Abstract
Background:
The aim of this study was to evaluate the effects of alumina nanowires as reinforcement phases in polyhydroxybutyrate-chitosan (PHB-CTS) scaffolds to apply in cartilage tissue engineering.
Methods:
A certain proportion of polymers and alumina was chosen. After optimization of electrospun parameters, PHB, PHB-CTS, and PHB-CTS/3% Al2O3 nanocomposite scaffolds were randomly electrospun. Scanning electron microscopy, Fourier transform infrared spectroscopy, water contact angle measurement, tensile strength, and chondrocyte cell culture studies were used to evaluate the physical, mechanical, and biological properties of the scaffolds.
Results:
The average fiber diameter of scaffolds was 300–550 nm and the porosity percentages for the first layer of all types of scaffolds were more than 81%. Scaffolds' hydrophilicity was increased by adding alumina and CTS. The tensile strength of scaffolds decreased by adding CTS and increased up to more than 10 folds after adding alumina. Chondrocyte viability and proliferation on scaffolds were better after adding CTS and alumina to PHB.
Conclusion:
With regard to the results, electrospun PHB-CTS/3% Al2O3 scaffold has the appropriate potential to apply in cartilage tissue engineering.
Keywords: Alumina nanowires, cartilage tissue engineering, chitosan, electrospinning, polyhydroxybutyrate
Introduction
Trauma and disease have important roles in damaging or losing the ability of cartilage. One of the attractive areas for the reproduction of cartilage tissue is tissue engineering.[1] Tissue engineering is a combination of medical science and engineering that is aimed at restoring, improving, or maintaining the biological function of damaged tissues. Scaffold, cells, and the environmental factors are the three key points; proper interaction between which can lead to an appropriate biological function. Since scaffolds in tissue engineering have a mimicking extracellular matrix (ECM) role, methods of scaffolds preparation as well as selecting biomaterials are of great importance. Tissue engineering scaffolds should have appropriate biocompatible and biodegradable properties. Furthermore, their biodegradation products should be nontoxic. In addition, since cartilages are mostly under load, these scaffolds should also have appropriate mechanical and physical properties. Today, because of its ability to mimic the ECM, the electrospinning method is used to design tissue engineering scaffolds. In addition to economic considerations and its simplicity, the most important feature of this technique is the high level of cells attaching and rapid transfer of food and metabolic products from the scaffolds.[1,2] Studies have shown that each polymer has some drawbacks, in addition to its merits, which limit the application of polymers in pure form and thus composites are introduced. For example, although polyhydroxybutyrate (PHB) has positive features such as biocompatibility, high mechanical properties, and nontoxic degradation products, the hydrophobic nature, low degradation, and brittleness of the PHB limit its sole application in tissue engineering.[2] It seems that using a natural and hydrophilic polymer such as chitosan (CTS), undesirable properties of PHB can be overcome. Hydrophilicity was increased after adding CTS to PHB.[3] In addition, intrinsic brittleness of PHB-CTS was improved, and the cell response was better.[3] However, some reports also show that mechanical strength is reduced after adding CTS to PHB.[4,5] One of the best methods to improve the mechanical properties of polymers is to make composites of them using ceramics as reinforcement phases.[4,5,6] One-dimensional nanostructures (nanowires, nanowhiskers, and nanotubes) have shown to be the most effective in enhancing mechanical properties due to their interdiffusion and entanglement in host polymer.[7] One of the most merits of electrospinning methods is the capacity of aligning one-dimensional nanostructures in the fibers.[8,9] In addition to improving the physical and mechanical properties, these nanostructures can also enhance the cell response.[4,5,6] Alumina, as the first bioceramics, can be a proper choice due to its low cost, good compatibility, and high modulus. Alumina nanostructures have shown a significant increase in mechanical properties of poly (ε-caprolactone) and polymethylmethacrylate (PMMA).[7,10] The effects of alumina in different dimensions to enhance mechanical properties of composites based on PMMA were compared, and it was reported that one-dimensional reinforcements (such as whiskers and fibers) can be more effective compared to spherical nanoparticles of alumina.[7] There are also reports of the positive effects of alumina nanostructures on differentiation, growth, and proliferation of mesenchymal stem cells.[11,12] From alumina nanowires, no genotoxic, cytotoxic, and nuclear damage were observed in fibroblastic studies.[13]
In this work, PHB, PHB-CTS, and PHB-CTS scaffolds reinforced with alumina nanowires and are fabricated using electrospinning method. The surface morphology, hydrophilicity, and mechanical properties are evaluated. For rabbit chondrocytes cells, cell viability and cell attachment were assessed and compared on PHB, PHB-CTS, and PHB-CTS/3% Al2O3 composite scaffolds.
Methods
PHB (Mw = 300,000 g/mol), CTS (Mw = 1526.454 g/mol, deacetylation degree = 75%–85%), and alumina nanowires (200–400 nm in length and 2–4 nm in diameters) were purchased from Sigma Aldrich (USA). Trifluoroacetic acid (TFA = CF3COOH, Mw = 169.87 g/mol, density = 1.49 g/ml, and purity percent = 99%) was purchased from Merck (Germany). In the cell studies, the rabbit chondrocyte cells (Pasteur Institute/Iran), culture media (F12, GIBCO/USA), and MTT powder (Sigma Aldrich/USA) were used.
Preparation of electrospun scaffolds
As proposed by Sadeghi et al.,[3] 20% CTS was added to a 9% wt solution of PHB.[3] To prevent the agglomeration, Al2O3 nanowires suspension (3% wt of alumina nanowires in 0.2 cc of TFA) was sonicated for 12 s. PHB, PHB-CTS, and PHB-CTS/3% Al2O3 random nanofibers were electrospun. The optimum electrospinning parameters were 25 cm as the distance between the collecting plate and the tip of the needle, and 0.01 mL/min as the flow rate and 22 kV as the high voltage were applied.
Scanning electron microscopy evaluations
The morphologies of the fibers are assessed using scanning electron microscopy (SEM, SERONTECHNOLOGIES, AIS2100/South Korea) at ×10,000 magnifications. The mean of the fiber diameters and the porosity is measured through Image J (Wayne Rasband, National Institute of Health/USA) and MATLAB (R2016a) software, respectively.
Fourier-transform infrared analyses
Attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR) (Bruker, Tensor 27/Germany) was done to evaluate chemical bonds in electrospun scaffolds.
Water contact angle
The angle of double-deionized water drop is recorded after 10 s using contact angle meter (CA-ES10, Fars EOR/Iran) to determine the hydrophilicity of the scaffolds.
Analysis of mechanical properties of scaffolds
Mechanical properties of scaffolds were analyzed by a tensile device (INSTRON-5566/USA) in accordance with DIN EN ISO 05/1995. Scaffolds were cut in 3 cm × 0.5 cm, and the test was done in triplicate for each specimen. The load cell was adjusted in 20 (N), the extension rate was kept at 1 mm/min, and the distance between the two jaws was 20 mm.
Cell behavior assay
PHB, PHB-CTS, and PHB-CTS/3% Al2O3 composite scaffolds were cut into circular sheets and sterilized through PBS (for 15 min), ethanol (for 15 min), and ultraviolet light (for 60 min) before cell culture; then, they were placed on 24-well plates. The chondrocyte cells were seeded with the density 5 × 103 on each scaffold and incubated at 37°C, and their medium was refreshed every other day. The morphology and adhesion of rabbit chondrocyte cells were assessed by SEM on days 1 and 7 after culturing. MTT assay determined the viable cell count on days 1, 3, and 7 according to ISO-10993-5 standard.
Statistical analysis
All experiments were performed in at least three replications. The results were expressed as the means ± standard error. The value of *P < 0.05 was considered to be statistically significant. The one-way ANOVA analysis was applied to compare the samples.
Results
Fibers' morphology evaluation
SEM images are shown in Figure 1. The average fiber diameters and their porosity percentage are tabulated in Table 1. All of the scaffolds were fully porous, uniform, and bead free. The fiber diameters of scaffolds were increased by increasing CTS and alumina to PHB. More than 80% porosities for the first layer, nearly 50% for the second layer, and 20% for the third layer were evaluated through MATLAB software in all three types of scaffolds.
Figure 1.
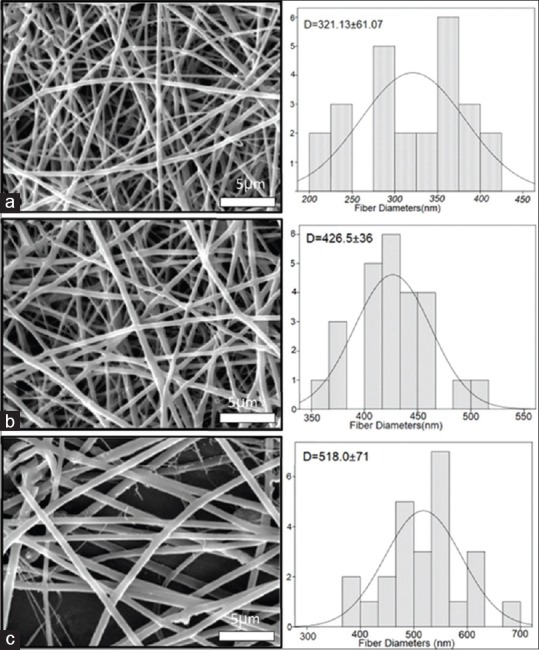
Scanning electron microscopy images and histograms illustrating the diameter distribution of (a) polyhydroxybutyrate, (b) polyhydroxybutyrate-chitosan, and (c) polyhydroxybutyrate-chitosan/3% Al2O3
Table 1.
The average fiber diameters of the scaffolds, the porosity percentage, water contact angle, and tensile strength for polyhydroxybutyrate, polyhydroxybutyrate-chitosan, and polyhydroxybutyrate-chitosan/3% Al2O3
| Samples | Average fiber diameters (nm) | Porosity percentage | Water contact angle | Tensile strength (MPa) | ||
|---|---|---|---|---|---|---|
| First layer | Second layer | Third layer | ||||
| PHB | 321.1±61 | 81.70 | 51.71 | 20.14 | 80.5±2.0 | 2.81±0.15 |
| PHB-CTS | 426.5±36 | 81.51 | 46.61 | 22.09 | 54.3±0.9 | 0.89±0.26 |
| PHB-CTS/3% Al2O3 | 518.0±71 | 81.38 | 48.11 | 25.95 | 31.1±0.7 | 11.18±1.24 |
PHB-CTS – Polyhydroxybutyrate-chitosan
Fourier transform infrared spectrometry
FTIR-ATR spectrum of PHB, PHB-CTS, and PHB-CTS/3% Al2O3 is shown in Figure 2a-c, respectively. The characteristic band of PHB at 1720 cm−1, related to stretching absorption of carbonyl group (υC = O), was observed.[14] In addition, peaks at 980, 1227, and 1276 cm−1, corresponding to crystalline phases of PHB and 1184 cm−1, were attributed to the amorphous phase of PHB.[15] The carboxyl group was also absorbed from 3041 to 2794 cm−1, and the hydroxyl group was absorbed at 3433 cm−1.[16] After adding CTS to PHB, a small shoulder appeared at 1670 cm−1 (near 1724 cm−1), which was related to amide I group in CTS.[17] After adding alumina to PHB-CTS, the peak at 1720 cm−1 was sharper. Furthermore, it was noteworthy that the intensity of absorption spectroscopy in the carboxylic peaks of 3041-2704 cm−1 decreased after adding CTS and alumina nanowires to PHB.
Figure 2.
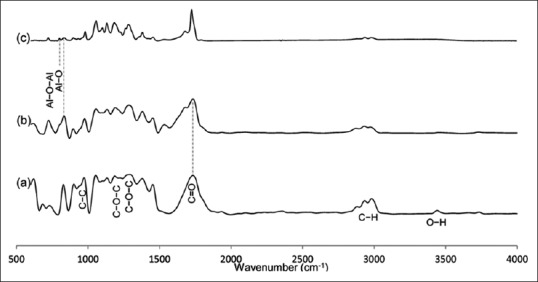
Fourier transform infrared spectroscopy attenuated total reflection spectrum of (a) polyhydroxybutyrate, (b) polyhydroxybutyrate-chitosan, and (c) polyhydroxybutyrate-chitosan/3% Al2O3 scaffolds
Hydrophilicity
The water contact angle for PHB scaffold was measured at 80.5° ± 2.0°. The hydrophilicity of PHB scaffolds significantly increased after adding the CTS and alumina (P < 0.05), and the results are tabulated in Table 1.
Mechanical properties
The addition of CTS to PHB led to a reduction in tensile strength from 2.81 ± 0.15 MPa to 0.89 ± 0.26 MPa significantly (P < 0.05). After adding alumina, the tensile strength for PHB-CTS/3% Al2O3 was enhanced more than 10 folds, that is, 11.18 ± 1.24 MPa (P < 0.05), and the results are tabulated in Table 1.
Cell studies
To assess the adhesion and proliferation of chondrocyte cells, SEM images on day 1 and on day 7 were taken from PHB, PHB-CTS, and PHB-CTS/3% Al2O3 scaffolds [Figure 3], and cell viability of PHB, PHB-CTS, and PHB-CTS/3% Al2O3 is shown in Figure 4. The minimum of chondrocyte cell viability was observed for PHB scaffolds.
Figure 3.
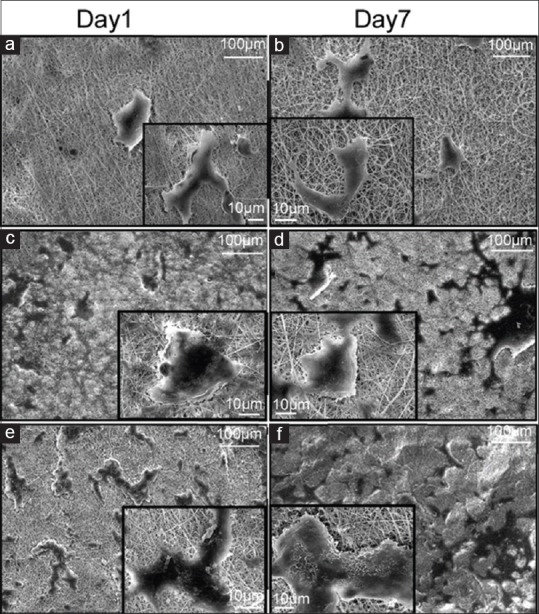
Scanning electron microscopy images of chondrocyte cells seeded on electrospun (a and b) polyhydroxybutyrate, (c and d) polyhydroxybutyrate-chitosan, and (e and f) polyhydroxybutyrate-chitosan/3% Al2O3 nanowires, left column at day 1 and right column on day 7
Figure 4.
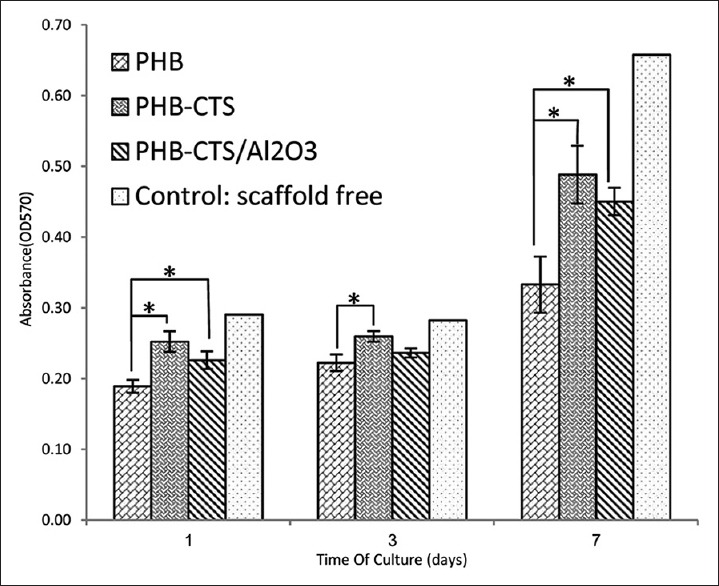
Cell viability as indicated by MTT assay of chondrocyte cells seeded on electrospun polyhydroxybutyrate, polyhydroxybutyrate-chitosan, and polyhydroxybutyrate-chitosan/3% Al2O3 nanowires in days 1, 3, and 7 (*P < 0.05)
Discussion
After adding CTS and Al2O3, scaffolds had appropriate fiber diameters and porosity percentages as the initial requirement in tissue engineering. Porosity percentages in three layers also showed an appropriate interconnectivity in the scaffolds.[18] The fiber diameters of scaffolds were increased by increasing CTS to PHB. Formation of hydrogen bond between CTS and PHB is responsible for it. This increase was also observed after CTS was added to PHB and added to PLA.[6,14] The same trend was observed after adding alumina to PHB-CTS, which can result from some existing interactions between alumina and polymers. Formation of polar bonds and hydrogen bonding between alumina nanoparticles and polyesters was reported by Rodríguez-Lorenzo et al.[19] In a similar study, after adding alumina whiskers in polycaprolactone (PCL), the same trend followed about average fiber diameters.[10]
There are some evidence of the formation of hydrogen bonding between PHB and CTS, and alumina nanowires can be seen from the reduction in the absorption intensity of carboxylic groups in FTIR [Figure 2]. The change from 1720 cm−1 to 1724 cm−1 can also explain by miscibility and distribution of crystalline portion, which resulted from appropriate interactions of two polymers.[17] Adding alumina to the PHB-CTS was confirmed by observing two closed peaks at 806 cm−1 and 820 cm−1 in Figure 2c. The peak at 820 cm−1 was related to PHB-CTS, while 806 cm−1 was attributed to alumina nanowires.[20]
Surface hydrophilicity assessment is very important in the cellular behavior of scaffolds. PHB scaffolds, because of their low hydrophilic nature, present the major contact angle among specimens. The hydrophilic nature of CTS[21] and alumina[22] leads to a decrease in the water contact angle. Furthermore, the effect of hydrogen bonds, which formed after adding CTS and Al2O3 to PHB confirmed by FTIR, should not be ignored.
After adding CTS to PHB, the tensile strength was reduced, resulting from the CTS amorphous nature. This decrease was observed by other researchers.[4] Increase in the tensile strength of PHB-CTS/3% Al2O3 nanocomposite scaffold was observed, and the high intrinsic strength of alumina was attributed to it. The PCL electrospun fibers also showed a significant increase in tensile strength after adding alumina nanowhiskers to them as the reinforcement phase.[10] It was reported that elastic modulus of healthy cartilage in regions with meniscus cover is in the range from 2.13 ± 0.74 to 5.13 ± 1.91 MPa.[23] Thus, alumina, as reinforcement phase, can be promising materials to enhance mechanical properties of scaffolds in tissue engineering.
For cell assessments, as it can be observed, the cell morphology still in round shape on the PHB scaffold which is attributed to low hydrophilicity of PHB reported by other researchers.[16] However, the cells spread on PHB-CTS scaffolds and PHB-CTS/3% Al2O3 scaffolds by their pseudopodia. The hydrophilic natures of CTS and alumina were attributed to it. Adding natural polymers such as gelatin and CTS to PHB can contribute to more cell growth and proliferation.[3,24] The PHB-CTS scaffolds reinforced with alumina nanowires and maintained the appropriate cell morphology and proliferation.[10] The results are confirmed by MTT. The minimum of chondrocyte cell viability was observed for PHB scaffolds, while PHB-CTS and PHB-CTS/3% Al2O3 had higher amounts (P < 0.05). Although cell viability on PHB-CTS scaffold was higher than PHB-CTS/3% Al2O3, this difference is not statistically significant (P < 0.05).
Conclusions
Alumna nanowires were added to PHB-CTS solution and were randomly electrospun. Adding CTS and alumina nanowires has no undesirable effects on the fiber diameters and porosities. The hydrophilicity of scaffolds was enhanced after adding CTS and alumina to PHB. The presence of alumina nanowires significantly increased the tensile strength of PHB and PHB-CTS scaffolds. The results of cell studies showed that the chondrocyte cells spread on PHB-CTS and PHB-CTS/3% Al2O3 scaffolds more than PHB scaffolds. These results indicate that PHB-CTS/3% Al2O3 is a promising material for applying as a cartilage tissue engineering scaffold.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
BIOGRAPHIES

Elahe Bahremandi Toloue received a B.S. degree in Physics from the University of Isfahan in 2007 and M.S. degree in solid state Physics from the Islamic Azad University of Tehran in 2012. She is a M.Sc. graduated from the department of biomedical engineering at Isfahan University of Medical Sciences (MUI), Iran. Her research interests include biomaterials, tissue engineering, and nanobiomaterials.
Email: e.bahremandi@yahoo.com

Saeed Karbasi received his BS degree in Material Engineering from Shiraz University, Iran, in 1994 and MS and PhD degrees in Biomedical Engineering from Amirkabir University, Iran, in 1997 and 2005, respectively. He is currently full professor at Isfahan University of Medical Sciences, Iran. His research interests include biomaterials, tissue engineering, and nanobiomaterials.
Email: karbasi@med.mui.ac.ir

Hossein Salehi is an associated professor in the department of anatomical sciences at the Isfahan university of medical sciences where he has been a faculty member since 2011. he completed his Ph.D. in anatomical sciences at the Isfahan university of medical sciences. his research interests lie in the area of stem cell biology.
Email: psalehi2002@gmail.com

Mohammad Rafienia received his BS degree in Material Engineering from Isfahan University of technology, Iran, in 1998 and MS and PhD degrees in Biomedical Engineering from Amirkabir University, Iran, in 2001 and 2007, respectively. He is currently an Assosciate Professor at Isfahan University of Medical Sciences. His research interests include biomaterials, tissue engineering, and drug delivery.
Email: m_rafienia@med.mui.ac.ir
References
- 1.Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I, et al. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater Sci Eng C Mater Biol Appl. 2017;78:1246–62. doi: 10.1016/j.msec.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. The Biomaterials: Silver Jubilee Compendium. Elsevier Science. 2000:175–89. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 3.Sadeghi D, Karbasi S, Razavi S, Mohammadi S, Shokrgozar MA, Bonakdar S. Electrospun poly (hydroxybutyrate)/chitosan blend fibrous scaffolds for cartilage tissue engineering. Journal of Applied Polymer Science. 2016;133:47. [Google Scholar]
- 4.Karbasi S, Alizadeh ZM. Effects of multi-wall carbon nanotubes on structural and mechanical properties of poly (3-hydroxybutyrate)/chitosan electrospun scaffolds for cartilage tissue engineering. Bull Mater Sci. 2017;40:1247–53. [Google Scholar]
- 5.Khoroushi M, Foroughi MR, Karbasi S, Hashemibeni B, Khademi AA. Effect of polyhydroxybutyrate/chitosan/bioglass nanofiber scaffold on proliferation and differentiation of stem cells from human exfoliated deciduous teeth into odontoblast-like cells. Mater Sci Eng C Mater Biol Appl. 2018;89:128–39. doi: 10.1016/j.msec.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Karbasi S, Zarei M, Foroughi M. Effects of multi-wall carbon nano-tubes (MWNTs) on structural and mechanical properties of electrospun poly (3-hydroxybutyrate) scaffold for tissue engineering applications. Sci Iranica Trans F Nanotechnol. 2016;23:3145. [Google Scholar]
- 7.Alzarrug FA, Dimitrijević MM, Heinemann RM, Radojević V, Stojanović DB, Uskoković PS, et al. The use of different alumina fillers for improvement of the mechanical properties of hybrid PMMA composites. Mater Des. 2015;86:575–81. [Google Scholar]
- 8.Fu SY, Lauke B. The elastic modulus of misaligned short-fiber-reinforced polymers. Compos Sci Technol. 1998;58:389–400. [Google Scholar]
- 9.Li D, Xia Y. Electrospinning of nanofibers: Reinventing the wheel? Adv Mater. 2004;16:1151–70. [Google Scholar]
- 10.Dong Z, Wu Y, Wang Q, Xie C, Ren Y, Clark RL, et al. Reinforcement of electrospun membranes using nanoscale al2O3 whiskers for improved tissue scaffolds. J Biomed Mater Res A. 2012;100:903–10. doi: 10.1002/jbm.a.34027. [DOI] [PubMed] [Google Scholar]
- 11.Song Y, Ju Y, Song G, Morita Y. In vitro proliferation and osteogenic differentiation of mesenchymal stem cells on nanoporous alumina. Int J Nanomedicine. 2013;8:2745–56. doi: 10.2147/IJN.S44885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson M, Pålsgård E, Wilshaw PR, Di Silvio L. Initial in vitro interaction of osteoblasts with nano-porous alumina. Biomaterials. 2003;24:3039–46. doi: 10.1016/s0142-9612(03)00146-7. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto M, Sasaki J, Imazato S. Investigation of the cytotoxicity of aluminum oxide nanoparticles and nanowires and their localization in L929 fibroblasts and RAW264 macrophages. J Biomed Mater Res B Appl Biomater. 2016;104:241–52. doi: 10.1002/jbm.b.33377. [DOI] [PubMed] [Google Scholar]
- 14.Asran AS, Razghandi K, Aggarwal N, Michler GH, Groth T. Nanofibers from blends of polyvinyl alcohol and polyhydroxy butyrate as potential scaffold material for tissue engineering of skin. Biomacromolecules. 2010;11:3413–21. doi: 10.1021/bm100912v. [DOI] [PubMed] [Google Scholar]
- 15.Naveen N, Kumar R, Balaji S, Uma T, Natrajan T, Sehgal P. Synthesis of nonwoven nanofibers by electrospinning – A promising biomaterial for tissue engineering and drug delivery. Adv Eng Materials. 2010;12:B380–7. [Google Scholar]
- 16.Zhijiang C, Yi X, Haizheng Y, Jia J, Liu Y. Poly (hydroxybutyrate)/cellulose acetate blend nanofiber scaffolds: Preparation, characterization and cytocompatibility. Mater Sci Eng C Mater Biol Appl. 2016;58:757–67. doi: 10.1016/j.msec.2015.09.048. [DOI] [PubMed] [Google Scholar]
- 17.Medvecky L, Giretova M, Stulajterova R. Properties and in vitro characterization of polyhydroxybutyrate-chitosan scaffolds prepared by modified precipitation method. J Mater Sci Mater Med. 2014;25:777–89. doi: 10.1007/s10856-013-5105-0. [DOI] [PubMed] [Google Scholar]
- 18.Ghasemi-Mobarakeh L, Semnani D, Morshed M. A novel method for porosity measurement of various surface layers of nanofibers mat using image analysis for tissue engineering applications. J Applied Polym Sci. 2007;106:2536–42. [Google Scholar]
- 19.Rodríguez-Lorenzo LM, Salinas AJ, Vallet-Regí M, San Román J. Composite biomaterials based on ceramic polymers. I. Reinforced systems based on Al2O3/PMMA/PLLA. J Biomed Mater Res. 1996;30:515–22. doi: 10.1002/(SICI)1097-4636(199604)30:4<515::AID-JBM10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Patel AK, Balani K. Dispersion fraction enhances cellular growth of carbon nanotube and aluminum oxide reinforced ultrahigh molecular weight polyethylene biocomposites. Mater Sci Eng C Mater Biol Appl. 2015;46:504–13. doi: 10.1016/j.msec.2014.10.075. [DOI] [PubMed] [Google Scholar]
- 21.Bagheripour E, Moghadassi A, Hosseini S, Van der Bruggen B, Parvizian F. Novel composite graphene oxide/chitosan nanoplates incorporated into PES based nanofiltration membrane: Chromium removal and antifouling enhancement. J Ind Eng Chem. 2018;62:311–20. [Google Scholar]
- 22.Ishak NF, Hashim NA, Othman MH, Monash P, Zuki FM. Recent progress in the hydrophilic modification of alumina membranes for protein separation and purification. Ceram Int. 2017;43:915–25. [Google Scholar]
- 23.Thambyah A, Nather A, Goh J. Mechanical properties of articular cartilage covered by the meniscus. Osteoarthritis Cartilage. 2006;14:580–8. doi: 10.1016/j.joca.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Nagiah N, Madhavi L, Anitha R, Srinivasan NT, Sivagnanam UT. Electrospinning of poly (3-hydroxybutyric acid) and gelatin blended thin films: Fabrication, characterization, and application in skin regeneration. Polym Bull. 2013;70:2337–58. doi: 10.1016/j.msec.2013.06.042. [DOI] [PubMed] [Google Scholar]


