Significance
Host innate immune cells, such as macrophages, can endocytose the invading pathogens and induce inflammatory innate responses to fight infection. In this study, we show that a Cav1-interacting protein, LAPF, promotes the endocytosis of bacteria and production of proinflammatory cytokines by inducing Src-LAPF-Caveolin complex formation in response to innate stimuli. Our results reveal a host defense strategy against bacterial infection by increasing macrophage endocytosis and induction of innate response. We propose that pharmacological activation of LAPF and Src could potentially be applied to the control of bacterial infections.
Keywords: LAPF, Caveolin-1, endocytosis, macrophage, inflammatory response
Abstract
Macrophages can internalize the invading pathogens by raft/caveolae and/or clathrin-dependent endocytosis and elicit an immune response against infection. However, the molecular mechanism for macrophage endocytosis remains elusive. Here we report that LAPF (lysosome-associated and apoptosis-inducing protein containing PH and FYVE domains) is required for caveolae-mediated endocytosis. Lapf-deficient macrophages have impaired capacity to endocytose and eliminate bacteria. Macrophage-specific Lapf-deficient mice are more susceptible to Escherichia coli (E. coli) infection with higher bacterial loads. Moreover, Lapf deficiency impairs TLR4 endocytosis, resulting in attenuated production of TLR-triggered proinflammatory cytokines. LAPF is localized to early endosomes and interacts with caveolin-1. Phosphorylation of LAPF by the tyrosine kinase Src is required for LAPF-Src-Caveolin complex formation and endocytosis and elimination of bacteria. Collectively, our work demonstrates that LAPF is critical for endocytosis of bacteria and induction of inflammatory responses, suggesting that LAPF and Src could be potential targets for the control of infectious diseases.
Innate immune cells detect invading pathogens and launch appropriate inflammatory responses to eliminate infections. These cells express many kinds of pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), which can recognize pathogen-associated molecular patterns (PAMPs) and activate downstream signaling cascades to induce inflammation (1). TLR4, the PRR that specifically recognizes lipopolysaccharide (LPS) of Gram-negative bacteria, activates nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) through myeloid differentiation factor 88 (MyD88) to induce the expression of proinflammatory cytokines (2, 3). In addition, TLR4 is internalized into endosomes upon recognizing LPS to induce IRF3-dependent type-I IFN production through the adaptor proteins Toll-IL-1 resistance domain-containing adaptor-inducing IFN-β (TRIF) and TRAM (TRIF-related adaptor molecule, refs. 4 and 5). However, the molecular mechanism by which innate signaling induces efficient innate responses needs further investigation.
Innate immune cells, such as macrophages, dendritic cells (DCs), monocytes, and neutrophils, can internalize the invading pathogens by endocytosis. After being internalized, pathogens become targets of a series of vesicular trafficking at organelles ranging from early endosomes to lysosomes, where they are killed by Mst1-Mst2-Rac signaling-induced reactive oxygen species (ROS) and subsequently degraded by hydrolytic enzymes. The antigens derived from those pathogens are then presented on major histocompatibility complex (MHC) molecules, which are subsequently recognized by T cell receptors and activate CD4+ and CD8+ T cells of the adaptive immune system (6). Endocytosis occurs via a variety of mechanisms, specifically clathrin-mediated endocytosis, caveolae-mediated endocytosis, macropinocytosis, and phagocytosis (7, 8). However, the mechanisms linking the endocytosis of invading pathogens and activation of innate signaling remain to be fully explored.
Caveolae, a kind of specialized lipid rafts, are bulb-shaped plasma membrane invaginations first described in the 1950s (9). Since then, caveolae have been reported to be broadly involved in many cell processes, such as endocytosis, transcytosis (a specialized form of endocytosis), lipid homeostasis, and signal transduction (10, 11). Pathogens that are internalized via caveolae-mediated endocytosis include FimH-expressing Escherichia coli, Simian Virus 40 (SV-40), Group A streptococci, and Brucella abortus (12). Caveolae are also involved in the endocytosis of receptors, such as TLR4 (13, 14), and this is an essential regulatory mechanism for innate immune responses and signal transduction. The main component proteins of caveolae are caveolins and cavins. Caveolin family consists of three members, namely, caveolin-1 (Cav1), caveolin-2 (Cav2), and caveolin-3 (Cav3). Cav1 and Cav2 are expressed in most cell types (15, 16). Cav3 is specifically expressed in muscle cells (17). Cav1 in caveolae binds TLR4, endothelial nitric oxide synthase (eNOS), MAPK, cyclooxygenase (COX), and integrin signaling molecules to initiate different signaling pathways (18–21). However, the innate function of Cav1 in bacterial infection and the underlying mechanism are yet to be determined.
In this study, we identified LAPF (lysosome-associated and apoptosis-inducing protein containing PH and FYVE domains), which was cloned by our laboratory (22, 23), as a Cav1-interacting protein by mass spectrometry. LAPF has been reported to act as an adaptor protein that recruits phosphorylated p53 to lysosomes to trigger lysosomal destabilization during apoptosis (22, 23). We now find that LAPF is critically involved in inducing innate immune responses and in enhancing bacterial endocytosis and the bactericide capacity of macrophages by inducing Src-LAPF-Caveolin complex formation.
Results
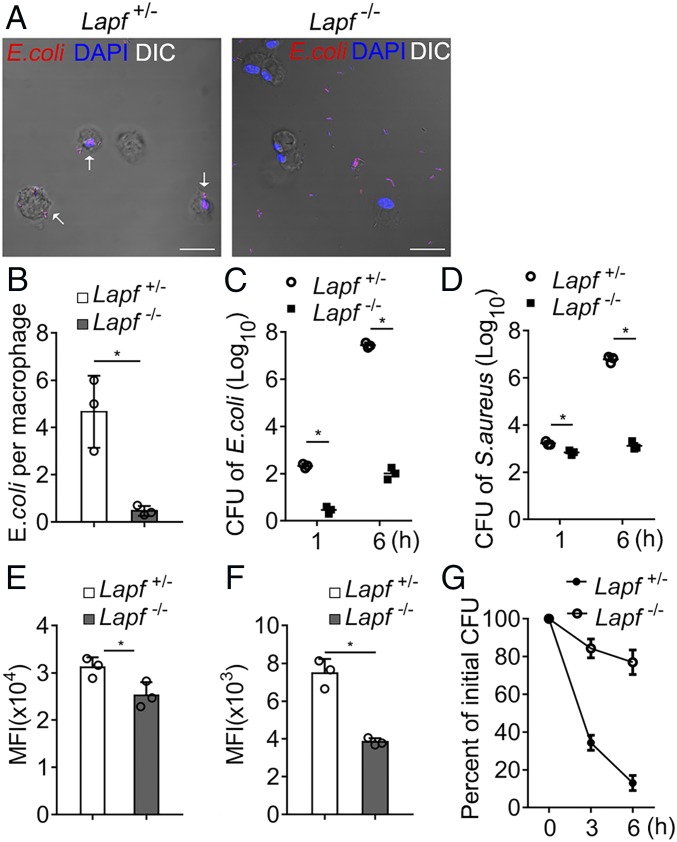
Lapf Deficiency Impairs Bacterial Endocytosis and Compromises the Bactericidal Ability of Macrophages.
To explore the molecular mechanism of caveolae-medicated endocytosis, we first screened for Cav1-interacting proteins by mass spectrometry (MS). LAPF was identified in the assay as a possible Cav1-interacting protein (SI Appendix, Fig. S1). We generated Lapffl/fl -LysMcre (Lapf−/−) mice, in which Lapf was deleted conditionally and efficiently in macrophages, using the littermate Lapffl/+-LysMcre (Lapf+/−) mice as a control group (SI Appendix, Figs. S2 and S3). We stimulated Lapf+/− and Lapf−/− peritoneal macrophages with heat-killed and PI (propidium iodide)-labeled E. coli for 1 h and visualized the internalization of bacteria particles using confocal microscopy (Fig. 1A and SI Appendix, Fig. S4). Lapf deficiency significantly impaired endocytosis of E. coli by macrophages (Fig. 1B). We incubated macrophages with viable E. coli and Staphylococcus aureus (S. aureus). Lapf−/− macrophages showed significantly decreased endocytosis of E. coli or S. aureus in comparison with Lapf+/− macrophages, as measured by colony-forming units (CFUs) (Fig. 1 C and D). We then incubated Lapf+/− and Lapf−/− macrophages with Zymosan and latex beads for 1 h, respectively. We found that the mean fluorescence intensity of Lapf−/− macrophages was significantly lower than that of Lapf+/− macrophages (Fig. 1 E and F). These results indicate that Lapf deficiency impairs the endocytosis of various pathogen particles by macrophages.
Fig. 1.
Lapf-deficient macrophages endocytose less E. coli and S. aureus and eliminate less endocytosed E. coli. (A) Confocal microscopy of immunofluorescence staining of heat-killed PI-labeled E. coli (red) endocytosed by Lapf+/− and Lapf−/− macrophages 1 h after incubation. (Scale bars, 25 µm.) (B) Counts of E. coli engulfed per Lapf+/− or Lapf−/− macrophages as in A. (C and D) CFU assays of E. coli (C) and S. aureus (D) engulfed by Lapf+/− and Lapf−/− macrophages 1 h after infection. CFU, colony-forming units. (E and F) MFI of BMDMs (bone marrow-derived macrophages) after incubation with Zymosan (E) and latex beads (F). MFI, mean fluorescence intensity. (G) Relative percent of live E. coli counts in Lapf+/− or Lapf−/− macrophages at indicated times compare with initial endocytosed E. coli (0 h) counts. Data are presented as mean ± SD of three independent experiments (B–G) or shown for one representative experiment from three independent experiments with similar results (A). *P < 0.05.
To directly evaluate the microbicidal capacity of macrophages, we exposed macrophages to E. coli for 6 h and set that as the initial internalization phase (shown as 0 h in Fig. 1G). We then counted the number of surviving E. coli inside the cells 3 or 6 h later by CFU assay (shown as 3 and 6 h in Fig. 1G). The percentage of live E. coli counts to initially internalized counts was significantly higher in Lapf−/− macrophages than in Lapf+/− macrophages (Fig. 1G), suggesting that Lapf deficiency impairs bacterial endocytosis and compromises the bactericide ability of macrophages.
Macrophage-Specific Lapf Deficiency Impairs the Elimination of Bacteria in Vivo.
We further investigated whether LAPF was required for the elimination of bacteria in vivo. Lapf−/− mice were more susceptible to E. coli infection than Lapf+/− mice (Fig. 2A). Remarkably, the bacterial loads in spleen and liver of Lapf−/− mice were significantly higher compared with control mice (Fig. 2 B and C). Concomitantly, we also measured the production of E. coli-induced inflammatory cytokines in vivo. Productions of TNFα, IFN-β, and IL-6 in serum significantly decreased in Lapf−/− mice in comparison with Lapf+/− mice (Fig. 2D). Infiltration of inflammatory cells into lungs of Lapf−/− mice also decreased (Fig. 2E). These results indicate that LAPF is essential for the clearance of bacterial infection and the induction of inflammatory response in vivo.
Fig. 2.
Lapf-deficient mice are more susceptible to E. coli infection with less inflammatory cytokines. (A) Survival of Lapf+/− and Lapf−/− mice (n = 10 per genotype), monitored daily after i.p. injection with E. coli (1 × 109/kg body weight). (B and C) Bacterial load in spleen (B) and liver (C) of Lapf+/− and Lapf−/− mice after injection as in A. (D) ELISA of TNF-α, IL-6, and IFN-β in serum collected 4 or 8 h after the injection as in A. (E) Hematoxylin-and-eosin staining of lung sections from Lapf+/− and Lapf−/− mice after injection as in A. (Scale bars, 400 μm.) Data are presented as mean ± SD of three independent experiments (B–D) or shown for one representative experiment from three independent experiments with similar results (A and E). *P < 0.05.
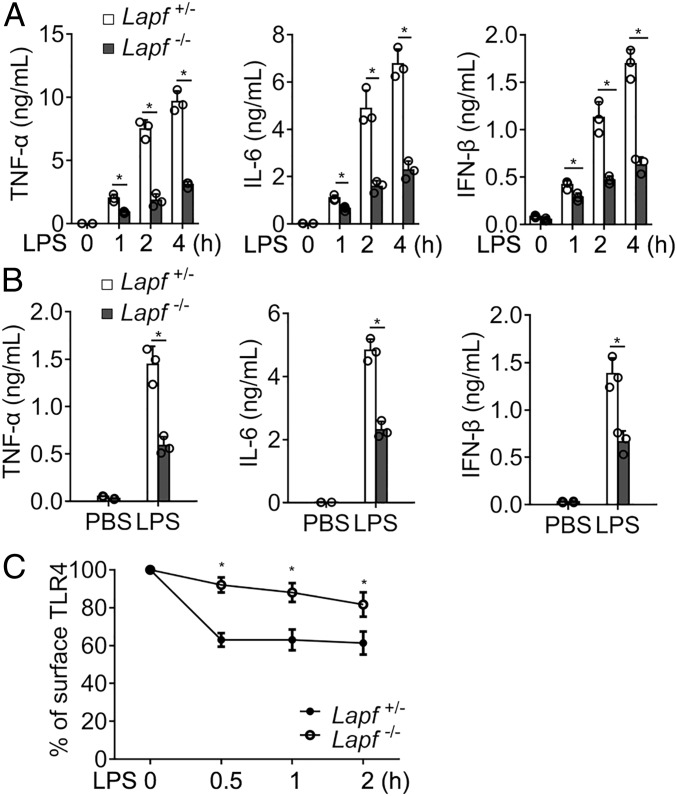
Lapf Deficiency Impairs TLR-Triggered Cytokine Productions by Decreasing TLR4 Endocytosis.
We further investigated how Lapf deficiency in macrophages affects the inflammatory response to bacterial infection. We found that the cytokine productions (TNF-α, IL-6, and IFN-β) of Lapf-deficient macrophages significantly decreased upon E. coli stimulation compared with control macrophages (SI Appendix, Fig. S5). To rule out the effect of bacterial endocytosis on cytokines production, we stimulated Lapf+/− and Lapf−/− macrophages with LPS. LPS-induced productions of TNF-α, IL-6, and IFN-β were significantly lower in Lapf−/− macrophages than in Lapf+/− macrophages (Fig. 3A). Likewise, the expression of Tnfa, Ifnb, and Il6 mRNA was also significantly decreased in Lapf−/− macrophages (SI Appendix, Fig. S6). We further examined whether Lapf deficiency affected TLR4-triggered signal transduction and found that Lapf deficiency decreased TLR4-triggered activation of TBK1, IRF3, IKKα/β, p65, JNK, ERK1/2, and p38 (SI Appendix, Fig. S7). When mice were challenged with LPS, we found that inflammatory cytokines in serum of Lapf−/− mice were significantly dampened compared with Lapf+/− mice (Fig. 3B).
Fig. 3.
Lapf deficiency impairs LPS-activated endocytosis of TLR4 and production of TNF-α, IL-6, and IFN-β. (A) ELISA of TNF-α, IL-6, and IFN-β in the supernatant of Lapf+/− and Lapf−/− macrophages stimulated with LPS (100 ng/mL) for the indicated times. (B) ELISA of TNF-α, IL-6, and IFN-β in serum of Lapf+/− and Lapf−/− mice challenged by LPS (10 mg/kg) for 3 h. (C) Percentage of surface TLR4 on Lapf+/− and Lapf−/− BMDMs (bone marrow-derived macrophages) upon LPS (100 ng/mL) stimulation. Data are presented as mean ± SD of three independent experiments. *P < 0.05.
We previously showed that endocytosis and membrane translocation of cytoplasmic of TLR4 and IFNγR2 were critical for the activation of innate response against intracellular bacterial infection in macrophages (24, 25). We thus evaluated whether Lapf deficiency affects endocytosis of TLR4. Indeed, TLR4 expression on Lapf−/− macrophages surface upon LPS stimulation was significantly higher than that on control macrophages surface (Fig. 3C and SI Appendix, Fig. S8).
We next investigated whether LAPF was involved in MyD88/TIRAP-dependent signaling. Neither Lapf deficiency nor LAPF overexpression affected the interaction of Myd88 with TLR4 or TRAF6 (SI Appendix, Fig. S9). Collectively, these results demonstrate that Lapf deficiency impairs TLR4-triggered signal transduction and inflammatory response by decreasing TLR4 endocytosis.
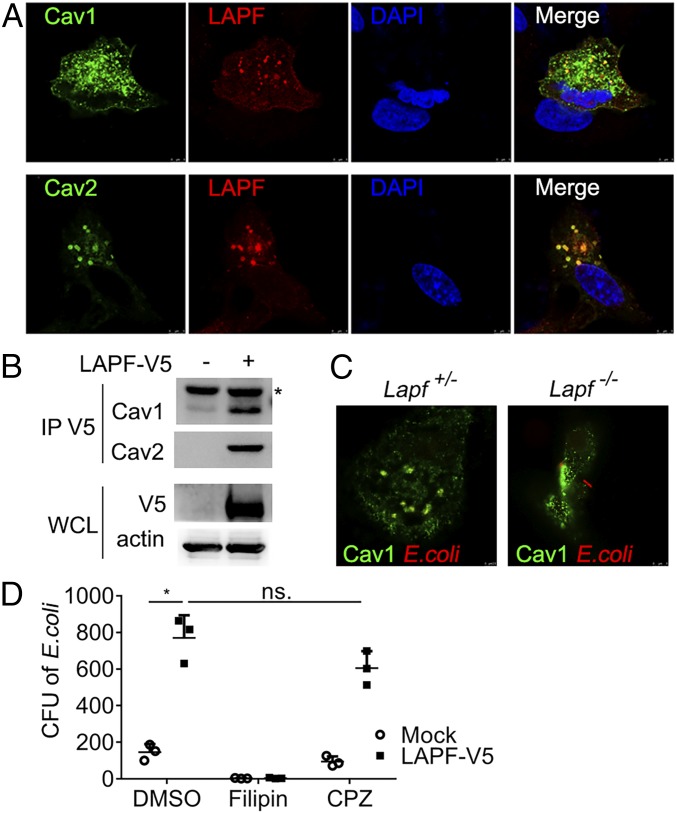
LAPF Promotes Internalization of E.coli by Caveolae-Mediated Endocytosis.
To investigate the underlying mechanism of LAPF in promoting bacterial internalization, we decided to examine the potential interaction of LAPF with Cav1 and Cav2 in more detail as LAPF was identified as a possible Cav1-binding protein in our MS experiment (SI Appendix, Fig. S1). Ectopically overexpressed LAPF colocalized with both Cav1 and Cav2 in A549 human adenocarcinoma cells (Fig. 4A). Likewise, overexpressed LAPF interacted with endogenous Cav1 and Cav2 in HEK293T human embryonic kidney cells, as detected by coimmunoprecipitation and immunoblotting analysis (Fig. 4B).
Fig. 4.
LAPF interacts with Cav1/2 and promotes caveolae-dependent endocytosis. (A) Confocal microscopy of the immunofluorescence staining of overexpressed LAPF and Cav1/2 in A549 cells. (Magnification, 252×.) (B) Immunoblot analysis of anti-V5 immunoprecipitated lysates of 293T cells. (C) Confocal microscopy of the immunofluorescence staining of Cav1 and E. coli in Lapf+/− and Lapf−/− macrophages. (Magnification, 252×.) (D) CFU assay after E. coli infection in macrophages pretreated with Filipin (3 μM) or CPZ (10 μM). Data are presented as mean ± SD of three independent experiments (D), or shown for one representative experiment from three independent experiments with similar results (A–C). *P < 0.05. ns., no significant differences.
Considering that caveolae-mediated endocytosis is critical for E. coli internalization (26), we investigated whether Lapf deficiency would impact the caveolae-dependent endocytosis of E. coli. We incubated Lapf+/− or Lapf−/−macrophages with heat-killed PI-labeled E. coli for indicated times and found that endocytosed E. coli colocalized with Cav1 in Lapf+/− macrophages, but not in Lapf−/−macrophages (Fig. 4C and SI Appendix, Fig. S10). We treated LAPF-overexpressed RAW264.7 cells with Filipin (caveolae-mediated endocytosis inhibitor) or Chlorpromazine (CPZ, clathrin-mediated endocytosis inhibitor) before E. coli infection. The endocytosis of E. coli by LAPF-overexpressed RAW264.7 cells was significantly inhibited by Filipin, but not by CPZ (Fig. 4D). These data demonstrate that LAPF promotes the endocytosis of bacteria by a caveolae-dependent mechanism.
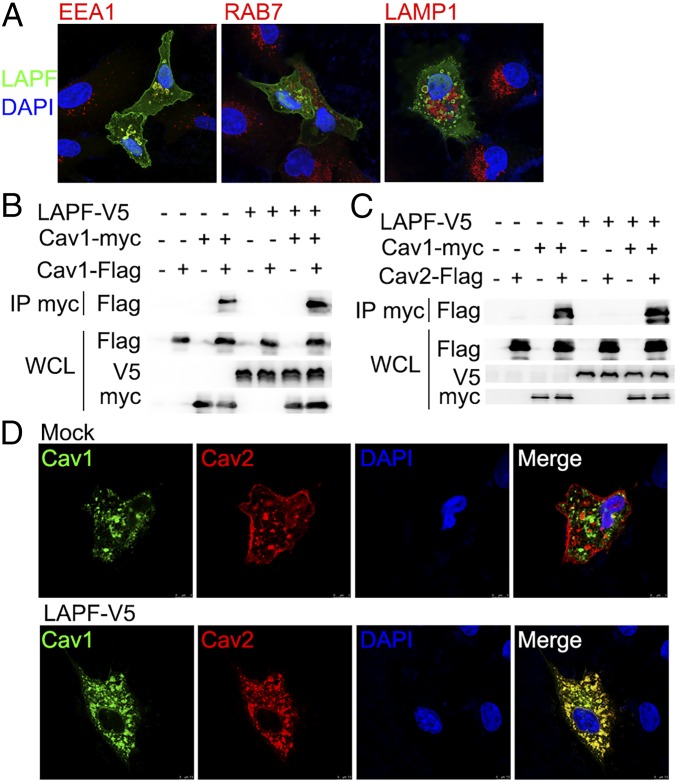
LAPF Promotes Caveolae Formation.
To investigate the mechanism of LAPF in caveolae-mediated endocytosis, we first examined the subcellular localization of LAPF. Overexpressed LAPF colocalized with EEA1, a marker of early endosomes, but not RAB7 (markers of late endosomes) or LAMP1 (markers of lysosomes) (Fig. 5A and SI Appendix, Fig. S11). Since there was no obvious difference between the expression of Cav1/Cav2 in Lapf+/− and Lapf−/− macrophages (SI Appendix, Fig. S3), we examined whether LAPF influenced caveolae formation, which was indicated by caveolins aggregation (27). Indeed, LAPF overexpression promoted both the oligomerization of Cav1 and the aggregation of Cav1/2 (Fig. 5 B and C), and LAPF overexpression substantially increased the colocalization of Cav1/2 (Fig. 5D). These results suggest that LAPF can interact with Cav1 and Cav2 and increase caveolae-mediated endocytosis by promoting caveolae formation.
Fig. 5.
LAPF locates on early endosomes and promotes aggregation of Cav1/2. (A) Confocal microscopy of the immunofluorescence staining of EEA1, RAB7, LAMP1, and overexpressed LAPF. (Magnification, 126×.) (B) Immunoblot analysis of anti-myc immunoprecipitated lysates of 293T cells cotransfected with V5-tagged LAPF and Flag-tagged Cav1. (C) Immunoblot analysis of anti-myc immunoprecipitated lysates of 293T cells cotransfected with V5-tagged LAPF and Flag-tagged Cav2. (D) Immunofluorescence analysis of overexpressed Cav1, Cav2, and LAPF in A549 cells. (Magnification, 252×.) Data are shown for one representative experiment from three independent experiments with similar results.
Phosphorylated LAPF Is Critical for Caveolae Formation and Bacterial Endocytosis.
We next examined the role of LAPF in caveolae formation. It has been reported that Cav1 can be phosphorylated by the tyrosine kinase Src and that activated Cav1 promotes caveolae-mediated endocytosis (27, 28). On the other hand, Cav2 could not be phosphorylated by Src (SI Appendix, Fig. S12). We found that overexpressed LAPF colocalized with Src (Fig. 6A and SI Appendix, Fig. S13). Furthermore, Src promoted tyrosine phosphorylation of LAPF (Fig. 6B), as well as oligomerization of LAPF (SI Appendix, Fig. S14).
Fig. 6.
LAPF induces Src-Cav1 complex upon E. coli stimulation. (A) Confocal microscopy of the immunofluorescence staining of overexpressed-LAPF and constitutively active Src (CA-Src). (Magnification, 252×.) (B) Immunoblot analysis of anti-Flag immunoprecipitated lysates of 293T cells cotransfected with LAPF-Flag and myc-tagged CA-Src with HA-tagged Akt as tyrosine phosphorylation negative control. (C) Immunoblot analysis of anti-myc immunoprecipitated lysates of 293T cells cotransfected with V5-tagged LAPF wild type (WT) or LAPF point mutant (Y64F, Y74F, Y208F, and Y268F). (D) Immunoblot analysis of anti-V5 immunoprecipitated lysates of 293T cells cotransfected with myc-tagged CA-Src. (E) Immunoblot analysis of anti-myc immunoprecipitated lysates of 293T cells cotransfected with Flag-tagged Cav2, V5-tagged LAPF wild type (WT), or LAPF point mutant (Y64F, Y74F, Y208F, and Y268F). (F) Confocal microscopy of the immunofluorescence staining of EEA1 and overexpressed-LAPF (Y208F or Y268F). (Magnification, 252×.) (G) Confocal microscopy of the immunofluorescence staining of overexpressed-Cav1 and LAPF-WT and LAPF mutants (Y208F or Y268F). (Magnification, 252×.) (H) Immunoblot analysis of LAPF and Cav1 in anti-Src immunoprecipitated lysates of macrophages stimulated with E. coli, with Src as a loading control and IgG as an immunoprecipitation control. (I) Immunoblot analysis of Cav1 in anti-Src immunoprecipitated lysates of Lapf+/− and Lapf−/− macrophages stimulated with E. coli, with Src as a loading control and IgG as an immunoprecipitation control. (J) CFU assays of LAPF-WT or LAPF-Y268F overexpressed RAW264.7 cells 1 h after E. coli infection. Data are presented as mean ± SD of three independent experiments (J) or shown for one representative experiment from three independent experiments with similar results (A–I). *P < 0.05.
LAPF contains four tyrosine residues that can be potential Src phosphorylation sites. We replaced each tyrosine in LAPF with phenylalanine (Y64F, Y74F, Y208F, or Y268F point mutation) and cooverexpressed each mutant together with Src. Interaction of LAPF with Src was impaired by tyrosine mutation of LAPF (Fig. 6C). Phosphorylation of LAPF mutants was substantially decreased compared with wild-type LAPF, especially of the Y208F and Y268F mutants (Fig. 6D). LAPF mutants were also unable to increase the aggregation of Cav1 and Cav2 (Fig. 6E). LAPF-Y208F and LAPF-Y268F still colocalized with early endosomal marker EEA1 (Fig. 6F and SI Appendix, Fig. S15), but no longer colocalized with Cav1 (Fig. 6G). These results indicate that phosphorylation of LAPF is required for its colocalization with Src and Cav1, as well as its function of promoting caveolae formation.
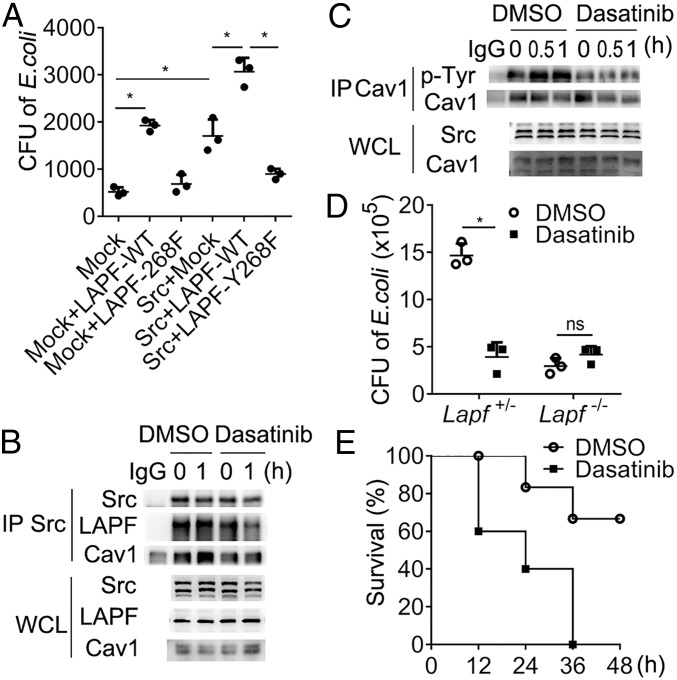
Next, we investigated the function of LAPF phosphorylation in bacterial endocytosis. We detected Src-LAPF-Cav1 formation in macrophages upon E. coli stimulation by immunoprecipitation and found that Src-LAPF-Cav1 complex formation peaked at 1 h after E. coli infection (Fig. 6H). We challenged the Lapf+/− and Lapf−/− macrophages with E. coli and found that Lapf deficiency impaired the interaction between Src and Cav1 (Fig. 6I). Overexpression of LAPF-WT in RAW264.7 cells increased E. coli endocytosis, whereas overexpression of LAPF-Y268F did not (Fig. 6J). These results indicate that phosphorylation of LAPF by Src is required for Src-Cav1 complex formation and bacterial endocytosis.
Src-LAPF-Cav1 Complex Is Critical for Endocytosis of Bacteria in Vivo and in Vitro.
Finally, we investigated whether the Src-LAPF-Cav1 complex is required for bacterial endocytosis. Overexpression of Src or LAPF-WT alone increased the endocytosis of bacteria in A549 cells, which was further increased by cooverexpression of Src and LAPF-WT together, but not by cooverexpression of Src and LAPF-Y268F (Fig. 7A). These results suggest that Src-LAPF-Cav1 complex was required for the endocytosis of bacteria in vitro. Src inhibitor dasatinib markedly inhibited the interaction of Src with LAPF and Cav1 (Fig. 7B), as well as phosphorylation of Cav1 (Fig. 7C). Moreover, dasatinib decreased bacteria endocytosis in Lapf+/− macrophages, but not in Lapf−/− macrophages (Fig. 7D). Furthermore, dasatinib-treated mice were more susceptible to E. coli infection compared with control-treated mice (Fig. 7E). These data indicate that Src-mediated LAPF phosphorylation and formation of Src-LAPF-Cav1 complex is critical for endocytosis of bacteria in vivo and in vitro.
Fig. 7.
Disruption of Src-LAPF-Cav1 complex formation impairs endocytosis of bacteria. (A) CFU analysis of E. coli in A549 cells overexpressing Src, LAPF-WT, or LAPF-268 as indicated. (B) Immunoblot analysis of Cav1 and LAPF in anti-Src immunoprecipitated lysates of macrophages pretreated with dasatinib as indicated and then stimulated with E. coli, with Src as a loading control and IgG as an immunoprecipitation control. (C) Immunoblot analysis of pTyr in anti-Cav1 immunoprecipitated lysates of dasatinib (1 μM) treated macrophages stimulated with E. coli, with Src as a loading control and IgG as an immunoprecipitation control. (D) CFU analysis of E. coli in Lapf+/− and Lapf−/− macrophages pretreated with dasatinib (1 μM). (E) Survival of mice (n = 10 per group) pretreated with DMSO or dasatinib (15 mg/kg body weight) and then intraperitoneally injected with E. coli (1 × 109/kg body weight). Data are presented as mean ± SD of three independent experiments (A and D), or shown for one representative experiment from three independent experiments with similar results (B, C, and E). ns, not significant. *P < 0.05.
Discussion
Innate immune cells can eliminate pathogens by internalizing them and inducing inflammatory innate responses. In this study, we found that LAPF functioned as a Cav1-interacting protein and promoted the endocytosis and elimination of bacteria and the production of proinflammatory cytokines. Lapf-deficient mice were susceptible to E. coli infection in vivo with higher bacteria load and less lung inflammation, highlighting the functional significance of LAPF-mediated balance of endocytosis and inflammation in host defense. Our results uncover a host defense strategy against bacterial infection both by increasing endocytosis and elimination of bacteria and by amplifying the innate immune response.
Caveolins, the main proteins of caveolae, broadly participate in both endocytosis of bacteria and the signaling of inflammation. Cav1 deficiency suppressed LPS-induced lung inflammation by decreasing eNOS expression (29), and Cav1 inhibited the upstream regulators of antioxidant defense enzymes to further exacerbate the inflammatory response in nonimmune cells, such as endothelial cells (30). Deletion of Cav1 attenuated phagocytosis in macrophages by decreasing the expression of CD36 and TLR4 (31). These results indicate that Cav1 may promote the inflammatory responses. Recently, ZNRF1 was shown to promote LPS and bacterial-induced proinflammatory cytokine production by ubiquitinating and degrading Cav1 (32), suggesting that Cav1 also has a negative role in TLR4 signaling. Thus, the role of caveolae and caveolins in inflammation or infection requires further clarification in different contexts of cell types and/or infection models. We discovered that Src-LAPF-Caveolin complex was required for endocytosis and elimination of bacteria in both epithelia cells and macrophages, suggesting a positive role of Cav1 in regulating inflammatory response.
It is known that TLR4 translocates to early endosomes where it activates the TRAM/TRIF pathway. Subsequently, TLR4 is sorted either to lysosomes for degradation to terminate the signaling or to the plasma membrane for receptor recycling (33). Endocytosis of TLR4 is required to amplify inflammatory responses (33). Our current work showed Lapf deficiency significantly impaired the endocytosis of TLR4 and suggested that the LAPF-Src-Cav complex is a molecular mediator involved in the signaling transduction of TLR4. Further research to reveal the function of LAPF in the recycling of TLR4 from the endosome or lysosome to cell membrane and oligomerization of TLR4 on membrane is needed.
Src-family kinases have been broadly studied and shown to modulate a wide range of cellular processes and regulators, such as the regulation of immunoreceptors, C-type lectins, integrins, G protein-coupled receptors, and many others through phosphorylating of immunoreceptor tyrosine-based inhibitory motifs (ITIMs), immunoreceptor tyrosine-based activation motifs (ITAM), or others (34). Src was also found to induce “inhibitory” signaling pathway on TLR-induced inflammatory responses from low avidity of integrin CD11b, DAP12, or FcRg (35). In the present study, a Src-LAPF-Caveolin complex is found to be critical for bactericide and inducing inflammatory responses. Moreover, inhibition of Src in vivo and in vitro by dasatinib aggravates bacterial infection and mortality. These results further suggest that activation of Src could be a therapeutic strategy for infectious diseases.
Our study suggests that LAPF is located in early endosomes. Considering that both LAPF and Cav1 have lipid-binding domains in sequence, further studies are needed to reveal which kind of lipid or membrane components, such as phosphatidylinositol, are required for their interactions. Our study found that Lapf deficiency impaired the bactericidal efficiency of macrophages. We speculate that early endosome-located LAPF might promote macrophages to kill intracellular bacteria by increasing acidification of endosome and lysosome (36). In addition, we changed our strategy to conditional knockout of Lapf instead of whole genome knockout because of the embryonic lethality. However, taking into account that LAPF plays a critical role in cell apoptosis (22, 23), the functions of LAPF in other cells or organs should be further determined using different conditional gene knockout strategies.
In conclusion, LAPF is required for endocytosis and elimination of bacteria, which provides host resistance to the invading bacteria. Our results reveal a molecular mechanism for how the innate immune cells exert their function against bacterial infection and propose that activation of LAPF and Src may be a potential approach to the control of bacterial infection.
Materials and Methods
Full details of materials and methods, including the generation and genotype identification of mice, cell culture, ELISA, plasmid constructs and transfection, RNA quantification, bacterial infection and CFU assay, immunoprecipitation and immunoblot analysis, immunofluorescent confocal microscopy, flow cytometry, and statistical analysis are provided in SI Appendix.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Key Research and Development Program of China (2018YFA0507401); National Natural Science Foundation of China (31522019, 81471568, 80178101, and 31770945); and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016-12M-1-003). We thank Ms. Xiaofei Li and Dr. Xingguang Liu for technical assistance.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1903896116/-/DCSupplemental.
References
- 1.Cao X., Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 16, 35–50 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Liu J., Cao X., Cellular and molecular regulation of innate inflammatory responses. Cell. Mol. Immunol. 13, 711–721 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J., Qian C., Cao X., Post-translational modification control of innate immunity. Immunity 45, 15–30 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Husebye H., et al. , Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 25, 683–692 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald K. A., et al. , LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J. Exp. Med. 198, 1043–1055 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauwels A. M., Trost M., Beyaert R., Hoffmann E., Patterns receptors and signals: Regulation of phagosome maturation. Trends Immunol. 38, 407–422 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doherty G. J., McMahon H. T., Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902 (2009). [DOI] [PubMed] [Google Scholar]
- 8.El-Sayed A., Harashima H., Endocytosis of gene delivery vectors: From clathrin-dependent to lipid raft-mediated endocytosis. Mol. Ther. 21, 1118–1130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada E., The fine structure of the gall bladder epithelium of the mouse. J. Biophys. Biochem. Cytol. 1, 445–458 (1955). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamaze C., Tardif N., Dewulf M., Vassilopoulos S., Blouin C. M., The caveolae dress code: Structure and signaling. Curr. Opin. Cell Biol. 47, 117–125 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Parton R. G., Caveolae: Structure function and relationship to disease. Annu. Rev. Cell Dev. Biol. 34, 111–136 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez N. E., Gaur U., Wilson M. E., Role of caveolae in Leishmania chagasi phagocytosis and intracellular survival in macrophages. Cell. Microbiol. 8, 1106–1120 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Płóciennikowska A., Hromada-Judycka A., Borzęcka K., Kwiatkowska K., Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 72, 557–581 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascual-Lucas M., Fernandez-Lizarbe S., Montesinos J., Guerri C., LPS or ethanol triggers clathrin- and rafts/caveolae-dependent endocytosis of TLR4 in cortical astrocytes. J. Neurochem. 129, 448–462 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Rothberg K. G., et al. , Caveolin, a protein component of caveolae membrane coats. Cell 68, 673–682 (1992). [DOI] [PubMed] [Google Scholar]
- 16.Maceckova M., et al. , Bone marrow-derived macrophages exclusively expressed caveolin-2: The role of inflammatory activators and hypoxia. Immunobiology 220, 1266–1274 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Way M., Parton R. G., M-caveolin, a muscle-specific caveolin-related protein. FEBS Lett. 376, 108–112 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Chidlow J. H. Jr, Sessa W. C., Caveolae, caveolins, and cavins: Complex control of cellular signalling and inflammation. Cardiovasc. Res. 86, 219–225 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin L., et al. , Caveolae and Caveolin-1 integrate reverse cholesterol transport and inflammation in atherosclerosis. Int. J. Mol. Sci. 17, 429–445 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sotgia F., et al. , Caveolin-1 and cancer metabolism in the tumor microenvironment: Markers, models, and mechanisms. Annu. Rev. Pathol. 7, 423–467 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Feng H., Guo W., Han J., Li X. A., Role of caveolin-1 and caveolae signaling in endotoxemia and sepsis. Life Sci. 93, 1–6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W., et al. , The lysosome-associated apoptosis-inducing protein containing the pleckstrin homology (PH) and FYVE domains (LAPF), representative of a novel family of PH and FYVE domain-containing proteins, induces caspase-independent apoptosis via the lysosomal-mitochondrial pathway. J. Biol. Chem. 280, 40985–40995 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Li N., et al. , Adaptor protein LAPF recruits phosphorylated p53 to lysosomes and triggers lysosomal destabilization in apoptosis. Cancer Res. 67, 11176–11185 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., et al. , Lysosome-associated small Rab GTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood 110, 962–971 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Xu X., et al. , Phosphorylation-mediated IFN-gammaR2 membrane translocation is required to activate macrophage innate response. Cell 175, 1336–1351.e17 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Shin J. S., Gao Z., Abraham S. N., Involvement of cellular caveolae in bacterial entry into mast cells. Science 289, 785–788 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Parton R. G., del Pozo M. A., Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 14, 98–112 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Minguet S., et al. , Caveolin-1-dependent nanoscale organization of the BCR regulates B cell tolerance. Nat. Immunol. 18, 1150–1159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrean S., et al. , Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J. Immunol. 177, 4853–4860 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Layne J., Majkova Z., Smart E. J., Toborek M., Hennig B., Caveolae: A regulatory platform for nutritional modulation of inflammatory diseases. J. Nutr. Biochem. 22, 807–811 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai T. H., et al. , Impaired Cd14 and Cd36 expression, bacterial clearance, and Toll-like receptor 4-Myd88 signaling in caveolin-1-deleted macrophages and mice. Shock 35, 92–99 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Lee C. Y., et al. , The ubiquitin ligase ZNRF1 promotes caveolin-1 ubiquitination and degradation to modulate inflammation. Nat. Commun. 8, 15502 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosadini C. V., Kagan J. C., Early innate immune responses to bacterial LPS. Curr. Opin. Immunol. 44, 14–19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowell C. A., Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: Signaling cross talk. Cold Spring Harb. Perspect. Biol. 3, a002352 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han C., et al. , Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat. Immunol. 11, 734–742 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Mitchell G., Isberg R. R., Innate immunity to intracellular pathogens: Balancing microbial elimination and inflammation. Cell Host Microbe 22, 166–175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.