Abstract
Drug‐induced liver injury (DILI) due to complementary and alternative medicine (CAM) use is on the rise throughout the world by patients looking for “safer” alternatives. However, data on acute‐on‐chronic liver failure (ACLF) due to CAM are lacking. In a large cohort of patients with cirrhosis, we retrospectively studied CAM‐related health‐seeking behavior and attempted to identify those who developed possible CAM‐DILI‐related ACLF. In this study, we examine the clinical, biochemical, and liver histopathologic characteristics of possible CAM‐DILI‐related ACLF, describe implicated CAM agents, and discuss predictors of patient outcomes. Out of 1,666 patients with cirrhosis, 68% used CAM at some point. A total of 35.7% (n = 30/84) patients presented with CAM‐related DILI leading to ACLF in the whole CAM‐DILI‐related decompensation cohort. The most common CAM was unlabeled polyherbal Ayurvedic formulations. Of possible patients with ACLF, 63% self‐medicated with CAM based on social media sharing. Mean age ± SD was 51.9 ± 9.9 years, 83% were male patients, median follow‐up duration was 173 (range, 14‐584) days, median Child‐Turcotte‐Pugh score was 13 (range, 10‐14), Model for End‐Stage Liver Disease‐sodium score was 30.1 ± 4.8, median chronic liver failure‐organ failure (CLIF‐C‐OF) score was 11 (range, 8‐14), and median CLIF‐C‐ACLF score was 98 (range, 87‐127). Portal‐based neutrophilic predominant mixed inflammation, hepatocyte ballooning, autoimmune‐like features, and severe cholestasis were seen on liver biopsy. Overall, 53% of patients died (median survival 194 days). Baseline overt hepatic encephalopathy and CLIF‐C‐OF score, total bilirubin, hyponatremia and leukocytosis, and grade of ACLF predicted 1‐, 3‐, 6‐ and 12‐month mortality, respectively. Conclusion: Possible CAM‐DILI‐related ACLF has a high mortality. Strict monitoring and identification of CAM use among people with cirrhosis and an integrative public health educational practice can help ameliorate this modifiable risk factor that potentiates heavy liver disease burden and resource use.
Abbreviations
- ACLF
acute‐on‐chronic liver failure
- AHM
Ayurveda and herbal medicines
- ANUSH
Ayurveda, Naturopathy, Unani, Siddha, and Homeopathy
- AUC
area under the curve
- CAM
complementary and alternative medicine
- CI
confidence interval
- CLIF
chronic liver failure
- CLIF‐C‐OF
chronic liver failure‐organ failure
- DILI
drug‐induced liver injury
- HE
hepatic encephalopathy
- HR
hazard ratio
- MELD
Model for End‐Stage Liver Disease
- RUCAM
Roussel Uclaf Causality Assessment Method
The National Sample Survey analysis of 2014 in India revealed that the use of alternative systems of medicine, such as Ayurveda, Naturopathy, Unani, Siddha, and Homoeopathy (ANUSH) was around 5%‐7% in both urban and rural areas.1 A higher use of alternative and pseudoscientific treatments by urban male individuals than their rural counterparts was noted.2 In India, 2,458 Ayurvedic hospitals, with 44,820 beds, 15,353 dispensaries, and 478,750 registered practitioners, were serving the Indian population as of 2010.3 Information on disease burden reduction and improvement in survival from acute as well as chronic diseases directly related to ANUSH systems of medicine is nonexistent.4 Even in the wake of declining complementary and alternative medicine (CAM) integration into the public health system in India, health‐seeking behavior toward pseudoscientific medical practices revolving around ANUSH systems has been on the rise due to intense social media sharing of fake “safer” treatment‐related news.5 Acute‐on‐chronic liver failure (ACLF) is a catastrophic disease that can occur as a consequence of severe drug‐induced liver injury (DILI). Ayurvedic herbal drugs have been implicated as a cause for severe DILI.6 The potential role and outcomes of DILI‐related ACLF has not been studied in detail, and CAM‐DILI‐related ACLF data are not available.7
We performed a retrospective analysis at our center in a large cohort of patients with liver disease to study CAM‐related health‐seeking behavior and clinical outcomes among patients with cirrhosis. ACLF due to CAM‐DILI was challenging to assign to a retrospective cohort because the time linkage of cirrhosis and exposure to the toxic agent cannot be accurately ascertained. We therefore decided to use the term “possible” CAM‐DILI‐related ACLF. This was done to clarify that it was not clear that this exposure retrospectively is what precipitated the ACLF, even though stringent measures were undertaken to rule out all other etiologies for the acute event. We also outlined the clinical, biochemical, and histologic characteristics and reviewed the literature on implicated CAM agents, delineated reasons for CAM use, and analyzed patient outcomes.
Patients and Methods
From September 2016 to September 2018, electronic records of all patients tagged with “liver disease” were retrieved. Patient files were scrutinized for diagnosis of cirrhosis, ACLF, and history of CAM use at any point in time. The diagnosis of cirrhosis was based on clinical, investigational, radiologic, and when possible, histopathology assessments that were retrieved from the respective hospital documents. ACLF was defined per the European Association for the Study of the Liver–CLIF Consortium definition and scored and graded per the ACLF‐CLIF system into no ACLF (grade 0) and ACLF grades 1‐3, based on organ failure involvement.8 Cirrhosis was diagnosed on history and clinical examination and in accordance with radiologic or histologic evidence for the same. Complementary and alternative medicine use was defined as any of Ayurveda‐, Naturopathy‐, Unani‐, Siddha‐, or Homeopathy‐based oral medicine intake. Possible CAM‐DILI‐related ACLF was defined as use of CAM and development of ACLF, with no other clinically identifiable trigger (or triggers). Patients with clinically identifiable triggers for an acute event other than CAM were excluded from final analysis. After identifying all patients with cirrhosis and ACLF with a history of CAM intake, patients were further divided into those who took CAM before and after the diagnosis of cirrhosis. Patients with ACLF due to alcoholic hepatitis, acute viral hepatitis, reactivation of chronic viral infection, DILI due to prescription drugs, those undergoing surgical interventions, and those with trauma, primary hepatic and nonhepatic malignancies, and bacterial or fungal sepsis as inciting events were excluded. Transjugular liver biopsy was performed in those patients who were willing to have the procedure. Hepatic encephalopathy (HE) was graded as per West Haven criteria.9 Ascites was graded as no ascites, mild (grade 1, detected on ultrasonography), moderate (grade 2, shifting dullness), and severe (grade 3, fluid thrill) based on clinical and radiologic evaluation.10 CAM use in the included patients was further divided into Ayurveda‐, Naturopathy‐, Unani‐, Siddha‐, or Homeopathy‐based treatment that was taken from registered public and private institutes with documented prescriptions (R‐ANUSH), unregistered ANUSH‐based or folk medicine‐based traditional healers without documentation or prescriptions (UR‐ANUSH), and patient or patient–family driven self‐treatments that were shared on social media (SM‐ANUSH). The Roussel Uclaf Causality Assessment Method (RUCAM) was used to define a DILI event as probable, possible, or definite. We compared patient outcomes at 180 days between those with possible CAM‐DILI‐ (n = 28) and prescription drug‐ (n = 11) related ACLF. The study was performed to conform to the Helsinki Declaration of 1975, as revised in 2000 and 2008, concerning human and animal rights and was approved by the hospital ethical review board. Patient outcomes with respect to mortality were assessed at the end of 1, 3, and 6 months and the end of 1 year.
Statistical Analysis
Statistical analysis was performed using MedCalc Statistical Software (Ostend, Belgium). Data are given as mean and SD or as median and range as applicable. Pearson's correlation measure was used to determine the relationship of patient outcomes with respect to independent variables. When the distribution of variables was not normal, the degree of relationship between the variables was determined using Spearman's rank correlation. Backward multiple regression analysis with the automatic weighted regression procedure to correct for heteroscedasticity was used to examine the significant predictors associated with outcome at different time points. If there was more than one significant independent variable and the dependent variable was dichotomous, then logistic regression was used. Receiver operating characteristics analysis using the method of DeLong et al.11 (for calculating the SE of the area under the curve [AUC]) was performed to identify cut offs of independent nondichotomous variables that predicted mortality at study time periods. The maximal value of Youden's index (J statistic) was used as a criterion for selecting the optimum cut‐off point. The probability of survival at set time periods and between grouped independent variables was calculated using the Kaplan‐Meier method and graphically represented by the survival time curve. Comparison of survival curves was done using the log rank test, and P < 0.05 was considered significant.
Results
Patient Screening and Inclusion
From September 2016 to September 2018, records of 2,384 patients with liver disease underwent screening; of these, 1,666 were diagnosed to have cirrhosis. CAM was used at some point in time by 68% (n = 1,132). We excluded 271 patients with cirrhosis because of incomplete recording of pertinent data. Out of 861 patients with cirrhosis, 32% (n = 276) and 68% (n = 585) used CAM before and after diagnosis of cirrhosis, respectively. In the former group, 72% (n = 198/276) of patients presented as compensated cirrhosis while 28.2% (n = 78/276) had decompensation. We excluded 45 patients (n = 78) due to identifiable causes for decompensation, and 33 patients were found to have decompensation related to CAM use, of which 39% (n = 13/33) presented as possible CAM‐related ACLF. In the latter group, 81% (n = 476/585) of patients presented as compensated cirrhosis while 18.6% (n = 109/585) had decompensations. Fifty‐eight patients were excluded (n = 109) due to identifiable causes for decompensations, and 51 patients were found to have new onset and worsening of existing decompensation directly related to CAM use. Of these, 33% (n = 17/51) presented as ACLF. Hence, a total of 35.7% (n = 30/84) of patients presented with possible CAM‐DILI‐ACLF in the whole CAM‐DILI‐related decompensation cohort (Fig. 1).
Figure 1.
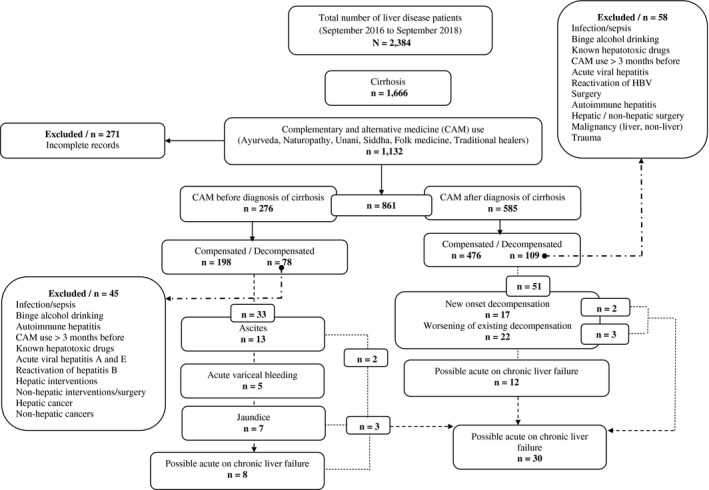
Consolidated Standards of Reporting Trials diagram. Abbreviation: HBV, hepatitis B virus.
Patient Demographics and Baseline Clinical and Investigational Characteristics
The mean age of the patient cohort (n = 30) was 51.9 ± 9.9 years, and 83% were male patients (n = 25). The median follow‐up duration was 173 (range, 14‐584) days. Diabetes mellitus was present in 66.7% (n = 20), dyslipidemia in 46.7% (n = 14), systemic hypertension in 23.3% (n = 7), hypothyroidism in 6.7% (n = 2), and obesity in 40% (n = 12). The most common underlying chronic etiology of liver disease was nonalcoholic fatty liver (66.7%, n = 20), followed by alcohol (23.3%, n = 7). Ascites was detected in all patients, with grade 1 ascites in 13% (n = 4), grade 2 in 47% (n = 14), and grade 3 in 40% (n = 12). Overt HE was seen in 66.7% of patients (n = 20) at admission, with grade 2 HE in 43% (n = 13) and grade 3 in 10% (n = 3). Acute variceal bleeding (AVB) at admission was seen in 10% (n = 3), while 36.7% (n = 11) developed AVB on follow‐up. Cirrhosis was diagnosed and known in 56.7% of patients (n = 17), while in the rest, possible CAM‐DILI‐related ACLF was the index presentation for the diagnosis of cirrhosis. Higher grades of ACLF were noted in 33.3% (n = 10, grade 2 in 6 patients and grade 3 in 4 patients). Mean ± SD serum total bilirubin (n = 30) was 11.2 ± 6.7 mg/dL, international normalized ratio was 2.5 ± 0.8, median Child‐Turcotte‐Pugh score was 13 (range, 10‐14), Model for End‐Stage Liver Disease‐sodium (MELD‐Na) score was 30.1 ± 4.8, median CLIF score was 11 (range, 8‐14), and median CLIF‐C‐ACLF score was 98 (range, 87‐127). Thirty patients completed 30 and 60 days follow‐up, while 28 and 26 patients completed 6 months and 12 months follow‐up, respectively (Table 1).
Table 1.
Baseline Investigational Features of Patients With Possible CAM‐Related ACLF
| Parameter (N = 30) | Minimum | Maximum | Mean | Median | SD |
|---|---|---|---|---|---|
| Age | 26 | 66 | 51.9 | 54.5 | 9.9 |
| Hemoglobin (g/L) | 6.8 | 14.8 | 10.2 | 10.2 | 1.59 |
| Total leucocyte count (per mm3) | 3,200 | 16,300 | 7,213.3 | 5,950 | 3,525.74 |
| Platelet count (×1000 per mm3) | 34 | 233 | 97.6 | 86 | 46.25 |
| Total bilirubin (mg/dL) | 5.8 | 32.8 | 12.5 | 11.2 | 6.78 |
| Alanine aminotransferase (IU/L) | 30 | 568 | 110.8 | 62.5 | 137.57 |
| Aspartate aminotransferase (IU/L) | 42 | 882 | 138.3 | 76.5 | 186.34 |
| Alkaline phosphatase (IU/L) | 68 | 274 | 156.7 | 139.5 | 53.51 |
| Gamma‐glutamyl transpeptidase (IU/L) | 49 | 384 | 144.7 | 112 | 80.35 |
| Serum albumin (mg/dL) | 1.8 | 3.2 | 2.6 | 2.7 | 0.35 |
| International normalized ratio | 1.12 | 5.1 | 2.5 | 2.3 | 0.83 |
| Serum blood urea (mg/dL) | 12 | 46 | 25.7 | 24 | 8.51 |
| Serum creatinine (mg/dL) | 0.6 | 2.3 | 1.4 | 1.3 | 0.45 |
| Serum sodium (meq/L) | 120 | 141 | 130.9 | 130.5 | 5.60 |
| Serum potassium (meq/L) | 2.9 | 4.4 | 3.7 | 3.7 | 0.40 |
| Child‐Turcotte‐Pugh score | 10 | 14 | 12.5 | 13 | 1.22 |
| MELD‐Na score | 20 | 40 | 30.1 | 29.5 | 4.84 |
| CLIF‐C‐ACLF score | 87 | 127 | 103.5 | 98 | 13.35 |
| CLIF score | 8 | 16 | 10.9 | 11 | 2.17 |
| Duration of CAM intake (days) | 8 | 92 | 33.2 | 28 | 20.94 |
| Duration of follow‐up (days) | 14 | 584 | 199.7 | 173 | 144.56 |
| Time to onset of liver injury (days) from start of CAM drug | 10 | 102 | 41.8 | 35 | 24.74 |
Complementary and Alternative Medicines
The most common type of CAM used by the patients in the analyzed cohort was Ayurveda (76.7%, n = 23), followed by Naturopathy (13.3%, n = 4) and Siddha (10%, n = 3). Multiple CAM use (four or more different types of drugs) was seen in 56.7% of patients (n = 17), while single, double, and triple medication use were noted in 20%, 16.7%, and 7%, respectively. The most common reason for CAM intake was for diabetes mellitus (33.3%, n = 10), followed by treatment of chronic liver disease (26.7%, n = 8). On RUCAM scoring, possible (score 3‐5) and probable (score 6‐8) CAM‐related DILI was seen in 43.3% and 56.6% (n = 17) of patients, respectively. Only 6.6% (n = 2) of patients had a documented prescription for CAM from R‐ANUSH practitioners; 30% (n = 9) took CAM from UR‐ANUSH practitioners (untrained, unregistered, traditional healers), and 63.3% (n = 19) self‐medicated with CAM (SM‐ANUSH) after coming across “safer medicine‐related health news” on social media (Table 2).
Table 2.
Details of CAMs Used by Patients Who Developed Possible ACLF
| Complementary and Alternative Medicines |
| Aloe vera extracts + unknown polyherbal powder mixtures * , * |
| Aquatic rotula + Phyllantus niruri (gale of the wind/stonebreaker) |
| Datura stramonium (Devil's weed) + Bengal velvet bean + Chlorophytum borivilianum (safed musli) |
| Gold‐containing metallomineral ash (Thanga bhasma) + Phyllantus niruri (gale of the wind/stonebreaker) |
| Holarrhena antidysentrica (bitter oleander) + unknown polyherbal powder mix |
| Magnesium sulfate + Scoparia dulcis (sweet broom weed) |
| Justicia adhatoda (Malabar nut tree) leaf extracts in capsule and syrup formulations† |
| Guava leaf extracts + aloe vera extracts |
| Passion fruit leaf + aloe vera + Phyllantus niruri (gale of the wind/stonebreaker) |
| Unlabeled polyherbal powder and syrup formulations + unlabeled herbal tablets‡ |
| Raw Carica papaya seed extracts |
| Boerhavia diffusa (spreading hogweed) extracts + polyherbal powder mixtures |
| Unlabeled metallomineral ash (Bhasma) formulation |
| Polygonum aviculare (wireweed) + aloe vera + polyherbal powder mixtures |
| Polygonum aviculare (wireweed) + Phyllantus niruri (gale of the wind/stonebreaker) |
| Reasons for using complementary and alternative medicines (N = 30) |
| Treatment for diabetes mellitus (n = 10) |
| “Cure” from cirrhosis (n = 8) |
| Treatment for fatty liver (n = 2) |
| Nonsurgical treatment of gall stones and liver and gall bladder cleanse in cirrhosis (n = 2) |
| Treatment of bronchitis, alcohol de‐addiction, high cholesterol level, cure from chronic hepatitis B virus infection, pruritus, treatment for loss of libido in cirrhosis, loss of appetite and dyspepsia, and weight loss (n = 1 each) |
1 patient each except.
4 patients;
3 patients;
11 patients.
Liver Histopathology
Out of 30 patients with RUCAM scores suggestive of probable and possible CAM‐DILI‐related ACLF, 12 consented to transjugular liver biopsy (Table 3; Figs. 2 and 3). Pertinent findings on liver histopathology included portal‐based moderate to severe mixed inflammation with a predominance of neutrophils, eosinophilic infiltration of portal tracts, cholangitis, intracanalicular and hepatocytic cholestasis, extensive ballooning and feathery degeneration of hepatocytes, fibrotic areas dissecting and extending into cirrhotic nodules, moderate to severe ductular reaction, and extensive siderosis.
Table 3.
Liver Histology of Patients With Possible ACLF Secondary to CAM‐Related DILI
| Patient | Complementary and Alternative Medicine | Liver Histopathology Findings*, * |
|---|---|---|
| 1 | Aloe vera extract + unknown polyherbal powder mixtures | Mild to moderate portal‐mixed cellular inflammation with neutrophilic predominance, marked ductular reaction with periportal hepatocyte destruction |
| 2 | Aquatic rotula + Phyllantus niruri (gale of the wind/stonebreaker) | Intracanalicular and hepatocellular cholestasis, neutrophilic portal inflammation with moderate to severe ductular reaction |
| 3 | Gold‐containing metallomineral ash (Thanga bhasma) + Phyllantus niruri (gale of the wind/stonebreaker) | Eosinophilic infiltrates, lymphocytes and occasional periportal neutrophils, moderate to severe cholestasis (canalicular and hepatocytic) with periportal hepatocyte destruction, and occasional ballooning with extensive sinusoidal fibrosis |
| 4 | Holarrhena antidysentrica (bitter oleander) + unknown polyherbal powder mix | Areas of incomplete nodule formation, marked infiltration of portal tract with neutrophils, lymphocytes and eosinophils, cholangiolar cholestasis, and Mallory hyaline |
| 5 | Magnesium sulfate + Scoparia dulcis (sweet broom weed) | Advanced bridging fibrosis with extension of fibrotic strands into the cirrhotic nodules, mixed inflammatory cells with neutrophilic predominance, mild to moderate eosinophilic infiltrates, moderate to severe canalicular and hepatocellular cholestasis |
| 6 | Guava leaf + aloe vera extract | Periportal necrosis, neutrophilic portal inflammation, cholestasis predominantly intracanalicular, and mild to moderate ductular reaction |
| 7 | Passion fruit leaf + aloe vera + Phyllantus niruri (gale of the wind/stonebreaker) | Interface hepatitis with lymphoplasmacytic cells, extensive feathery degeneration, severe siderosis, ballooning of hepatocytes, and ductal siderosis |
| 8 | Unknown polyherbal powder and syrup formulations + unlabeled herbal tablets | Extensive siderosis with mild to moderate lymphocytic portal‐based inflammation |
| 9 | Unknown polyherbal powder + unlabeled herbal tablets | Periportal and portal‐based neutrophilic inflammation, interface hepatitis, extensive ballooning of hepatocytes with occasional eosinophils |
| 10 | Unlabeled metallomineral ash (Bhasma) formulation | Marked ballooning of hepatocytes, feathery degeneration, multinucleation, irregular hepatic nodule formation, lymphoplasmacytic cells with occasional neutrophils |
| 11 | Malabar nut tree leaf + aloe vera extract | Moderate plasma cell‐rich portal‐based infiltration with mild to moderate neutrophilic inflammation, few lymphocytes, and severe canalicular cholestasis |
| 12 | Malabar nut tree leaf + root extract | Moderate to severe lympho‐plasmacytic portal‐based infiltration with occasional eosinophils and few neutrophils |
Cirrhosis or advanced fibrosis is a common finding.
Figure 2.
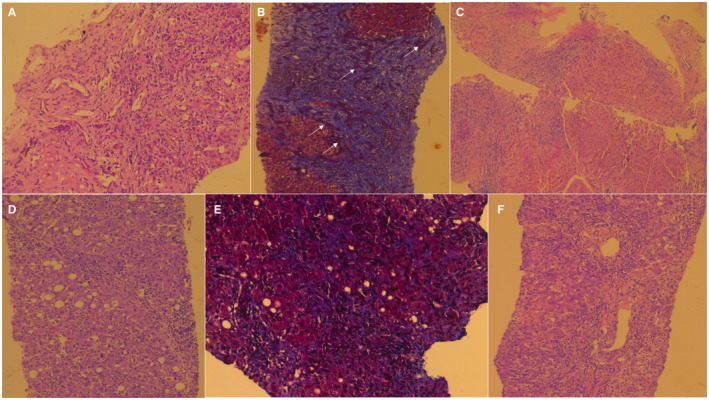
Liver biopsy findings of patients with possible CAM‐related ACLF. Cirrhosis with mild to moderate neutrophilic predominant mixed inflammation with periportal hepatocyte destruction. (A) Aloe vera extract along with unlabeled polyherbal powder, (H&E, magnification ×20) and (B) advanced fibrosis with irregular nodule formation with marked ductular reaction (white arrows) within areas of fibrosis (MT stain, magnification ×10). (C) Cirrhosis with moderate to severe lymphoplasmacytic inflammation of the portal tracts without interface hepatitis but with severe siderosis; unlabeled polyherbal powder, multi‐herb syrup, and unlabeled Ayurvedic tablets (H&E, magnification ×10). (D) Cirrhosis with severe plasma cell‐rich portal inflammation, few lymphocytes, mild to moderate neutrophilic infiltrates, and canalicular cholestasis, and areas of macrovesicular steatosis are also seen; aloe vera and Malabar nut tree leaf extract (H&E, magnification ×20). (E) Extensive and severe sinusoidal fibrosis; gold‐containing Siddha metallomineral ash and gale of the wind herb (MT, magnification ×20). (F) Marked infiltration of portal tract with neutrophils, lymphocytes, and eosinophils, with cholangiolar cholestasis; bitter oleander and unlabeled polyherbal powder (H&E, magnification ×20). Abbreviations: H&E, hematoxylin and eosin; MT, Masson‐Trichrome.
Figure 3.
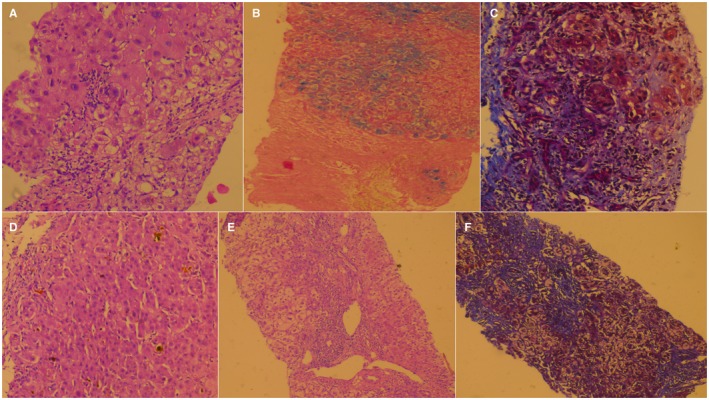
Liver biopsy findings of patients with possible CAM‐related ACLF. (A) Liver biopsy showing interface hepatitis with lymphoplasmacytic cells, extensive feathery degeneration, and ballooning of hepatocytes; passion fruit leaf concoction, aloe vera extract, and gale of the wind herb (H&E, magnification ×20). (B) Portal inflammation with severe siderosis in the absence of human hemochromatosis protein gene mutation; polyherbal powder and unlabeled Ayurvedic tablets (H&E, magnification ×20). (C) Extensive fibrosis with dissection of fibrotic strands into islands of hepatocyte nodules; magnesium sulfate and sweet broom weed (MT, magnification ×20). (D) Severe intracanalicular and hepatocellular cholestasis; metallomineral Ayurvedic preparations and unlabeled polyherbal powders (H&E, magnification ×40). (E,F) Marked ballooning of hepatocytes with multinucleation, feathery degeneration with extensive fibrosis, irregular hepatocyte nodules, and lymphoplasmacytic cellular inflammation and interface hepatitis; Malabar nut tree leaf extract (H&E and MT, magnification ×10 and ×20, respectively). Abbreviations: H&E, hematoxylin and eosin; MT, Masson‐Trichrome.
Outcomes and Significant Predictors
At the end of the median follow‐up of 173 (range, 14‐584) days, 53% (n = 16/30) died, with a median survival of 194 days. On multivariate analysis, only the ACLF grade (cut‐ off point >1; sensitivity 50%, specificity 86%; AUC, 70; 95% confidence interval [CI], 0.502‐0.850; z statistic, 2.03; P = 0.04) was found to be an independent predictor of overall mortality (P = 0.03; Fig. 4A). Forty percent of patients (n = 4/10 each) with ACLF grade 0 and 1 died, with a mean survival of 265.4 (SEM, 35.7) and 266.8 (SEM, 52.4) days, respectively; 67% and 100% of patients with ACLF grade 2 (n = 4/6) and 3 (n = 4/4) died, with a mean survival of 231.5 (SEM 84.4) and 119.5 (SEM 72.3) days, respectively. By grouping patients into lower (0+1) and higher (2+3) grades of ACLF, an overall higher mortality was noted in the latter group (P = 0.03; hazard ratio [HR], 2.72; 95% Confidence Interval (CI), 0.90‐8.24) (Fig. 4B). At the end of 1, 3, 6, and 12 months follow‐up, 13% (n = 4/30; mean survival, 28.7 days), 20% (n = 6/30; 79.6 days), 50% (n = 14/28; 134.9 days), and 61.5% (n = 16/26; 221.3 days) patients died, respectively. Although the presence of overt HE or HE grade did not predict overall mortality, the latter significantly predicted death at 1 month. All patients (n = 10/30) with HE grade 0 survived, while 67% (n = 2/3) with HE grade 3 at admission died the first month (P = 0.02; grouped [HE 0+1 versus 2+3] HR, 2.79; 95% CI, 0.39‐19.8) On multiple regression, the CLIF‐C‐OF score at baseline (cut‐off point, >10; sensitivity 100%, specificity 50%; AUC, 86.1; 95% CI, 0.685‐0.959; z statistic, 3.68; P < 0.001) was found to be an independent predictor of mortality (P = 0.04) at 1 month (Fig. 5A). Baseline total bilirubin (cut‐off point, >12.5 mg/dL; sensitivity 100%, specificity 75%; AUC, 85.4; 95% CI, 0.678‐0.956; z statistic, 5.19; P < 0.001) was found to be an independent predictor of mortality (P = 0.006) at 3 months. Presence of hyponatremia and high total leucocyte counts at admission significantly predicted mortality at 6 months (P < 0.05), while only ACLF grade at baseline (cut‐off point, >1; sensitivity 50%, specificity 90%; AUC, 70; 95% CI, 0.490‐0.862; z statistic, 1.96; P = 0.04) was a significant predictor of mortality at 1 year (P = 0.003). Higher ACLF grades (2+3) also significantly predicted mortality at 1 year (P = 0.02; HR, 2.81; 95% CI, 0.92‐8.55) (Fig. 5B) but not at 1, 3, and 6 months. The most common cause of death was sepsis in 44%, followed by progressive liver failure leading to multiple organ dysfunction in 38% and refractory acute variceal bleeding leading to renal failure and metabolic acidosis in 18%. On comparing outcomes at the end of 180 days between patients with possible CAM‐DILI and prescription‐drug‐related ACLF, mortality was found to be higher among patients in the CAM‐DILI cohort (50% versus 28%), but this did not reach statistical significance (Fig. 6).
Figure 4.
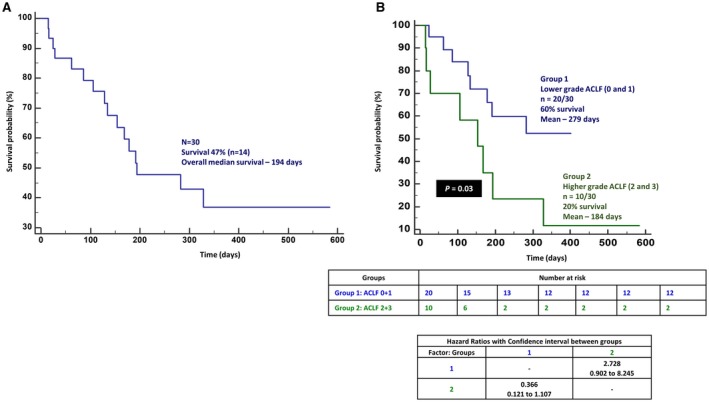
Overall survival in patients with possible CAM‐related ACLF. (A) Overall survival in patients with CAM‐related DILI and (B) lower and higher grades of ACLF.
Figure 5.
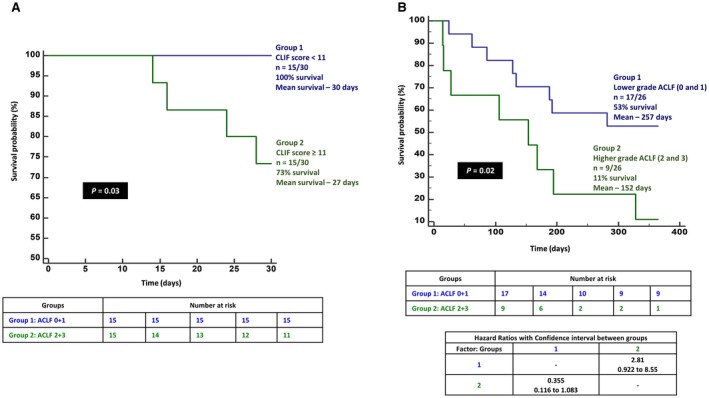
Survival of patients with possible CAM‐related ACLF. (A) Proportion of patients with possible CAM‐related ACLF surviving at 1 month, grouped according to CLIF scores. (B) Proportion of patients with CAM‐related DILI and lower and higher grades of ACLF surviving at the end of 1 year.
Figure 6.
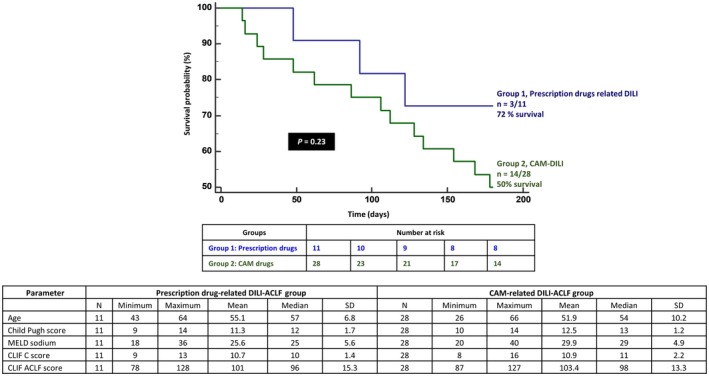
Proportion of patients with possible CAM‐related ACLF compared to those with prescription drug‐related ACLF surviving at 180 days and characteristics of both groups with respect to liver disease severity.
Discussion
In this retrospective, descriptive, observational study, we describe patterns of CAM use in a large cohort of patients with cirrhosis, identify and define a group that manifests CAM‐related DILI based on validated causality tools, and develop possible CAM‐related ACLF. We present novel data on pertinent predictors, histopathologic features, and outcomes associated with possible CAM‐related ACLF due to unregulated use of mostly Ayurveda formulations. In a study published in abstract form, Devarbhavi et al.12 analyzed 2,224 patients with ACLF and found that 6.5% had severe liver disease due to drugs of which the second most common cause was CAM. The authors also found that non‐anti‐tuberculosis medication‐related DILI leading to ACLF had a higher mortality. Seeff et al.13 showed that at least 42% of patients with chronic liver disease used some form of CAM on a regular basis and that herbal preparations were used by 20% of patients with liver disease without advice from or knowledge of treating physicians. In our study, overall 68% of patients with cirrhosis used some form of CAM at some time point during their disease, with 68% consuming CAM after the diagnosis of cirrhosis. It is important to note that social media‐based information was a powerful driving factor for CAM‐related health‐seeking behavior and that most treatments were met from unregistered and untrained traditional Ayurvedic healers. This alarming finding is also a potentially modifiable risk factor if uncompromising regulation from governmental public health agencies and coordination from practitioners of modern and alternative medicine for proper patient education is attempted.
In a recent study, Philips and colleagues14 analyzed 1,440 patients without cirrhosis and found 94 of these to have severe liver injury and associated Ayurvedic herbal medicine (AHM) use. Thirty‐three patients were suspected to have AHM‐DILI on RUCAM and to have HE, hypoalbuminemia, hepatic necrosis on liver biopsy; they found the presence of high levels of arsenic and mercury to be significantly associated with mortality. Some patients had liver fibrosis and steatosis with hepatic decompensation, supporting a diagnosis of ACLF. AHM intake from unregulated traditional healers was associated with higher mortality. Hayashi et al.15 showed that herbal and dietary supplements were important causes of liver injury requiring liver transplantation or causing liver‐related death. In their study, 21% of deaths were due to herbal supplements; ACLF was diagnosed in 33% of these cases. Higher bilirubin, coagulopathy, leukocytosis, and thrombocytopenia were found to be independent predictors of mortality in the whole DILI cohort. In our study, we found similar predictors of mortality in the short (1‐3 months), intermediate (6 months), and long (12 months) term. The CLIF–C‐OF score >10 and presence of overt HE at admission, total bilirubin >12.5 mg/dL at admission, hyponatremia and elevated leucocyte counts at admission, and grade of ACLF predicted mortality at 1, 3, 6, and 12 months, respectively. Our findings are in line with the current literature on ACLF but provide better insights into possible CAM‐related ACLF outcomes for patients at specific time points. The CLIF‐C‐ACLF, a good prognostic score for patients with ACLF and those who are critically ill with cirrhosis, was not found to significantly predict outcomes in our cohort of patients with possible CAM‐related ACLF, probably because most had very high severity scores and statistical significance was not met. In a study on AHM‐related DILI in patients without cirrhosis, the liver histopathology included mostly massive, submassive, and bridging necrosis and fibrosis with neutrophilic inflammation and cholestasis; the former significantly correlating with mortality.14 In the current study, we found that such patterns were not evident in the presence of cirrhosis and that portal‐based mixed inflammation, autoimmune hepatitis‐like inflammatory infiltrates, eosinophils, severe cholestasis, and marked hepatocyte ballooning and feathery degeneration predominated. Liver injury (typically hepatocellular type) associated with portal‐based severe neutrophilic inflammation and extensive ballooning change of hepatocytes has been described with aloe‐related DILI; these features were also prominent in our patient cohort.16, 17
The liver, renal, and cerebral toxicity of Devil's weed and the cytotoxicity of bitter oleander, guava, and passion fruit leaf extracts consumed by patients in our study are well described.18, 19, 20, 21 It was interesting to note that the liver biopsies of those consuming Malabar nut tree (or vasaka) extracts demonstrated autoimmune hepatitis‐like histologic features, probably because of the presence of quinazoline alkaloids, phytosteroids, chalcones, triterpenoids, and flavonoids with possible immunomodulatory properties.22 Hillman et al.23 showed that CAM‐induced DILI is at least as severe in presentation as prescription medicine‐related DILI, with higher rates of transplantation and lower transplant‐free survival (17%) in those who progress to acute liver failure. In our study, approximately 70% of patients with ACLF grade 2 and all patients with ACLF grade 3 died on follow‐up beyond 6 months. This may relate to the higher mortality among patients with possible ACLF due to CAM‐DILI than the mortality seen with other causes, such as prescription drug‐related ACLF, seen in our cohort of patients. However, larger studies and comparison with other acute etiologies in ACLF need to be performed to substantiate our findings.
The major limitation of our study was that it was retrospective in nature and from a single center. We were unable to absolutely assess the specific CAM time of exposure before and after in the whole cirrhosis cohort due to the retrospective nature of the study. There was difficulty in ascertaining the component responsible for causing liver injury because the majority of patients consumed unlabeled, over the counter, and traditional healer‐prescribed multiple medications. We did not analyze CAM samples for hepatotoxic substances. However, a previous study had linked liver injury to the presence of hepatotoxic heavy metals and volatile organic compounds that may have been components of the CAM or introduced as adulterants during processing in the absence of good manufacturing practices.14 Although RUCAM is not validated in CAM‐related DILI, we used RUCAM in our study because it is among the best tools currently available for ascertaining causality. It might seem that there is an over‐representation of nonalcoholic fatty liver disease (NAFLD) in the study cohort, but this was due to most patients consuming CAM for metabolic syndrome and may not represent NAFLD as a potential risk factor for CAM‐DILI. We could only describe the overall use of CAM in the whole cohort at “some point in time,” and patients with cirrhosis who had a history of CAM intake during the 3 months preceding clinical worsening were only included to better define outcomes and homogenize inclusion. We chose 3 months as a reasonable cut‐off point for an acute DILI event based on the literature.24, 25 Comparing all the patients with CAM intake to those without CAM intake in the hospital would have provided a broader outlook; however, this would have meant comparing those with exposure to CAM without any actual linked clinical events to a much larger group of patients with highly heterogeneous clinical events without addressing the limitation in the literature.
Possible CAM‐DILI‐related ACLF is associated with very poor intermediate and long‐term transplant‐free survival. Specific predictors at admission, such as overt HE, hyponatremia, leukocytosis, CLIF‐C‐OF score, and ACLF grade can help guide the treating physician dealing with this catastrophic disease in deciding timing, referral, and listing for liver transplantation. Social media‐based self‐medications and treatments from untrained and unregulated Ayurvedic traditional healers aimed at safer alternatives for chronic liver disease burden an unknowing population with a preventable cause for severe liver injury. Stringent monitoring, public health education in the wake of exaggerated CAM use, and integrative medical educational practices in regions entrenched in pseudoscientific beliefs are a necessity.
Potential conflict of interest: Nothing to report.
References
- 1. Jana A, Basu R. Examining the changing health care seeking behavior in the era of health sector reforms in India: evidences from the National Sample Surveys 2004 & 2014. Glob Health Res Policy 2017;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rao KD, Sundararaman T, Bhatnagar A, Gupta G, Kokho P, Jain K. Which doctor for primary health care? Quality of care and non‐physician clinicians in India. Soc Sci Med 2013;84:30‐34. [DOI] [PubMed] [Google Scholar]
- 3. Ministry of AYUSH . Summary of infrastructure facilities under AYUSH; 2010. http://www.ayush.gov.in/infrastructure/summary-infrastructure-facilities-under-ayush. Published February 15, 2016. Accessed November 18, 2018. [Google Scholar]
- 4. Rastogi S. Ayurveda for comprehensive healthcare. Indian J Med Ethics 2009;6:101‐102. [DOI] [PubMed] [Google Scholar]
- 5. Merchant RM, Asch DA. Protecting the value of medical science in the age of social media and “fake news”. JAMA 2018. 10.1001/jama.2018.18416. [DOI] [PubMed] [Google Scholar]
- 6. Devarbhavi H. Ayurvedic and herbal medicine‐induced liver injury: it is time to wake up and take notice. Indian J Gastroenterol 2018;37:5‐7. [DOI] [PubMed] [Google Scholar]
- 7. Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, et al. Acute‐on‐chronic liver failure in cirrhosis. Nat Rev Dis Primers 2016;2:16041. [DOI] [PubMed] [Google Scholar]
- 8. Hernaez R, Solà E, Moreau R, Ginès P. Acute‐on‐chronic liver failure: an update. Gut 2017;66:541‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferenci P. Hepatic encephalopathy. Gastroenterol Rep (Oxf) 2017;5:138‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moore CM, Van Thiel DH. Cirrhotic ascites review: pathophysiology, diagnosis and management. World J Hepatol 2013;5:251‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837‐845. [PubMed] [Google Scholar]
- 12. Devarbhavi H, Choudhury A, Reddy V, Sharma MK, Maiwall R, Jain P, et al.; APASL ACLF Working Party . Acute on chronic liver failure secondary to drugs: causes, outcome and predictors of mortality. J Hepatol 2016;64(Suppl.):S232. [Google Scholar]
- 13. Seeff LB, Lindsay KL, Bacon BR, Kresina TF, Hoofnagle JH. Complementary and alternative medicine in chronic liver disease. Hepatology 2001;34:595‐603. [DOI] [PubMed] [Google Scholar]
- 14. Philips CA, Paramaguru R, Joy AK, Antony KL, Augustine P. Clinical outcomes, histopathologic patterns and chemical analysis of Ayurveda and herbal medicine associated with severe liver injury‐a single‐center experience from southern India. Indian J Gastroenterol 2018;37:9‐17. [DOI] [PubMed] [Google Scholar]
- 15. Hayashi PH, Rockey DC, Fontana RJ, Tillmann HL, Klapowitz N, Barnhart H, et al. Death and liver transplantation within 2 years of onset of drug‐induced liver injury. Hepatology 2017;66:1275‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teschke R, Wolff A, Frenzel C, Schulze J, Eickhoff A. Herbal hepatotoxicity: a tabular compilation of reported cases. Liver Int 2012;32:1543‐1556. [DOI] [PubMed] [Google Scholar]
- 17. Yang HN, Kim DJ, Kim YM, Kim BH, Sohn KM, Choi MJ, et al. Aloe‐induced toxic hepatitis. J Korean Med Sci 2010;25:492‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Disel NR, Yilmaz M, Kekec Z, Karanlik M. Poisoned after dinner: dolma with Datura stramonium. Turk J Emerg Med 2016;15:51‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jamadagni PS, Pawar SD, Jamadagni SB, Chougule S, Gaidhani SN, Murthy SN. Review of Holarrhena antidysenterica (L.) Wall. ex A. DC.: pharmacognostic, pharmacological, and toxicological perspective. Pharmacogn Rev 2017;11:141‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Díaz‐de‐Cerio E, Verardo V, Gómez‐Caravaca AM, Fernández‐Gutiérrez A, Segura‐Carretero A. Health effects of Psidium guajava L. leaves: an overview of the last decade. Int J Mol Sci 2017;18:pii:E897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bandara KRV, Padumadasa C, Peiris DC. Potent antibacterial, antioxidant and toxic activities of extracts from Passiflora suberosa L. leaves. PeerJ 2018;6:e4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh TP, Singh OM, Singh HB. Adhatoda vasica Nees: phytochemical and pharmacological profile. Nat Prod J 2011;1:29‐39. [Google Scholar]
- 23. Hillman L, Gottfried M, Whitsett M, Rakela J, Schilsky M, Lee WM, et al. Clinical features and outcomes of complementary and alternative medicine induced acute liver failure and injury. Am J Gastroenterol 2016;111:958‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu YC, Mao YM, Chen CW, Chen JJ, Chen J, Cong W, et al.; Drug‐induced Liver Injury (DILI) Study Group; Chinese Society of Hepatology (CSH); Chinese Medical Association (CMA) . CSH guidelines for the diagnosis and treatment of drug‐induced liver injury. Hepatol Int 2017;11:221‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ, et al.; Practice Parameters Committee of the American College of Gastroenterology . ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug‐induced liver injury. Am J Gastroenterol 2014;109:950‐966. [DOI] [PubMed] [Google Scholar]


