaluate and direct the treatment of inducible ventricular arrhythmias and may play an important role in risk stratification of DM1 patients.9,10 Although our cohort size is modest, we continue to recruit myotonic dystrophy patients in our clinic, thereby expanding the size of our DM1 patient cohort.
Conclusions
Conduction system disease with progression to complete AV block is a well-recognized complication of several neuromuscular disorders, including myotonic dystrophy. The presence of conduction disease characterized by first-degree AV block and underlying LBBB suggest a key pathogenic role of electrical dysynchrony in mediating the cardiomyopathy associated with DM1.11 In patients with DM1, implantation of a permanent pacemaker is recommended even in asymptomatic patients with an abnormal resting ECG or with HV interval prolongation during electrophysiological study.12 Cardiac resynchronization therapy allows for complete pharmacological therapy use, namely the use of beta-blockers, thereby allowing both anti-arrhythmic effects and therapeutic responses with the concomitant LBBB and cardiomyopathy. Given the inherent risk of SCD in this vulnerable patient population, it is crucial that physicians recognize the risk of ventricular arrhythmias in DM1 patients and consider the use of medical therapy and device intervention in a timely manner.3
Lead author biography

Anish Nikhanj is currently a PhD candidate at the University of Alberta in Canada. His research is focused on understanding heart disease in patients with muscular dystrophies. As a proponent of translational medicine, Anish maintains the objective of using his research to augment the patient care process and improve patient outcomes.
Supplementary Material
Acknowledgements
We acknowledge the Neuromuscular Multidisciplinary (NMMD) clinic and the patients and the families at the Kaye Edmonton Clinic, University of Alberta.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Gagnon C, Chouinard MC, Laberge L, Veillette S, Begin P, Breton R, Jean S, Brisson D, Gaudet D, Mathieu J.. Health supervision and anticipatory guidance in adult myotonic dystrophy type 1. Neuromuscul Disord 2010;20:847–851. [DOI] [PubMed] [Google Scholar]
- 2. Wahbi K, Meune C, Porcher R, Becane HM, Lazarus A, Laforet P, Stojkovic T, Behin A, Radvanyi-Hoffmann H, Eymard B, Duboc D.. Electrophysiological study with prophylactic pacing and survival in adults with myotonic dystrophy and conduction system disease. JAMA 2012;307:1292–1301. [DOI] [PubMed] [Google Scholar]
- 3. Wahbi K, Babuty D, Probst V, Wissocque L, Labombarda F, Porcher R, Becane HM, Lazarus A, Behin A, Laforet P, Stojkovic T, Clementy N, Dussauge AP, Gourraud JB, Pereon Y, Lacour A, Chapon F, Milliez P, Klug D, Eymard B, Duboc D.. Incidence and predictors of sudden death, major conduction defects and sustained ventricular tachyarrhythmias in 1388 patients with myotonic dystrophy type 1. Eur Heart J 2017;38:751–758. [DOI] [PubMed] [Google Scholar]
- 4. Tanawuttiwat T, Wagner KR, Tomaselli G, Nazarian S.. Left ventricular dysfunction and conduction disturbances in patients with myotonic muscular dystrophy type i and ii. JAMA Cardiol 2017;2:225–228. [DOI] [PubMed] [Google Scholar]
- 5. Groh WJ, Groh MR, Saha C, Kincaid JC, Simmons Z, Ciafaloni E, Pourmand R, Otten RF, Bhakta D, Nair GV, Marashdeh MM, Zipes DP, Pascuzzi RM.. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med 2008;358:2688–2697. [DOI] [PubMed] [Google Scholar]
- 6. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO; American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, Heart Rhythm Society. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2013;127:e283–352. [DOI] [PubMed] [Google Scholar]
- 7. McNally EM, Sparano D.. Mechanisms and management of the heart in myotonic dystrophy. Heart (British Cardiac Society) 2011;97:1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Auffret V, Martins RP, Daubert C, Leclercq C, Le Breton H, Mabo P, Donal E.. Idiopathic/iatrogenic left bundle branch block–induced reversible left ventricle dysfunction. J Am Coll Cardiol. 2018;72:3177–3188. [DOI] [PubMed] [Google Scholar]
- 9. Ezri M, Lerman BB, Marchlinski FE, Buxton AE, Josephson ME.. Electrophysiologic evaluation of syncope in patients with bifascicular block. Am Heart J 1983;106:693–697. [DOI] [PubMed] [Google Scholar]
- 10. Twidale N, Heddle WF, Ayres BF, Tonkin AM.. Clinical implications of electrophysiology study findings in patients with chronic bifascicular block and syncope. Aust N Z J Med 1988;18:841–847. [DOI] [PubMed] [Google Scholar]
- 11. Kirk JA, Kass DA.. Cellular and molecular aspects of dyssynchrony and resynchronization. Heart Fail Clin 2017;13:29–41. [DOI] [PubMed] [Google Scholar]
- 12. Lazarus A, Varin J, Babuty D, Anselme F, Coste J, Duboc D.. Long-term follow-up of arrhythmias in patients with myotonic dystrophy treated by pacing: a multicenter diagnostic pacemaker study. J Am Coll Cardiol 2002;40:1645–1652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.