Significance
Several species of Caenorhabditis nematodes, including Caenorhabditis elegans, have recently evolved self-fertile hermaphrodites from female/male ancestors. These hermaphrodites can either self-fertilize or mate with males, and the extent of outcrossing determines subsequent male frequency. Using experimental evolution, the authors show that a gene family with a historical role in sperm competition plays a large role in regulating male frequency after self-fertility evolves. By reducing, but not completely eliminating outcrossing, loss of the mss genes contributes to adaptive tuning of the sex ratio in a newly self-fertile species.
Keywords: sex ratio, androdioecy, nematodes, sperm
Abstract
The maintenance of males at intermediate frequencies is an important evolutionary problem. Several species of Caenorhabditis nematodes have evolved a mating system in which selfing hermaphrodites and males coexist. While selfing produces XX hermaphrodites, cross-fertilization produces 50% XO male progeny. Thus, male mating success dictates the sex ratio. Here, we focus on the contribution of the male secreted short (mss) gene family to male mating success, sex ratio, and population growth. The mss family is essential for sperm competitiveness in gonochoristic species, but has been lost in parallel in androdioecious species. Using a transgene to restore mss function to the androdioecious Caenorhabditis briggsae, we examined how mating system and population subdivision influence the fitness of the mss+ genotype. Consistent with theoretical expectations, when mss+ and mss-null (i.e., wild type) genotypes compete, mss+ is positively selected in both mixed-mating and strictly outcrossing situations, though more strongly in the latter. Thus, while sexual mode alone affects the fitness of mss+, it is insufficient to explain its parallel loss. However, in genetically homogenous androdioecious populations, mss+ both increases male frequency and depresses population growth. We propose that the lack of inbreeding depression and the strong subdivision that characterize natural Caenorhabditis populations impose selection on sex ratio that makes loss of mss adaptive after self-fertility evolves.
Evolutionary transitions in sexual systems are common in many animal and plant taxa (1). One of the most extreme is the adoption of self-fertility. Historically hermaphroditic organisms, such as flowering plants, evolve selfing through loss of mechanisms that prevent union of gametes of the same individual (2–4). However, ancestrally male/female (dioecious or gonochoristic) organisms can also evolve selfing when the female sex evolves male function to become a neohermaphrodite (5–7). This process creates an androdioecious mating system in which hermaphrodites and males coexist (8–10). Androdioecious populations can have highly skewed sex ratios, as males are optional for reproduction.
In Caenorhabditis nematodes, Caenorhabditis elegans, Caenorhabditis briggsae, and Caenorhabditis tropicalis have evolved self-fertility independently from male/female ancestors (11–13). Selfing increases homozygosis and unmasks deleterious mutations (14–16). With the right amount of selfing, however, deleterious mutations can be purged from the genome (17, 18). Indeed, selfing Caenorhabditis have healthy, naturally homozygous genotypes and are actually susceptible to outbreeding depression (19, 20). Nevertheless, experimental evolution studies reveal that the frequency of males and outcrossing become elevated with increased mutation rate or novel environmental challenges, such as coevolving parasites (21–23). A similar benefit for outcrossing in facilitating adaptation has also been observed in hermaphroditic snails (24).
In addition to possible fitness differences between selfed and outcross progeny, the frequency of males is strongly influenced by male mating ability and hermaphrodite receptivity, which vary among species and populations (21, 25). To successfully produce outcrossed progeny, a male must locate a hermaphrodite, initiate a complex suite of copulating behaviors, and successfully transfer sperm (26, 27). After insemination, male sperm must compete with the hermaphrodite’s self-sperm for fertilization. The XX–XO system of genetic sex determination of Caenorhabditis means that variation in pre- or postmating male mating success will impact the frequency of males (28). However, the genetic basis of male mating ability and how it links to the difference in male frequency remains unclear.
XO Caenorhabditis males are produced from either outcrossing or from the spontaneous nondisjunction of the X chromosome during meiosis. The frequency of males varies in different species and populations (21, 29, 30), but it is always below 0.5. N2 Bristol, the common C. elegans laboratory strain, has a male frequency of ≤0.002 (8, 30), near the rate of production by nondisjunction (31–33). The low frequency of males in these selfing lineages may often be adaptive. Under benign conditions, hermaphrodites enjoy a population growth rate advantage that allows invasion of male/female populations by eliminating the cost of males (34, 35). In addition, selfing provides reproductive assurance where mates are limited, facilitating colonization of new habitat patches (36–38). Finally, the process of mating with males can physically and physiologically damage the hermaphrodites (39–42).
The simultaneous benefits of selfing and need for at least occasional outcrossing may create an intermediate optimum sex ratio in androdioecious species like C. elegans. The modulation of sex ratio could be implemented at the level of mating success. Previously we identified a Caenorhabditis gene family, mss (for “male secreted short”), that can affect male frequency. mss genes are conserved in outcrossing species, but are consistently lost in the androdioecious species (43). MSS proteins are heavily glycosylated surface factors that confer male sperm competitiveness. In the outcrossing Caenorhabditis remanei, mutant males lacking all mss paralogs are fully fertile, yet sire fewer progeny than wild-type (WT) mss+ males in competition. In the selfing C. briggsae, transgenic males in which mss has been restored sire more outcrossed progeny than mss− (i.e., WT) males, and are more effective at suppressing hermaphrodite self-sperm use. The strong advantage conferred by mss thus presents a mystery: Why would the males of androdioecious species consistently lose mss if it benefits them? One possibility is that the reduction of sexual selection leads to loss of mss purely through drift. Here, we consider an alternative, namely that mss loss was adaptive in the incipient selfing ancestors of C. elegans and C. briggsae because it resolved a sexual conflict over mating (44).
Hamilton (45) proposed that in isolated small populations where only mated females disperse, interdemic selection favors a female-biased sex ratio. The inbreeding associated with this mating system imposes additional selection to minimize male frequency (46). Though these models were produced with fig wasps and other gonochoristic organisms in mind, they are also highly relevant to Caenorhabditis, which colonize spatially isolated fruits in small numbers (13, 47, 48). These circumstances resemble Hamilton’s local mate competition (LMC) scenario, but offer the extreme case of zero males as a viable option to selfing hermaphrodites.
We have used simple population models and experimental cultures to simulate natural populations of the sort that may have existed as C. briggsae was evolving, and adapting to, selfing. We find that the change to partial self-fertility is insufficient to explain the parallel loss of the mss genes under a model of global panmixia. We therefore hypothesized that perhaps C. briggsae populations without mss (which we will refer to as mss−) would grow faster than others with mss+, owing to a lower male frequency and increased egg output in the former. We provide theoretical and experimental support for the plausibility of this scenario. Overall, our results suggest that mating system and population structure have interacted to promote the adaptive loss of the mss family in selfing Caenorhabditis species.
Methods
C. briggsae Strains.
AF16, a C. briggsae wild isolate originally isolated in India, was obtained from the Caenorhabditis Genetics Center. CP161 (nmIs7[Cni-mss-1(+) Cni-mss-2(+) Cbr-myo-2::GFP unc-119(+)]; Cbr-unc-119(nm67) III) and CP162 (nmIs8[Cni-mss-1(+) Cni-mss-2(+) Cbr-myo-2::GFP unc-119(+)]; Cbr-unc-119(nm67) III) are independent mss+ chromosomally integrated transgenic strains generated through microparticle bombardment as described previously (43). In addition to mss genes from Caenorhabditis nigoni, the construct has a dominant Cbr-myo-2::GFP reporter and Cbr-unc-119(+), which serves as a marker for identification of successful transgenic lines. CP164 was generated by crossing Cbr-she-1(v49) (49) to CP161(nmIs7), followed by selfing and sibling mating to isolate an outcrossing strain that is homozygous for both mss+ transgene and she-1(v49). Since worms carrying she-1 alleles are inherently temperature sensitive, the strains were maintained at 25 °C to prevent selfing.
Modeling Interaction of Male Frequency, Mating Success, and mss+ Allele Frequency.
We used a deterministic, discrete-generation model with no resource limitation, which should be accurate in early stages of population expansion. The male frequency in a given generation, m’, is calculated from male frequency at the previous generation (m) and male fertilization success (α) as m’ = mα/2. Hermaphrodite frequency equals (1 − m). The presence of the mss+ transgene is assumed to only impact α, through its effect on sperm competitiveness. For the mixed-genotype models (Fig. 1A), we assumed dominance of mss+ in mss−/mss+ males and no impact of mss genotype on hermaphrodites or females (43). For the gonochoristic version of the model (Fig. 1B), we assume all eggs are cross-fertilized, so that a male genotype’s contribution to the next generation is the product of its frequency and a sperm competition factor, C, associated with that genotype.
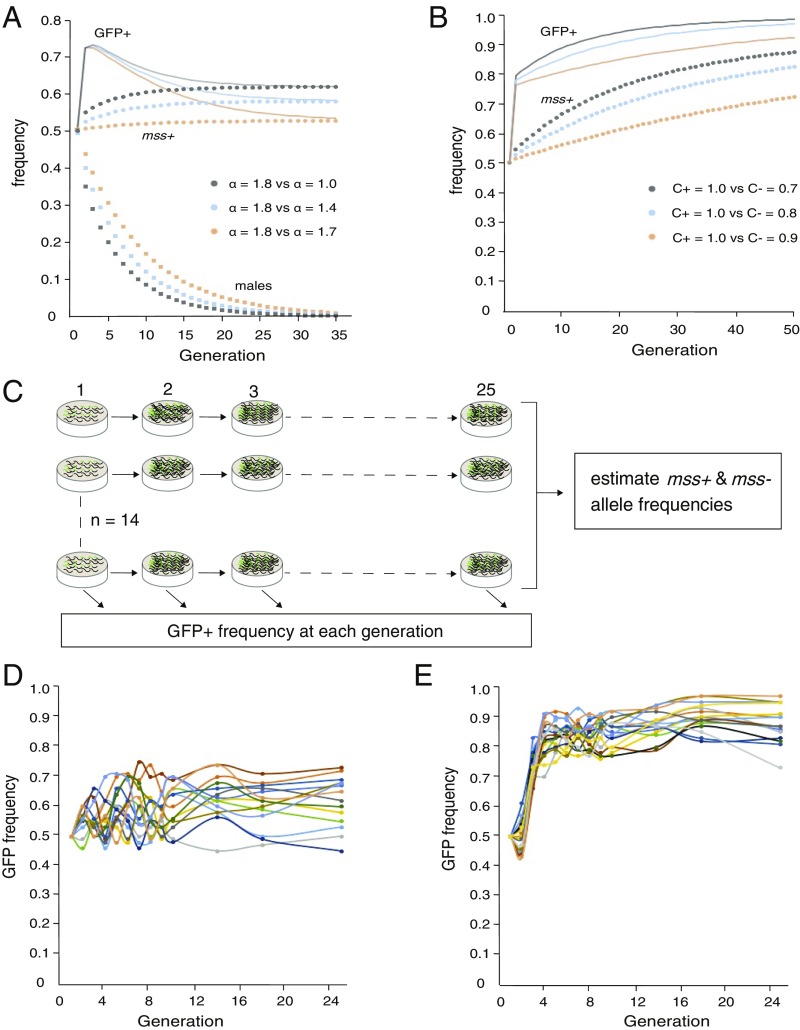
Fig. 1.
Relative fitness of mss+ and mss− genotypes in androdioecious and gonochoristic populations. (A) Modeling the competition between mss+ and mss− genotypes in a panmictic population, founded with equal proportions of selfing hermaphrodites and males, half of which are homozygous for each genotype. Over time the mss+ allele frequency (Upper colored dots) increases, but slows as males are eliminated (because α < 2). The expected sum of mss+/+ and mss+/− diploid genotype frequencies is also shown (GFP+, thin solid lines) for comparison with experimental data. (B) Modeling the competition between mss+ and mss− genotypes in a panmictic, obligately outcrossing (i.e., male/female) population. The sperm competitiveness factor (C) for mss+ is set at 1, while that for mss− males can vary from 0 (sterility) to 1 (equal to mss+). (C) Experimental evolution scheme for assessing mss+ fitness in androdioecios and gonochoristic population. (D) In a mixed population of C. briggsae WT AF16 (mss−, GFP−) and CP161 (mss+, GFP+), where selfing and outcrossing coexist, GFP+ (sum of mss+/+ and mss+/− diploid genotype) frequency increases from 50 to a median of 63.5% over 25 generations. The estimated mean and median mss frequency at the 25th generation, 0.59 and 0.57, are significantly higher than 0.50 (P = 0.0287; one-tailed sign test, n = 14). (E) Competition in a mixed population of C. briggsae she-1(v49); mss−, GFP− and CP164 (she-1(v49); mss+, GFP+), where all XX animals lack self-sperm. Median GFP+ frequency increased to 0.72 at the third generation, and was 0.87 at the 25th generation. The GFP+ frequency distribution at generation 25 is significantly higher than 0.75 (P = 0.0002594; one-tailed sign test, n = 16). Similarly, the mean mss+ allele frequency at the 25th generation is 0.67, significantly higher than the initial 0.50. (P = 7.629e-06; one-tailed sign test, n = 16).
Experimental Evolution.
For mss+ vs. mss− competition experiments, bulk crosses were first established separately for the AF16 and from CP161 strains. Fifty L4 males and 50 L4 hermaphrodites were allowed to cross for 24 h. Next, five mated hermaphrodites and five males from each strain were transferred to a new plate to form a mixed-genotype population of 20 adults. Fourteen replicates were grown at 25 °C on 6-cm nematode growth medium (NGM) agar plates seeded with Escherichia coli strain OP50. Every 3 d, roughly 3% of a culture was transferred to a new plate by excising a 1-cm square of agar. The number of worms with and without GFP expression were counted every generation through the 10th generation, and then at the 14th, 18th, and 25th generation (Dataset S1). Cbr-she-1(v49) and CP164 experiments were set up in the same way except there were 16 replicates.
For the experiments described in Fig. 1 C–E, we determined the fractions of GFP+ and GFP− animals by scoring 100 randomly chosen animals per experimental line with a fluorescence stereoscope. This also provides an estimate of the frequency of the mss−/− genotype. To estimate the frequencies of mss+/+ and mss+/− genotypes at generation 25, we isolated 6–8 individual virgin GFP+ hermaphrodites or females from each replicate, and either allowed them to lay self-progeny or mated them with Cbr-she-1 (GFP−) males. Offspring were scored for GFP expression, and in all cases broods were either all GFP+ (indicating the test mother was mss+/+) or segregating for GFP+ and GFP− (indicating the mother was mss+/−). Genotype counts and estimated allele frequencies for both sets of experimental lines are provided in Dataset S1. One-tailed sign tests were used to test whether the observed increase in mss+ was significant, compared with the initial frequency, and were computed using R version 3.5.1. χ2 tests were performed on the deviation between observed and expected frequency based on Hardy–Weinberg equilibrium.
Measuring Sex Ratios and Population Growth in Genetically Homogenous Cultures.
For each strain (AF16, CP161, and CP162), 50 L4 males and 50 L4 hermaphrodites were set up for crossing for 24 h, after which an individual hermaphrodite (as verified by mating plug) was transferred to a 6-cm NGM plate seeded with E. coli strain OP50 (3-cm diameter) and allowed to lay embryos for 40 h before the hermaphrodite mother was removed. After 3 d of incubation at room temperature, the number of F1 males and hermaphrodites (L4 and adult stages) was counted. Worms were washed off of the plates in M9 buffer with 0.05% Tween (to prevent worms sticking to pipette tips) and resuspended in 200 µL in a 2-mL microfuge tube. The entire suspension was transferred onto a plain glass slide (AmScope) as thin streaks of liquid for counting with a stereoscope.
F2 populations were cultured as described above, except the mated P0 hermaphrodite was transferred to a 10-cm NGM plate (OP50 bacterial spot diameter: 6 cm). The larger plates ensured the worms did not run out of food after two generations. After 5 d at room temperature, the worms were washed into a 15-mL Falcon tube. For sex ratio estimation, ∼100 worms per plate were transferred onto a plain glass slide. For automated counting of entire cultures, a separate set of culture plates was used. Each worm suspension was diluted 10-fold with M9 buffer and aliquoted to 96-well microtiter plates at a density of approximately 10 worms per microliter. The entire population of animals in the 96-well microtiter plate was then counted using the large particle nematode sorter (COPAS Biosort, Union Biometrica). Initially no specific size gating was set, so that worms of all sizes and stages, including young L1, L2, and embryos, were included in the counts. Subsequently, we realized F2 animals were beginning to lay embryos and these were hatching in L1 larvae. Smith et al. (50) demonstrated extinction (EXT) values below 39 (log EXT <3.669) correspond to L1 larvae and detritus. We therefore used this as a cutoff to distinguish F2 adults from young F3 larva. Only older F2 worms were used in the summary statistics boxplots and calculation of its statistical significance.
Results
Fitness of mss+ Allele in Competitive Context Varies with Mating System.
In extant C. briggsae, traces of mss genes remain as pseudogenes (43), indicating they were lost recently. To assess how the shift to selfing may have impacted mss fitness, we first modeled the population dynamics of an mss-like modifier of male fertilization success in both the derived self-fertile condition and in the ancestral, obligately outcrossing system. Our approach was similar to previous models of the maintenance of males in androdioecious nematodes (35, 51, 52). In principle, selfing rate, male fertilization success (α), inbreeding depression, the rate of nondisjunction at the X chromosome, and relative viability difference between males and hermaphrodites all can impact the frequency of males. However, since inbreeding depression is low in long-selfing species (19, 20, 53), X nondisjunction is rare, and male and hermaphrodite viability is equal in the early, reproductive phase of life, a simplified model for the change of male frequency overtime would be m′ = αm/2, where m and m′ are male frequency at current and subsequent generation, respectively, and α is the male fertilization success. α combines both male mate-finding (premating) and sperm precedence (postmating) success, and can be anywhere from 0 to 2. αm is the proportion of hermaphrodite eggs that are outcrossed.
When α is maximal, m′ = m, and males are maintained. If males additionally start as half the population, all progeny are cross-fathered and the population is a male/female equivalent. Assuming a constant value of α at all male frequencies, any value of less than two leads to eventual loss of males. There is some indication that α may vary as a function of male frequency (35), but in common laboratory strains (e.g., C. elegans N2, C. briggsae AF16) populations enriched for males steadily lose them (8, 35, 43). However, even in this case, mss+ males still have a transient advantage over mss− males, and the mss+ genotype is expected to increase in frequency whenever males are present in appreciable numbers (Fig. 1A). In the extreme case of obligate outcrossing, mss+ should steadily increase in frequency until it is fixed in the population (Fig. 1B), consistent with its consistent presence in gonochoristic Caenorhabditis.
To experimentally assess how the change to selfing impacts the fitness of the mss+ genotype, we carried out two experimental evolution studies. One employed self-fertile stocks mixed with males, and the other a self-sterile, obligately outcrossing strain homozygous for a she-1 (spermless hermaphrodite) loss-of-function mutation (49). she-1 mutants reproduce effectively as a male/female strain, similar to fog-2 mutant C. elegans (54). The frequency of the mss+ transgene, which is marked with a dominant myo-2::GFP reporter, was tracked in mixed-genotype populations for 25 generations (Fig. 1C). Each replicate began with equal numbers of mated AF16 (mss−/−, GFP−) and CP161 (mss+/+, GFP+) hermaphrodites.
In the mixed-mating populations, GFP+ frequency is expected to increase from the initial 0.50 due to the formation of heterozygotes by crosses between the parental strains, and potentially by selection for the mss+ allele. However, only with positive selection will the mss+ allele frequency be consistently elevated above the initial 0.50. In our experiment, GFP+ frequency rose to a median of 63.5% (Fig. 1D). Guided by an empirical estimate of heterozygote and homozygote fractions, we converted observed GFP+ frequencies at generation 25 to mss+ allele frequencies. These ranged from 0.42 to 0.67, had a mean of 0.57 (SEM ± 0.02), and for 11 of 14 replicates were greater than 0.50. This distribution is shifted significantly above the starting frequency of 0.50. We conclude that the mss+ allele is under modest but detectable positive selection in the mixed-mating case. Male frequency at generation 25 (0.09) remained above what our simple model would predict (see Discussion).
Next we asked how the frequency of mss+ would change in a C. briggsae strain that can only outcross, approximating the ancestral gonchoristic condition. When mixed populations were established with equal numbers of mss−; she-1 (GFP−) and mss+; she-1 (GFP+) worms, GFP+ frequency increased to a median 0.72 at the third generation. This initial increase can be fully explained by the formation of the first heterozygotes. However, the frequency of GFP+ genotypes continued to rise, to a median value of 0.87 at the 25th generation (Fig. 1E). This is significantly higher than the 0.75 expected from genetic drift alone. In addition, the mean mss+ allele frequency at the 25th generation is 0.67, significantly higher than the initial 0.50. These results indicate that mss+ also enjoys higher fitness than mss− in she-1 mutants and confirm that obligate outcrossing selects more strongly for mss+ than the mixed-mating case.
mss+ Increases Male Production and Depresses Growth.
Given the measurable positive selection for mss+ in large mixed-mating populations, we considered an alternative situation, in which small, genetically homogenous subpopulations compete against each other. We previously observed that CP161 and CP162, two transgenic mss+ C. briggsae strains, were better at maintaining males compared with the mss− AF16 WT strain (43). Modeling predicts that in populations of initially identical size, those that are mss+ (i.e., whose males have a higher value of α) would produce more male offspring (and thus fewer hermaphrodites) and grow more slowly than those that are mss− (Fig. 2 A and B). To test the first prediction, mated hermaphrodites of the AF16 wild-type, CP161, and CP162 strains were used to found single-genotype populations (Fig. 2C). As expected, the percentage of males in the AF16 (mss−) population was significantly lower than in the two mss+ populations in the second (i.e., F1; Fig. 2D) and third (i.e., F2; Fig. 2E) generations.
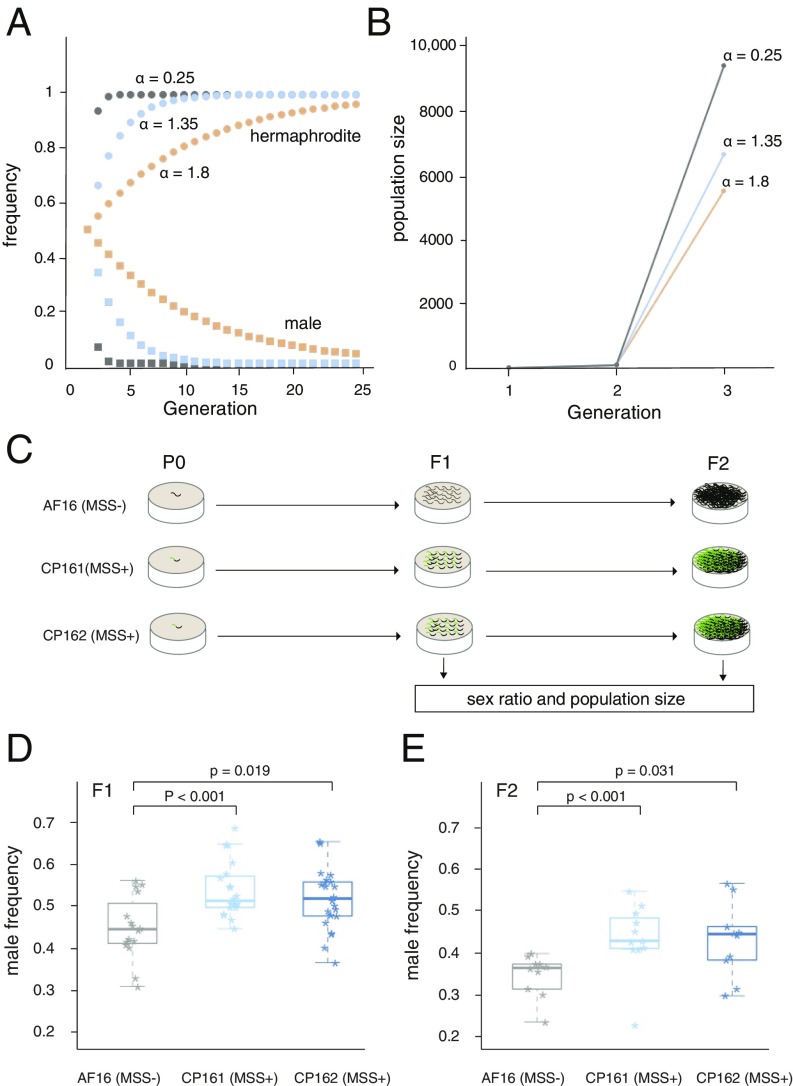
Fig. 2.
mss+ C. briggsae produce more males. (A) Modeling the impact of varying fertilization success (α) on sex ratio over time in a genetically homogenous population. Males and hermaphrodites are both at 50% frequency in the first generation. At any value of α < 2 male frequency declines and hermaphrodite frequency increases. (B) Expected impact of α on population growth. At generation one, the population is founded with one mated hermaphrodite. At generation two, population size is determined solely by the fecundity of this hermaphrodite (here we assume each produces 100 eggs). The advantage of low male fertilization success is seen in the substantially larger number of grand-offspring in generation three. (C–E) Experimental examination of male frequency. (C) Schematic of experimental design. Replicated populations of the indicated genotype were started with mated hermaphrodites and subsequently scored for male frequency in the first (F1) or second (F2) generation. (D) In F1 populations (progeny of a single mated hermaphrodite), CP161(mss+) (n = 20) and CP162 (mss+) (n = 22) both have a significantly higher ratio of males compared with AF16 (wild type) (n = 16). The median percentage is 0.448, 0.516, and 0.521 for each strain. (E) In the F2 generation, CP161 (mss+) (n = 12) and CP162 (mss+) (n = 10) again both showed a significantly higher ratio of males compared with AF16 wild type (Kolmogorov–Smirnov test). Individual data points represent the frequency of males in each replicate population.
Using these results, the proportion of hermaphrodite eggs that are outcrossed (αm) can be estimated for each of the genotypes. From P0 to F1, they are 89.2% (AF16), 100% (CP161), and 100% (CP162), respectively. From F1 to F2, they are 72.6% (AF16), 86% (CP161), and 89.2% (CP162). In the P0 to F1 generation, α for the three strains is estimated to be 1.79 (AF16), 2 (CP161), and 2 (CP162). Because only hermaphrodites that bore copulatory plugs were picked for the P0 founders, these values reflect only postmating sperm competition. From the F1 to F2 generations, where successful sperm transfer by the F1 males was not guaranteed, α was estimated to be 1.62 for AF16, 1.67 for CP161, and 1.71 for CP162. Thus, male fertilization success is consistently higher in the mss+ populations than in the mss− AF16. Nevertheless, by the F2 generation some hermaphrodites were not outcrossed despite the presence of many males.
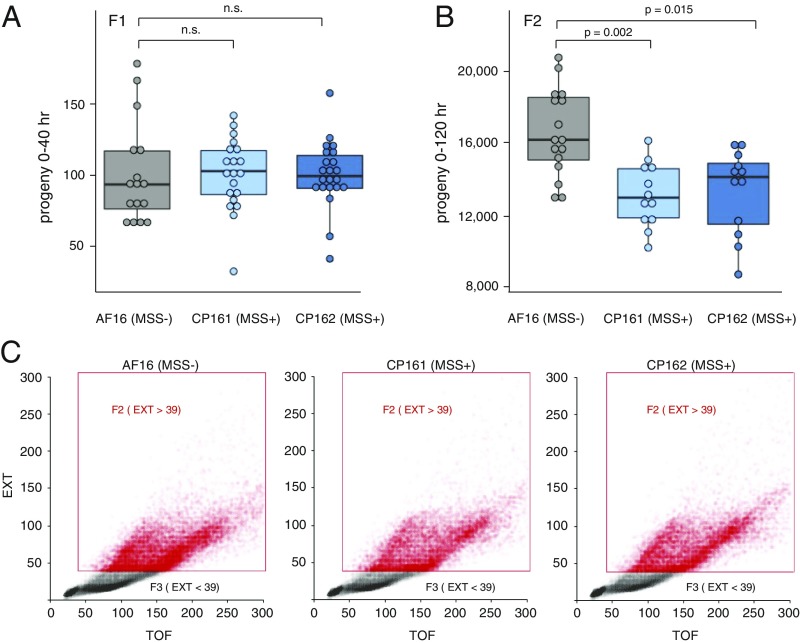
We next examined the prediction of slower population growth in mss+ cultures. The number of progeny laid by a mated hermaphrodite in 40 h was not significantly different between mss− AF16 and the two mss+ transgenic lines (Fig. 3A). Thus, an intrinsic fertility difference between mss+ C. briggsae and mss− C. briggsae can be ruled out, validating a key assumption of our model. After the F1s produced F2s, we used an automated worm sorter to precisely count all progeny in these much larger populations. Wild-type AF16 produced a significantly higher number of F2s (n = 16 cultures, median = 16,140) than CP161 (n = 12, median = 12,870) and CP162 (n = 12, median = 14,050) (Fig. 3 B and C). These results confirm the expected reduction of population growth incurred by high male mating success. To better understand the dynamics of population growth difference as a function of sex ratio difference, we can model population growth as p′ = (p) × (h) × (f), where p′ is the population size at generation N, p is the population size at generation N-1, h is the fraction of hermaphrodites, and f is the fecundity of each hermaphrodite. The median fractions of hermaphrodites at the first generation (0.552 for AF16 WT, 0.484 for CP161, and 0.479 for CP162) predict that in the second generation AF16 WT will have 1.14 times more progeny than CP161, and 1.15 times more than CP162. These are close to our experimental finding: AF16 has 1.25 times more progeny than CP161 (expected 1.14), and 1.15 times more progeny than CP162 (expected 1.15). We conclude the F2 growth of these experimental cultures can be predicted by F1 sex ratios.
Fig. 3.
mss+ populations grow slower. (A) The F1 progeny produced by a single mated hermaphrodite of strains AF16 (n = 16), CP161 (n = 20), and CP162 (n = 21) are not significantly (n.s.) different. (B) The mss+ strains CP161 (n = 12) and CP162 (n = 12) both produced significantly smaller numbers of F2 progeny than the mss− AF16 (n = 16). P value indicated for each comparison is by the Kolmogorov–Smirnov test. (C) Examples of population size determination by automated worm sorter. Eight replicates with the number of worms closest to the medium value were used to make the scatterplots of extinction (EXT) vs. time-of-flight (TOF) data for individual worms. Data points in gray represent small, translucent embryos, larvae, which are F3 progeny. Data points in red represent animals developed enough to be F2 (or older) after 5 d of growth.
Discussion
The repeated evolution of self-fertility suggests that it benefits species that adopt it, likely through reproductive assurance and a large boost in intrinsic growth rate (55, 56). The ongoing role of males, however, is less clear. While Caenorhabditis hermaphrodites lay more embryos after crossing (25), total brood size is not likely to be the most significant contributor to the growth of the population. First, the reproductive value of late progeny is discounted compared with the progeny laid earlier (57). Furthermore, C. elegans and C. briggsae hermaphrodites evolved traits that actually reduce their chance of mating. They secrete less potent sex pheromones (8, 58–60), are less receptive to males that do locate them (27), and can eject the male sperm after transfer (61, 62). While these traits make outcrossing inefficient, they create more hermaphrodite-biased sex ratios and reduce the potential for sterilizing matings with males of sympatric, gonochoristic congeners (41, 63). It appears that outcrossing is minimized in androdioecious species by a suite of changes in both sexes, yet complete male inviability or sterility has not evolved (with exceptions; ref. 64). Our results suggest that loss of mss+ was one of these changes.
Effects of Mating System on mss Fitness.
We predicted that in both mixed-mating populations with selfing hermaphrodites and males, and in obligately outcrossing populations, the mss+ transgene will increase in frequency (Fig. 1 A and B). This is expected to continue to fixation in the outcrossing competition, but be muted and dependent on the presence of males in the mixed-mating situation. Our experimental populations generally support these conclusions. In the male/female (she-1) mixed population, the mss+ allele had a strong advantage, consistent with the observed retention of mss+ in obligately outcrossing species (43). In the mixed-mating case, we also found an increase in mss+ frequency, and as expected it was less pronounced than in the outcrossing case. However, we did not observe an initial sharp increase in GFP+ frequency as expected (compare Fig. 1 A and D), indicating that mating between mss− and mss+ animals in the first generations was less than the values of α estimated from other experiments would predict.
Given the initially low mating inferred (by GFP+ frequency) in the first few generations of the mixed-mating experiment, it is surprising that male frequency (m) remained as high as it did (median of 0.09) at generation 25. Using the empirically calculated value of α for the mss+ CP161 strain in the second generation (1.67) for all subsequent generations, m is predicted to be 0.007 in generation 25, an order of magnitude smaller than observed. A likely explanation is that α (fertilization success) of males varies as a function of male frequency, as suggested by Stewart and Phillips (35). Rare males may encounter virgin hermaphrodites (instead of mated hermaphrodites or other males) more reliably, allowing more matings per male. It is also possible that some assortative mating occurred with regard to mss genotype, though no mechanism to mediate this is apparent.
Loss of mss Boosts Population Growth.
The parallel loss of mss in three distinct lineages is striking. However, the measurable positive selection for mss+ we observed in partially selfing populations indicates that this loss is unlikely to be via drift alone. Our results are consistent with a model whereby loss of the mss family was a response to LMC-like conditions that selected for minimal outcrossing. To approximate the LMC scenario, we compared male frequencies and population growth rates in populations of pure mss− and mss+. While mss+ and mss− C. briggsae hermaphrodites do not differ in their intrinsic fecundity or viability (Fig. 3A), we found higher frequencies of males in mss+ populations (Fig. 2 D and E). Presumably as a direct consequence of this elevated male frequency, by the third generation mss+ populations had lower census sizes (Fig. 3B), as predicted (Fig. 2 A and B). With a transient food supply and in the presence of other competing species, this enhanced growth would produce more dispersive L3-dauer larvae. The loss of the mss+ allele could therefore increase outmigration and the probability of successful colonization of the next patch. In contrast, similar small populations of strict outcrossers would gain nothing from mss loss, because male frequency is a constant 50%.
Conclusions
Males are rare in natural populations of androdioecious Caenorhabditis, often rarer than observed in the laboratory conditions (65, 66). Outcrossing is associated with growth-retarding effects of males addressed above, as well as outcrossing depression and other genetic signatures (19, 67–69). Nevertheless, males persist and are generally fertile worldwide. Exposure to the dauer lifestage, environmental stresses such as heat shock, mutational load, and novel pathogens all can lead to increased outcrossing (22, 70). Thus, the sex ratio of an androdioecious population likely represents a balance between selection for outcrossing under stressful conditions and selection to maximize hermaphrodite selfing under more benign conditions. We propose that mss loss represents a mechanism to help tune the sex ratio to a hermaphrodite-biased value while still allowing outcrossing.
Supplementary Material
Acknowledgments
We thank Gerald Wilkinson, Thomas Kocher, and Patrick Phillips for helpful discussions, as well as Xiaojing Yuan and Iqbal Hamza for help with COPAS worm sorter. This work was supported by National Science Foundation Awards IOS-1755379 and IOS-1355119 (to E.S.H.) and an Ann G. Wylie Dissertation Fellowship from the University of Maryland Graduate School (to D.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1903925116/-/DCSupplemental.
References
- 1.Leonard J. L. Ed., Transitions Between Sexual Systems: Understanding the Mechanisms of, and Pathways Between, Dioecy, Hermaphroditism and Other Sexual Systems (Springer, New York, NY, ed. 1, 2019). [Google Scholar]
- 2.Igic B., Lande R., Kohn J. R., Loss of self‐incompatibility and its evolutionary consequences. Int. J. Plant Sci. 169, 93–104 (2008). [Google Scholar]
- 3.Tsuchimatsu T., Kaiser P., Yew C.-L., Bachelier J. B., Shimizu K. K., Recent loss of self-incompatibility by degradation of the male component in allotetraploid Arabidopsis kamchatica. PLoS Genet. 8, e1002838 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarne P., Auld J. R., Animals mix it up too: The distribution of self-fertilization among hermaphroditic animals. Evolution 60, 1816–1824 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Ellis R. E., Lin S.-Y., The evolutionary origins and consequences of self-fertility in nematodes. F1000Prime Rep. 6, 62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatarenkov A., Lima S. M. Q., Taylor D. S., Avise J. C., Long-term retention of self-fertilization in a fish clade. Proc. Natl. Acad. Sci. U.S.A. 106, 14456–14459 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weeks S. C., Chapman E. G., Rogers D. C., Senyo D. M., Hoeh W. R., Evolutionary transitions among dioecy, androdioecy and hermaphroditism in limnadiid clam shrimp (Branchiopoda: Spinicaudata). J. Evol. Biol. 22, 1781–1799 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Chasnov J. R., Chow K. L., Why are there males in the hermaphroditic species Caenorhabditis elegans? Genetics 160, 983–994 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haag E. S., The evolution of nematode sex determination: C. elegans as a reference point for comparative biology. WormBook Online Rev C Elegans Biol, 1–14 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas C. G., Woodruff G. C., Haag E. S., Causes and consequences of the evolution of reproductive mode in Caenorhabditis nematodes. Trends Genet. 28, 213–220 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Félix M.-A., Braendle C., Cutter A. D., A streamlined system for species diagnosis in Caenorhabditis (Nematoda: Rhabditidae) with name designations for 15 distinct biological species. PLoS One 9, e94723 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiontke K., et al. , Caenorhabditis phylogeny predicts convergence of hermaphroditism and extensive intron loss. Proc. Natl. Acad. Sci. U.S.A. 101, 9003–9008 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiontke K. C., et al. , A phylogeny and molecular barcodes for Caenorhabditis, with numerous new species from rotting fruits. BMC Evol. Biol. 11, 339 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlesworth D., Wright S. I., Breeding systems and genome evolution. Curr. Opin. Genet. Dev. 11, 685–690 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Cutter A. D., Wasmuth J. D., Washington N. L., Patterns of molecular evolution in Caenorhabditis preclude ancient origins of selfing. Genetics 178, 2093–2104 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morton N. E., Theoretical aspects of population genetics. Am. J. Hum. Genet. 24, 488–489 (1972). [Google Scholar]
- 17.Crnokrak P., Barrett S. C. H., Perspective: Purging the genetic load: A review of the experimental evidence. Evolution 56, 2347–2358 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Lande R., Schemske D. W., Schultz S. T., High inbreeding depression, selective interference among loci, and the threshold selfing rate for purging recessive lethal mutations. Evolution 48, 965–978 (1994). [DOI] [PubMed] [Google Scholar]
- 19.Dolgin E. S., Charlesworth B., Baird S. E., Cutter A. D., Inbreeding and outbreeding depression in Caenorhabditis nematodes. Evolution 61, 1339–1352 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Gimond C., et al. , Outbreeding depression with low genetic variation in selfing Caenorhabditis nematodes. Evolution 67, 3087–3101 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Anderson J. L., Morran L. T., Phillips P. C., Outcrossing and the maintenance of males within C. elegans populations. J Hered 101, (suppl. 1):S62–S74 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morran L. T., Parmenter M. D., Phillips P. C., Mutation load and rapid adaptation favour outcrossing over self-fertilization. Nature 462, 350–352 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slowinski S. P., et al. , Coevolutionary interactions with parasites constrain the spread of self-fertilization into outcrossing host populations. Evolution 70, 2632–2639 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noël E., et al. , Experimental evidence for the negative effects of self-fertilization on the adaptive potential of populations. Curr. Biol. 27, 237–242 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Hodgkin J., Doniach T., Natural variation and copulatory plug formation in Caenorhabditis elegans. Genetics 146, 149–164 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emmons S. W., Sexual behavior of the Caenorhabditis elegans male. Int. Rev. Neurobiol. 69, 99–123 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Garcia L. R., LeBoeuf B., Koo P., Diversity in mating behavior of hermaphroditic and male-female Caenorhabditis nematodes. Genetics 175, 1761–1771 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wegewitz V., Schulenburg H., Streit A., Experimental insight into the proximate causes of male persistence variation among two strains of the androdioecious Caenorhabditis elegans (Nematoda). BMC Ecol. 8, 12 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sivasundar A., Hey J., Sampling from natural populations with RNAI reveals high outcrossing and population structure in Caenorhabditis elegans. Curr. Biol. 15, 1598–1602 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Teotónio H., Manoel D., Phillips P. C., Genetic variation for outcrossing among Caenorhabditis elegans isolates. Evolution 60, 1300–1305 (2006). [PubMed] [Google Scholar]
- 31.Cutter A. D., Payseur B. A., Rates of deleterious mutation and the evolution of sex in Caenorhabditis. J. Evol. Biol. 16, 812–822 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Hodgkin J. Male phenotypes and mating efficiency in CAENORHABDITIS ELEGANS. Genetics 103, 43–64 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodgkin J., Horvitz H. R., Brenner S., Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics 91, 67–94 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katju V., LaBeau E. M., Lipinski K. J., Bergthorsson U., Sex change by gene conversion in a Caenorhabditis elegans fog-2 mutant. Genetics 180, 669–672 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart A. D., Phillips P. C., Selection and maintenance of androdioecy in Caenorhabditis elegans. Genetics 160, 975–982 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker H. G., Self-Compatibility and establishment after “Long-Distance” dispersal. Evolution 9, 347–349 (1955). [Google Scholar]
- 37.Theologidis I., Chelo I. M., Goy C., Teotónio H., Reproductive assurance drives transitions to self-fertilization in experimental Caenorhabditis elegans. BMC Biol. 12, 93 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zierold T., Hanfling B., Gómez A., Recent evolution of alternative reproductive modes in the ‘living fossil’ Triops cancriformis. BMC Evol. Biol. 7, 161 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palopoli M. F., et al. , Natural and experimental evolution of sexual conflict within Caenorhabditis nematodes. BMC Evol. Biol. 15, 93 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi C., Murphy C. T., Mating induces shrinking and death in Caenorhabditis mothers. Science 343, 536–540 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ting J. J., et al. , Intense sperm-mediated sexual conflict promotes reproductive isolation in Caenorhabditis nematodes. PLoS Biol. 12, e1001915 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodruff G. C., Knauss C. M., Maugel T. K., Haag E. S., Mating damages the cuticle of C. elegans hermaphrodites. PLoS One 9, e104456 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin D., et al. , Rapid genome shrinkage in a self-fertile nematode reveals sperm competition proteins. Science 359, 55–61 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chasnov J. R., The evolution from females to hermaphrodites results in a sexual conflict over mating in androdioecious nematode worms and clam shrimp. J. Evol. Biol. 23, 539–556 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Hamilton W. D., Extraordinary sex ratios. A sex-ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology. Science 156, 477–488 (1967). [DOI] [PubMed] [Google Scholar]
- 46.Herre E. A., Sex ratio adjustment in fig wasps. Science 228, 896–898 (1985). [DOI] [PubMed] [Google Scholar]
- 47.Cutter A. D., Caenorhabditis evolution in the wild. Bioessays 37, 983–995 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Woodruff G. C., Phillips P. C., Field studies reveal a close relative of C. elegans thrives in the fresh figs of Ficus septica and disperses on its Ceratosolen pollinating wasps. BMC Ecol. 18, 26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo Y., Lang S., Ellis R. E., Independent recruitment of F box genes to regulate hermaphrodite development during nematode evolution. Curr. Biol. 19, 1853–1860 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Smith M. V., et al. , A discrete time model for the analysis of medium-throughput C. elegans growth data. PLoS One 4, e7018 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hedgecock E. M., The mating system of Caenorhabditis elegans: Evolutionary equilibrium between self- and cross-fertilization in a facultative hermaphrodite. Am. Nat. 110, 1007–1012 (1976). [Google Scholar]
- 52.Otto S. P., Sassaman C., Feldman M. W., Evolution of sex determination in the Conchostracan shrimp Eulimnadia texana. Am. Nat. 141, 329–337 (1993). [DOI] [PubMed] [Google Scholar]
- 53.Johnson T. E., Hutchinson E. W., Absence of strong heterosis for life span and other life history traits in Caenorhabditis elegans. Genetics 134, 465–474 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schedl T., Kimble J., fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics 119, 43–61 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bell G., The Masterpiece of Nature: The Evolution and Genetics of Sexuality (CUP Archive, 1982). [Google Scholar]
- 56.Smith J. M., The Evolution of Sex (CUP Archive, 1978). [Google Scholar]
- 57.Hoogendyk C. G., Estabrook G. F., The consequences of earlier reproduction in declining populations. Math. Biosci. 71, 217–235 (1984). [Google Scholar]
- 58.Chasnov J. R., So W. K., Chan C. M., Chow K. L., The species, sex, and stage specificity of a Caenorhabditis sex pheromone. Proc. Natl. Acad. Sci. U.S.A. 104, 6730–6735 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leighton D. H. W., Choe A., Wu S. Y., Sternberg P. W., Communication between oocytes and somatic cells regulates volatile pheromone production in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 111, 17905–17910 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simon J. M., Sternberg P. W., Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 99, 1598–1603 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barker D. M., Copulatory plugs and paternity assurance in the nematode Caenorhabditis elegans. Anim. Behav. 48, 147–156 (1994). [Google Scholar]
- 62.Kleemann G. A., Basolo A. L., Facultative decrease in mating resistance in hermaphroditic Caenorhabditis elegans with self-sperm depletion. Anim. Behav. 74, 1339–1347 (2007). [Google Scholar]
- 63.Ting J. J., Cutter A. D., Demographic consequences of reproductive interference in multi-species communities. BMC Ecol. 18, 46 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lints R., Emmons S. W., Regulation of sex-specific differentiation and mating behavior in C. elegans by a new member of the DM domain transcription factor family. Genes Dev. 16, 2390–2402 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barrière A., Félix M.-A., High local genetic diversity and low outcrossing rate in Caenorhabditis elegans natural populations. Curr. Biol. 15, 1176–1184 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Barrière A., Félix M.-A., Temporal dynamics and linkage disequilibrium in natural Caenorhabditis elegans populations. Genetics 176, 999–1011 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baird S. E., Stonesifer R., Reproductive isolation in Caenorhabditis briggsae: Dysgenic interactions between maternal- and zygotic-effect loci result in a delayed development phenotype. Worm 1, 189–195 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ross J. A., et al. , Caenorhabditis briggsae recombinant inbred line genotypes reveal inter-strain incompatibility and the evolution of recombination. PLoS Genet. 7, e1002174 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seidel H. S., Rockman M. V., Kruglyak L., Widespread genetic incompatibility in C. elegans maintained by balancing selection. Science 319, 589–594 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manoel D., Carvalho S., Phillips P. C., Teotónio H., Selection against males in Caenorhabditis elegans under two mutational treatments. Proc. Biol. Sci. 274, 417–424 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.