INTRODUCTION
Eating disorders are characterized by a combination of disturbances in body image and maladaptive eating behaviors. The neural mechanisms remain unclear, and perhaps less clear than for other psychiatric illnesses with a similar public health impact. Early approaches to understanding the neurobiology underlying these complex disorders focused on neurotransmitters and identified differences in the presence and metabolism of dopamine and serotonin.1,2 As neuroscience and neuroimaging have advanced, it has become increasingly possible to examine the neural systems that govern human behavior. These advances can be leveraged in psychiatry to link brain activity with maladaptive behavior. One perspective focuses on neural systems that have been well-characterized and tests whether these systems function normally in the setting of illness—a bottom-up approach. An alternative perspective starts with clinical phenomena and abnormal behavior and tests hypotheses about neural under-pinnings—a top-down approach (Fig. 1). Both methods use brain-behavior models to develop and systematically test hypotheses.
Fig. 1.
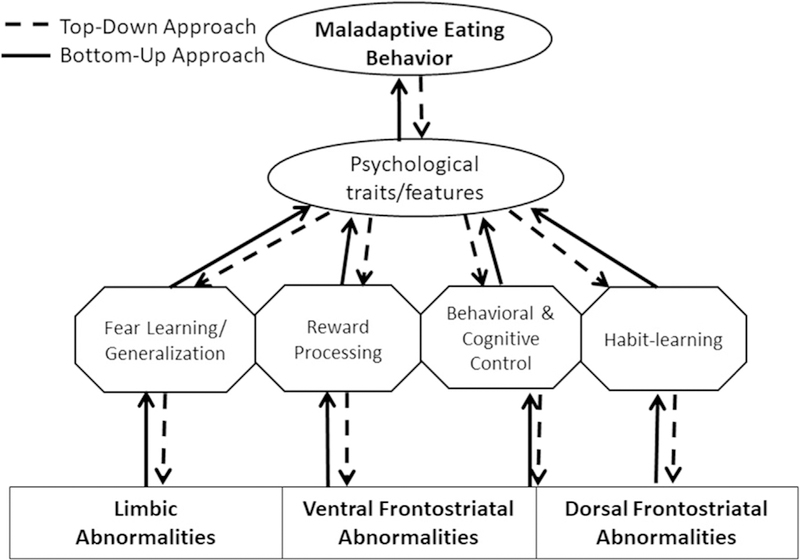
Modeling the links between brain and behavior to create specific testable hypotheses. Maladaptive eating behavior consists of food avoidance and rigid dieting practices in anorexia nervosa (AN) and in bulimia nervosa (BN), and out-of-control binge eating and compensatory behaviors in BN and the binge-eating/purging subtype of AN. These maladaptive behaviors are associated with numerous psychological traits, such as high anxiety and high obsessionality. These traits, in turn, are associated with specific neurocognitive processes that are the behavioral output associated with neural circuits. Fear learning processes are related to limbic circuits and are associated with anxiety and avoidance behaviors. Reward processing and learning are associated with ventral frontostriatal circuits and relate to hedonic processes as well as the development of learned behaviors (perhaps including dieting). Control processes and habit learning are associated with dorsal frontostriatal circuits and are related to disinhibition and obsessionality, which are also related to eating disordered behaviors.
In this review, the authors describe the existing data on neural mechanisms of eating disorders, focusing on 3 areas that suggest paths forward: reward, behavioral and cognitive control, and decision making. To date, the majority of research in these areas comes from anorexia nervosa (AN) and bulimia nervosa (BN); therefore, this review focuses on the neural mechanisms for these disorders.
Each of these areas includes a range of cognitive–behavioral processes and associated neural systems. Reward has been parsed into hedonic value (or liking), motivational salience (or wanting), and reinforcement learning.3,4 The reward system in the brain is composed of the midbrain/ventral tegmental area, ventral striatum (including the nucleus accumbens), medial prefrontal cortex (prefrontal cortex), and orbitofrontal cortex (OFC). Reward-centered models in AN have hypothesized both hyporesponsiveness to reward and aberrant reward attribution (ie, positive stimuli become aversive and vice versa, such that hunger becomes rewarding).5 In BN, theoretic models posit that increased expectancy of reward, but decreased experienced reward from eating may contribute to maladaptive behavior patterns.6
Behavioral and cognitive control processes include response inhibition (or inhibitory control), attentional control, and cognitive flexibility. These processes recruit overlapping frontostriatal, cinguloopercular, and frontoparietal networks7–10; the PFC are generally viewed as the central executors of control.11,12 In AN, patterns of restrictive eating are interpreted as extremes of self-control. BN is associated with impulsivity and the tendency to act rashly in the context of negative emotion.13 Such observations raise questions about the functioning of neural systems associated with behavioral and cognitive control across eating disorders.14
Decision making can be considered an end-result of reward and control processes. In cognitive neuroscience, this phenomenon is often studied via choice-based paradigms. This rapidly advancing field of neuroscience is newly being applied to psychiatry, using neuroimaging and computational approaches.15
REWARD SYSTEMS
Reward is a broad construct that can encompass innate rewards like food, as well as learned or complex reinforcers. Among healthy individuals, food and money are commonly used to evaluate reward responsiveness. This pattern creates a complexity in adapting experimental designs, because the reward value of food cannot be assumed in eating disorders. Reward learning can theoretically include both positive and negative feedback, although learning from positive feedback is better understood, to date.
Numerous studies have measured the degree to which individuals with eating disorders find food rewarding, or pleasurable, as compared with healthy controls. Individuals with AN rate food as less pleasurable than healthy controls, especially high-calorie foods.16 Some have hypothesized that individuals with AN do not experience food as rewarding, and that this experience results in decreased intake.17–19
Individuals with BN have high scores on self-report measures of novelty, pleasure, and sensation-seeking behaviors.20 Moreover, the self-reported drive to eat for pleasure in the absence of an energy deficit predicts the development of loss-of-control eating21 and is associated with binge eating frequency.22
Patients with AN and BN consume large amounts of artificial sweetener through gum, diet beverages, and sweetener packets as compared with healthy controls.23 Together, these observations have led to the bottom-up examination of reward systems through behavioral and neuroimaging experiments.
Behavioral Studies: Anorexia Nervosa
To assess the reward value of food in AN, some studies measure motivation to pursue food, assessed as speed of responding to food (faster response indicates greater motivation). Patients with AN reported less wanting of high-calorie foods and response times were slower (than healthy controls) when selecting between 2 high-calorie foods.17,24 In 1 study (but not the other), individuals with AN were faster than healthy controls to select low-calorie foods.17
Approach–avoidance paradigms assume that it is easier to approach than to avoid stimuli with motivational value. Two studies suggest less motivation to pursue food by finding reduced approach bias (approach responding minus avoidance responding) to food stimuli in AN.25,26 Adolescents with AN displayed an approach bias toward low-calorie, but not high-calorie, food stimuli.27 Individuals with AN, and the binge eating/ purging subtype in particular, showed greater motivation than healthy controls to obtain artificial sweetener.28
Reward learning is understudied in AN, especially using behavioral tasks. One study has demonstrated that individuals with AN are impaired at learning from feedback, both before and after weight restoration.29
Brain Studies: Anorexia Nervosa
Many functional MRI (fMRI) studies have presented images of food (presumed to be innately rewarding) and noted differential activation among AN in areas of the brain that are considered parts of the reward system (ventral striatum, middle frontal gyrus, ventromedial PFC), and dorsolateral PFC (dlPFC), although specific regions and the direction of neural activation differ across studies (eg, hypoactivity vs hyperactivity).30–33 Some studies have shown differences between AN and healthy controls in response to sweet taste, although in regions less central to reward.34–36
In monetary tasks, underweight adolescents with AN appropriately differentiated between wins and losses in the ventral striatum,37 whereas adults recovered from AN demonstrated hyporesponsiveness to monetary rewards in the ventral striatum compared with healthy controls.38
The reward system has also been probed by measuring neural response to the differences between received and expected rewards, the so-called prediction error. In eating disorders, this phenomenon has been studied by training associations between neutral stimuli and delivery of aliquots of sweet liquid (presumed reward). When prediction error occurred, there were differences between patients with AN and healthy controls in neural activation patterns in the striatum, as well as in the OFC.39,40
Indications of reward system abnormalities also come from PET studies, which have identified increased dopamine receptor (D2/D3) binding in the ventral striatum among individuals recovered from AN.41,42 Although these data hint at an abnormality in dopamine pathways, 1 PET study of dopamine receptors found no difference between patients with AN and healthy controls.43 Diffusion tensor imaging approaches and resting state functional connectivity studies have shown increased connectivity within the reward system, specifically the nucleus accumbens–OFC connectivity.44
Summary
Together, behavioral and neuroimaging data suggest decreased reward properties of food, abnormal value and/or processing of reward value of food, and hints of abnormal dopamine functioning. Although intriguing, these findings have not been linked directly to disturbances in eating behavior in AN.
Behavioral Studies: Bulimia Nervosa
To measure the reward value of food among individuals with BN, 2 studies have assessed how hard patients will work for food (ie, motivation). In 1 study, women with BN worked harder than healthy controls for chocolate candies, which were delivered and consumed as they were earned, providing immediate reinforcement.45 In another, women with BN pressed buttons to earn points to be cashed in at the end of the experiment for aliquots of a palatable yogurt shake. When instructed to binge eat, individuals with BN worked harder for the shake than healthy controls. However, when specifically told to work toward a comfortable level of intake and not to binge eat, individuals with BN did not work as hard as healthy controls for the yogurt shake portions.46 To measure the reinforcing value of taste without ingestion, 1 study used a modified sham feeding procedure during which participants sipped and spit solutions as much as they wanted in 1-minute increments. Women with BN sipped roughly 50% more than healthy controls, regardless of solution sweetness.47 These experiments indicate an increased reinforcing value of food and of the orosensory aspects of eating among women with BN. There are currently no experiments with a nonfood reward, leaving a gap in understanding reward processing and reward learning in BN.
Brain Studies: Bulimia Nervosa
Neural responses to pictures of food or actual tastes have been somewhat inconsistent across studies of BN. Participants with BN often show greater activation in the medial OFC, anterior cingulate cortex (ACC), visual cortex, and insula in response to pictures of palatable foods relative to healthy controls,48,49 and this increased activation has been correlated with symptom severity.49 Negative mood states before scanning have been associated with increased neural activation in anticipation of a palatable milkshake, suggesting a link between affect and food evaluation.50
In contrast, some research suggests a reduced reward response both to expected sweet taste receipt and to unexpected taste receipt in BN relative to healthy controls.51–53 Women remitted from BN have been found to have increased neural response to predictable receipt of palatable foods and an abnormal failure to devalue taste stimuli after eating.54,55 In a fed state compared with a fasted state, individuals remitted from BN do not show the decrease in putamen and amygdala responses to liquid tastants (sucrose or water) that healthy controls do.56
Unlike healthy controls, individuals remitted from BN did not differentiate between wins and losses in ventral striatum response during a monetary choice task.57 When visual stimuli were associated with later food and later monetary rewards, individuals with BN (and binge eating disorder) showed altered activation to food-associated stimuli, but no significant group differences related to monetary reward.58
In the prediction error paradigm, adults with BN showed decreased activation in the bilateral ventral putamen, amygdala, insula, and lateral OFC associated with unexpected outcomes related to receipt of sucrose and prediction errors.53 Thus, neural signals involved in reward-based learning may be altered in both adolescent and adult BN.
Structurally, voxel-based morphometry has shown increased59 or normal60 volumes of ventral striatum (nucleus accumbens) among individuals with BN compared with healthy controls. PET and single photon emission computed tomography studies have identified decreased striatal dopamine transporter availability and decreased dorsal striatal dopamine release associated with binge eating frequency among individuals with BN.61,62 Very few published studies have focused on resting state functional connectivity in individuals with BN.63–66 These studies have focused on somatosensory, dorsal ACC, and cerebellar seeds and networks, not reward circuitry. However, integrations of graph theory network analysis approaches with resting state data suggest that women with BN show reduced nodal strength in reward-related areas (the medial OFC and putamen).66 Moreover, increases in reward-circuit connectivity (from the ACC to ventral striatum and anterior insula) after repetitive transcranial magnetic stimulation was associated with symptom remission, suggesting that increases in reward circuity connectivity may mediate symptom improvement.67
Summary
Together, behavioral and neuroimaging studies suggest an increased reinforcing value of food and changes in the brain reward system in association with food. There is, as yet, little evidence to suggest non-food-related reward system abnormalities.
BEHAVIORAL AND COGNITIVE CONTROL
The broad constructs of behavioral and cognitive control include motor inhibition and attentional control.68 Behavioral paradigms that assess behavioral and cognitive control typically require individuals to inhibit a response or ignore interfering information. For example, go/no-go tasks measure action restraint and require the inhibition of a button-pressing go response when no-go stimuli appear on a screen.69 Stop-signal tasks require withholding of a button-pressing go response when a rare auditory or visual stop signal sounds or appears.68,70 The delay between the go and the stop signal varies across the task, resulting in easy (short delay) and more difficult (long delay) trials. The Stroop task71 and Simon task72 require individuals to override a prepotent response when 2 pieces of information are inconsistent (eg, naming a word’s ink color instead of reading the color word such as blue, pressing a button that matches the direction in which an arrow is pointing rather than the side of the screen on which it is presented). Overlapping corticostriatothalamocortical loops, including lateral PFC, ACC, dorsal striatum, the presupplementary motor area, insula, and parietal regions, are involved in these processes.73,74
Behavioral control deficits may be particularly relevant to the pathophysiology of binge eating, because the sense of loss of control over eating is a defining element of binge episodes in the binge eating/purging subtype of AN and in BN.75 In AN, recent theories posit that increased cognitive control and difficulties with set shifting may promote or maintain excessive control over food intake.76 In addition to difficulty controlling eating, high rates of other dysregulated, often impulsive behaviors among individuals with BN suggest that the disorder may also be characterized by impairment in inhibitory control across multiple domains.77–81
Behavioral Studies: Anorexia Nervosa
Neuropsychological studies of individuals with AN have consistently found executive function deficits in some domains related to cognitive control. Patients with AN show difficulty changing responses when instructions changed, as evidenced on set shifting tasks and Stroop tasks.82 Although attention is often impaired owing to starvation, studies of attention bias do not suggest deficits in attentional control among individuals with AN.83 Behavioral control tasks, in which participants must engage inhibitory control to prevent an active motor response, generally do not show a difference between individuals with AN and healthy controls.84
Brain Studies: Anorexia Nervosa
Despite the numerous reports of cognitive inflexibility in AN, the neural correlates are not clear. One study that required shifting of behavioral responses found less activation in the frontostriatal circuits and associated increased set shifting errors among individuals with AN.85 Several studies found decreased neural activation in regions that are considered part of control networks during tasks that involve inhibition of motor responses. On the stop signal task, there are data suggesting decreased medial pre-frontal activity during difficult trials; however, there is no evidence of behavioral differences between patients with AN and healthy controls.84,86,87
Summary
Behavior in AN is highly suggestive of aberrant self-control processes; neuroimaging suggests that the neural mechanisms of AN symptoms may be more complex than solely excessive self-control.
Behavioral Studies: Bulimia Nervosa
Behavioral data from classic neurocognitive paradigms support the hypothesis that BN is characterized by deficits in both behavioral and cognitive control. Results of a metaanalysis that included mostly studies using Stroop task variants suggest deficits in cognitive control, or interference control, in individuals with BN that are more pronounced when food or body-related stimuli are used.88
Brain Studies: Bulimia Nervosa
In food-related fMRI studies, individuals with BN show altered neural activation in control-related regions. Individuals with BN showed decreased activation compared with healthy controls in the left dlPFC in response to food pictures and trend-level decreased left dlPFC activation during chocolate shake receipt.49,52 However, because there is no explicit control-related demand during these tasks, decreased activation in PFC cannot be linked explicitly to control-related processes.
To date, 4 studies have used cognitive and behavioral tasks during fMRI scanning to measure the neural correlates of control in BN. Two studies found that both adolescents and adults with BN showed decreased activation in the frontostriatal control circuitry during the resolution of cognitive conflict on the Simon task, and this decreased activation was associated with more frequent bulimic symptoms.89,90 Machine learning approaches using these altered patterns of activation can reliably distinguish BN cases from healthy controls.91,92 One study has used a go/no-go task to examine differences in response inhibition to food and neutral images and found that only a BN subgroup with the most frequent binge eating showed decreased activation during response inhibition to neutral images in dorsal striatum, with additional hypoactivation in sensorimotor areas.92 One emotional go/no-go study found that individuals with BN showed age-dependent deficits in PFC activation during emotional go/no-go task response inhibition relative to healthy controls.93
Structural neuroimaging findings also suggest that alterations in control-related circuitry in BN may be age dependent and reflect an abnormal neurodevelopmental trajectory. Cross-sectional data from large samples of adolescents and adults with BN indicate age-associated decreases in the volume and cortical thickness of frontal and parietal regions.94,95 The only longitudinal structural imaging study of adolescent BN to date reported that reduced cortical thickness compared with healthy controls in the right ventrolateral PFC persisted over 2 years of follow-up, even among those who achieved symptom remission.96 Between-subject variations in cortical thickness of the ventrolateral PFC were inversely associated with specific BN symptoms, suggesting consistently more pronounced cortical thinning in this control-associated region in individuals with more frequent BN symptoms. Subcortical findings have been more inconsistent, and decreased60,97 or normal98,99 volumes of dorsal striatum, specifically the caudate nucleus, have been documented in BN compared with control participants.
The only published study of brain connectivity at rest using a control-related seed (in the left dlPFC) did not report any difference in resting state functional connectivity in executive control circuitry in women with BN compared with healthy controls.63
Summary
Behavioral data in BN indicate deficits in inhibitory control that are particularly pronounced in the context of food cues. Limited existing neuroimaging data suggest that functional and structural alterations in control circuits occur early in the course of BN and may contribute to the disorder’s persistence over time.
DECISION MAKING
Several important advances in cognitive neuroscience have come from examining decision making. Examining maladaptive behavior in eating disorders creates top-down models and testable hypotheses about neural mechanisms of illness. This approach has been less commonly used to date, but holds a lot of promise. Decision making is often measured as a choice behavior, which in many ways is the result of reward and control as well as other cognitive processes such as attention, learning, and memory. This approach, which integrates behavioral and brain studies, provides complementary insights to the bottom-up probes described elsewhere in this article.
Activation in corticostriatal regions (caudate, anterior putamen, ventromedial PFC, and dlPFC) is integral to goal-directed action.4,100–104 Computational approaches have allowed for differentiation between goal-oriented and more automatic decisions.105 Value-based decision making has been shown to be mediated by cortical systems.106,107 When decisions are more automatic, or habitual, these processes are related to the dorsal striatum and associated cortical regions.108
Monetary Decisions (Temporal Discounting)
One well-characterized behavior involves the trade-off between rewards available immediately, and rewards available only after a delay—termed temporal discounting. Among healthy controls, there is individual variability in the rate at which the value of the reward decreases with the time needed to wait to receive it. This complex behavior incorporates reward systems and control systems, as well as the experience of time.109 The discount rate, a quantification of the loss of value over time, has been shown to relate to real-life behaviors, such as academic performance and risk-taking behaviors. Among individuals with eating disorders, temporal discounting has been of interest because of the potential analogy to trade-offs between the immediate food reward and longer term weight- or shape-related goals.110 Several studies have found that individuals with AN discount the value of money less steeply than healthy controls,111–114 although some have not.115,116 Associated neuroimaging findings have suggested that there are frontostriatal system differences between patients with AN and healthy controls during temporal discounting. Acutely ill individuals with AN showed decreased ventral striatal and dorsal ACC activation associated with decreased (ie, less steep) discounting of money. After weight restoration, normalization of behavior was associated with increased activation of the striatum and dlPFC (as well as other cortical regions).112 In a different paradigm, while healthy controls showed different activation patterns in reward and cognitive control-related circuitry depending on metabolic state (ie, hunger vs satiety), individuals recovered from AN did not.117
Among individuals with BN, temporal discounting rates are greater than those of healthy controls, suggesting a preference for immediate reward, difficulty delaying gratification, and perhaps poor control over reward.118,119 Transcranial direct current stimulation over the dlPFC decreased temporal discounting rates and temporarily decreased urges to binge eat.120 Although no published fMRI studies of BN have used delay discounting tasks, these noninvasive brain stimulation data support the hypothesis that lateral PFC dysfunction in BN contributes to difficulty delaying gratification.
Food-Based Decisions
Disturbances in eating are the central behavioral phenomena defining eating disorders (see Fig. 1). Examining the neural mechanisms of these maladaptive behaviors are therefore the main focus of a top-down approach. To address this in AN, a food choice task asks participants to make choices about what to eat, knowing that they will receive one of their choices after the task.106,121 Choice of high-fat foods during the task has been shown to correlate with actual caloric intake in a laboratory meal, providing external validation that this task measures maladaptive restrictive intake.122 One proposed model suggests that these maladaptive behaviors are habitual, or over-trained, choices and not governed by value-based decision-making systems.123 A key prediction of this model is that, like neural mechanisms of habit, restricted food intake in AN is guided by activity in the dorsal striatum and associated cortical regions, such as the dlPFC. fMRI scanning during this food choice task indicated that among those with AN, but not healthy controls, active food choice was associated with neural activation in the dorsal striatum.
SUMMARY
Research on the neural mechanisms of AN and BN have yet to converge on 1 clear underlying pathophysiology. Yet, current directions in research are promising. The most compelling data come from studies that began with a biological model and tested specific hypotheses. These behavioral, functional, and structural MRI, and PET studies have indicated that the reinforcing value of food is reduced in AN, whereas individuals with BN experience greater reinforcement from properties of food that are associated with binge eating. There are hints of reward processing and reward learning abnormalities that indicate that reward system dysfunction may be an important path forward. Current data suggest that control systems—broadly focused on corticostriatothalamocortical pathways—are affected in BN, and may reflect altered neurodevelopment. This direction has been less fruitful for understanding AN. Direct examination of maladaptive behavior in AN has indicated that there are differences in neural mechanisms of decision making about food. By testing hypotheses about the neural mechanisms of maladaptive behavior and about illness-related dysfunction in reward and control systems, the field may advance in understanding the pathophysiology of eating disorders.
Considerations and Limitations of Existing Research
Neurocognitive and neuroimaging research in AN and BN have often been limited by small sample sizes and differences in task designs that impact interpretation. For example, probes of the reinforcing value of food tend to find decreased reward from food in AN. Yet, when an active response to a reward or an actual ingestion is the outcome, there is less evidence of decreased responding (or sensitivity) to reward. Predictability of the stimulus delivered, the reward stimulus used (eg, pictures of money or food; glucose, sucrose, or sweet–fat combination solutions; artificial saliva; noncaloric sweet taste), and responses measured (eg, passive receipt, approach or avoidance responses) contribute to challenges in clarifying dysfunction in reward systems.
Studies of control mechanisms have used complex tasks with multiple subcomponents. For example, because the Stroop and Simon tasks simultaneously assess inhibitory and attentional control and conflict monitoring, it is unclear whether high error rates on these tasks indicate deficits in one or all of these dimensions of control, making it difficult to clarify mechanism dysfunction in eating disorders.
Probing decision making around monetary choices has its limitations, because this is not a direct reflection of the pathology of eating disorders. It may be that probing discounting in the setting of more illness-specific choices will be fruitful.124
There are also universal challenges in interpreting neural correlates in the setting of illness. If a clinical group performs differently from healthy controls on a task, differences in brain activation may simply reflect that behavior—without relevance for disease processes. When groups perform similarly on a task, several factors (eg, a different cognitive process, a different neural computation) could explain reduced activation in a clinical group, and again relevance to disease processes cannot be assumed automatically.125 Throughout the literature, differences in the stage of illness of the studied population creates a challenge. The field would benefit from clearer definitions of clinical stages, to improve our ability to identify markers of disease.
Future Directions
To move the field forward, new tasks, analytical approaches, and longitudinal designs are needed. Novel paradigms that permit a top-down approach may better elucidate the neural mechanisms of illness. Many existing paradigms have not demonstrated relevance to actual maladaptive behavior. Several studies report statistical correlations between eating disorder symptoms and control-related measures, yet it is unknown whether the processes assessed by these tasks directly promote salient pathology in AN and BN. Tasks that measure eating-specific planning126 and decision making, and food approach followed by consumption will be important to more accurately model the neural bases of dysregulated eating in AN and BN.
Tasks with greater specificity around subcomponents of broad neurocognitive constructs could help to inform future top-down studies and isolate targets for novel intervention. For example, within the realm of cognitive control, some data suggest that investigation of attentional control in eating disorders is warranted.79,94,127,128 This process may contribute to difficulty in planning and organizing eating behavior in BN.127
Advanced analytical approaches such as computational models show a lot of promise for understanding circuit-level abnormalities and individual variability that may promote eating disorder symptoms. Models from other areas of computational psychiatry could be developed to specifically understand reward, control, and decision-making processes in food and non-food-based paradigms.
All of these directions need to be incorporated into longitudinal imaging studies with large samples to fully understand the neuropathology of eating disorders.
KEY POINTS.
Cognitive neuroscience offers research approaches that can test hypotheses about the link between maladaptive eating behavior and underlying neural systems, thereby helping to further our understanding of eating disorders.
Reward-focused approaches have identified reward processing and learning abnormalities in anorexia nervosa, which have been less studied in bulimia nervosa.
Control-focused approaches suggest corticostriatal abnormalities in bulimia nervosa associated with dysfunction in cognitive and behavioral control.
Decision-making approaches show that the neural mechanisms of food choice among patients with anorexia nervosa differ from healthy individuals.
Abbreviations
- ACC
Anterior cingulate cortex
- AN
Anorexia nervosa
- BN
Bulimia nervosa
- dlPFC
Dorsolateral PFC
- fMRI
Functional MRI
- OFC
Orbitofrontal cortex
- PFC
Prefrontal cortex
Footnotes
Disclosures: J.E. Steinglass and E. Attia receive royalties from UpToDate.
REFERENCES
- 1.Kaye W Neurobiology of anorexia and bulimia nervosa. Physiol Behav 2008; 94(1):121–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaye WH, Wierenga CE, Bailer UF, et al. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci 2013;36(2):110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol 2009;9(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 1998;37: 407–19. [DOI] [PubMed] [Google Scholar]
- 5.Keating C, Tilbrook AJ, Rossell SL, et al. Reward processing in anorexia nervosa. Neuropsychologia 2012;50(5):567–75. [DOI] [PubMed] [Google Scholar]
- 6.Pearson CM, Wonderlich SA, Smith GT. A risk and maintenance model for bulimia nervosa: from impulsive action to compulsive behavior. Psychol Rev 2015;122(3):516–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev 2009;33(5):631–46. [DOI] [PubMed] [Google Scholar]
- 8.Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry 2011;69(12): e55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A 2007;104(26): 11073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haber SN. Corticostriatal circuitry In: Pfaff DW, Volkow ND, editors. Neuroscience in the 21st century. New York: Springer New York; 2016. p. 1–21. [Google Scholar]
- 11.Hampshire A, Chamberlain SR, Monti MM, et al. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 2010;50:1313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001;24:167–202. [DOI] [PubMed] [Google Scholar]
- 13.Fischer S, Smith GT, Cyders MA. Another look at impulsivity: a meta-analytic review comparing specific dispositions to rash action in their relationship to bulimic symptoms. Clin Psychol Rev 2008;28(8):1413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry 2009; 166(6):664–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maia TV, Frank MJ. From reinforcement learning models to psychiatric and neurological disorders. Nat Neurosci 2011;14(2):154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd CE, Steinglass JE. What can food-image tasks teach us about anorexia nervosa? A systematic review. J Eat Disord 2018;6(31):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowdrey FA, Finlayson G, Park RJ. Liking compared with wanting for high- and low-calorie foods in anorexia nervosa: aberrant food reward even after weight restoration. Am J Clin Nutr 2013;97(3):463–70. [DOI] [PubMed] [Google Scholar]
- 18.Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav 2009;97(5):537–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis C, Woodside DB. Sensitivity to the rewarding effects of food and exercise in the eating disorders. Compr Psychiatry 2002;43(3):189–94. [DOI] [PubMed] [Google Scholar]
- 20.Wagner A, Barbarich N, Frank G, et al. Personality traits after recovery from eating disorders: do subtypes differ? Int J Eat Disord 2006;39(4):276–84. [DOI] [PubMed] [Google Scholar]
- 21.Lowe MR, Arigo D, Butryn ML, et al. Hedonic hunger prospectively predicts onset and maintenance of loss of control eating among college women. Health Psychol 2016;35(3):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witt AA, Lowe MR. Hedonic hunger and binge eating among women with eating disorders. Int J Eat Disord 2014;47(3):273–80. [DOI] [PubMed] [Google Scholar]
- 23.Klein DA, Boudreau GS, Devlin MJ, et al. Artificial sweetener use among individuals with eating disorders. Int J Eat Disord 2006;39(4):341–5. [DOI] [PubMed] [Google Scholar]
- 24.Scaife JC, Godier LR, Reinecke A, et al. Differential activation of the frontal pole to high vs low calorie foods: the neural basis of food preference in anorexia nervosa? Psychiatry Res 2016;258:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veenstra EM, de Jong PJ. Reduced automatic motivational orientation towards food in restricting anorexia nervosa. J Abnorm Psychol 2011;120(3):708–18. [DOI] [PubMed] [Google Scholar]
- 26.Paslakis G, Kuhn S, Schaubschlager A, et al. Explicit and implicit approach vs. avoidance tendencies towards high vs. low calorie food cues in patients with anorexia nervosa and healthy controls. Appetite 2016;107:171–9. [DOI] [PubMed] [Google Scholar]
- 27.Neimeijer RA, de Jong PJ, Roefs A. Automatic approach/avoidance tendencies towards food and the course of anorexia nervosa. Appetite 2015;91:28–34. [DOI] [PubMed] [Google Scholar]
- 28.Schebendach J, Klein DA, Mayer LES, et al. Assessment of the motivation to use artificial sweetener among individuals with an eating disorder. Appetite 2017; 109:131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foerde K, Steinglass JE. Decreased feedback learning in anorexia nervosa persists after weight restoration. Int J Eat Disord 2017;50(4):415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuglset TS, Landro NI, Reas DL, et al. Functional brain alterations in anorexia nervosa: a scoping review. J Eat Disord 2016;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowdrey FA, Park RJ, Harmer CJ, et al. Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biol Psychiatry 2011;70(8):736–43. [DOI] [PubMed] [Google Scholar]
- 32.Ellison Z, Foong J, Howard R, et al. Functional anatomy of calorie fear in anorexia nervosa. Lancet 1998;352:1192. [DOI] [PubMed] [Google Scholar]
- 33.Holsen LM, Lawson EA, Blum J, et al. Food motivation circuitry hypoactivation related to hedonic and nonhedonic aspects of hunger and satiety in women with active anorexia nervosa and weight-restored women with anorexia nervosa. J Psychiatry Neurosci 2012;37(5):322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oberndorfer TA, Frank GK, Simmons AN, et al. Altered insula response to sweet taste processing after recovery from anorexia and bulimia nervosa. Am J Psychiatry 2013;170(10):1143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner A, Aizenstein H, Mazurkewicz L, et al. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology 2008;33(3):513–23. [DOI] [PubMed] [Google Scholar]
- 36.Monteleone AM, Monteleone P, Esposito F, et al. Altered processing of rewarding and aversive basic taste stimuli in symptomatic women with anorexia nervosa and bulimia nervosa: an fMRI study. J Psychiatr Res 2017;90:94–101. [DOI] [PubMed] [Google Scholar]
- 37.Bischoff-Grethe A, McCurdy D, Grenesko-Stevens E, et al. Altered brain response to reward and punishment in adolescents with anorexia nervosa. Psychiatry Res 2013;214(3):331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner A, Aizenstein H, Venkatraman VK, et al. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry 2007;164(12): 1842–9. [DOI] [PubMed] [Google Scholar]
- 39.Frank GK, Reynolds JR, Shott ME, et al. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology 2012; 37(9):2031–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank GKW, DeGuzman MC, Shott ME, et al. Association of brain reward learning response with harm avoidance, weight gain, and hypothalamic effective connectivity in adolescent anorexia nervosa. JAMA Psychiatry 2018; 75(10):1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frank GK, Bailer UF, Henry SE, et al. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol Psychiatry 2005;58(11):908–12. [DOI] [PubMed] [Google Scholar]
- 42.Bailer UF, Frank GK, Henry SE, et al. Serotonin transporter binding after recovery from eating disorders. Psychopharmacology (Berl) 2007;195(3):315–24. [DOI] [PubMed] [Google Scholar]
- 43.Broft A, Slifstein M, Osborne J, et al. Striatal dopamine type 2 receptor availability in anorexia nervosa. Psychiatry Res 2015;233(3):380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cha J, Ide JS, Bowman FD, et al. Abnormal reward circuitry in anorexia nervosa: a longitudinal, multimodal MRI study. Hum Brain Mapp 2016;37(11):3835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bodell L, Keel P. Weight suppression in bulimia nervosa: associations with biology and behavior. J Abnorm Psychol 2015;124(4):994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schebendach J, Broft A, Foltin R, et al. Can the reinforcing value of food be measured in bulimia nervosa? Appetite 2013;62:70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein D, Schenbendach J, Brown A, et al. Modified sham feeding of sweet solutions in women with and without bulimia nervosa. Physiol Behav 2009;96(1): 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brooks S, O’Daly O, Uher R, et al. Differential neural responses to food images in women with bulimia versus anorexia nervosa. PLoS One 2011;6(7):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uher R, Murphy T, Brammer M, et al. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry 2004;161(7): 1238–46. [DOI] [PubMed] [Google Scholar]
- 50.Bohon C, Stice E. Negative affect and neural response to palatable food intake in bulimia nervosa. Appetite 2012;58(3):964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frank G, Wagner A, Brooks-Achenbach S, et al. Altered brain activity in women recovered from bulimic type eating disorders after a glucose challenge. A pilot study. Int J Eat Disord 2006;39(1):76–9. [DOI] [PubMed] [Google Scholar]
- 52.Bohon C, Stice E. Reward abnormalities among women with full and subthreshold bulimia nervosa: a functional magnetic resonance imaging study. Int J Eat Disord 2011;44(7):585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frank G, Reynolds J, Shott M, et al. Altered temporal difference learning in bulimia nervosa. Biol Psychiatry 2011;70(8):728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oberndorfer T, Frank G, Fudge J, et al. Altered insula response to sweet taste processing after recovery from anorexia and bulimia nervosa. Am J Psychiatry 2013;214(2):132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radeloff D, Willmann K, Otto L, et al. High-fat taste challenge reveals altered striatal response in women recovered from bulimia nervosa: a pilot study. World J Biol Psychiatry 2014;15(4):307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ely A, Wierenga C, Bischoff-Grethe A, et al. Response in taste circuitry is not modulated by hunger and satiety in women remitted from bulimia nervosa. J Abnorm Psychol 2017;126(5):519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner A, Aizeinstein H, Venkatraman V, et al. Altered striatal response to reward in bulimia nervosa after recovery. Int J Eat Disord 2010;43(4):289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simon J, Skunde M, Walther S, et al. Neural signature of food reward processing in bulimic-type eating disorders. Soc Cogn Affect Neurosci 2016;11(9): 1393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schafer A, Vaitl D, Schienle A. Regional grey matter volume abnormalities in bulimia nervosa and binge-eating disorder. Neuroimage 2010;50(2):639–43. [DOI] [PubMed] [Google Scholar]
- 60.Frank GK, Shott ME, Hagman JO, et al. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry 2013;170(10):1152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broft A, Shingleton R, Kaufman J, et al. Striatal dopamine in bulimia nervosa: a PET imaging study. Int J Eat Disord 2012;45(5):648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tauscher J, Pirker W, Willeit M, et al. [123I] beta-CITand single photon emission computed tomography reveal reduced brain serotonin transporter availability in bulimia nervosa. Biol Psychiatry 2001;49(4):326–32. [DOI] [PubMed] [Google Scholar]
- 63.Lavagnino L, Amianto F, D’Agata F, et al. Reduced resting-state functional connectivity of the somatosensory cortex predicts psychopathological symptoms in women with bulimia nervosa. Front Behav Neurosci 2014;8:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee S, Ran Kim K, Ku J, et al. Resting-state synchrony between anterior cingulate cortex and precuneus relates to body shape concern in anorexia nervosa and bulimia nervosa. Psychiatry Res 2014;221(1):43–8. [DOI] [PubMed] [Google Scholar]
- 65.Amianto F, D’Agata F, Lavagnino L, et al. Intrinsic connectivity networks within cerebellum and beyond in eating disorders. Cerebellum 2013;12(5):623–31. [DOI] [PubMed] [Google Scholar]
- 66.Wang L, Kong Q-M, Li K, et al. Altered intrinsic functional brain architecture in female patients with bulimia nervosa. J Psychiatry Neurosci 2017;42(6):414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dunlop K, Woodside B, Lam E, et al. Increases in frontostriatal connectivity are associated with response to dorsomedial repetitive transcranial magnetic stimulation in refractory binge/purge behaviors. Neuroimage Clin 2015;8:611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eagle D, Bari A, Robbins T. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology 2008;199:439–56. [DOI] [PubMed] [Google Scholar]
- 69.Rubia K, Russelol T, Overmeyer S, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage 2001;13(2):250–61. [DOI] [PubMed] [Google Scholar]
- 70.Logan G, Schachar R, Tannock R. Impulsivity and inhibitory control. Psychol Sci 1997;8:60–4. [Google Scholar]
- 71.Stroop J Studies of interference in serial verbal reactions. J Exp Psychol 1935; 18:643–62. [Google Scholar]
- 72.Simon JR. Reactions toward the source of stimulation. J Exp Psychol 1969; 81(1):174–6. [DOI] [PubMed] [Google Scholar]
- 73.Aron AR, Herz DM, Brown P, et al. Frontosubthalamic circuits for control of action and cognition. J Neurosci 2016;36(45):11489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci 2012;35:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: fifth edition (DSM-5). Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 76.Wierenga C, Ely A, Bischoff-Grethe A, et al. Are extremes of consumption in eating disorders related to an altered balance between reward and inhibition? Front Behav Neurosci 2014;9(8):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fischer S, Smith GT, Anderson KG. Clarifying the role of impulsivity in bulimia nervosa. Int J Eat Disord 2003;33:406–11. [DOI] [PubMed] [Google Scholar]
- 78.Nasser J, Gluck M, Geliebter A. Impulsivity and test meal intake in obese binge eating women. Appetite 2004;43(3):303–7. [DOI] [PubMed] [Google Scholar]
- 79.Rosval L, Steiger H, Bruce K, et al. Impulsivity in women with eating disorders: problem of response inhibition, planning, or attention? Int J Eat Disord 2006; 39(7):590–3. [DOI] [PubMed] [Google Scholar]
- 80.Steiger H, Lehoux P, Gauvin L. Impulsivity, dietary control and the urge to binge in bulimic syndromes. Int J Eat Disord 1999;26(3):261–74. [DOI] [PubMed] [Google Scholar]
- 81.Yanovski S, Nelson J, Dubbert B, et al. Association of binge eating disorder and psychiatric comorbidity in obese subjects. Am J Psychiatry 1993;150(10): 1472–9. [DOI] [PubMed] [Google Scholar]
- 82.Smith KE, Mason TB, Johnson JS, et al. A systematic review of reviews of neuro-cognitive functioning in eating disorders: the state-of-the-literature and future directions. Int J Eat Disord 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schneier FR, Kimeldorf MB, Choo TH, et al. Attention bias in adults with anorexia nervosa, obsessive-compulsive disorder, and social anxiety disorder. J Psychiatr Res 2016;79:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bartholdy S, Dalton B, O’Daly OG, et al. A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task. Neurosci Biobehav Rev 2016;64:35–62. [DOI] [PubMed] [Google Scholar]
- 85.Zastrow A, Kaiser S, Stippich C, et al. Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. Am J Psychiatry 2009;166(5):608–16. [DOI] [PubMed] [Google Scholar]
- 86.Wierenga C, Bischoff-Grethe A, Melrose AJ, et al. Altered BOLD response during inhibitory and error processing in adolescents with anorexia nervosa. PLoS One 2014;9(3):e92017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oberndorfer TA, Kaye WH, Simmons AN, et al. Demand-specific alteration of medial prefrontal cortex response during an inhibition task in recovered anorexic women. Int J Eat Disord 2011;44(1):1–8. [DOI] [PubMed] [Google Scholar]
- 88.Wu M, Hartmann M, Skunde M, et al. Inhibitory control in bulimic-type eating disorders: a systematic review and meta-analysis. PLoS One 2013;8(12):e83412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marsh R, Steinglass JE, Gerber AJ, et al. Deficient activity in the neural systems that mediate self-regulatory control in bulimia nervosa. Arch Gen Psychiatry 2009;66(1):51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marsh R, Horga G, Wang Z, et al. An FMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. Am J Psychiatry 2011; 168(11):1210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cyr M, Yang X, Horga G, et al. Abnormal fronto-striatal activation as a marker of threshold and subthreshold bulimia nervosa. Hum Brain Mapp 2018;39(4): 1796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Skunde M, Walther S, Simon JJ, et al. Neural signature of behavioural inhibition in women with bulimia nervosa. J Psychiatry Neurosci 2016;41(5):E69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dreyfuss MFW, Riegel ML, Pedersen GA, et al. Patients with bulimia nervosa do not show typical neurodevelopment of cognitive control under emotional influences. Psychiatry Res Neuroimaging 2017;266:59–65. [DOI] [PubMed] [Google Scholar]
- 94.Berner LA, Stefan M, Lee S, et al. Altered cortical thickness and attentional deficits in adolescent girls and women with bulimia nervosa. J Psychiatry Neurosci 2018;43(3):151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marsh R, Stefan M, Bansal R, et al. Anatomical characteristics of the cerebral surface in bulimia nervosa. Biol Psychiatry 2015;77(7):616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cyr M, Kopala-Sibley DC, Lee S, et al. Reduced inferior and orbital frontal thickness in adolescent bulimia nervosa persists over two-year follow-up. J Am Acad Child Adolesc Psychiatry 2017;56(10):866–74.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coutinho J, Ramos AF, Maia L, et al. Volumetric alterations in the nucleus accumbens and caudate nucleus in bulimia nervosa: a structural magnetic resonance imaging study. Int J Eat Disord 2015;48(2):206–14. [DOI] [PubMed] [Google Scholar]
- 98.Joos A, Kloppel S, Hartmann A, et al. Voxel-based morphometry in eating disorders: correlation of psychopathology with grey matter volume. Psychiatry Res 2010;182(2):146–51. [DOI] [PubMed] [Google Scholar]
- 99.Amianto F, Caroppo P, D’Agata F, et al. Brain volumetric abnormalities in patients with anorexia and bulimia nervosa: a voxel-based morphometry study. Psychiatry Res 2013;213(3):210–6. [DOI] [PubMed] [Google Scholar]
- 100.de Wit S, Corlett PR, Aitken MR, et al. Differential engagement of the ventromedial prefrontal cortex by goal-directed and habitual behavior toward food pictures in humans. J Neurosci 2009;29(36):11330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Wit S, Watson P, Harsay HA, et al. Corticostriatal connectivity underlies individual differences in the balance between habitual and goal-directed action control. J Neurosci 2012;32(35):12066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 2009;35(1):48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Valentin VV, Dickinson A, O’Doherty JP. Determining the neural substrates of goal-directed learning in the human brain. J Neurosci 2007;27(15):4019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tanaka SC, Balleine BW, O’Doherty JP. Calculating consequences: brain systems that encode the causal effects of actions. J Neurosci 2008;28(26):6750–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Doll BB, Shohamy D, Daw ND. Multiple memory systems as substrates for multiple decision systems. Neurobiol Learn Mem 2015;117:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science 2009;324(5927):646–8. [DOI] [PubMed] [Google Scholar]
- 107.Pisauro MA, Fouragnan E, Retzler C, et al. Neural correlates of evidence accumulation during value-based decisions revealed via simultaneous EEG-fMRI. Nat Commun 2017;8:15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith KS, Graybiel AM. Habit formation coincides with shifts in reinforcement representations in the sensorimotor striatum. J Neurophysiol 2016;115(3): 1487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lempert KM, Steinglass JE, Pinto A, et al. Can delay discounting deliver on the promise of RDoC? Psychol Med 2018;1–10 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 110.McClelland J, Dalton B, Kekic M, et al. A systematic review of temporal discounting in eating disorders and obesity: behavioural and neuroimaging findings. Neurosci Biobehav Rev 2016;71:506–28. [DOI] [PubMed] [Google Scholar]
- 111.Steinglass JE, Lempert KM, Choo TH, et al. Temporal discounting across three psychiatric disorders: anorexia nervosa, obsessive compulsive disorder, and social anxiety disorder. Depress Anxiety 2017;34(5):463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Decker JH, Figner B, Steinglass JE. On weight and waiting: delay discounting in anorexia nervosa pretreatment and posttreatment. Biol Psychiatry 2015;78(9): 606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Steinglass JE, Figner B, Berkowitz S, et al. Increased capacity to delay reward in anorexia nervosa. J Int Neuropsychol Soc 2012;18(4):773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Steward T, Mestre-Bach G, Vintro-Alcaraz C, et al. Delay discounting of reward and impulsivity in eating disorders: from anorexia nervosa to binge eating disorder. Eur Eat Disord Rev 2017;25(6):601–6. [DOI] [PubMed] [Google Scholar]
- 115.King JA, Geisler D, Bernardoni F, et al. Altered neural efficiency of decision making during temporal reward discounting in anorexia nervosa. J Am Acad Child Adolesc Psychiatry 2016;55(11):972–9. [DOI] [PubMed] [Google Scholar]
- 116.Ritschel F, King JA, Geisler D, et al. Temporal delay discounting in acutely ill and weight-recovered patients with anorexia nervosa. Psychol Med 2015;45(6): 1229–39. [DOI] [PubMed] [Google Scholar]
- 117.Wierenga CE, Bischoff-Grethe A, Melrose AJ, et al. Hunger does not motivate reward in women remitted from anorexia nervosa. Biol Psychiatry 2015;77(7): 642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 2013;108:44–79. [DOI] [PubMed] [Google Scholar]
- 119.Kekic M, Bartholdy S, Cheng J, et al. Increased temporal discounting in bulimia nervosa. Int J Eat Disord 2016;49(12):1077–81. [DOI] [PubMed] [Google Scholar]
- 120.Kekic M, McClelland J, Bartholdy S, et al. Single-session transcranial direct current stimulation temporarily improves symptoms, mood, and self-regulatory control in bulimia nervosa: a randomised controlled trial. PLoS One 2017;12(1): e0167606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steinglass J, Foerde K, Kostro K, et al. Restrictive food intake as a choice–a paradigm for study. Int J Eat Disord 2015;48(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Foerde K, Steinglass JE, Shohamy D, et al. Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nat Neurosci 2015;18(11):1571–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Walsh BT. The enigmatic persistence of anorexia nervosa. Am J Psychiatry 2013;170(5):477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schreyer CC, Berry MS, Hansen JL, et al. Delay discounting in patients with anorexia nervosa: examining caloric restriction as a reward. International Conference on Eating Disorders Chicago, IL, April 21, 2018. [Google Scholar]
- 125.Poldrack RA. Is “efficiency” a useful concept in cognitive neuroscience? Dev Cogn Neurosci 2015;11:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pearson CM, Chester DS, Powell D, et al. Investigating the reinforcing value of binge anticipation. Int J Eat Disord 2016;49(6):539–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seitz J, Kahraman-Lanzerath B, Legenbauer T, et al. The role of impulsivity, inattention and comorbid ADHD in patients with bulimia nervosa. PLoS One 2013; 8(5):e63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. 3rd edition New York: Oxford University Press; 2006. [Google Scholar]


