ABSTRACT.
Transcranial direct current stimulation (tDCS) is a non-invasive, painless and easy-to use-technology. It can be used in depression, schizophrenia and other neurological disorders. There are no studies about longer usage protocols regarding the ideal duration and weekly frequency of tDCS.
Objective:
to study the use of tDCS twice a week for longer periods to improve memory in elderly with MCI.
Methods:
a randomized double-blind controlled trial of anodal tDCS on cognition of 58 elderly aged over 60 years was conducted. A current of 2.0 mA was applied for 30 minutes for 10 sessions, twice a week. The anode was placed over the left dorsolateral prefrontal cortex (LDLFC). Subjects were evaluated before and after 10 sessions by the following tests: CAMCOG, Mini-Mental State Examination (MMSE), Trail Making, Semantic Verbal Fluency (Animals), Boston naming, Clock Drawing Test, Word list memory (WLMT), Direct and Indirect Digit Order (WAIS-III and WMS-III) and N-back.
Results:
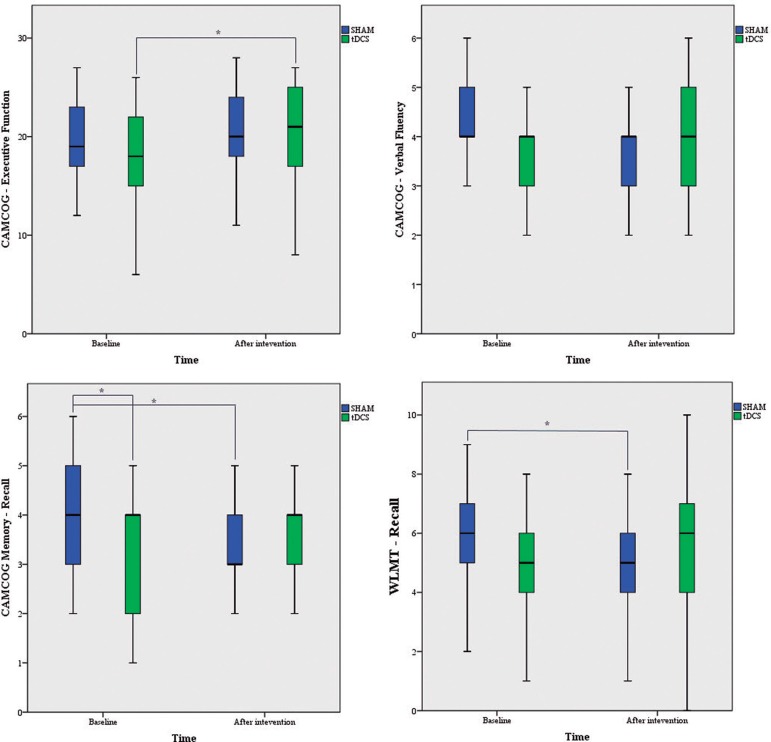
After 10 sessions of tDCS, significant group-time interactions were found for the CAMCOG - executive functioning (χ2 = 3.961, p = 0.047), CAMCOG - verbal fluency (χ2 = 3.869, p = 0.049), CAMCOG - Memory recall (χ2 = 9.749, p = 0.004), and WMLT - recall (χ2 = 7.254, p = 0.007). A decline in performance on the CAMCOG - constructional praxis (χ2 = 4.371, p = 0.037) was found in the tDCS group after intervention. No significant differences were observed between the tDCS and Sham groups for any other tasks.
Conclusion:
tDCS at 2 mA for 30 min twice a week over 5 consecutive weeks proved superior to placebo (Sham) for improving memory recall, verbal fluency and executive functioning in elderly with MCI.
Key words: mild cognitive impairment, elderly, tDCS, memory improvement
RESUMO.
A ETCC (estimulação transcraniana por corrente contínua) é uma tecnologia não-invasiva, indolor e de fácil utilização. Pode ser usada na depressão, esquizofrenia e outros distúrbios neurológicos. Não há orientações ideais sobre o uso de protocolos mais longos quanto à duração e frequência semanal da ETCC.
Objetivo:
estudar o uso de ETCC duas vezes por semana por 5 semanas em idosos com CCL.
Métodos:
o estudo foi controlado, randomizado, duplo-cego com ETCC anódica em 58 idosos acima de 60 anos. Uma corrente de 2,0 mA foi aplicada por 30 minutos durante 10 sessões consecutivas, 2 vezes por semana. O ânodo foi colocado no córtex pré-frontal dorsolateral esquerdo (LDLFC). Os pacientes foram avaliados antes e após 10 sessões pelos testes: CAMCOG, Mini-Exame do Estado Mental (MMSE), Trilhas, Fluência Verbal Semântica - Animais, Boston, Relógio, Memória da Lista de Palavras (WLMT), Dígitos - ordem direta e indireta (WAIS-III e WMS-III) e N-back.
Resultados:
foram encontradas interações significativas (tempo/grupo) para CAMCOG - funcionamento executivo (χ2 = 3,961, p = 0,047), CAMCOG - fluência verbal (χ2 = 3,869, p = 0,049), CAMCOG - recuperação da memória (χ2 = 9.749, p = 0,004), WMLT - recordação (χ2 = 7,254, p = 0,007). Foi observado um declínio no grupo ETCC após a intervenção para CAMCOG - praxia construtiva (χ2 = 4,371, p = 0,037). Não encontramos diferenças significativas entre os grupos ETCC e placebo para outros testes.
Conclusão:
A ETCC de 2 mA por 30 min, 2x por semana, por 5 semanas consecutivas, é superior ao placebo (Sham) na melhoria da recuperação de memória, fluência verbal e funcionamento executivo em idosos com CCL.
Palavras-chave: comprometimento cognitivo leve, idosos, ETCC, melhora da memória
Transcranial Direct Current Stimulation (tDCS) is associated with cognitive improvements in healthy individuals,1 , 2 modulating cortical excitability through synaptic long-term potentiation/depression rate.3 The most important objective of tDCS is to modulate neuronal activity of some specific brain areas in a polarity-dependent pathway.4 During stimulation, current flows into the brain between the electrodes, modulating the brain such that the region beneath the anode undergoes depolarization resulting in excitation, while the area beneath the cathode undergoes hyperpolarization and inhibition.5 Although many authors have studied the effects of tDCS for mental disorders,6 there is no clear consensus on applying this technique in dementia-related disorders.7 Mild Cognitive Impairment (MCI) may represent a prodromal stage of Alzheimer’s dementia.8 Many studies have suggested a progression rate of MCI to dementia averaging around 10% to 15% per year, particularly in amnestic MCI, where executive cognition disabilities are prevalent.9
In the complex physiopathology of MCI, many authors describe a dorsolateral prefrontal cortex (DLPFC) dysfunction. They suggest that there is altered DLPFC functional connectivity with various cortical and subcortical regions during the resting state.10 DLPFC function is very important for maintaining executive memory cognition and working memory. DLPFC dysfunction affects incoming sensory information, language comprehension, reasoning and learning. Neurophysiological and neuroimaging studies have shown altered DLPFC functioning as one of the possible neural bases responsible for the cognitive deficits, such as poor episodic memory retrieval and executive function, noted in MCI patients.11 Anode placement over the left DLPFC and cathode over the right supraorbital region is the most common tDCS protocol for improving working memory.
There is a lack of effective treatments to prevent progression to dementia. Only a few studies have examined the efficacy of neuromodulation strategies for treatment of deficits associated to MCI or dementia. A single session of 1mA anodal tDCS improved word-retrieval of a group of 18 MCI patients in a study with a crossover design.12 Moreover, four sessions of 2mA anodal tDCS were also associated with cognitive improvement in mild vascular dementia.13 However, there are no studies about the effects of a longer protocol which might be suitable for current clinical practice, in terms of duration and weekly frequency.
METHODS
Participants
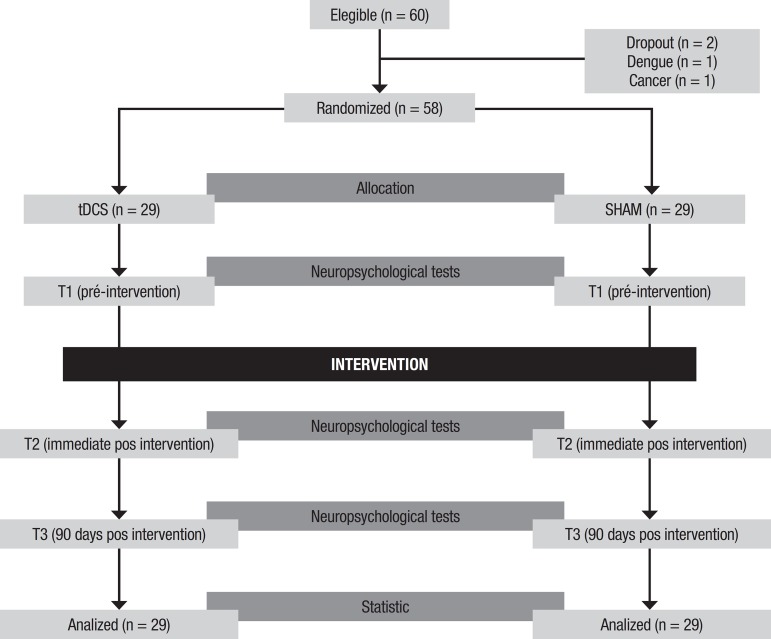
Figure 1 shows the general study design. Sixty individuals aged over 60 years with MCI were recruited, of which 58 (20 males and 38 females) completed the study. Participants were assigned in order of spontaneous arrival at a medical clinic by a geriatric specialist, until a total of 60 participants was reached. The participants were then randomized into an active or sham group. The trial started after 60 individuals had been recruited, in order to achieve a 95% confidence interval with 12.75% confidence interval. Clinical diagnosis was based on the Mayo Clinic Criteria.14 Two individuals, one from each group, dropped out due to medical conditions unrelated to the study. Patients with unstable medical conditions, dementia and axis I psychiatric disorders, as well as subjects on psychotropic or anticholinergic drugs, were not included in the study.
Figure 1. General study design.
Ethics
The present study was approved by the UNIFESP ethics committee under number CAAE: 54213115.7.0000.5505. The study was not registered on clinical trials.
Materials
Stimulation was delivered by a specialized device (brand Ibramed, model STRIAT GMES) with 25cm2 square rubber electrodes in a saline-soaked sponge. TDCS stimulation was administered by a trained biomedic, with no contact with the other evaluators. The instruments below were used for neuropsychological assessment. The Cambridge Cognitive Examination (CAMCOG) is a battery of psychological tests for cognitive assessment, comprised of several subscales to evaluate the following domains: orientation, language, memory, attention, praxis, perception, calculation and abstract thinking.15 The Mini-Mental State Examination test (MMSE) is a cognitive screening instrument assessing six dimensions: orientation, memory, attention, calculus, language and praxis.16 The Trail Making Test, comprising two versions, is a test which evaluates visual attention and task switching.17 The Semantic Verbal Fluency test (Animal word version) (SVF) evaluates verbal fluency by asking the individual to name as many different animals they can in one minute.18 The Boston naming test assesses verbal memory by presenting pictures of everyday objects and asking the subject to name them.19 The Clock Drawing Test entails a task where the individual is asked to draw a clock, used to assess visuospatial and praxis abilities.20 The Word List Memory Test (WLMT) comprises three phases, in which the individual is presented 10 words and has to recall them after 90 seconds and after 15 minutes from among 10 other distractors.21 The Digital Symbol-Coding test is a subtest from the Wechsler Adult Intelligence Scale which assesses processing speed, associative memory and graphomotor speed. The Forward and Backward Digit Span test is a subtest from the Wechsler Memory Scale which assesses verbal working memory and attention.22 The N-back test comprises a computer-test in which the individual is presented a sequence of stimuli, displayed one by one, and performs the task of matching the current stimulus with another presented n steps earlier in the sequence. We also applied the Hamilton Depression Rating Scale (HAM-D). These neuropsychological tests were administered by a blinded trained neuropsychologist who had no contact with the other evaluators.
Procedures
We report the results of a randomized double-blind controlled trial of anodal tDCS assessing cognition. A current of 2.0 mA was applied for 30 minutes for 10 sessions, twice a week. The anode was placed over the left dorsolateral prefrontal cortex (LDLFC) and the cathode in the right supraorbital area. Sham stimulation involved the same set-up, but the current was turned off after a 30-second ramp. Figure 1 depicts the patient allocation and procedure protocol.
Statistical analysis
Group comparisons were performed using the Mann-Whitney test and Pearson’s Chi square test. Differences between groups involving neuropsychological measures at baseline and after intervention were assessed with generalized estimating equations (GEE) (Gamma distribution and first-order autoregressive correlation matrix). Post-hoc pairwise comparison was corrected for multiple comparisons using least significant difference.
RESULTS
Groups were matched for age (u(56) = 455; p = 0.591), gender (χ2(1) = 0.605; p = 0.581) and education level (χ2(2) = 4.971; p = 0.083). No significant differences were found in blood pressure, laboratory blood measures, cranial MRI aspects or HAM-D scores. Table 1 shows the clinical characteristics of the tDCS and Sham groups. Table 2 shows comparisons involving neuropsychological parameters between baseline and after 10 sessions of tDCS/Sham stimulation. After 10 sessions of tDCS, significant group-time interactions for the CAMCOG - executive functioning (χ2 = 3.961, p = 0.047), CAMCOG - verbal fluency (χ2 = 3.869, p = 0.049), CAMCOG Memory - recall (χ2 = 9.749, p = 0.004), and WMLT - recall (χ2 = 7.254, p = 0.007) were evident. A decline in performance for the CAMCOG - constructional praxis (χ2 = 4.371, p = 0.037) was found in the tDCS group after intervention. No significant effects involving the interaction between time and group were found for any other tasks. Figure 2 shows effects on neuropsychological parameters after tDCS × Sham interventions
Table 1. Summary of clinical characteristics and test results for group comparison.
| Active group (29) | SHAM group (29) | Sig. | |
|---|---|---|---|
| Age in years (mean ± SD) | 73.0 ± 9.2 | 71.6 ± 7.9 | 0.38 |
| Sex – no. of women (%) | 20 (69.0) | 22 (75.9) | 0.42 |
| Systolic arterial pressure (mean ± SD) | 127.3 ± 9.4 | 129.3 ± 7.7 | 0.35 |
| Diastolic arterial pressure (mean ± SD) | 79.2 ± 5.7 | 80.4 ± 4.6 | 0.54 |
| Hemoglobin (mean ± SD) | 13.0 ± 0.9 | 13.5 ± 0.7 | 0.35 |
| Blood glucose (mean ± SD) | 91.6 ± 11.4 | 89.7 ± 8.9 | 0.60 |
| TSH (mean ± SD) | 2.5 ± 2.1 | 5.0 ± 0.2 | 0.06 |
| Sodium (mean ± SD) | 140.7 ± 2.6 | 141.3 ± 2.7 | 0.44 |
| Vitamin B12 (mean ± SD) | 461.6 ± 216.5 | 527.3 ± 391.6 | 0.52 |
| PCR (mean ± SD) | 5.4 ± 5.7 | 2.88 ± 3.2 | 0.45 |
| Cholesterol (mean ± SD) | 190.4 ± 47.9 | 180.9 ± 36.2 | 0.82 |
| HDL-C (mean ± SD) | 55.8 ± 14.5 | 51.6 ± 3.5 | 0.15 |
| Educational level | . | . | 0.11 |
| Middle school. n (%) | 4 (13.8) | 9 (31.1) | . |
| High school. n (%) | 6 (20.6) | 6 (20.6) | . |
| University. n (%) | 19 (65.5) | 14 (48.3) | . |
| Cranial MRI | . | . | 0.08 |
| RMC 0 n (%) | 1 (3.4) | 7 (24.1) | . |
| RMC 1 n (%) | 23 (79.3) | 19 (65.5) | . |
| RMC 2 n (%) | 5 (17.3) | 3 (10.4) | . |
| BDNF polymorphism | . | . | 0.05 |
| Genotype G/G | 20 (69) | 19 (65.5) | . |
| Genotype A/G | 9 (31) | 10 (34.5) | . |
Table 2. Summary of cognitive test results comparing pre and post-intervention for each group, derived from repeated measures GEE.
| Test | Group | Pre-intervention | Post-intervention | Ptime | Pgroup | Pgroup time | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard error | Mean | Standard error | ||||||
| CAMCOG | |||||||||
| Executive functioning | SHAM | 113.55 | 1487 | 116 | 1.271 | 0.709 | 0.001 | 0.047 | |
| Active | 111.17 | 2130 | 115.31 | 2.334 | |||||
| Constructional praxis | SHAM | 2.28 | 0.137 | 2.69 | 0.139 | 0.146 | 0.609 | 0.037 | |
| Active | 2.43 | 0.132 | 2.36 | 0.145 | |||||
| Total language | SHAM | 27.45 | 0.27 | 27.28 | 0.344 | 0.374 | 0.963 | 0.116 | |
| Active | 27.03 | 0.401 | 27.66 | 0.332 | |||||
| Motor response | SHAM | 3.86 | 0.064 | 3.86 | 0.064 | 0.315 | 0.632 | 0.315 | |
| Active | 3.83 | 0.098 | 3.97 | 0.034 | |||||
| Verbal answer | SHAM | 2.93 | 0.047 | 3 | 0.049 | 0.763 | 0.698 | 0.362 | |
| Active | 2.97 | 0.059 | 2.93 | 0.047 | |||||
| Reading | SHAM | 2 | 0 | 2.03 | 0.034 | 0.980 | 0.096 | 0.150 | |
| Active | 1.97 | 0.034 | 1.93 | 0.047 | |||||
| Settings | SHAM | 5.62 | 0.133 | 5.66 | 0.132 | 0.130 | 0.733 | 0.215 | |
| Active | 5.41 | 0.192 | 5.76 | 0.093 | |||||
| Picture naming | SHAM | 7.86 | 0.08 | 7.76 | 0.18 | 0.527 | 0.519 | 0.750 | |
| Active | 7.9 | 0.057 | 7.86 | 0.064 | |||||
| Verbal fluency | SHAM | 4.17 | 0.162 | 3.9 | 0.171 | 0.772 | 0.876 | 0.049 | |
| Active | 3.97 | 0.192 | 4.17 | 0.201 | |||||
| Memory | SHAM | 20.59 | 0.456 | 21.48 | 0.432 | 0.006 | 0.731 | 0.766 | |
| Active | 20.24 | 0.666 | 21.34 | 0.655 | |||||
| Memory recall | SHAM | 4 | 0.213 | 3.32 | 0.202 | 0.303 | 0.361 | 0.004 | |
| Active | 3.28 | 0.228 | 3.58 | 0.186 | |||||
| Memory recognition | SHAM | 5.21 | 0.165 | 5.41 | 0.134 | 0.409 | 0.394 | 0.560 | |
| Active | 5.14 | 0.181 | 5.17 | 0.169 | |||||
| Remote Memory | SHAM | 4.31 | 0.213 | 4.83 | 0.176 | 0.003 | 0.434 | 0.345 | |
| Active | 4.62 | 0.198 | 4.9 | 0.209 | |||||
| Recent memory | SHAM | 3.69 | 0.139 | 3.79 | 0.113 | 0.752 | 0.151 | 0.253 | |
| Active | 3.62 | 0.124 | 3.45 | 0.151 | |||||
| Fixing address | SHAM | 3.61 | 0.234 | 4.28 | 0.145 | <0.001 | 0.696 | 0.881 | |
| Active | 3.72 | 0.219 | 4.34 | 0.164 | |||||
| Heads up | SHAM | 6.17 | 0.234 | 6 | 0.218 | 0.918 | 0.621 | 0.321 | |
| Active | 5.83 | 0.32 | 6.03 | 0.251 | |||||
| Calculation | SHAM | 1.85 | 0.067 | 1.83 | 0.07 | 0.478 | 0.939 | 0.300 | |
| Active | 1.79 | 0.075 | 1.9 | 0.077 | |||||
| Praxis | SHAM | 10.79 | 0.185 | 11.14 | 0.193 | 0.125 | 0.157 | 0.626 | |
| Active | 10.52 | 0.252 | 10.69 | 0.224 | |||||
| Ideational praxis | SHAM | 3.76 | 0.093 | 3.76 | 0.079 | 0.810 | 0.584 | 0.810 | |
| Active | 3.83 | 0.07 | 3.79 | 0.09 | |||||
| Constructional praxis | SHAM | 2.28 | 0.137 | 2.69 | 0.139 | 0.146 | 0.609 | 0.037 | |
| Active | 2.43 | 0.132 | 2.36 | 0.145 | |||||
| Ideomotor praxis | SHAM | 4.76 | 0.079 | 4.69 | 0.11 | 0.694 | 0.089 | 0.269 | |
| Active | 4.41 | 0.158 | 4.55 | 0.115 | |||||
| Tactile perception | SHAM | 2 | 0 | 1.97 | 0.034 | 0.565 | 0.556 | 0.565 | |
| Active | 1.97 | 0.034 | 1.97 | 0.034 | |||||
| Visual sense | SHAM | 7.52 | 0.143 | 6.93 | 0.188 | <0.001 | 0.812 | 0.908 | |
| Active | 7.59 | 0.15 | 6.97 | 0.21 | |||||
| Abstract thinking | SHAM | 6.1 | 0.317 | 6.38 | 0.332 | 0.011 | 0.691 | 0.156 | |
| Active | 5.63 | 0.301 | 6.59 | 0.303 | |||||
| Time orientation | SHAM | 4.72 | 0.096 | 4.86 | 0.064 | 0.994 | 0.510 | 0.112 | |
| Active | 4.79 | 0.075 | 4.66 | 0.132 | |||||
| Spatial orientation | SHAM | 4.83 | 0.07 | 4.9 | 0.057 | 0.701 | 0.905 | 0.08 | |
| Active | 4.9 | 0.057 | 4.93 | 0.047 | |||||
| Total | SHAM | 113.55 | 1.487 | 116 | 1,271 | <0.001 | 0.536 | 0.295 | |
| Active | 111.17 | 2.130 | 115.31 | 2,334 | |||||
| Final | SHAM | 93.93 | 0.979 | 95.83 | 0.686 | 0.001 | 0.579 | 0.742 | |
| Active | 92.83 | 1.447 | 95.14 | 1,602 | |||||
| Trail Making Test | |||||||||
| Version A – time | SHAM | 0.5697 | 0.06614 | 0.6462 | 0.08633 | 0.631 | 0.104 | 0.09 | |
| Active | 0.8269 | 0.11021 | 0.7707 | 0.09325 | |||||
| Version A – errors | SHAM | 1.13 | 0.117 | 1.12 | 0.122 | 0.780 | 0.623 | 0.765 | |
| Active | 1.25 | 0.23 | 1.29 | 0.352 | |||||
| Version B – time | SHAM | 24.872 | 0.29003 | 24,503 | 0.3288 | 0.874 | 0.962 | 0.929 | |
| Active | 24.559 | 0.25994 | 24,455 | 0.25501 | |||||
| Version B – errors | SHAM | 3 | 0.403 | 2.49 | 0.889 | 0.497 | 0.665 | 0.610 | |
| Active | 3 | 1,156 | 1.84 | 0.563 | |||||
| Word List Memory Task | |||||||||
| WLMT-A1 | SHAM | 4.9 | 0.241 | 5.05 | 0.298 | 0.503 | 0.404 | 0.694 | |
| Active | 4.69 | 0.244 | 4.86 | 0.307 | |||||
| WLMT-A2 | SHAM | 6.24 | 0.222 | 6.52 | 0.279 | 0.061 | 0.724 | 0.310 | |
| Active | 6.1 | 0.29 | 6.17 | 0.289 | |||||
| WLMT-A3 | SHAM | 6.79 | 0.282 | 7.17 | 0.285 | 0.263 | 0.838 | 0.524 | |
| Active | 6.86 | 0.242 | 6.97 | 0.295 | |||||
| WLMT-recall | SHAM | 5.85 | 0.302 | 5.14 | 0.361 | 0.987 | 0.694 | 0.007 | |
| Active | 4.97 | 0.351 | 5.66 | 0.451 | |||||
| WLMT-recall test: intrusions | SHAM | 1.28 | 0.171 | 1.22 | 0.139 | 0.941 | 0.205 | 0.851 | |
| Active | 1.57 | 0.396 | 1.6 | 0.357 | |||||
| WLMT-recognition test | SHAM | 9.1 | 0.171 | 8.93 | 0.212 | 0.412 | 0.615 | 0.995 | |
| Active | 8.97 | 0.21 | 8.79 | 0.343 | |||||
| WLMT-recognition test – intrusions |
SHAM | 1.28 | 0.176 | 0.96 | 0.103 | 0.395 | 0.021 | 0.091 | |
| Active | 1.65 | 0.284 | 1.81 | 0.363 | |||||
| WLMT-total | SHAM | 17.93 | 0.589 | 18.59 | 0.697 | 0.373 | 0.593 | 0.789 | |
| Active | 17.66 | 0.66 | 18 | 0.801 | |||||
| Other tests | |||||||||
| Semantic Verbal Fluency
test (Animal word version) |
SHAM | 17.31 | 0.867 | 16.86 | 0.903 | 0.81 | 0.874 | 0.268 | |
| Active | 16.55 | 0.972 | 17.24 | 0.971 | |||||
| Mini-Mental
State Examination test |
SHAM | 27.31 | 0.369 | 27.31 | 0.297 | 0.751 | 0.578 | 0.751 | |
| Active | 26.93 | 0.5 | 27.14 | 0.48 | |||||
| Boston Naming test | SHAM | 13.31 | 0.342 | 13.48 | 0.34 | 0.179 | 0.682 | 0.816 | |
| Active | 13.1 | 0.31 | 13.34 | 0.273 | |||||
| Hamilton Depression Rating Scale |
SHAM | 8.66 | 1.003 | 6.74 | 0.712 | 0.033 | 0.117 | 0.459 | |
| Active | 10.31 | 1.394 | 9.13 | 1,259 | |||||
| Clock Drawing Test | SHAM | 8.759 | 0.2881 | 9,241 | 0.1883 | 0.012 | 0.401 | 0.743 | |
| Active | 8.345 | 0.4059 | 8,948 | 0.3957 | |||||
| N-back | SHAM | 508 | 0 | 508 | 0 | 0.312 | 0.312 | 0.312 | |
| Active | 500.48 | 7.386 | 508 | 0 | |||||
| WAIS III - Code | SHAM | 39.07 | 3.225 | 38.93 | 3.276 | 0.7 | 0.613 | 0.77 | |
| Active | 41.76 | 3.525 | 40.69 | 3.129 | |||||
| WAIS III – Digit span DO | SHAM | 7.38 | 0.403 | 7.45 | 0.435 | 0.785 | 0.734 | 0.959 | |
| Active | 7.52 | 0.337 | 7.62 | 0.385 | |||||
| WAIS III – Digit span IO | SHAM | 4.17 | 0.201 | 3.79 | 0.277 | 0.184 | 0.45 | 0.595 | |
| Active | 4.31 | 0.316 | 4.14 | 0.283 | |||||
Figure 2. Boxplot showing results for both groups at pre and post intervention.
*Significant differences (corrected with LSD).
DISCUSSION
Our results suggest that tDCS can improve some aspects of memory impairment in elderly with MCI. We found significant changes in memory recall and long-term memory after administration of 10 sessions of tDCS twice a week.
Some authors have demonstrated advantages with the use of tDCS in treatment of mental disorders, particularly depression and cognitive impairment.23 , 24 Although most studies demonstrate that tDCS is a safe and effective method in depression and possibly Alzheimer disease,25 there are important issues to be considered. First, there is a lack of studies on tDCS efficacy for Mild Cognitive Impairment in the elderly. There are also doubts about the best techniques relating to the intensity of current, stimulation time, electrode placement and number and frequency of sessions. When studying actual results, we note variability of findings and conclusions, suggesting that numerous different factors may affect the results. Besides the variability of protocols, there is evidence in literature that genetic factors, such as Brain-Derived Neurotrophic Factor (BDNF) polymorphism, may influence the improvement in cognition after brain stimulation.26 We believe that a better understanding of neuroplasticity genes will be important to predict outcomes in tDCS.
Another important practical consideration is that trials usually involve daily sessions, which span a period of 4 weeks. This protocol is not affordable for most patients. In this sense, our premise in testing the efficacy of 30 min sessions, twice a week over 5 weeks was precisely to verify whether a more economical paradigm could also lead to positive results. Our results suggest that, using a current of 2 mA for 30 min twice a week over 5 consecutive weeks, tDCS is superior to placebo (Sham) for improvement of memory recall, verbal fluency and executive functioning in elderly with MCI. This study has some limitations: it was not possible to calculate the sample size because this was a pilot study. Nevertheless, the confidence interval was calculated for a sample of 60 individuals considering a 95% significance level and population of 209.3 million population. Although Fisher’s LSD was used, the statistical analysis did not employ more conservative methods for multiple comparison corrections such as Bonferroni or Sidak. The protocol was not registered in clinical trials, but was approved and followed by the Ethics Committee of the Unifesp (São Paulo Federal University). Despite other limitations of the study, including time and frequency of stimulation and number of subjects, results indicate a positive and promising therapeutic role for tDCS use in aging-related working memory dysfunction.
Further research involving larger trials and comparing different clinical protocols for this cohort is needed until translation to clinical practice can occur. More systematic research into this treatment alternative might help improve cognitive dysfunction in aging and related disorders.
Footnotes
This study was conducted at the Department of Psychiatry, Federal University of São Paulo, SP, Brazil.
REFERENCES
- 1.Bennabi D, Pedron S, Haffen E, Monnin J, Peterschmitt Y, Waes VV. Transcranial direct current stimulation for memory enhancement: from clinical research to animal models. Front Syst Neurosci. 2014;doi:10–10. doi: 10.3389/fnsys.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fertonani A, Rosini S, Cotelli M, Rossini PM, Miniussi C. Naming facilitation induced by transcranial direct current stimulation. Behav Brain Res. 2010;208(2):311–318. doi: 10.1016/j.bbr.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 3.Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5(3):175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor córtex through the scalp. Neuroreport. 1998;9:2257–2260. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- 5.Nitsche MA, Paulus W. Excitability changes induced in the human motor córtex by weak transcranial direct current stimulation. Pt3J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boggio PS1, Rigonatti SP, Ribeiro RB, Myczkowski ML, Nitsche MA, Pascual-Leone A, Fregni F. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsycopharmacol. 2008;11(2):249–254. doi: 10.1017/S1461145707007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khedr EM, Gamal NF, El-Fetoh NA, Khalifa H, Ahmed EM, Ali AM, et al. A double-blind randomized clinical trial on the efficacy of cortical direct current stimulation for the treatment of Alzheimer 's disease. Front Aging Neurosci. 2014;275(6):1–12. doi: 10.3389/fnagi.2014.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly E, Zaitchik D, Copeland M, Schmahmann J, Gunther J, Albert M. Predicting conversion to Alzheimer disease using standardized clinical information. Arch Neurol. 2000;57(5):675–680. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- 9.Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29:753–772. doi: 10.1016/j.cger.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang P, Wang Z, Yang Y, Jia X, Li K. Functional disconnection and compensation in mild cognitive impairment Evidence from DLPFC connectivity using resting-state fMRI. PLoS One. 2011;6(7):e22153. doi: 10.1371/journal.pone.0022153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Liang P, Lu S, Li K, Zhong N. The role of the DLPFC in inductive reasoning of the MCI patients and normal agings: an fMRI study. Sci China C Life Sci. Sci China C Life Sci. 2009;52(8):789–795. doi: 10.1007/s11427-009-0089-1. [DOI] [PubMed] [Google Scholar]
- 12.Meinzer M, Lindenberg R, Phan MT, Ulma L, Volkb C, Agnes Flöel A. Transcranial direct current stimulation in mild cognitive impairment: behavioral effects and neural mechanisms. Alzheimer Dement. 2015;11(9):1032–1040. doi: 10.1016/j.jalz.2014.07.159. [DOI] [PubMed] [Google Scholar]
- 13.André S, Heinrich S, Kayser F, Menzler K, Kesselring J, Khader PH. At-home tDCS of the left dorsolateral prefrontal cortex improves visual shot-term memory in mild vascular dementia. J Neurol Sci. 2016;369:185–190. doi: 10.1016/j.jns.2016.07.065. [DOI] [PubMed] [Google Scholar]
- 14.Petersen RC1, Smith GE, Ivnik RJ, Tangalos EG, Kokmen E. Mild Cognitive Impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 15.Paradela EM, Lopes CS, Lourenço RA. Cad Saude. Publica. 2009;25(12):2562–2570. doi: 10.1590/s0102-311x2009001200004. [DOI] [PubMed] [Google Scholar]
- 16.Bertolucci PHF, Brucki SMD, Campacci SR, Juliano Y. O Mini-exame do estado Mental em uma população geral. Impacto da escolaridade. Arq Neuropsiquiatr. 1994;52:1–7. [PubMed] [Google Scholar]
- 17.Cahn D, Salmon D, Butters N, Widerholt W, Corey-Bloom J, Edelstein S, Barret-Connor E. Detection of Dementia of the Alzheimer type in a population-based sample: Neuropsychological test performance. J Int Neuropsychol Soc. 1995;1(3):252–260. doi: 10.1017/s1355617700000242. [DOI] [PubMed] [Google Scholar]
- 18.Ardila A, Bernal B. Cognitive testing toward the future: The example of Semantic Verbal Fluency (Animals) Int J Psychol. 2006;41(5):324–332. [Google Scholar]
- 19.Kaplan EF, Goodglass H, Weintraub S. The Boston Namimg Test. 2nd ed. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 20.Agrell B, Dehlin O. The Clock-drawing test. Age Aging. 1998;27:399–403. doi: 10.1093/ageing/afs149. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson RC, Shiffrin RM. The control of short-term memory. Sci Am. 1971;221:82–90. doi: 10.1038/scientificamerican0871-82. [DOI] [PubMed] [Google Scholar]
- 22.Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. 5th Ed. Oxford: Oxford University Press; 2012. [Google Scholar]
- 23.Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Salvoro B, Giacopuzzi M. Transcranial direct current stimulation in severe, drug-resistant major depression. J Afffect Disord. 2009;118(1-3):215–219. doi: 10.1016/j.jad.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Andrews SC, Hoy KE, Enticott PG, Daskalakis ZJ, Fitzgerald PB. Improving working memory the effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimul. 2011;4(2):84–89. doi: 10.1016/j.brs.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 25.George MS, Aston-Jones G. Noninvasive techniques for probing neurocircuitry and treating illness: vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) Neuropsycopharmacology. 2010;35(1):301–316. doi: 10.1038/npp.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]