Significance
Mitochondria generate the cellular fuel, adenosine triphosphate, or ATP, to sustain complex life. Production of ATP depends on the oxidation of energy-rich compounds to produce a chemical potential difference for hydrogen ions (or proton motive force, pmf), across the inner mitochondrial membrane (IMM). Disruption of the IMM, dissipation of the pmf, and cell death occur if the total concentration of calcium inside mitochondria is elevated sufficiently to open a pore in the IMM. It has been proposed that the pore is in the membrane sector of the ATP synthase. Here, we show that five membrane proteins associated with the enzyme’s stator are not involved in the pore, and that the pore persists in the absence of the enzyme complex.
Keywords: human mitochondria, permeability transition, ATP synthase
Abstract
The opening of the permeability transition pore, a nonspecific channel in inner mitochondrial membranes, is triggered by an elevated total concentration of calcium ions in the mitochondrial matrix, leading to disruption of the inner membrane and necrotic cell death. Cyclosporin A inhibits pore opening by binding to cyclophilin D, which interacts with the pore. It has been proposed that the pore is associated with the ATP synthase complex. Previously, we confirmed an earlier observation that the pore survives in cells lacking membrane subunits ATP6 and ATP8 of ATP synthase, and in other cells lacking the enzyme’s c8 rotor ring or, separately, its peripheral stalk subunits b and oligomycin sensitive conferral protein. Here, we investigated whether the pore is associated with the remaining membrane subunits of the enzyme. Individual deletion of subunits e, f, g, and 6.8-kDa proteolipid disrupts dimerization of the complex, and deletion of DAPIT (diabetes-associated protein in insulin sensitive tissue) possibly influences oligomerization of dimers, but removal of each subunit had no effect on the pore. Also, we removed together the enzyme’s membrane bound c8 ring and the δ-subunit from the catalytic domain. The resulting cells assemble only a subcomplex derived from the peripheral stalk and membrane-associated proteins. Despite diminished levels of respiratory complexes, these cells generate a membrane potential to support uptake of calcium into the mitochondria, leading to pore opening, and retention of its characteristic properties. It is most unlikely that the ATP synthase, dimer or monomer, or any component, provides the permeability transition pore.
For more than 40 y, it has been recognized that mitochondria contain the permeability transition pore (PTP), a nonspecific channel which opens in response to elevated levels of Ca2+ ions in the mitochondrial matrix (1). In consequence, the mitochondria take in water, their cristae swell, and their membranes rupture. Similar observations have been made in many vertebrates from humans to lampreys and also in plants and yeasts (2). Besides elevated Ca2+ ions, PTP opening is promoted by phosphate, adenine nucleotide depletion, and reactive oxygen species (3). This sundering of the mitochondrial membranes disrupts the synthesis of ATP and ion homeostasis in the organelle and leads to cell death by necrosis (4, 5). Because the human PTP has been implicated in neurodegeneration, ischemia reperfusion injury, muscle dystrophy, and cancer (6), the pore has been studied extensively in various cell types and also in isolated mitochondria. Under experimental conditions, opening of the PTP in cells can be provoked by high concentrations of thapsigargin, an inhibitor of the Ca2+-ATPase in the sarcoplasmic and endoplasmic reticula, or by a direct effect on mitochondria (7). Another way is to make the plasma membrane of cultured cells permeable, either with ionophores such as ferutinin (8) or with the mild detergent digitonin (9), to provide a route for exogenous Ca2+ ions to enter the cells. The Ca2+ uniporter, a component of the inner mitochondrial membrane, takes the elevated cytoplasmic Ca2+ ions into the mitochondrial matrix, which can accumulate large amounts of Ca2+ in the presence of phosphate (10, 11). However, when the total concentration of Ca2+ in the mitochondrial matrix is sufficiently elevated, the PTP opens (12). Inhibition of the Ca2+ uniporter with the oxygen-bridged dinuclear ruthenium amine complex, Ru-360, or genetic disruption of the uniporter, reduces ingress of Ca2+ ions into the matrix space and the pore remains closed (13). The PTP is defined experimentally by a number of properties including the following. First, cyclosporin A (CsA) inhibits opening of the PTP by binding to cyclophilin D, a prolyl cis-trans isomerase found in the mitochondrial matrix (14), which is considered to interact with and modulate the PTP rather than being an integral component. Second, the opening of the PTP is inhibited by bongkrekic acid and stimulated by carboxyatractylate (15). Third, the pore allows hydrophilic molecules up a molecular mass of 1,500 Da to pass (16).
Several proteins have been proposed as components of the PTP and have been rejected subsequently (17). Another proposal investigated here and previously is that the PTP is associated with the dimeric ATP synthase complex in the inner mitochondrial membrane (18). Each constituent monomer is an assembly of 28 proteins of 18 different types organized into a membrane extrinsic domain, where synthesis of ATP takes place, and a membrane intrinsic domain, where the turning of the enzyme’s rotor is generated from the proton motive force (Fig. 1) (19). The domains are linked by a central stalk (subunits γ, δ, and ε) and a peripheral stalk (subunits b, d, F6, and the oligomycin sensitivity conferral protein or OSCP). The subcomplex of the central stalk and a ring of eight c subunits in the membrane domain constitute the enzyme’s rotor. One suggestion is that the PTP is associated with this c8 ring (20–22), and another is that the OSCP provides the site of interaction of cyclophilin D with the PTP (18). However, neither suggestion is tenable. The characteristic properties of the PTP persist in one human cell line where the three genes encoding the c subunit have been disrupted, making them devoid of the c subunit, and in others, which lack a peripheral stalk by virtue of disruption of the gene for either the OSCP or subunit b (23, 24). Recently, we investigated the pathway of assembly of the membrane arm of ATP synthase by removing individual supernumerary subunits e, f, g, DAPIT, and 6.8-kDa proteolipid (6.8PL) and characterizing the vestigial ATPase complexes (25). Here, we have taken advantage of this knowledge to study whether individual removal of these supernumerary subunits, which influence formation of dimers and their oligomerization, has any effect on the PTP, and in other experiments to see whether mitochondria devoid of an assembled ATP synthase still retain the characteristics of a functional PTP.
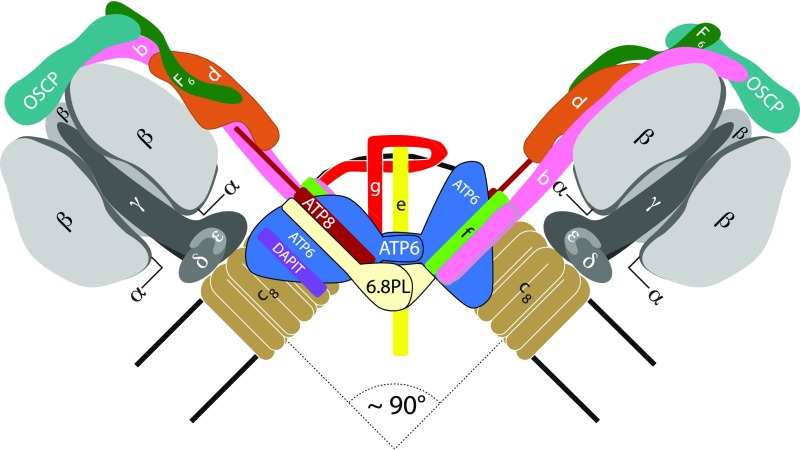
Fig. 1.
Organization of subunits in the dimeric ATP synthase complex in mammalian mitochondria. The dimers form rows along the edges of the cristae. Black lines represent the limits of the inner membrane between the matrix and the IMS (intermembrane space). The F1 catalytic domain (subunit composition α3β3γδε) including the central stalk is above; one α-subunit has been removed from each monomer to expose the γ-subunit (black), lying approximately along the central axis of the spherical α3β3 domain. The γ-, δ- and ε-subunits are bound to the c8 ring (brown), and together these subunits constitute the rotor. Rotation is generated by the translocation of protons through the interface between the c8 ring and ATP6 (blue). The peripheral stalks are made of single copies of subunits OSCP, b, d, and F6. Subunit b has two N-terminal transmembrane α-helices. The membrane domain also contains subunits ATP8 (brick red), e (yellow), f (green), g (red), DAPIT (purple), and 6.8PL (cream), each with a single transmembrane α-helix. The C-terminal region of ATP8, extends into the peripheral stalk. Subunits e, f, g, DAPIT, and 6.8PL are “supernumerary” with no known roles in the generation or hydrolysis of ATP. In the dimeric complex, subunits ATP6 and 6.8PL provide the protein interface between monomers. Subunits e and g make a separate domain not in contact with ATP6 and 6.8PL. One e-g pair at the front of the dimeric structure has been removed for clarity. The dimer interface and arrangement of supernumerary membrane subunits is based upon the structure of dimeric Fo from S. cerevisiae (33).
Results
Effect of Removal of Supernumerary Subunits.
According to the pathway of assembly of the membrane arm of human ATP synthase (SI Appendix, Fig. S1), the individual removal of any one of supernumerary subunits e, f, g, and 6.8PL prevents the vestigial complexes from dimerizing (25). In contrast, dimers persist to some extent when DAPIT is removed. PTP opening in cells lacking individual supernumerary subunits was assessed by three independent methods. They were: first, calcein-cobalt quenching and depletion of the fluorescence of tetramethylrhodamine methyl ester (TMRM) in response to the treatment of intact cells with either thapsigargin (TG) or the calcium ionophore ferutinin (FT); second, measurement of calcium retention capacity (CRC) by the mitochondria in cells permeabilized with digitonin; and third, the swelling of mitochondria in response to exogenous Ca2+ in permeabilized cells monitored by light absorbance (23, 24). As assayed by these methods, the removal of each of these supernumerary subunits did not prevent the opening of the PTP or the inhibition of opening by CsA (SI Appendix, Figs. S2–S5). Therefore, the disruption of dimers of ATP synthase by removal of any of subunits e, f, g, and 6.8PL does not influence the function of the PTP, nor does the removal of DAPIT, and the individual subunits are not part of the PTP.
Human Mitochondria Devoid of Assembled ATP Synthase.
Yeast ATP synthase is assembled from preformed modules of the F1 domain, the peripheral stalk, and the c10 ring (26, 27). The process of assembly of the human enzyme is similar, but not identical (25), and the assembly of its F1 domain has not been studied in detail yet. However, depletion of any of the three components of the central stalk, the γ-, δ-, ε-subunits (Fig. 1), affects the assembly of the entire domain, accompanied by a reduction in the levels of peripheral stalk and membrane subunits (28, 29), and the accumulation of an uncharacterized subcomplex containing the c subunit. Homozygous missense mutations in ATP5F1D, the single human gene encoding the δ-subunit, are associated with metabolic disorder and are accompanied by a reduction in the level of ATP synthase and changes in the morphology of the cristae (30). Therefore, we disrupted ATP5F1D in HAP1-A12 cells, which lack the c subunit (23). Deletion of a single base pair in exon II of this gene produced a frame shift, which changed the mature δ-subunit after residue 88 and terminated the chain after a further 10 residues unrelated to the sequence of the δ-subunit (SI Appendix, Figs. S6 and S7). The resulting clonal cell line, known as HAP1-Δ(c+δ), lacks both the c and δ-subunits (Fig. 2) and grows at about the same rate as HAP1-WT cells (SI Appendix, Fig. S8A), although the mutant cells contain 40% more mitochondrial DNA molecules than HAP1-WT cells (SI Appendix, Fig. S8B). However, as with HAP1-A12 cells (23), the HAP1-Δ(c+δ) cells have a reduced respiratory capacity (SI Appendix, Fig. S8C), consistent with the reduced levels of complexes I, III, and IV, but not complex II, in their mitochondria (SI Appendix, Fig. S9). As expected, the HAP1-Δ(c+δ) cells contain no intact ATP synthase (Fig. 2 and SI Appendix, Fig. S10), and there was no evidence of any vestigial complex containing the F1 domain; such vestigial complexes have been observed previously in human ρ0-cells and in HAP1-A12 cells, and in HAP1 cells lacking the capability to express individually the OSCP, b, e, f, g, and 6.8PL subunits (23–25). However, there was evidence in both HAP1-WT and HAP1-Δ(c+δ) cells of an uncharacterized subcomplex containing at least subunit g (s1 in SI Appendix, Fig. S10) that may be related to an assembly intermediate containing subunits b, e, and g (31).
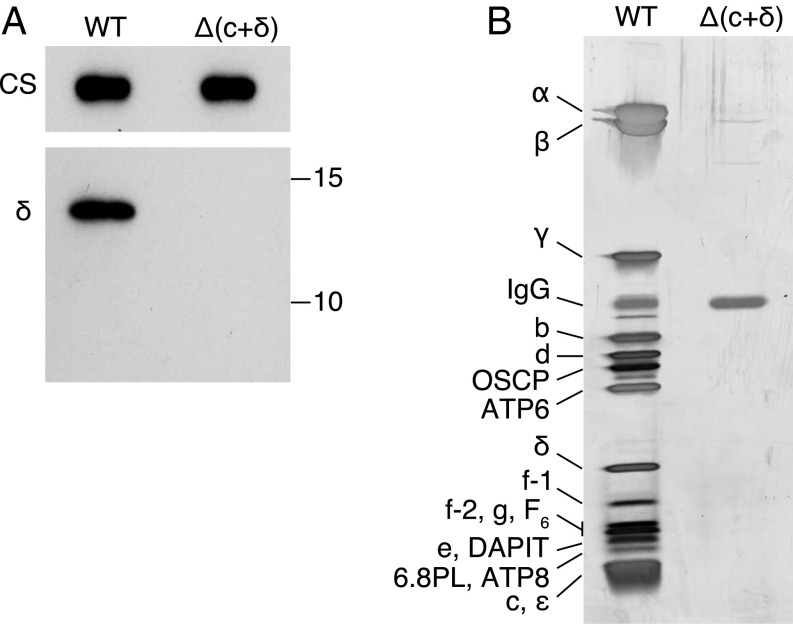
Fig. 2.
Absence of the δ-subunit and of the assembly of ATP synthase from clonal HAP1-Δ(c+δ) cells. (A) Analysis by Western blotting of extracts of mitoplasts from HAP1 wild-type (WT) and HAP1-Δ(c+δ) cells made with n-dodecyl-β-d-maltoside and fractionated by SDS/PAGE. The antibodies were against the δ-subunit, and citrate synthase (CS) provided a loading control. The positions of molecular mass markers (in kilodaltons) are shown on the right. (B) Analysis by SDS/PAGE of ATP synthase immunopurified from mitoplasts derived from HAP1-WT and HAP1-Δ(c+δ) cells. Material derived from the same amount of starting material was applied in both lanes. The gel was stained with silver. The positions of subunits are indicated on the left.
The absence of intact ATP synthase and vestigial complexes containing the F1 catalytic domain from HAP1-Δ(c+δ) cells was confirmed by quantitative MS of material immunocaptured from cells under conditions employed for purification of ATP synthase from HAP1-WT cells (Fig. 3A, SI Appendix, Fig. S11, and Datasets S1 and S2). The Δ(c+δ) material contained no trace of some subunits of ATP synthase and very low levels of others relative to HAP1-WT cells. It is noteworthy that the relative abundance of IF1 in the immunocaptured material had decreased drastically also, in contrast to observations in mutant HAP1 cells lacking individually supernumerary subunits (excepting DAPIT), peripheral stalk subunits OSCP or b, and subunit c (23–25). In all of these mutant cells, where different vestigial ATPase complexes remain (SI Appendix, Fig. S1), each with an intact F1 domain, the level of IF1 is elevated relative to HAP1-WT cells, presumably to prevent the F1 domain from hydrolyzing ATP. Thus, the absence of IF1 from the immunocaptured material from HAP1-Δ(c+δ) cells is consistent with the absence of an assembled F1 domain. Quantitative MS analysis of mitoplasts confirmed the loss of the three central stalk subunits, γ, δ, and ε, and the two mitochondrially encoded subunits, ATP6 and ATP8 (and the c subunit is absent also), but significant levels (25–55%) of all of the other subunits were measured (Fig. 3B, SI Appendix, Fig. S11, and Datasets S3 and S4). Residual levels of subunits were detected also by fractionation of mitoplasts by SDS/PAGE, followed by Western blotting with subunit-specific antibodies (SI Appendix, Fig. S12), albeit at apparently lower levels than those measured in stable isotope labeling using amino acids in cell culture (SILAC) analyses. The quantitative MS experiments also confirm reduced levels of subunits of complexes I, III, and IV (SI Appendix, Fig. S13 and Dataset S3).
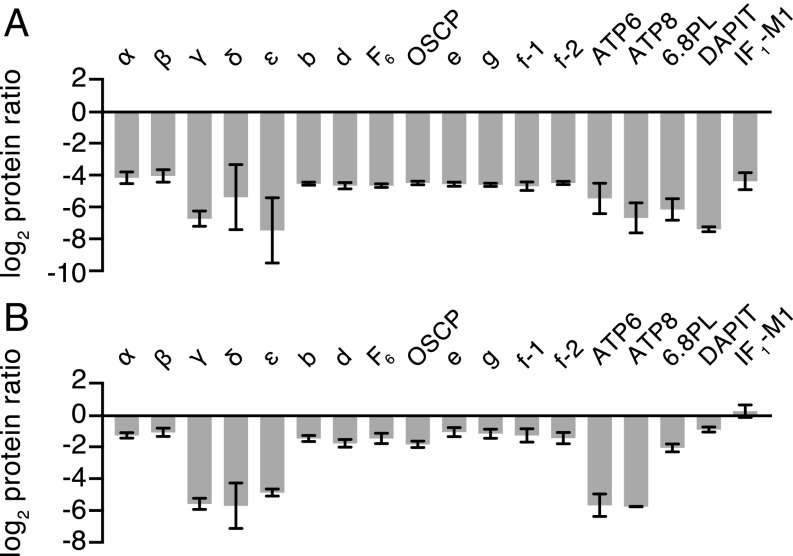
Fig. 3.
Relative quantitation of ATP synthase subunits in HAP1-Δ(c+δ) cells. Relative abundances of subunits and the inhibitor protein IF1 are shown. (A) Immunopurified ATP synthase and vestigial complexes. (B) Mitoplasts. The complexes were purified from mitoplasts from 1:1 mixtures of differentially SILAC-labeled cells. Complexes and mitoplasts were fractionated by SDS/PAGE, and proteins were stained with Coomassie blue dye. Tryptic digests were analyzed by mass spectrometry. The histograms are the median values of both relative abundance ratios determined for proteins found in complementary SILAC experiments. The data for all of the identified proteins are given in SI Appendix, Fig. S11 and Datasets S1–S4. Error bars show the range of the two values. IF1-M1 is a specific mature form of IF1 (23), and f-1 and f-2 are isoforms of subunit f (Swiss-Prot P56134). The relative abundance of the c subunit was not determined.
Characteristics of the PTP in HAP1-Δ(c+δ) Cells.
The function of the PTP was compared in HAP1-WT and HAP1-Δ(c+δ) cells by the same three independent assays described above.
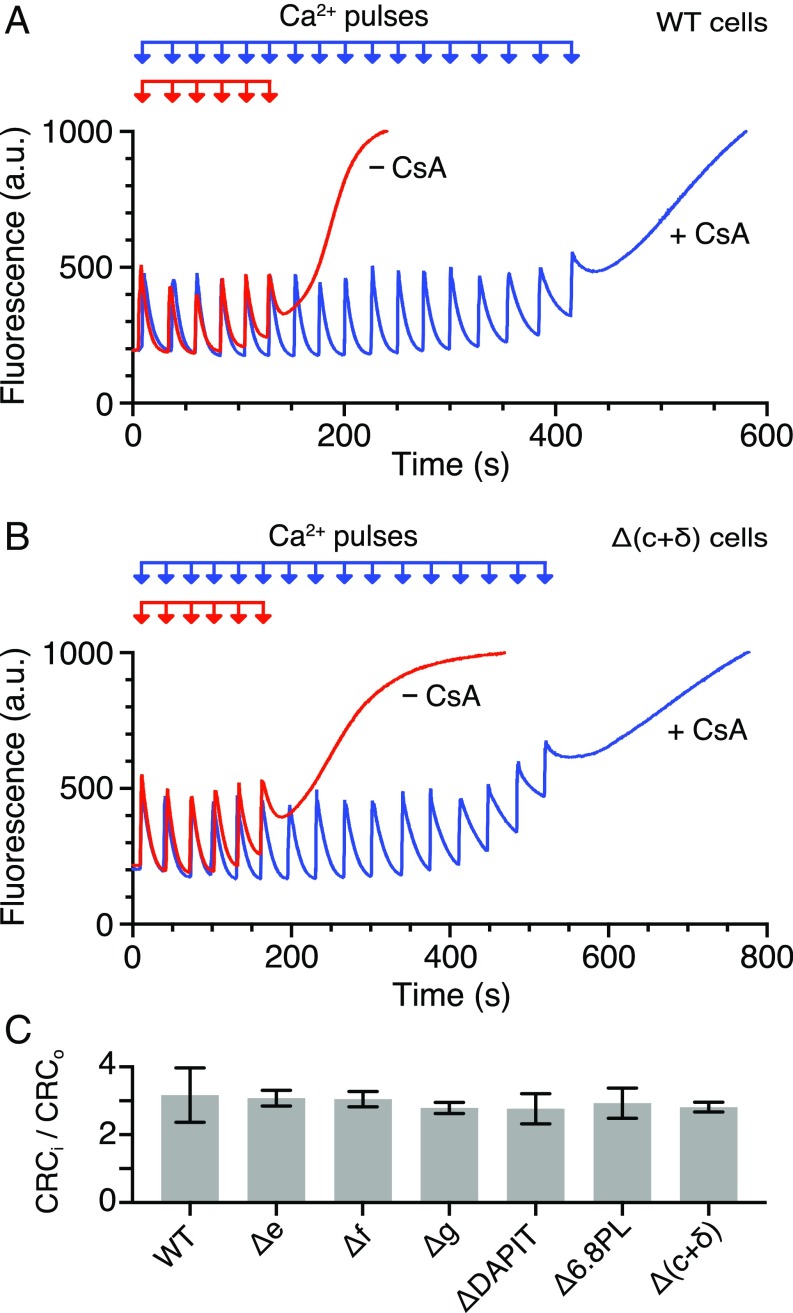
By the first assay, the opening of the pore was demonstrated by calcein-cobalt quenching and loss of TMRM fluorescence in both intact HAP1-WT and HAP1-Δ(c+δ) cells in the presence of either thapsigargin or the calcium ionophore ferutinin. With both reagents, opening of the pore was inhibited by CsA (Fig. 4). In the second assay, the capacity of mitochondria to retain calcium was followed in HAP1-WT and HAP1-Δ(c+δ) cells, where the plasma membrane had been permeabilized with digitonin. The responses of the cells to successive pulses of Ca2+ were monitored in the absence and in the presence of CsA (Fig. 5). On average, the ratio of the number of calcium pulses needed to induce the opening of the PTP in the presence and absence of CsA was very similar, and also to the values in HAP1-Δe, -Δf, -Δg, -ΔDAPIT, and -Δ6.8PL cells (Fig. 5C and SI Appendix, Table S3). In both HAP1-WT (24) and HAP1-Δ(c+δ) cells, inhibition of the mitochondrial calcium uniporter with Ru360 immediately after a single calcium injection prevented further uptake of Ca2+ by mitochondria (SI Appendix, Fig. S14). Also, there was no difference in HAP1-WT and HAP1-Δ(c+δ) cells to the effects of either bongkrekic acid or carboxyatractylate, on the opening of the PTP in response to pulses of exogenous Ca2+ (SI Appendix, Fig. S15). Thus, in response to pulses of exogenous Ca2+, there was no qualitative difference in pore opening in the presence and in the absence of an assembled ATP synthase complex. However, the rate of uptake of calcium was slower in the HAP1-Δ(c+δ) cells than in HAP1-WT cells (SI Appendix, Fig. S16), despite the former having slightly elevated levels of components of the calcium uniporter (Dataset S3). The slower uptake presumably arises because of the reduced levels of respiratory complexes I, III, and IV in HAP1-Δ(c+δ) cells (SI Appendix, Figs. S9 and S13). As expected, first, with the introduction of each calcium pulse, the membrane potential fell (SI Appendix, Fig. S17), and then recovered as calcium accumulated in the mitochondria, but more slowly in the mutant than in the wild-type cells, until, in both wild-type and mutant cells, the PTP opened with release of accumulated calcium and complete collapse of the membrane potential. An independent estimate of maximal respiratory capacity based on cellular oxygen consumption following treatment with the ionophore, carbonyl cyanide-4-(trifluoromethoxy)-phenylhydrazone, provides additional confirmation that HAP1-Δ(c+δ) cells retain the ability to generate a proton flux sufficient to sustain uptake of Ca2+ (SI Appendix, Table S4).
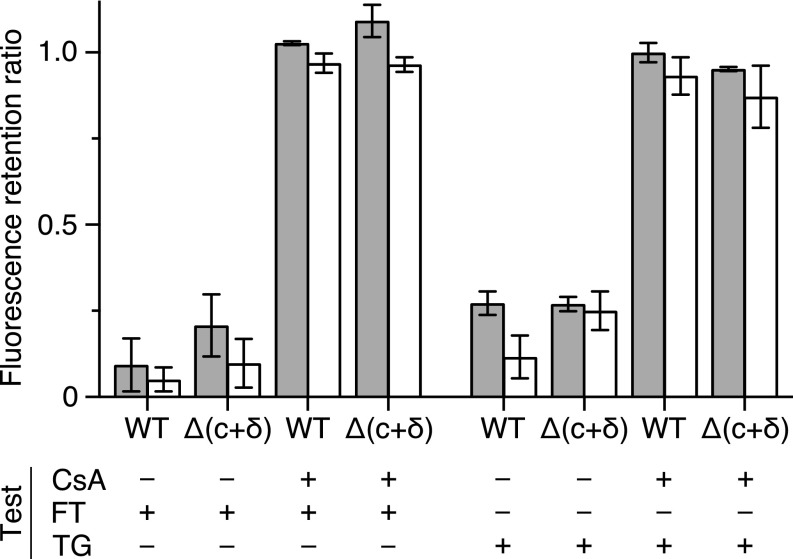
Fig. 4.
Influence of FT or TG on the opening of the PTP in HAP1 cells. HAP1-WT and HAP1-Δ(c+δ) cells were stained with calcein and TMRM and then kept at 37 °C for 30 min in the presence of 25 μM FT, or for 1 h with 40 μM TG. Duplicate samples were similarly stained and treated with 5 μM CsA and then with either FT or TG. Fluorescence intensities of calcein and TMRM were measured in a cell-counting fluorescence microscope (see SI Appendix, Fig. S3 for an example). Gray and white columns correspond to the fluorescence retention ratios for calcein and TMRM, respectively, calculated as shown in SI Appendix, Fig. S3. The data shown are the mean values ± SD (n = 4). The data providing the basis for this summary are presented in SI Appendix, Tables S1 and S2. In each instance, the fluorescence retention ratios for treatment in the absence or presence of CsA are significantly different (t test, P < 0.01).
Fig. 5.
Calcium induced opening of the PTP in permeabilized HAP1 cells. The calcium retention capacity of mitochondria was determined in digitonin permeabilized cells (2 × 107 cells per mL) in response to pulses of 10 μM CaCl2, in the absence and presence of 1 μM CsA. Mitochondrial uptake of extramitochondrial Ca2+ was monitored by the fluorescence of Calcium green-5N given in arbitrary units (a.u.). The collapse of the fluorescence signal corresponds to the opening of the PTP. (A) HAP1-WT cells. (B) HAP1-Δ(c+δ) cells. (C) Quantitation of the PTP displayed as the ratio of the number of calcium pulses required to induce the PTP in CsA inhibited (CRCi) and untreated (CRCo) samples (±SD). The experimental data for the number of calcium pulses required to induce the PTP are shown in SI Appendix, Table S3, and representative traces for HAP1-Δe, -Δf, -Δg, -ΔDAPIT, and -Δ6.8PL cells are given in SI Appendix, Fig. S5.
The third assay is based upon monitoring the change in absorbance of mitochondria in permeabilized HAP1 cells accompanying the opening of the PTP and mitochondrial swelling, induced by a single calcium addition, and inhibited by CsA (SI Appendix, Fig. S18). As in HAP1-Δb and -ΔOSCP cells (24), in cells lacking individual subunits e, f, g, DAPIT, and 6.8PL, and also in the HAP1-Δ(c+δ) cells, Ca2+-induced changes in their mitochondria monitored by absorption of light were generally slower and less extensive than in HAP1-WT cells (SI Appendix, Figs. S4 and S18). The Ca2+-induced swelling of mitochondria in HAP1-WT and HAP1-Δ(c+δ) cells was carried out also in the presence of PEGs 200, 600, 1000, 1500, 2000, and 4000 at concentrations that generate an equivalent osmotic pressure. In both wild-type and mutant cells, the PTP allowed PEGs 200 and 600 to pass, and in both cases, PEGS 1500, 2000, and 4000 were excluded (SI Appendix, Fig. S19), and PEG 1000 was close to the cutoff limit for pore permeability in both cell types. Therefore, the pores in the wild-type and mutant cells have similar, probably the same, sizes.
Discussion
The human PTP is characterized by its ability to open in response to the elevation of the total concentration of Ca2+ in the mitochondrial matrix, and by the inhibition of opening by CsA, mediated via the binding of the drug to cyclophilin D, and the interaction of cyclophilin D with the PTP. In previous work, the removal of neither of the membrane subunits b or c had any significant impact on the opening of the pore in response to elevation of matrix Ca2+ in three independent assays and in every instance pore opening remained sensitive to the action of CsA (23, 24). Similarly, the absence of mitochondrial DNA from ρ0-143B cells and, thus, of their ability to synthesize membrane subunits ATP6 and ATP8, had no effect on pore opening or on the inhibitory effect of CsA (23). Similar observations have been made before with another ρ0-143B cell line (32).
Here, it has been shown that there was a similar lack of impact on the opening of the PTP in response to elevated matrix Ca2+ by the same three independent assays employed previously when the remaining membrane subunits e, f, g, 6.8PL, and diabetes-associated protein in insulin sensitive tissue (DAPIT) of ATP synthase were removed individually from HAP1 cells by gene disruption. As yet, there is no detailed structure of the interface between the monomers in the dimeric mammalian ATP synthase complex, but the ATP synthase in S. cerevisiae has the same subunit composition as the mammalian complex, yeast subunits j and k, respectively, being the orthologs of mammalian 6.8PL and DAPIT (25). In a structure of the dimeric membrane domain of the ATP synthase from S. cerevisiae (33), the interface between monomers is formed by interactions between the ATP6 subunits and between the j subunits in each monomer, and no other subunit appears to be involved directly in forming the dimer interface, although the structures of some membrane subunits are incomplete (33). We have shown previously that the removal of any of subunits OSCP, b, c, e, f, g, and 6.8PL individually, and of ATP6 and ATP8 together, stalls the assembly of the complex, and various vestigial partially assembled ATPase complexes accumulate that all lack the dimer interface forming proteins, ATP6 and 6.8PL (25). Similarly, the removal of DAPIT probably disrupts the oligomerization of dimers into the long rows along the cristae edges. Hence, the proposal that the dimeric form of the ATP synthase provides the PTP (18) is extremely unlikely.
In a more drastic experiment, the genes for the c and δ-subunits were disrupted in the clonal cell line, HAP1-Δ(c+δ). Their mitochondria lack not only the c ring, ATP6, and ATP8 and associated subunits, DAPIT and 6.8PL, but also there is no assembled F1 domain, and associated OSCP subunit, and yet they retain a PTP, which opens characteristically in response to elevation of the concentration of matrix Ca2+, and opening, as usual, is inhibited by CsA. The only remaining vestige of the ATP synthase in HAP1-Δ(c+δ) cells may be an incompletely characterized subcomplex containing subunits b, e, and g, and possibly other subunits, and the participation of each of these subunits in the PTP has been eliminated in the past and present work (24). In HAP1-Δ(c+δ) cells, the levels of respiratory complexes I, III, and IV and oxygen consumption are reduced markedly relative to HAP1-WT cells. A similar reduction in respiratory complexes and oxygen consumption has been noted also in HAP1-Δc, -Δb, and -ΔOSCP cells (23, 24), leading to the ability to generate a membrane potential to drive the uptake of Ca2+ being questioned (34), despite clear experimental evidence that they were able to do so (23, 24). Therefore, to remove any possible residual doubts about the ability of the mitochondria of HAP1-Δ(c+δ) cells to maintain a membrane potential and to accumulate pulses of exogenous Ca2+, it was shown here explicitly that they do, as expected, take up Ca2+ more slowly than mitochondria in HAP1-WT cells, but the membrane potential recovers, and they accumulate Ca2+ to the point where the PTP opens and the membrane potential collapses (SI Appendix, Figs. S16 and S17). Moreover, a simple calculation provides additional confirmation that these mitochondria generate a sufficient proton flux to sustain uptake of Ca2+ (SI Appendix, Table S4).
Similar to HAP1-Δb and HAP1-ΔOSCP cells (24), cells lacking individually subunits e, f, g, DAPIT, and 6.8PL, and also the HAP1-Δ(c+δ) cells, the Ca2+-induced changes in their mitochondria monitored by absorption of light were slower and less extensive than in HAP1-WT cells (SI Appendix, Figs. S4 and S18). This behavior has been used to infer that the deletion of the individual b and OSCP subunits does have an impact on the size of the PTP (34). However, it is well-known that aberrant assembly of human ATP synthase has far-reaching effects, both on the formation of mitochondrial networks and on the ultrastructure of the mitochondria themselves (30, 35–41). Likewise, the removal of either subunit e or g from the ATP synthase in S. cerevisiae prevents the complex from dimerizing with an accompanying profound impact on the morphology of the mitochondria (42, 43). They lose their characteristic cristae, and cross-sectional views of the inner membranes consist of concentric circular structures, likened in appearance to the cross-section of an onion. Thus, the dimerization of ATP synthase and the oligomerization of the dimers in long rows are major determinants in the formation of the cristae (44, 45), and changes (mutations; subunit deletions) that disrupt the assembly of the ATP synthase complex, such as those described here, will affect the structure and light absorbing or scattering properties of the mitochondria. So far, the impact of the removal of the various subunits of the ATP synthase on the structure of the mitochondria networks in HAP1 cells has not been studied by detailed microscopic analysis, but it is likely that the structures of the mitochondrial networks and of their cristae, and the light absorbing and scattering properties of the mitochondria will all have been affected profoundly. Also, the induction of the PTP might be slower in mutant mitochondria where metabolic differences arise because the ATP synthase is defective, leading to a shift in the NAD(P)H/NAD(P)+ and ADP/ATP ratios and a less than favorable environment for PTP induction (46, 47). Changes in the structure of the mitochondria and the cristae and in their metabolism provide a more plausible explanation of the observed impacts on the apparent slowing of the opening of the PTP as assayed by light absorbance. A similar slower light absorbance change in yeast mitochondria lacking subunits e and g has also been interpreted as demonstrating an involvement of these subunits in the formation of the PTP (48). The matrix of the mitochondria (or at least in some of the mitochondria) from the yeast cells lacking subunits e and g, still had a dense (i.e., not swollen) appearance in electron micrographs in negative stain under conditions that would be expected to open the PTP (48). However, the opening of the PTP is an all-or-nothing effect; the pore is either closed or open, and the mitochondria are either dense or swollen (16), and a slower rate of opening probably produces a mixed population of dense and swollen states.
Another hallmark of the PTP that has been characterized consistently is its ability to allow PEGs of increasing molecular weight up to a specific maximum to pass, and to prevent the passage of larger PEG polymers (16, 49, 50). Here, it has been shown that in HAP1-WT and HAP1-Δ(c+δ) cells the pore allows PEG polymers up to PEG 1000 to pass and excludes larger polymers. Therefore, the diameter of the pore and its other characteristic features are the same in the HAP1-Δ(c+δ) cells and HAP1-WT cells. These data provide a crude estimate of the pore diameter of about 2 nm, and this estimate is similar to values of pore diameter estimated by the same and related methods in various cell types (SI Appendix, Table S5). A recent attempt to reinvoke the c8 ring of the ATP synthase as providing the PTP (51) takes no account of the estimated diameter of the PTP being about 20 Å or greater (SI Appendix, Table S5), whereas the central nonprotein space in the c8 ring is 4 Å in diameter at its narrowest point in the center of the lipid bilayer, widening to 12 Å at the membrane surface (23). Nor are the chemical and physical aspects of the central pore of the c8 ring considered; it has an almost entirely hydrophobic surface, is filled with lipids and is blocked on the matrix side of the ring by the central stalk of the ATP synthase. There is no evidence that the c8 ring exists on its own in wild-type mitochondrial membranes or that the c8 ring and the catalytic F1 domain can be dissociated from the intact ATP synthase in the inner mitochondrial membrane. These physical, chemical, and biological properties are all inconsistent with the proposal that the c8 ring provides the aqueous channel of the PTP that allows Ca2+, water, and PEG molecules up to PEG 1000 to pass (23, 52).
In conclusion, the experiments presented here, combined with others published previously (23, 24), now provide overwhelming evidence that the proposal that the PTP is associated with the ATP synthase is not sustainable.
Materials and Methods
The derivation and characterization of human cell lines HAP1-Δe, -Δf, -Δg, -Δ6.8PL, and -ΔDAPIT where, respectively, the genes for subunits e, f, g, 6.8PL, and DAPIT of ATP synthase were disrupted, have been described (25). HAP1-Δ(c+δ) cells were generated by CRISPR-Cas9 gene editing (53) in HAP1-A12 cells of ATP5F1D, the gene encoding the δ-subunit of ATP synthase. Growth and oxygen consumption rates of cells were measured as before (23, 24). The oligomeric state of ATP synthase was examined by BN-PAGE (54). ATP synthase and other respiratory complexes were transferred to membranes and detected by Western blotting. ATP synthase and its subcomplexes were immuno-captured from mitoplasts (23) and analyzed by SDS/PAGE. Proteins were subject to SILAC and quantitated by mass spectrometry (23, 55). The opening of the PTP was assayed by calcein-cobalt chloride quenching, and mitochondrial membrane potential measurements with TMRM, as before (23), with the PTP inducers thapsigargin and ferutinin (7, 8). The mitochondrial CRC assay was performed as described previously (23), except that calcium levels were measured with 0.2 or 0.25 μM calcium green-5N. Calcium-induced opening of the PTP and mitochondrial swelling were monitored in digitonin permeabilized cells as described before (24), with 2 × 107 cells per mL and single injections of either 100 or 150 μM CaCl2. The CRC assay solution was used with HAP1-Δ(c+δ) cells for mitochondrial swelling experiments. The size exclusion limit of the PTP was determined by performing the mitochondrial swelling assay in the presence of polyethylene glycols of varying molecular weights (49, 50). For additional details, see SI Appendix.
Supplementary Material
Acknowledgments
We thank M. G. Montgomery [Medical Research Council (MRC) Mitochondrial Biology Unit] for producing Fig. 1 and SI Appendix, Fig. S1. This work was supported by MRC UK Grants MR/M009858/1 and MC_UU_00015/8 (to J.E.W.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904005116/-/DCSupplemental.
References
- 1.Hunter D. R., Haworth R. A., Southard J. H., Relationship between configuration, function, and permeability in calcium-treated mitochondria. J. Biol. Chem. 251, 5069–5077 (1976). [PubMed] [Google Scholar]
- 2.Azzolin L., et al. , The mitochondrial permeability transition from yeast to mammals. FEBS Lett. 584, 2504–2509 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoratti M., Szabò I., The mitochondrial permeability transition. Biochim. Biophys. Acta 1241, 139–176 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa T., et al. , Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434, 652–658 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Baines C. P., et al. , Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Rasola A., Bernardi P., The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis 12, 815–833 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Korge P., Weiss J. N., Thapsigargin directly induces the mitochondrial permeability transition. Eur. J. Biochem. 265, 273–280 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Abramov A. Y., Duchen M. R., Actions of ionomycin, 4-BrA23187 and a novel electrogenic Ca2+ ionophore on mitochondria in intact cells. Cell Calcium 33, 101–112 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Chauvin C., et al. , Rotenone inhibits the mitochondrial permeability transition-induced cell death in U937 and KB cells. J. Biol. Chem. 276, 41394–41398 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Lehninger A. L., Mitochondria and calcium ion transport. Biochem. J. 119, 129–138 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholls D. G., Chalmers S., The integration of mitochondrial calcium transport and storage. J. Bioenerg. Biomembr. 36, 277–281 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Chalmers S., Nicholls D. G., The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J. Biol. Chem. 278, 19062–19070 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Pan X., et al. , The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 15, 1464–1472 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanveer A., et al. , Involvement of cyclophilin D in the activation of a mitochondrial pore by Ca2+ and oxidant stress. Eur. J. Biochem. 238, 166–172 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Hunter D. R., Haworth R. A., The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch. Biochem. Biophys. 195, 453–459 (1979). [DOI] [PubMed] [Google Scholar]
- 16.Haworth R. A., Hunter D. R., The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch. Biochem. Biophys. 195, 460–467 (1979). [DOI] [PubMed] [Google Scholar]
- 17.Biasutto L., Azzolini M., Szabò I., Zoratti M., The mitochondrial permeability transition pore in AD 2016: An update. Biochim. Biophys. Acta 1863, 2515–2530 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Giorgio V., et al. , Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. U.S.A. 110, 5887–5892 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker J. E., The ATP synthase: The understood, the uncertain and the unknown. Biochem. Soc. Trans. 41, 1–16 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Bonora M., et al. , Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle 12, 674–683 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alavian K. N., et al. , An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. U.S.A. 111, 10580–10585 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azarashvili T., et al. , Potential role of subunit c of F1Fo-ATPase and subunit c of storage body in the mitochondrial permeability transition. Effect of the phosphorylation status of subunit c on pore opening. Cell Calcium 55, 69–77 (2014). [DOI] [PubMed] [Google Scholar]
- 23.He J., et al. , Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase. Proc. Natl. Acad. Sci. U.S.A. 114, 3409–3414 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He J., Carroll J., Ding S., Fearnley I. M., Walker J. E., Permeability transition in human mitochondria persists in the absence of peripheral stalk subunits of ATP synthase. Proc. Natl. Acad. Sci. U.S.A. 114, 9086–9091 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He J., et al. , Assembly of the membrane domain of ATP synthase in human mitochondria. Proc. Natl. Acad. Sci. U.S.A. 115, 2988–2993 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rak M., Gokova S., Tzagoloff A., Modular assembly of yeast mitochondrial ATP synthase. EMBO J. 30, 920–930 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naumenko N., Morgenstern M., Rucktäschel R., Warscheid B., Rehling P., INA complex liaises the F1Fo-ATP synthase membrane motor modules. Nat. Commun. 8, 1237 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Havlícková V., Kaplanová V., Nůsková H., Drahota Z., Houstek J., Knockdown of F1 epsilon subunit decreases mitochondrial content of ATP synthase and leads to accumulation of subunit c. Biochim. Biophys. Acta 1797, 1124–1129 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Pecina P., et al. , Role of the mitochondrial ATP synthase central stalk subunits γ and δ in the activity and assembly of the mammalian enzyme. Biochim. Biophys. Acta Bioenerg. 1859, 374–381 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Oláhová M., et al. , Biallelic mutations in ATP5F1D, which encodes a subunit of ATP synthase, cause a metabolic disorder. Am. J. Hum. Genet. 102, 494–504 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujikawa M., Sugawara K., Tanabe T., Yoshida M., Assembly of human mitochondrial ATP synthase through two separate intermediates, F1-c-ring and b-e-g complex. FEBS Lett. 589, 2707–2712 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Masgras I., Rasola A., Bernardi P., Induction of the permeability transition pore in cells depleted of mitochondrial DNA. Biochim. Biophys. Acta 1817, 1860–1866 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Guo H., Bueler S. A., Rubinstein J. L., Atomic model for the dimeric FO region of mitochondrial ATP synthase. Science 358, 936–940 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernardi P., Why F-ATP synthase remains a strong candidate as the mitochondrial permeability transition pore. Front. Physiol. 9, 1543 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonckheere A. I., et al. , Restoration of complex V deficiency caused by a novel deletion in the human TMEM70 gene normalizes mitochondrial morphology. Mitochondrion 11, 954–963 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Cameron J. M., et al. , Complex V TMEM70 deficiency results in mitochondrial nucleoid disorganization. Mitochondrion 11, 191–199 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Habersetzer J., et al. , Human F1Fo ATP synthase, mitochondrial ultrastructure and OXPHOS impairment: A (super-)complex matter? PLoS One 8, e75429 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mourier A., Ruzzenente B., Brandt T., Kühlbrandt W., Larsson N. G., Loss of LRPPRC causes ATP synthase deficiency. Hum. Mol. Genet. 23, 2580–2592 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson C. B., et al. , A novel mitochondrial ATP6 frameshift mutation causing isolated complex V deficiency, ataxia and encephalomyopathy. Eur. J. Med. Genet. 60, 345–351 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Siegmund S. E., et al. , Three-dimensional analysis of mitochondrial crista ultrastructure in a patient with Leigh syndrome by in situ cryoelectron tomography. iScience 6, 83–91 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bajzikova M., et al. , Reactivation of dihydroorotate dehydrogenase-driven pyrimidine biosynthesis restores tumor growth of respiration-deficient cancer cells. Cell Metab. 29, 399–416.e10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnold I., Pfeiffer K., Neupert W., Stuart R. A., Schägger H., Yeast mitochondrial F1Fo-ATP synthase exists as a dimer: Identification of three dimer-specific subunits. EMBO J. 17, 7170–7178 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paumard P., et al. , The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 21, 221–230 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dudkina N. V., Sunderhaus S., Braun H. P., Boekema E. J., Characterization of dimeric ATP synthase and cristae membrane ultrastructure from Saccharomyces and Polytomella mitochondria. FEBS Lett. 580, 3427–3432 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Davies K. M., et al. , Macromolecular organization of ATP synthase and complex I in whole mitochondria. Proc. Natl. Acad. Sci. U.S.A. 108, 14121–14126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunter D. R., Haworth R. A., The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch. Biochem. Biophys. 195, 468–477 (1979). [DOI] [PubMed] [Google Scholar]
- 47.Haworth R. A., Hunter D. R., Allosteric inhibition of the Ca2+-activated hydrophilic channel of the mitochondrial inner membrane by nucleotides. J. Membr. Biol. 54, 231–236 (1980). [DOI] [PubMed] [Google Scholar]
- 48.Carraro M., et al. , High-conductance channel formation in yeast mitochondria is mediated by F-ATP synthase e and g subunits. Cell. Physiol. Biochem. 50, 1840–1855 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Pfeiffer D. R., Gudz T. I., Novgorodov S. A., Erdahl W. L., The peptide mastoparan is a potent facilitator of the mitochondrial permeability transition. J. Biol. Chem. 270, 4923–4932 (1995). [DOI] [PubMed] [Google Scholar]
- 50.Brustovetsky N., Dubinsky J. M., Limitations of cyclosporin A inhibition of the permeability transition in CNS mitochondria. J. Neurosci. 20, 8229–8237 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neginskaya M. A., et al. , ATP synthase c-subunit-deficient mitochondria have a small cyclosporine A-sensitive channel, but lack the permeability transition pore. Cell Rep. 26, 11–17.e2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou W., Marinelli F., Nief C., Faraldo-Gómez J. D., Atomistic simulations indicate the c-subunit ring of the F1Fo ATP synthase is not the mitochondrial permeability transition pore. eLife 6, e23781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ran F. A., et al. , Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wittig I., Carrozzo R., Santorelli F. M., Schägger H., Supercomplexes and subcomplexes of mitochondrial oxidative phosphorylation. Biochim. Biophys. Acta 1757, 1066–1072 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Ong S. E., et al. , Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 1, 376–386 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.