Abstract
Purpose of Review
In this review, we summarized the current knowledge of connecting tubule-glomerular feedback (CTGF), a novel mechanism of renal microcirculation regulation that integrates sodium handling in the connecting tubule (CNT) with kidney hemodynamics.
Recent Findings
Connecting tubule-glomerular feedback is a crosstalk communication between the CNT and the afferent arteriole (Af-Art), initiated by sodium chloride through the epithelial sodium channel (ENaC). High sodium in the CNT induces Af-Art vasodilation, increasing glomerular pressure and the glomerular filtration rate and favoring sodium excretion. CTGF antagonized and reset tubuloglomerular feedback and thus increased sodium excretion. CTGF is absent in spontaneous hypertensive rats and is overactivated in Dahl salt-sensitive rats. CTGF is also modulated by angiotensin II and aldosterone.
Summary
CTGF is a feedback mechanism that integrates sodium handling in the CNT with glomerular hemodynamics. Lack of CTGF could promote hypertension, and CTGF overactivation may favor glomerular damage and proteinuria. More studies are needed to explore the alterations in renal microcirculation and the role of these alterations in the genesis of hypertension and glomerular damage in animals and humans.
Keywords: Connecting tubule-glomerular feedback, Tubuloglomerular feedback, ENaC, Hypertension, Proteinuria
Introduction
The kidney is an organ implicated in blood pressure (BP) regulation by controlling sodium and water excretion [1, 2]. Renal microcirculation has some unique characteristics that differentiate this organ from other tissues. Thus, the afferent arteriole (Af-Art) gives rise to a group of capillaries (glomerulus) that finally converge in other arterioles, the efferent arteriole (Ef-Art) [3]. The Ef-Art will finally give rise to a new series of capillaries called peritubular capillaries to finish in the venous system. The glomerulus is a special capillary bed due to a specialized wall and the relatively high capillary pressure (~ 40 mmHg vs. 10 mmHg in other capillary beds) [4]. The glomerulus is responsible for ultrafiltrating the plasma that ultimately will be transformed into urine along the tubule segments. Positioned between two arterioles, the glomerular pressure can be regulated with great precision by the resistance of both the Af-Art and the Ef-Art [5]. The Af-Art regulates the flow and pressure going into the glomerulus and, for instance, is a key variable in the amount of ultrafiltrate generated or the glomerular filtration rate (GFR) [6]. Af-Art resistance is the most efficient way to regulate GFR and, consequently, electrolyte and water excretion. Similar to other tissues, arteriole resistance is controlled by several mechanisms, some of which are systemic (nervous system and hormones), paracrine (autacoids), and local, such as the myogenic response [7••]. However, the kidney has at least two extra mechanisms that control Af-Art resistance: tubuloglomerular feedback (TGF) and connecting tubule-glomerular feedback (CTGF) [8•]. These two feedback mechanisms regulate Af-Art resistance according to the amount of sodium inside the tubules [7••]. TGF and CTGF are part of the crosstalk mechanism between the tubules and the hemodynamics of the kidney [8•]. The term feedback implies that these mechanisms are part of a system where the output signal will modify (positive or negative) the input, closing a loop. TGF is a negative feedback that occurs when the sodium inside the tubule increases. Thus, TGF activation induces Af-Art contraction, decreasing blood flow and pressure into the glomerulus, thereby reducing the GFR [8•]. TGF occurs at the macula densa (MD), a region between the loop of Henle and the distal convoluted tubule. MD is a specialized region inside the tubule, with taller cells specialized in sensing sodium. When a high amount of sodium is detected by MD, ATP/adenosine is released to the adjacent Af-Art (and the extracellular mesangium) that will induce Af-Art contraction by activating the adenosine type 1 receptors [7••]. MD detects sodium by the sodium channel NKCC2 and in minor contribution through the NHE type 3 [9–11]. On the other hand, the CTGF is a positive feedback factor that increases the Af-Art diameter (reducing Af-Art resistance) when a high amount of sodium is detected in the connecting tubule (CNT) [8•]. Af-Art vasodilation will increase the flow and pressure in the glomerulus, thereby increasing the GFR and sodium and water filtration. Thus, CTGF is a mechanism that tends to increase sodium excretion, while TGF tends to prevent sodium loss.
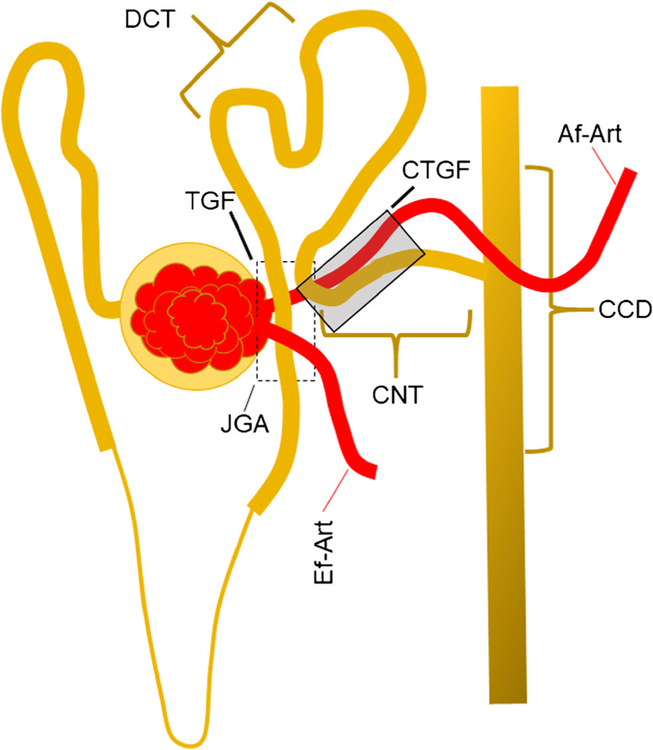
These two feedback mechanisms have an anatomical basis in the nephron. Figure 1 shows a schematic representation of the contacts between the Af-Art and the tubular segments. The tubule segments of a nephron contact their own Af-Art at least two times, once at the MD level and then downstream of the distal convoluted tubule, in the CNT, where the tubule returns to the Af-Art to attach for short distance. The anatomical relationship between the Af-Art and MD is part of the juxtaglomerular apparatus and has been described very well elsewhere [7••, 12]. More recently, the relationship between the CNT and the Af-Art [13, 14] has been described. The physiological significance of this contact is not well understood, but it is markedly constant. Af-Arts contact CNTs in 90% of the superficial nephrons and decrease in deeper nephrons, reaching 73.3% in the medullary region [13, 14]. Connecting tubules are a very important portion of the nephron where the final regulation of the sodium chloride balance is performed [15, 16]. At least two types of cells are present in the CNT: the principal cells and the intercalated cells. Principal cells express the epithelial sodium channel (ENaC) responsible for sodium reabsorption [16]. Intercalated cells participate in acid-base regulation and chloride reabsorption and indirectly modulate sodium reabsorption [17]. Both types of cells are important in blood pressure regulation. Liddle syndrome is characterized by hypertension and hypokalemia, and a gain of function mutation in ENaC is responsible for this phenotype. Recently, it has been shown that the overexpression of the Cl/HCO3 exchanger Pendrin in the intercalated cells of rodents also increases blood pressure, which is more related to the amount of chloride intake [18, 19]. These findings highlight the importance of this region in sodium and water balance regulation. Therefore, it is not surprising that a feedback mechanism to regulate renal hemodynamics occurs in CNTs.
Fig. 1.
Interaction between tubules and arterioles in the nephron. The tubule gets contact with afferent arteriole (AF-Art) at least in two sites: at the juxtaglomerular apparatus (JGA) (dashed line box) where the macula densa (MD) interact with the Af-Art. The JGA is the site for the tubule-glomerular feedback (TGF). The second contact between tubules and Af-Art occurs at the connecting tubule (CNT); there is the anatomical site of the connecting tubule-glomerular feedback (CTGF) (dark box). Ef-Art efferent arteriole, DCT distal convoluted tubule, CCD cortical collecting ducts
Under normal conditions, TGF and CTGF are permanently interacting. As we will explain later, CTGF modulates TGF. The net results of these two feedbacks are a pattern of oscillation in the Af-Art diameter [20•]. This oscillation can be evaluated in the renal blood flow, GFR, and the intratubular pressure. The oscillations have two components: a high-frequency component (~ 30 mHz) and a more slow component detected at < 10 mHz [20•]. In animals lacking the A1 adenosine receptor, the high-frequency oscillation disappeared [21]. Thus, TGF is the high-frequency component of the oscillation. Recently, it has been proposed that the low-frequency component is due to the CTGF mechanism [20•].
Connecting Tubule-Glomerular Feedback Mediators
To explore the role of the attached connecting tubule to Af-Art, we dissected the nephron from a rabbit kidney, and using a microperfusion system, we perfused the Af-Art and the attached CNT tubule. We found that the preconstricted Af-Art becomes dilated when the amount of sodium in the CNT is increased [8•]. This was the first evidence of cross-communication between the CNT and the Af-Art, which we named connecting tubule-glomerular feedback. The epithelial sodium channel (ENaC) is the more abundant sodium channel in the distal nephron [16]. To investigate the role of the ENaC in initiating CTGF, we inhibited the ENaC channel in the CNT during changes in sodium concentration. We found that amiloride and the more specific ENaC inhibitor, benzamil, both inhibited the Af-Art vasodilation induced by sodium. These results indicate that ENaC initiates the CTGF [22]. After ENaC activation, both prostaglandin E2 (PGE2) and epoxyeicosatrienoic acids (EETs) are released from the epithelial cells of the CNT and induce Af-Art vasodilation. PGE2, through the EP4 receptor, induces half of the CTGF effects, while EETs, derived from epoxigenase, mediate the remaining effects [23].
In vivo, several modulators can affect CTGF activity. We have shown that angiotensin II increases CTGF through the AT1 receptor [24]. Similarly, aldosterone through the GPRC receptor increases the CTGF response [25]. Nitric oxide has an inhibitory effect on CTGF, possibly due to the inhibition of sodium transport in epithelial cells [8•].
Connecting Tubule-Glomerular Feedback Modulates TGF
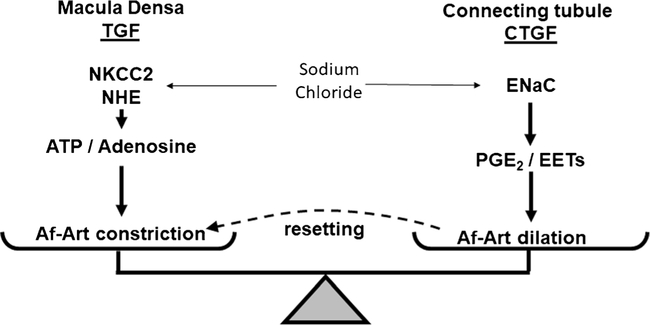
The glomerular pressure is a key determinant of GFR and sodium excretion [5]. Decreasing GFR to a critical level may lead to sodium and water retention and hypertension [1, 26]. We explained that when the amount of sodium increased in the MD, the GFR decreased because of the TGF. In the hypothetical situation of TGF being the only mechanism to regulate sodium excretion, each time that homeostasis is challenged with an extra load of sodium, for example, highly salted meat, the GFR would decrease, the sodium would not be excreted, and the blood pressure would increase. Finally, high BP will induce pressure natriuresis to reach the balance equilibrium [1]. However, most mammals do not experience this situation. In contrast, when a high-salt diet occurs, TGF resetting has been described [27]. Thus, for the same amount of sodium, TGF is less sensitive and permissive to excrete more sodium. Although the mechanism behind TGF resetting is not fully understood, there is evidence that CTGF participates in TGF resetting. Wistar-Kyoto rats experience an attenuation of the TGF response (TGF resetting) when they are fed a high-salt diet [28]. In rats fed a high-salt diet, CTGF was increased, and by blocking CTGF with benzamil, TGF was restored. These results indicate that at least part of the TGF resetting is due to the CTGF that modulates the TGF response. More dramatic TGF resetting is observed in the remnant kidney after unilateral nephrectomy (UNx) [29]. When renal parenchyma is reduced, the remnant nephron must compensate by increasing the amount of sodium and water excretion. This compensation includes an increase in the single nephron GFR and subsequent glomerular and tubular hypertrophy. After UNx, TGF is reset, and CTGF is very active [29]. The inhibition of CTGF after UNx restored the TGF response. A recent mathematical model simulation study showed the importance of CTGF in maintaining the dynamic stability of TGF [20•]. These experiments suggest an important role for CTGF in water and sodium excretion by modulating the TGF response. Figure 2 shows the proposed interaction between TGF and CTGF.
Fig. 2.
Schematic representation of tubuloglomerular feedback (TGF) and connecting tubule-glomerular feedback (CTGF). TGF occurs at the macula densa when sodium chloride is detected through the sodium-potassium-two chloride cotransporter (NKCC2) and secondary to the sodium proton exchanger (NHE). TGF is mediated by adenosine triphosphate and/or adenosine inducing the afferent arteriole (Af-Art) vasoconstriction. CTGF occurs at the connecting tubule by the epithelial sodium channel (ENaC) which detects the distal sodium load. Prostaglandins E2 (PPGE2) and epoxyeicosatrienoic acids (EETs) are released and induce the Af-Art vasodilation. CTGF participates in the TGF resetting, where the sodium load must be higher to induce the same vasoconstriction, due to the vasodilation induced by CTGF
Connecting Tubule-Glomerular Feedback in Hypertension
African-Americans with essential hypertension are frequently salt-sensitive and have abnormal renal hemodynamics [30]. The filtration fraction and intraglomerular pressure are increased in these individuals during high sodium intake. These alterations in renal hemodynamics may be partly responsible for the greater propensity to renal failure in hyper-tensive African-Americans [31]. In Dahl SS rats, glomerular capillary pressure increases in response to high-salt intake, and this effect is accompanied by significant glomerular injury compared to SHR with similar blood pressure [32]. Glomerular capillary pressure is controlled mainly by Af-Art resistance, which is regulated by the vasoconstrictor TGF and the vasodilator CTGF [7••]. Compared to SHR, we showed that Dahl SS rats had lower TGF responses in normal and high-salt diets and greater CTGF responses in both diets [28]. We also showed that Dahl SS rats have greater TGF resetting, which was at least in part mediated by CTGF. Compared with normal Wistar Kyoto rats, CTGF is decreased in SHR (almost absent) and enhanced in Dahl SS rats [28].
The exaggerated CTGF in Dahl SS may be a consequence of a high-salt diet, favoring sodium excretion. However, it has been shown that in the CNT/CCD of Dahl SS rats, the expression and activity of ENaC is increased [33]. We showed that CTGF in Dahl SS rats is exaggerated, even without a high-salt diet, suggesting that this effect could be a consequence of the exaggerated ENaC activity [34].
In contrast, CTGF is almost absent in SHR [28]. SHRs present a higher vascular tone, especially in the Af-Art, with an exaggerated myogenic response and an exaggerated TGF response [7••]. At this point, we do not know if the absence of CTGF in SHR is a consequence of a primary alteration in the CTGF pathway or if an unresponsive Af-Art (hypertonic) does not dilate, despite CTGF activation.
In conclusion, our studies show that CTGF is a novel mechanism of regulation of Af-Art resistance. Its exaggerated response is responsible for more than 50% of the increase in PGC in salt-sensitive hypertension, and the absence of CTGF may participate in the pathogenesis of hypertension in SHR. Understanding the mechanisms of renal autoregulation may lead to both the prevention and better treatment of renal disease and hypertension.
Could CTGF Absence Induce Hypertension?
We stated the role of CTGF in TGF resetting and its potential contribution to salt and water excretion. We can speculate about the consequence of CTGF absence or whether CTGF is not working properly. According to the TGF-CTGF interaction explained above, the absence of CTGF may lead to a disequilibrium in favor of TGF, inducing Af-Art vasoconstriction and sodium and water retention. This effect would lead to hypertension, and finally, pressure natriuresis will be responsible for the new homeostatic equilibrium. This association, low CTGF with a high TGF response, was observed in SHR [28]. We have shown that SHR rats have a strong TGF response and that CTGF is almost absent, even in the high-salt diet. In these rats, the high (TGF) and low (CTGF) oscillatory patterns of the renal blood flow are lost and are replaced by a chaotic fluctuation of the renal blood flow with a net increase in renal resistance [35]. These alterations allow us to speculate that the absence of CTGF contributes to hypertension; however, more studies, particularly studies to reestablish CTGF in this strain, are necessary. SHR has several physiological alterations that can explain the hypertensive phenotype, including alterations in smooth muscle signaling in arterioles throughout the body; however, the kidney may have a preponderant role in the genesis of hypertension because the transplant of normal kidneys in SHR rats ameliorates hypertension [36].
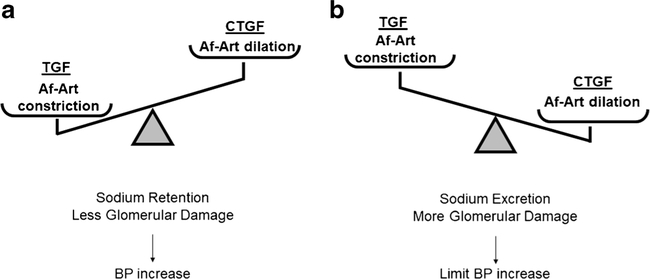
Another approach to modify the CTGF-TGF equilibrium may be increasing the sensitivity of TGF. Recently, the Liu group increased the sensibility of the TGF response in mice by genetically ablating the cilia in the macula densa [37] or directly by knocking out the nitric oxide synthase NOS1β gene [38]; as a consequence, in both models, the nitric oxide production is reduced, and the TGF response is exaggerated. In both models, the animals developed salt-sensitive hypertension, with a difference of more than 10–20 mmHg in the mean arterial pressure by telemetry. Another study showed that the overexpression of the adenosine type 1 receptor in Af-Art increases the TGF response [27]. In this study, animals with an exaggerated TGF response showed a BP increase in the dark period of the day, when rodent animals usually eat (salt load), but no differences were found in the average mean blood pressure. Unfortunately, the CTGF was not evaluated in those experiments. Preliminary data from our group show that the inhibition of CTGF with benzamil for 3 weeks after the UNx prevents the compensatory renal blood flow increase and that the mean blood pressure increases by ~ 9 mmHg using the tail cuff BP measurement method [39]. Despite the limitations of this preliminary study and considering previous evidence, we believe that the alteration of the CTGF-TGF equilibrium should be further investigated as a potential cause of hypertension to confirm the hypothesis that the CTGF-TGF equilibrium may open possibilities for a new therapeutic approach by inhibiting TGF or potentiating CTGF mechanisms. Figure 3 explains the proposed hypothetical alteration of CTGF-TGF equilibrium. More studies exploring the CTGF inhibition of animals as well as more translational investigations are needed to confirm the role of CTGF in blood pressure regulation.
Fig. 3.
Alteration of the TGF-CTGF equilibrium. a Data suggest that if the TGF response is exaggerated, the kidney will increase sodium retention by decreasing the glomerular filtration (GFR) and activating the renin-angiotensin system. These changes would increase the blood pressure (BP) that finally will increase GFR restoring the sodium filtration but a new homeostatic BP level. At the same time, the glomerulus will be more protected to barotrauma due to the Af-Art vasoconstriction. b Contrarily, an excess of CTGF will allow the transmission of systemic BP to the glomerulus, favoring the barotrauma in the presence of systemic hypertension. At the same time, the exaggerated CTGF will induce a high GFR (hyperfiltration) that will favor sodium excretion, ameliorating the increase in BP. TGF tubule glomerular feedback, CTGF connecting tubule-glomerular feedback, Af-Art afferent arteriole
Role of CTGF in Proteinuria
CTGF is a key determinant of glomerular pressure by modifying Af-Art resistance. Glomerular pressure is one of the main causes of glomerular damage in kidney diseases [31]. Proteinuria is an initial marker of glomerular damage and is related to glomerular pressure. Thus, most therapeutic approaches to decrease proteinuria and prevent the progression of glomerular damage involve reducing the glomerular pressure by decreasing systemic BP, particularly by using angiotensin II inhibitors or ang II antagonist [40]. An exaggerated CTGF activation that would dilate the Af-Art may favor the transmission of systemic BP into the glomerulus and induce glomerular damage and protein-uria. In this regard, Dahl SS rats presented greater glomerular damage and proteinuria than SHR rats for the same level of pressure [32]. We observed an exaggerated CTGF response in Dahl SS rats compared with that in Wistar Kyoto rats fed normal and high-salt diets [28]. We also showed that Dahl SS rats have a greater CTGF than SHR rats, which may help to explain the exaggerated glomerular damage observed in Dahl SS rats [28].
Obesity is associated with proteinuria and CKD progression in humans and animals [41–43]. The precise mechanism is not well understood and includes the activation of the RAS system and inflammation. However, glomerular hyperfiltration plays a key role as a main cause of renal damage and proteinuria [44]. Obesity is associated with reduced Af-Art resistance and increased glomerular flow and pressure [45]; these hemodynamic changes result in glomerular hyper-tension, glomerulomegaly, and glomerulosclerosis [43]. Recently, we explored the TGF and CTGF mechanisms in Zucker obese rats, an animal model of obesity without diabetes. We found that CTGF was elevated in obese animals compared to lean animals, while TGF was less responsive in obese rats due to CTGF overactivation [45]. This result suggests a role for CTGF in the proteinuria and renal damage associated with obesity. More studies are necessary to investigate the nexus between obesity and exaggerated CTGF.
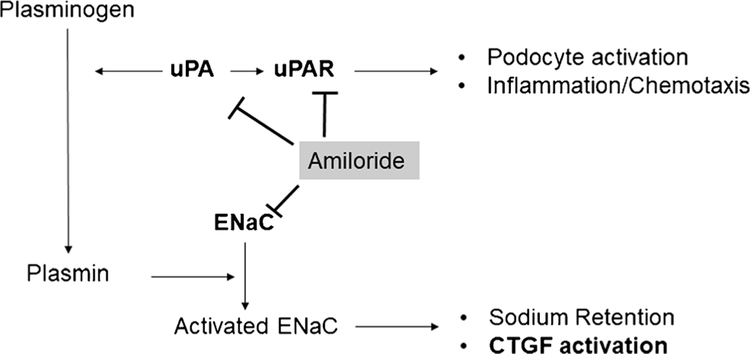
Amiloride is a potassium-sparing diuretic that blocks the epithelial sodium channel (ENaC). Amiloride was the first drug used to inhibit CTGF [8•]. Recent evidence shows the beneficial effect of amiloride on proteinuria in hypertension and renal diseases [46, 47]. The mechanism behind these properties is not fully understood. One possible mechanism is associated with the decrease in systemic blood pressure that may protect the glomerulus. However, amiloride is not a potent diuretic or BP-lowering drug. In fact, amiloride is used in combination with other diuretics to spare potassium losses [48]. Moreover, some animal studies show marked glomerular protection and proteinuria reduction independent of BP effects [49]. The mechanism behind amiloride protection independent of blood pressure is not well understood. One possibility is that amiloride has shown effects on podocyte stabilization both in vitro and in vivo by reducing urokinase-type plasminogen activator receptor (uPAR) [50, 51]. Amiloride also decrease urokinase-type plasminogen activator by a direct effect [52]. Thus, the urokinase-type plasminogen activator converts plasminogen to plasmin, and in the tubular fluid, plasmin may proteolytically activate ENaC in the distal nephron [53]. Plasminogen and plasmin are massively filtrated across the glomerular barrier in proteinuria, and plasminogen can also be activated in the tubular fluid [54]. Recently, plasmine-activated ENaC has been proposed as a mechanism of sodium and water retention in nephrotic syndrome [54, 55] and as a cause of resistant hypertension [56, 57]. However, the amiloride effect observed in proteinuria is dramatic compared with the relative changes in urokinase-type plasminogen activator activity [57], suggesting that other concomitant effects can occur. Analyzing the data, we observed that amiloride also increased the creatinine level (decreasing GFR), probably by decreasing glomerular pressure. Thus, it may be possible that the anti-proteinuric effects observed in amiloride-treated patients and animals can be a consequence of less activated CTGF due to ENaC inhibition. We can speculate that the inhibited CTGF increases Af-Art resistance by decreasing glomerular pressure and proteinuria and, in some cases, GFR. Indeed, a serious adverse effect observed in these patients, other than hyperkalemia, was acute kidney injury due to a dramatic decrease in GFR. Unfortunately, in these clinical trials, the effect of amiloride on renal blood flow, renal arterial resistance, or GFR was not systematically evaluated. Figure 4 shows the possible effects of amiloride in proteinuria.
Fig. 4.
Effects of amiloride in the kidneys. Amiloride is an epithelial sodium channel (ENaC) inhibitor, used as a potassium sparing diuretic in the clinic. Amiloride, by inhibiting ENaC, decrease sodium retention and connecting tubule-glomerular feedback (CTGF). Despite the direct ENaC effects, amiloride inhibits the urokinase-type plasminogen activator (uPA) and its receptor uPAR. uPA converts plasminogen in plasmin, a serine protease that can cleave the gamma sub-unit of ENaC increasing the ENaC activity. uPA can act directly or by attaching to the uPAR that will allow a more physically limited activity of uPA. uPAR also have effects independent of uPA, such as podocyte activation and inflammation. Amiloride also is an uPAR blocker
All these data indicate a protective effect of amiloride on glomerular proteinuria. CTGF inhibition may be an alternative mechanism through which amiloride decreases albuminuria. Additionally, CTGF inhibition may explain why a few patients (those whose GFR depends on maximal Af-ART dilation) experience an acute drop in the glomerular filtration rate and secondary AKI.
Conclusion
The kidney is an important organ in hypertension diseases, both in pathogenesis and as target organ damage. Renal microcirculation regulation has a significant consequence in water and sodium homeostasis and in protecting renal damage. The alteration of the renal microcirculation is present in several animal models of hypertension. CTGF is a recently described mechanism that participates in Af-Art vasodilation and sodium excretion. CTGF also regulates the TGF response, a mechanism that tends to retain sodium. Thus, the interaction between CTGF-TGF may be critical in hypertension. CTGF is exaggerated and almost absent in two genetic models of hypertension, highlighting the importance of this interaction. Moreover, the overactivity of TGF also induces hypertension in animals. TGF and CTGF are modulated by angiotensin II and aldosterone, hormones that participate in the pathogenesis of hypertension and renal damage. We conclude that the study of the CTGF-TGF interaction may suggest some novel mechanism underlying the “essential” hypertension and renal damage and the possibility of new therapeutic approaches.
Key Points.
CTGF is a vasodilator mechanism that regulates afferent arteriole resistance.
CTGF is absent in spontaneous hypertensive rats and overactivated in Dahl salt-sensitive rats.
CTGF in excess may promote glomerular damage and proteinuria, while the absence may participate in sodium retention and hypertension.
Acknowledgments
We would like to thank Dr. Tengis Pavlov for the assistance in the figure preparation.
Funding
This study was funded by the Heart, Lung, and Blood Institute of the National Institutes of Health under award number HL-028982. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Guyton AC. Blood pressure control—special role of the kidneys and body fluids. Science (New York, NY). 1991;252(5014):1813–6. [DOI] [PubMed] [Google Scholar]
- 2.Frame AA, Wainford RD. Renal sodium handling and sodium sensitivity. Kidney Res Clin Pract. 2017;36(2):117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neal CR, Arkill K, Bell JS, Betteridge KB, Bates DO, Winlove CP, et al. Novel haemodynamic structures in the human glomerulus. Am J Physiol Ren Physiol. 2018;315:F1370–84. 10.1152/ajprenal.00566.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner BM, Troy JL, Daugharty TM. The dynamics of glomerular ultrafiltration in the rat. J Clin Investig. 1971;50:1776–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deen WM, Robertson CR, Brenner BM. Glomerular ultrafiltration. FedProc. 1974;33:14–20. [PubMed] [Google Scholar]
- 6.Brenner BM, Troy JL, Daugharty TM, Deen WM, Robertson CR. Dynamics of glomerular ultrafiltration in the rat. II. Plasma-flow dependence of GFR. Am J Physiol. 1972;223:1184–90. [DOI] [PubMed] [Google Scholar]
- 7.••.Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev. 2015;95(2):405–511 [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an extensive review that describe the renal autoregulation mechanisms with very precised details.
- 8.•.Ren Y, Garvin JL, Liu R, Carretero OA. Crosstalk between the connecting tubule and the afferent arteriole regulates renal micro-circulation. Kidney Int. 2007;71(11):1116–21 [DOI] [PubMed] [Google Scholar]; This is the first description of CTGF.
- 9.Peti-Peterdi J, Bebok Z, Lapointe JY, Bell PD. Novel regulation of cell [Na(+)] in macula densa cells: apical Na(+) recycling by H-KATPase. Am J Physiol Ren Physiol. 2002;282(2):F324–9. [DOI] [PubMed] [Google Scholar]
- 10.Komlosi P, Peti-Peterdi J, Fuson AL, Fintha A, Rosivall L, Bell PD. Macula densa basolateral ATP release is regulated by luminal [NaCl] and dietary salt intake. Am J Physiol Ren Physiol. 2004;286:F1054–F8. [DOI] [PubMed] [Google Scholar]
- 11.Ren Y, Garvin JL, Liu R, Carretero OA. Role of macula densa adenosine triphosphate (ATP) in tubuloglomerular feedback. Kidney Int. 2004;66(4):1479–85. [DOI] [PubMed] [Google Scholar]
- 12.Kirk KL, Bell PD, Barfuss DW, Ribadeneira M. Direct visualization of the isolated and perfused macula densa. Am J Physiol. 1985;248:F890–F4. [DOI] [PubMed] [Google Scholar]
- 13.Barajas L, Powers K, Carretero OA, Scicli AG, Inagami T. Immunocytochemical localization of renin and kallikrein in the rat renal cortex. Kidney Int. 1986;29(5):965–70. [DOI] [PubMed] [Google Scholar]
- 14.Dorup J, Morsing P, Rasch R. Tubule-tubule and tubule-arteriole contacts in rat kidney distal nephrons. A morphologic study based on computer-assisted three-dimensional reconstructions. Lab Investig. 1992;67(6):761–9. [PubMed] [Google Scholar]
- 15.Loffing J, Korbmacher C. Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC). Pflugers Arch Eur J Physiol. 2009;458(1):111–35. [DOI] [PubMed] [Google Scholar]
- 16.Frindt G, Palmer LG. Na channels in the rat connecting tubule. Am J Physiol Ren Physiol. 2004;286(4):F669–74. [DOI] [PubMed] [Google Scholar]
- 17.Wall SM, Lazo-Fernandez Y. The role of pendrin in renal physiology. Annu Rev Physiol. 2015;77:363–78. [DOI] [PubMed] [Google Scholar]
- 18.Jacques T, Picard N, Miller RL, Riemondy KA, Houillier P, Sohet F, et al. Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension. J Am Soc Nephrol. 2013;24(7): 1104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wall SM. Renal intercalated cells and blood pressure regulation. Kidney Res Clin Pract. 2017;36(4):305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.•.Liu R, Layton AT. Modeling the effects of positive and negative feedback in kidney blood flow control. Math Biosci. 2016;276:8–18 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study explore the effects of CTGF on TGF.
- 21.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, et al. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A. 2001;98:9983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren Y, D’Ambrosio MA, Garvin JL, Wang H, Carretero OA. Possible mediators of connecting tubule glomerular feedback. Hypertension. 2009;53(part 2):319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren Y, D’Ambrosio MA, Wang H, Garvin JL, Carretero OA. Participation of prostaglandin E 2 and EP4 receptors in connecting tubule glomerular feedback (CTGF) [abstract]. Hypertension. 2012;60(3 Supplement): A33. [Google Scholar]
- 24.Ren Y, D’Ambrosio MA, Wang H, Peterson EL, Garvin JL, Carretero OA. Mechanisms of angiotensin II-enhanced connecting tubule glomerular feedback. Am J Physiol Ren Physiol. 2012;303(2):F259–F65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren Y, D’Ambrosio MA, Garvin JL, Leung P, Kutskill K, Wang H, et al. Aldosterone sensitizes connecting tubule glomerular feedback via the aldosterone receptor GPR30. Am J Physiol Ren Physiol. 2014;307(4):F427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero CA, Peixoto AJ, Orias M. Estimated GFR or albuminuria: which one is really associated with resistant hypertension? Semin Nephrol 2014;34(5):492–7. [DOI] [PubMed] [Google Scholar]
- 27.Brown R, Ollerstam A, Persson AE. Neuronal nitric oxide synthase inhibition sensitizes the tubuloglomerular feedback mechanism after volume expansion. Kidney Int. 2004;65(4):1349–56. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, D’Ambrosio MA, Garvin JL, Ren Y, Carretero OA. Connecting tubule glomerular feedback in hypertension. Hypertension. 2013;62(4):738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monu SR, Ren Y, Masjoan-Juncos JX, Kutskill K, Wang H, Kumar N, et al. Connecting tubule glomerular feedback mediates tubuloglomerular feedback resetting after unilateral nephrectomy. Am J Physiol Ren Physiol. 2018;315(4):F806–F11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frohlich ED, Messerli FH, Dunn FG, Oigman W, Ventura HO, Sundgaard-Riise K. Greater renal vascular involvement in the black patient with essential hypertension. A comparison of systemic and renal hemodynamics in black and white patients. Miner Electrolyte Metab. 1984;10(3):173–7. [PubMed] [Google Scholar]
- 31.Weir MR. Salt intake and hypertensive renal injury in African-Americans. A therapeutic perspective. Am J Hypertens. 1995;8(6):635–44. [DOI] [PubMed] [Google Scholar]
- 32.Raij L, Azar S, Keane WF. Role of hypertension in progressive glomerular immune injury. Hypertension. 1985;7(3 Pt 1):398–404. [PubMed] [Google Scholar]
- 33.Pavlov TS, Staruschenko A. Involvement of ENaC in the development of salt-sensitive hypertension. Am J Physiol Ren Physiol. 2017;313(2):F135–F40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Romero CA, Masjoan Juncos JX, Monu SR, Peterson EL, Carretero OA. Effect of salt intake on afferent arteriolar dilatation: role of connecting tubule glomerular feedback (CTGF). Am J Physiol Ren Physiol. 2017;313(6):F1209–F15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holstein-Rathlou NH, Leyssac PP. TGF-mediated oscillations in the proximal intratubular pressure: differences between spontaneously hypertensive rats and Wistar-Kyoto rats. Acta Physiol Scand. 1986;126(3):333–9. [DOI] [PubMed] [Google Scholar]
- 36.Bianchi G, Fox U, Di Francesco GF, Giovanetti AM, Pagetti D. Blood pressure changes produced by kidney cross-transplantation between spontaneously hypertensive rats and normotensive rats. Clin Sci Mol Med. 1974;47(5):435–48. [DOI] [PubMed] [Google Scholar]
- 37.Song J, Wang L, Fan F, Wei J, Zhang J, Lu Y, et al. Role of the primary cilia on the macula Densa and thick ascending limbs in regulation of sodium excretion and hemodynamics. Hypertension. 2017;70(2):324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y, Wei J, Stec DE, Roman RJ, Ge Y, Cheng L, et al. Macula Densa nitric oxide synthase 1beta protects against salt-sensitive hypertension. J Am Soc Nephrol. 2016;27(8):2346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romero CA, Monu S, Knight R, Carretero OA, editors. Connecting tubule-glomerular feedback (CTGF) in renal hemodynamics and blood pressure (BP) after unilateral nephrectomy (UNX). Hypertension; 2016: Vol 68, Issue Suppl_1 (Abstract P148), USA. [Google Scholar]
- 40.Stevens PE, Levin A. Kidney disease: improving global outcomes chronic kidney disease guideline development work group M. evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30. [DOI] [PubMed] [Google Scholar]
- 41.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498–509. [DOI] [PubMed] [Google Scholar]
- 42.Praga M, Morales E. The fatty kidney: obesity and renal disease. Nephron. 2017;136(4):273–6. [DOI] [PubMed] [Google Scholar]
- 43.Maheshwari M, Romero CA, Monu SR, Kumar N, Liao TD, Peterson EL, et al. Renal protective effects of N-acetyl-Serylaspartyl-Lysyl-proline (ac-SDKP) in obese rats on a high-salt diet. Am J Hypertens. 2018;31(8):902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chagnac A, Herman M, Zingerman B, Erman A, Rozen-Zvi B, Hirsh J, et al. Obesity-induced glomerular hyperfiltration: its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol Dial Transplant. 2008;23(12):3946–52. [DOI] [PubMed] [Google Scholar]
- 45.Monu SR, Maheshwari M, Peterson EL, Carretero OA. Role of connecting tubule glomerular feedback in obesity related renal damage. Am J Physiol Ren Physiol. 2018. 10.1152/ajprenal.00227.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersen H, Hansen PB, Bistrup C, Nielsen F, Henriksen JE, Jensen BL. Significant natriuretic and antihypertensive action of the epithelial sodium channel blocker amiloride in diabetic patients with and without nephropathy. J Hypertens. 2016;34(8):1621–9. [DOI] [PubMed] [Google Scholar]
- 47.Williams B, MacDonald TM, Morant SV, Webb DJ, Sever P, McInnes GT, et al. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6(6):464–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Phys 2015;92(6). [PubMed] [Google Scholar]
- 49.Sepehrdad R, Chander PN, Oruene A, Rosenfeld L, Levine S, Stier CT Jr. Amiloride reduces stroke and renalinjury in stroke-prone hypertensive rats. Am J Hypertens. 2003;16(4): 312–8. [DOI] [PubMed] [Google Scholar]
- 50.Zhang B, Xie S, Shi W, Yang Y. Amiloride off-target effect inhibits podocyte urokinase receptor expression and reduces proteinuria. Nephrol Dial Transplant. 2012;27(5):1746–55. [DOI] [PubMed] [Google Scholar]
- 51.Xu LB, Chi N, Shi W. Amiloride, a urokinase-type plasminogen activator receptor (uTPA) inhibitor, reduces proteinurea in podocytes. Genet Mol Res. 2015;14(3):9518–29. [DOI] [PubMed] [Google Scholar]
- 52.Vassalli JD, Belin D. Amiloride selectively inhibits the urokinase-type plasminogen activator. FEBS Lett. 1987;214(1):187–91. [DOI] [PubMed] [Google Scholar]
- 53.Svenningsen P, Andersen H, Nielsen LH, Jensen BL. Urinary serine proteases and activation of ENaC in kidney—implications for physiological renal salt handling and hypertensive disorders with albuminuria. Pflugers Arch Eur J Physiol. 2015;467(3):531–42. [DOI] [PubMed] [Google Scholar]
- 54.Staehr M, Buhl KB, Andersen RF, Svenningsen P, Nielsen F, Hinrichs GR, et al. Aberrant glomerular filtration of urokinase-type plasminogen activator in nephrotic syndrome leads to amiloride-sensitive plasminogen activation in urine. Am J Physiol Ren Physiol. 2015;309(3):F235–41. [DOI] [PubMed] [Google Scholar]
- 55.Trimarchi H, Forrester M, Lombi F, Pomeranz V, Rana MS, Karl A, et al. Amiloride as an alternate adjuvant antiproteinuric agent in Fabry disease: the potential roles of plasmin and uPAR. Case Rep Nephrol. 2014;2014:854521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buhl KB, Oxlund CS, Friis UG, Svenningsen P, Bistrup C, Jacobsen IA, et al. Plasmin in urine from patients with type 2 diabetes and treatment-resistant hypertension activates ENaC in vitro. J Hypertens. 2014;32(8):1672–7 discussion 7. [DOI] [PubMed] [Google Scholar]
- 57.Oxlund CS, Buhl KB, Jacobsen IA, Hansen MR, Gram J, Henriksen JE, et al. Amiloride lowers blood pressure and attenuates urine plasminogen activation in patients with treatment-resistant hypertension. J Am Soc Hypertens. 2014;8(12):872–81. [DOI] [PubMed] [Google Scholar]