Abstract
Previous studies revealed associations of urinary Cd (U-Cd), a chronic Cd exposure biomarker, with blood pressure (BP) in non-pregnant adults. However, the evidence regarding trimester-specific blood pressure in pregnancy and U-Cd and effect modification by dietary intake of micronutrients is scarce. We randomly selected 653 women from the Omega Study cohort. U-Cd was quantified by inductively coupled plasma mass spectrometry. Trimester-specific, systolic (SBP) and diastolic blood pressure (DBP) were determined employing standard protocols and mean arterial pressure (MAP) was also calculated. Associations of SBP, DBP and MAP with U-Cd tertiles (≤0.21; 0.22–0.41; ≥0.42μg/g Cr) were assessed using multivariable linear regression models. We also explored effect modification by pre-pregnancy BMI (≤25 kg/m2 or >25 kg/m2) or low/high micronutrients intake. After adjusting confounders in, women with elevated (upper tertile) as compared with those with low (lowest tertile) U-Cd (≥ 0.42 μg/g Cr vs ≤0.21 μg/g Cr, respectively) had reduced third trimester MAP (−1.8; 95% CI: −3.1, −0.5 mmHg) and second trimester MAP (−1.1; 95% CI:−2.3,−0.03 mmHg). A significant decrease in third-trimester MAP associated with increased U-Cd was observed only among normal weight women (BMI≤25 kg/m2) and women with high dietary intake of micronutrients (calcium, magnesium, zinc and selenium). Notably, U-Cd concentrations increased with the increased consumption of zinc and non-heme iron food sources. No significant differences in U-Cd concentrations were found in preeclamptic women compared with non-preeclamptic women. Our study provides evidence that dietary intake of micronutrients should be taken into account when assessing the health effects of Cd in pregnant women.
Keywords: blood pressure, urinary cadmium, pregnancy, micronutrients
Background
Cadmium (Cd) is a toxic metal found in food, tobacco, and certain occupational environments. In the general nonsmoking population, diet is the most important source of Cd [1]. Leafy greens vegetables, grains, shellfish, root vegetables, legumes, seeds and nuts, and organ meats are typically considered to be the most significant dietary sources of Cd among non-smokers [2,3]. The effects of Cd on human health are diverse ranging from renal failure to osteoporosis, diabetes and cancer [4]. Moreover, Cd exposure has been associated with cardiovascular disease, such as coronary heart disease [5], peripheral artery disease [6] and heart rate variability [7]. Cd has a very long half-life in human -ranging from 5 to 30 years. Of note U-Cd mainly measures long-term exposure and blood Cd (B-Cd) recent exposure, but with noticeable overlap [8]. The association of either B-Cd or U-Cd with blood pressure has been inconsistent among non-pregnant adults with some studies showing a positive association of Cd with blood pressure while others finding non-significant or even inverse association [9].
Among adults, women constitute a risk group for Cd toxic effects partly because of higher concentrations of Cd in blood, urine and kidney and also they are able to absorb more Cd through the gastrointestinal tract due to higher prevalence of low iron stores [10]. Besides sex differences, pregnancy represents an increased susceptibility to Cd toxic effects for both the mother and the fetus [11,12].
In the course of normal pregnancy, mean arterial pressure (MAP) increases in the third trimester (37–40 weeks) compared with values in early pregnancy (17–20 weeks) (89 vs. 84 mmHg, respectively) [13]. Additional, preexisting cardiovascular conditions might be exacerbated by adaptations that occur during pregnancy such as circulating blood volume increase, systemic vascular resistance, cardiac output increase, among others [13]. Many other factors may increase preeclampsia risk including overweight/obesity [14], deficient intake of micronutrients (calcium, selenium, zinc and magnesium) [15–17] and Cd exposure [18,19]. At molecular level, Cd interferes with absorption of calcium, zinc, selenium and magnesium and deficiencies of these essential metals exaggerate cadmium toxicity due to increased absorption in the gut and greater retention in different organs [20,21].
To the best of our knowledge, the relation between U-Cd and trimester specific blood pressures have not been assessed. Therefore, we hypothesized that U-Cd will be associated with increased third trimester MAP, and this increase will be attenuated by higher dietary intake of micronutrients. We further hypothesized that the increase in MAP associated with U-Cd will be restricted to overweight/obese pregnant women.
Material and Methods
Study Population
The Omega Study, a large (N=4,344) prospective cohort study (1996–2008) based at the Center for Perinatal Studies at Swedish Medical Center in Seattle, Washington, USA was designed to investigate risk factors of pregnancy complications [22]. Participants were recruited from prenatal care clinics affiliated with Swedish Medical Center (Seattle, Washington) and Tacoma General Hospital (Tacoma, Washington). Women who spoke English and initiated prenatal care at a study clinic prior to 20 weeks gestation were eligible for participation. Women who were less than 18 years of age, did not intend to carry the pregnancy to term, or did not plan to deliver at study institutions were excluded. All procedures and study protocols were approved by the institutional review boards at the University of Washington and the study hospitals, and all participants provided written informed consent.
We selected a subcohort of 732 women randomly drawn from the full Omega study cohort. From the subcohort of 732 women, we excluded 29 women with multifetal pregnancies, 17 women with diabetes prior to pregnancy, 5 women with renal disease, 26 women with pre-gestational chronic hypertension, and 2 women with both conditions renal disease and hypertension. After exclusions, the analytic population for this study included 653 women.
Data Collection
Enrolled participants were asked to participate in a 45 to 60 minute interview during which trained research personnel used a structured questionnaire to elicit information regarding maternal sociodemographic characteristics, lifestyle habits (such as alcohol and tobacco use), and medical and reproductive histories. Three possible answers were employed to assess smoking status: 1) never smokers, 2) former smokers or 3) current smokers. Trained interviewers collected maternal anthropometry by using a structured questionnaire shortly after enrollment (15 gestational weeks, on average). Participants completed a self-administrated, validated, and semi-quantitative food-frequency questionnaire (FFQ) describing dietary intake over the prior six months (three months before pregnancy through the first three months of pregnancy, on average) [23]. For each food item, a standard portion size was specified and the participants answered how often, on average, they consumed food of that specified amount. There were 9 possible coding responses, ranging from “never or less than once per month” to “six or more times per day”. For example, zinc intake from food sources was calculated by multiplying the frequency of consumption of each food by zinc composition in the specified amount of that food and then summing up the zinc intake from each food item. Similarly, the food intake of calcium, magnesium, selenium and total iron was calculated [23]. In addition, we asked about the use of vitamin supplements during pregnancy.
Pre-pregnancy body mass index (BMI) was calculated as self-reported weight in kilograms divided by height in meters squared. Overweight/obese women were defined as having a BMI>25 kg/m2 and normal weight women as having a BMI≤25 kg/m2. Maternal medical records were abstracted by trained study personnel to confirm medical and reproductive histories and to ascertain pregnancy course, complications, and outcomes.
Urine Cadmium
Urine cadmium was assessed in early pregnancy at 15 weeks gestation, on average (standard deviation=2.9, interquartile range=13–17 gestational weeks), a clean-catch spot urine was collected in polyethylene containers, promptly separated into 2 mL aliquots, and stored at −80°C until analysis. Urine Cd and total arsenic concentrations were quantified using a validated method of inductively coupled mass-spectrometry (ICP-MS) following published protocols [24] at Metametrix Clinical Laboratory, a Clinical Laboratory Improvement Amendments (CLIA) certified facility in Duluth, Georgia.
Briefly, urine samples were shaken and 1 mL was acidified with 100 μl of concentrated HNO3. Then 500 μl of an internal standard solution, containing scandium, rhodium, and germanium (5 μg/L each) was added. Samples were diluted to 5 mL with deionized water. Polyatomic interferences were minimized by utilizing ICP-MS with a dynamic reaction cell (PerkinElmer SCIEX Elan DRC II with ESI SC-4, FAST Autosampler). The accuracy of ICP-MS was checked by conducting proficiency testing using urine reference material (New York Toxic/Trace Elements in Urine Event 3#1 2012). Urine creatinine (Cr) concentration was assessed using a commercially available kit (Genzyme Diagnostics, Catalogue #221–30/#221–50) with improved Jaffe Reaction. The limit of detection for U-Cd and total arsenic were 0.10 and 3.0 μg/g Cr, respectively. Laboratory personnel were double-blinded to pregnancy outcome information and other clinical characteristics.
Blood Pressure
During the study period, healthcare providers took BP readings as part of routine clinical practice. Although the measures were not strictly standardized as they would be in a clinical trial, BPs were taken twice using standard mercury sphygmomanometers (scaled to eleven numbers) and patients were rested and seated during examination. SBP and DBP were measured at first, second and third trimester of pregnancy and mean arterial pressure (MAP) was computed at each trimester of pregnancy. MAP considered an integrated parameter of BP, is known to be more reproducible than individual SBPs and DBPs [25] and has been employed to assess BP trajectories during pregnancy [26]. We therefore calculated trimester-specific MAP for each subject according with the following formula: MAP=2/3DBP+1/3SBP.
Preeclampsia and GDM Diagnosis
The diagnosis of PE was made according to American College of Obstetricians and Gynecologist guidelines [27]. These guidelines defined PE as new onset hypertension with proteinuria in women who are beyond 20 weeks of gestation. Hypertension was defined as sustained blood pressure readings of ≥140/90 mmHg taken ≥ 6h apart. Proteinuria was defined as urine protein concentrations ≥300 mg/dL on two or more random specimens collected at least 4h apart.
As part of routine antenatal follow-up among all women at participating clinics, a 50g, 1-hr oral glucose challenge test was administered between gestational weeks 24 and 28 to screen for gestational diabetes mellitus (GDM). Women were diagnosed with GDM if two or more 100-g, 3-hr oral glucose tolerance test levels exceeded the American Diabetes Association (ADA) criteria: fasting ≥ 5.3 mmol/L (≥ 95 mg/dL); 1-hr ≥ 10.0 mmol/L (≥ 180 mg/dL); 2-hr ≥ 8.6 mmol/L (≥ 155 mg/dL); 3-hr ≥ 7.8 mmol/L (≥ 140 mg/dL) [28].
Statistical Analysis
We compared the frequency distribution of relevant characteristics of the population according to tertiles of maternal urinary cadmium in the subcohort. Tertiles were defined based upon the distribution of urinary creatinine-corrected Cd (U-Cd). Chi-square test for categorical variables and ANOVA for continuous variables were computed to determine whether there were statistically significant differences in sociodemographic and reproductive characteristics across tertiles of U-Cd.
Multivariable linear regression analyses were performed to evaluate the associations between MAP or SBP or DBP (i.e., the dependent variables) and U-Cd (i.e., the independent variables) separately. Confounding was empirically assessed by entering covariates into each model one at a time, and by comparing the adjusted and unadjusted coefficients. Final linear regression models included covariates that altered unadjusted coefficients by at least 10%. Adjusted R square values, representing the total variation of each blood pressure measures explained by covariates in final models, were also reported. The following covariates were a priori considered as possible confounders based on their hypothesized relationships between outcomes (MAP) and exposure (U-Cd): maternal age (<25, 25–34, ≥35 years), race/ethnicity (Non-Hispanic White, African American, Asian, Other), multiparity (yes/no), gravidity, post high school education (yes/no), smoking during pregnancy (never smoker, former or current smoker), alcohol use during pregnancy (yes/no), no exercise during pregnancy (yes/no), prenatal vitamin intake (yes/no), pre-pregnancy body mass index (BMI) (<18.5, 18.5 to <25, 25 to <30, ≥30 kg/m2), income group (<30,000 dollars per year), marital status (married-yes/no), family history of diabetes (yes/no), family history of hypertension (yes/no), gestational diabetes case (GDM) (yes/no), urinary total arsenic (U-As) and iron-deficiency anemia during pregnancy (yes/no). Adjusted R-squares and 95% confidence intervals (95% CI) were calculated from the models.
Employing logistic regression adjusted for potential confounders, we evaluated the possible association between the incidence of preeclampsia and the U-Cd concentrations as a continuous variable. We considered a-priori the confounders previously listed for Cd and MAP associations.
Secondary Analysis
We explored the independent and joint effects of high U-Cd (≥0.42 μg/g Cr, representing the high tertile of U-Cd vs. ≤0.21 μg/g Cr) and pre-pregnancy overweight/obese status (BMI >25 vs. BMI ≤25 kg/m2) on trimester-specific MAP by adding a term for the interaction between high U-Cd and pre-pregnancy overweight/obese status to the multivariable model. Trimester-specific MAP among women with high U-Cd was compared to values among women with low U-Cd (≤0.21 μg/g Cr) within groups defined by pre-pregnancy overweight/obese status (pre-pregnancy BMI >25 vs. ≤25 kg/m2). Because micronutrient deficiencies might increase Cd absorption [29] and toxicity [21], we explored trimester-specific MAP and U-Cd associations in stratified analyses for micronutrients intake: above or below the mean values of the subcohort (1100 mg/day for calcium, 276 mg/day for magnesium, 11 mg/day for zinc, 13 mg/day for iron, 0.77 mg/day for heme iron, and 99 mg/day for selenium).
Because cigarette smoking is a major potential source of Cd exposure in the general population [30] and has been associated with adverse maternal and fetal outcomes [31], we performed sensitivity analysis including ever vs. never smoking and an interaction term for U-Cd and smoking status in a multivariable model. Further, we conducted sensitivity analyses excluding participants with samples that were too concentrated (urinary creatinine >300 mg/dl, 1 cohort member) or too dilute (<30 mg/dL of creatinine, 145 cohort members) according to the WHO guidelines [32]. We assessed the effect of U-Cd on blood pressure excluding observations with U-Cd above 1.0 μg/g creatinine (29 subcohort members), to assess whether high U-Cd concentrations had a large impact in the estimates.
Since we were not able to measure blood pressure for 10, 2 and 32 women at first, second and third trimester of pregnancy, respectively, we repeated the analyses excluding women with any missing blood pressure determination during pregnancy (N=44). Finally, hypertension and renal disease are conditions associated with Cd exposure in previous studies [9,33]. Thus, we examined the extent to which including women with chronic conditions prior to pregnancy such as hypertension, renal disease and diabetes impacts the results.
All statistical analyses were performed using Stata version 12.0 (StataCorp, College Station, Texas) and the alpha level of 0.05 was employed to define statistical significance.
Results
Select sociodemographic and lifestyle characteristics of the study cohort are summarized in Table 1. The mean age of study participants was 33 years (standard deviation = 4.4), and the mean gestational age at delivery was 39 weeks (standard deviation = 3.0). Members of the cohort tended to be married (84.7%), White (85.0%), never smokers (71.8%), and physically active during the year before pregnancy and pregnancy (76.4%). The 71.1% did not consume alcohol and 97% took daily vitamins during pregnancy. Approximately 44.4% and 14.2% of study participants reported family history of hypertension and diabetes, respectively.
Table 1.
Characteristics of the study cohort (N=653) according to tertiles of urinary Cd (U-Cd) concentrations, Seattle and Tacoma, WA 1996–2002
| Study Groups: Characteristics | Total cohort | U-Cd Tertile 1 (≤0.21 μg/g Cr) | U-Cd Tertile 2 (0.22–0.42 μg/g Cr) | U-Cd Tertile 3 (≥ 0.43 μg/g Cr) | p-Value |
|---|---|---|---|---|---|
| N | 653 | 230 | 211 | 212 | |
| Maternal age (years) | 32.9 ± 4.4 | 32.4 ± 0.29 | 32.7 ± 0.30 | 33.7 ± 0.30 | 0.002 |
| <25 | 20 (3.06) | 12 (5.22) | 5 (2.37) | 3 (1.42) | 0.048 |
| 25–34 | 402 (61.6) | 146 (63.5) | 134 (63.5) | 122 (57.6) | |
| ≥ 35 | 231 (35.4) | 72 (31.3) | 72 (34.1) | 87 (41.04) | |
| Pre-pregnancy body mass index (kg/m2) | 23.44 ± 5.03 | 23.7 ± 0.33 | 23.96 ± 0.34 | 22.68 ± 0.34 | 0.043 |
| Underweight (<18.5) | 42 (6.44) | 13 (5.68) | 13 (6.16) | 16 (7.55) | 0.404 |
| Normal weight (18.5 to <25.0) | 436 (66.9) | 150 (65.5) | 134 (63.5) | 152 (71.7) | |
| Overweight (25.0 to <30.0) | 125 (19.2) | 47 (20.5) | 47 (22.3) | 31 (14.6) | |
| Obese (≥30) | 49 (7.5) | 19 (8.3) | 17 (8.06) | 13 (6.13) | |
| Maternal race/ ethnicity | |||||
| Non-Hispanic White | 555 (85.0) | 201 (87.4) | 179 (84.8) | 175 (82.6) | 0.280 |
| African American | 14 (2.14) | 5 (2.17) | 7 (3.32) | 2 (0.94) | |
| Asian | 51 (7.81) | 12 (5.22) | 16 (7.58) | 23 (10.9) | |
| Other | 33 (5.05) | 12 (5.22) | 9 (4.27) | 12 (5.66) | |
| Education | |||||
| Post high school education | 594 (96.6) | 214 (97.3) | 190 (95.5) | 190 (96.9) | 0.568 |
| High school or less | 21 (3.41) | 6 (2.73) | 9 (4.52) | 6 (3.06) | |
| Annual household income <30,000 dollars | 23 (3.81) | 8 (3.72) | 8 (4.12) | 7 (3.61) | 0.246 |
| Married | 553 (84.7) | 197 (85.7) | 179 (84.83) | 177 (83.5) | 0.818 |
| Nulliparous | 394 (60.3) | 150 (65.2) | 119 (56.4) | 125 (59.0) | 0.148 |
| Gravidity | 2.11 ± 1.20 | 1.99 ± 0.08 | 2.11 ± 0.083 | 2.23 ± 0.08 | 0.033 |
| Gestational age at delivery (weeks) | 38.6 ± 2.98 | 38.7 ± 0.18 | 38.6 ± 0.12 | 38.5 ± 0.19 | 0.456 |
| Smoking statusa | |||||
| Never smokers | 441 (71.8) | 163 (74.1) | 141 (70.9) | 137 (70.3) | 0.658 |
| Former smokers | 130 (21.2) | 46 (20.91) | 41 (20.6) | 43 (22.05) | |
| Current smokers | 43 (7.0) | 11 (5.0) | 17 (8.5) | 15 (7.69) | |
| No-Alcohol use during pregnancy | 464 (71.06) | 169 (73.5) | 142 (67.3) | 153 (72.2) | 0.328 |
| Physical Active | 469 (76.4) | 174 (79.1) | 138 (69.4) | 157 (80.51) | 0.157 |
| Incident preeclampsiab | 13 (2) | 6 (2.61) | 5 (2.37) | 2 (0.95) | 0.415 |
| Family history of diabetesc | 93 (14.2) | 25 (10.9) | 35 (16.6) | 33 (15.6) | 0.183 |
| Family history of hypertensiond | 290 (44.4) | 101 (43.9) | 99 (46.9) | 90 (42.5) | 0.641 |
| Prenatal vitamin intake | 595 (96.9) | 216 (98.2) | 192 (96.5) | 187 (95.9) | 0.373 |
| Calories intake (Kcal)e | 1724 ± 667 | 1769 ± 45.5 | 1758 ± 48.03 | 1638 ± 48.5 | 0.054 |
| Micronutrients daily intakee,f | |||||
| Calcium (mg) | 1234 ± 637 | 1261 ± 29.5 | 1259 ± 31.1 | 1178 ± 31.5 | 0.821 |
| Magnesium (mg) | 289.4 ± 118.1 | 304.1 ± 4.19 | 287 ± 4.42 | 275.2 ± 4.47 | 0.118 |
| Zinc (mg) | 12.7 ± 6.2 | 12.46 ± 0.30 | 12.71 ± 0.31 | 12.81 ± 0.32 | 0.006 |
| Selenium (mg) | 102.4 ± 43.4 | 105.12 ± 1.27 | 103 ± 1.35 | 98.65 ± 1.36 | 0.545 |
| Total iron (mg) | 14.31 ± 6.9 | 14.2 ± 0.32 | 14.15 ± 0.34 | 14.65 ± 0.34 | 0.002 |
| Heme iron (mg) | 0.85 ± 0.58 | 0.83 ± 0.03 | 0.92 ± 0.04 | 0.79 ± 0.04 | 0.744 |
| No-Heme iron (mg) | 13.41 ± 6.70 | 13.28 ± 0.32 | 13.16 ± 0.34 | 13.81 ± 0.34 | 0.003 |
Data is presented as mean ± SE or number (%). P-values from one-way ANOVA for continuous variables and chi-square test for categorical variables.
SD=standard deviation, Cr=creatinine.
Self reported smoking status.
During study pregnancy.
Any primary or secondary family member with type 1 or type 2 diabetes.
Any primary or secondary family member with chronic hypertension.
Dietary intake estimated from semi-quantitative food frequency questionnaire (FFQ).
Adjusted by total calories intake.
Cd Concentrations in the Study Population
The geometric mean for U-Cd was 0.29 μg/g creatinine with a range of 0.1 to 8.2 μg/g creatinine. The mean, 25th and 75th percentile values for U-Cd concentration were 0.39, 0.12 and 0.48 μg/g creatinine, respectively. Older women had higher U-Cd concentrations and gravidity increased across U-Cd tertiles (Table 1). The U-Cd concentrations were not different according to maternal race/ethnicity, educational attainment, income groups, family history of diabetes or hypertension, physical activity or prenatal vitamin intake (Table 1). We did not observe statistically significant differences in U-Cd concentrations among women who reported smoking during pregnancy (N=43; 0.48±0.57 μg/g Cr), former smokers (N=130; 0.35±0.23 μg/g Cr) and never smokers (N=441; 0.39±0.5 μg/g Cr).
We next examined the associations between U-Cd with pre-pregnancy BMI and micronutrients daily intake using FFQ questionnaire. Compared with the first U-Cd tertile, mothers in the highest U-Cd tertile had lower BMI and reported higher intake of zinc, total iron and no-heme iron from dietary sources (Table 1).
Blood Pressure in relation to U-Cd Concentrations
Table 2 shows unadjusted and adjusted linear regression analysis of the trimester-specific MAP in relation to U-Cd tertiles. In multivariate adjusted regression models, we observed decreased in MAP at second (−1.1 mmHg; CI 95%: −2.3, −0.03) and third trimester (−1.8 mmHg; CI 95%: −3.1, −0.53) of pregnancy among women in the highest U-Cd tertile as compared with those in the lowest U-Cd tertile after adjustment for maternal age, race/ethnicity, parity, smoking, prenatal vitamin use, family history of hypertension, GDM status and physical activity. Contrary, first trimester MAP was not significantly associated with U-Cd when comparing extreme tertiles in adjusted models (β=−0.54 mmHg; CI95%: −1.9,0.81) (Table 2). All the models were further adjusted for pre-pregnancy BMI and we found BMI was not a confounder. However, the association U-Cd-MAP was restricted to normal weight women (pre-pregnancy BMI, ≤25 kg/m2).
Table 2.
Unadjusted and adjusted mean (SE) mean arterial pressure (MAP), according to trimester of pregnancy and U-Cd tertiles, Seattle and Tacoma, WA, 1996–2002
| First Trimester | Second Trimester | Third Trimester | ||||
|---|---|---|---|---|---|---|
| U-Cd tertiles (μg/g Cr) | Mean (SE) | Mean difference (95% CI) | Mean (SE) | Mean difference (95% CI) | Mean (SE) | Mean difference (95% CI) |
| Unadjusted | Unadjusted | Unadjusted | ||||
| ≤0.21 | 83.16 (0.45) | 0.0 (Reference) | 83.15 ± 0.38 | 0.0 (Reference) | 86.4 (0.44) | 0.0 (Reference) |
| 0.22–0.41 | 83.5 (0.48) | 0.31 (−0.98, 1.6) | 83.2 ± 0.39 | 0.05 (−1.02, 1.11) | 85.7 (0.46) | −0.72 (−1.97, 0.53) |
| ≥0.42 | 82.3 (0.47) | −0.87 (−2.16,0.42) | 81.61 ± 0.39 | −1.54 (−2.61, −0.47)a | 84.4 (0.46) | −2.09 (−3.35, −0.84)c |
| 1Adjusted | 1Adjusted | 1Adjusted | ||||
| ≤0.21 | 83.2 ± 0.46 | 0.0 (Reference) | 83.18 ± 0.38 | 0.0 (Reference) | 86.4 ± 0.44 | 0.0 (Reference) |
| 0.22–0.41 | 83.7 ± 0.49 | 0.49 (−0.85, 1.82) | 83.3 ± 0.40 | 0.25 (−0.84, 1.34) | 85.8 ± 0.45 | −0.54 (−1.79, 0.71) |
| ≥0.42 | 82.5 ± 0.49 | −0.54 (−1.89, 0.81) | 81.7 ± 0.40 | −1.14 (−2.25, −0.03)b | 84.4 ± 0.46 | −1.79 (−3.06, −0.53)d |
95% CI=95% confidence interval.
Adjusted for maternal age, race/ethnicity, parity, smoking, prenatal vitamin use, family history of hypertension, GDM case and physical activity. The adjusted mean values reported for Model 1 are from a regression model based on the entire study cohort (N=653) with indicators variables specified to represent Non-Hispanic white, nulliparous, never smokers, prenatal vitamin using women from 25–34 years of age, no family history of hypertension, no gestational diabetes and physical active during pregnancy.
P-value = 0.005
P-value= 0.043
P-value=0.001
P-value=0.006
Supplemental Table 1 shows the inverse association between trimester-specific SBP and DBP with U-Cd concentrations. At third trimester of pregnancy, a significant decrease in both SBP (−2.1 mmHg; CI95%: −3.74,−0.45) and DBP (−1.7 mmHg ; CI 95%: −2.9, −0.40) was associated with U-Cd when comparing extreme tertiles, whereas at second trimester only SBP was significantly lower in the third U-Cd compared with the first U-Cd tertile (−1.78 mmHg; CI 95%:−3.3, −0.26).
In the multivariable-adjusted logistic regression adjusted by maternal age, race/ethnicity, parity, smoking, family history of hypertension, GDM and physical activity, every 1 μg/g increase in U-Cd concentrations was not associated with higher risk of PE (RR 0.88; 95% CI: 0.12, 6.7).
Secondary Analyses
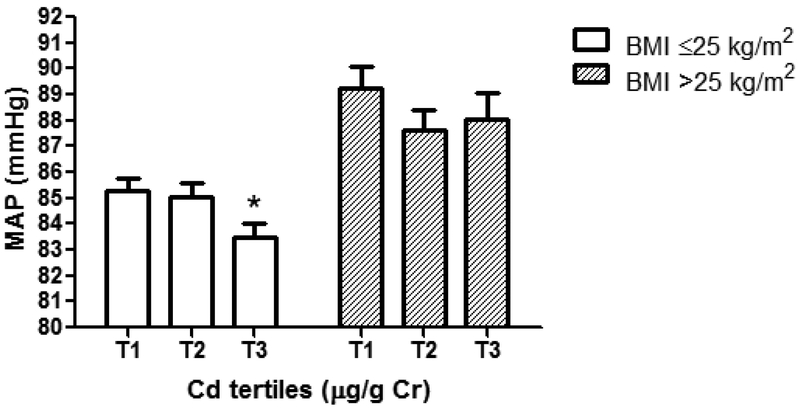
Associations of higher U-Cd with lower third trimester MAP appeared to be stronger among normal weight (BMI≤25 kg/m2) women as compared with overweight/obese women (Figure 1). Among normal weight women, third trimester MAP was lower (−1.5 mmHg; CI 95%: −2.96, −0.13) among those with U-Cd in the highest tertile (≥0.42 μg/g creatinine) as compared with those in the lowest tertile (≤0.21 μg/g creatinine). The interaction terms among U-Cd tertiles and BMI categories were not statistically significant.
Fig 1.
Associations between maternal third trimester mean arterial pressure (MAP) and U-Cd tertiles after stratification by maternal overweight/obese status. The bars represent Mean ± SE mean arterial pressure after adjustment for age, race/ethnicity, parity, smoking, prenatal vitamin use, family history of hypertension, GDM case and physical activity for women with BMI≤25 kg/m2 (white bars) and for women with BMI>25 kg/m2 (grey bars). Third trimester MAP decrease of 1.5 mmHg [(83.5 vs. 85.0 mmHg) (95% CI: −2.96, −0.13)] in the U-Cd third tertile (T3) compared with U-Cd first tertile (T1); *p-Value<0.05; T1, U-Cd ≤0.21 μg/g Cr; T2, U-Cd 0.22–0.41 μg/g Cr and T3, ≥0.42 μg/g Cr
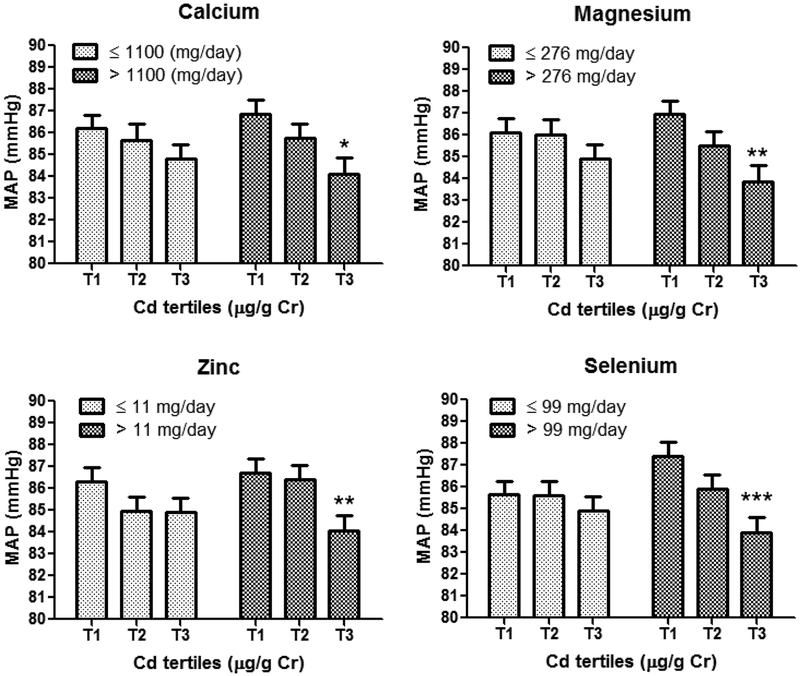
We analyzed each micronutrient separately and we observed that the inverse association between MAP and U-Cd was significant in the groups with higher Se, Ca, Mg or Zn daily intake (>99 mg Se,>1100 mg Ca, >276 mg Mg, >11 mg Zn) and null among lower intake groups (≤99, ≤1100, ≤276, ≤11, respectively) (Table 3; Figure 2). For instance, women in the third U-Cd tertile (vs. first tertile) with high Se intake (>99 mg/day) had lower third trimester MAP (−3.6 mmHg; 95% CI: −5.5, −1.60; p<0.001), whereas MAP was not significantly associated with U-Cd in the low selenium intake group (≤99 mg/day). The interaction terms in multivariable models were only significant for high selenium daily intake (>99 mg) and third U-Cd tertile (−3.15 mmHg; 95%CI: −5.77, −0.52; p=0.019) after adjustment for maternal age, race/ethnicity, parity, smoking, prenatal vitamin use, GDM case, physical activity and total calories intake.
Table 3.
Adjusted mean (SE) mean arterial pressure (MAP) for the third trimester of pregnancy, according to U-Cd tertiles after stratification by micronutrients daily intake, Seattle and Tacoma, WA, 1996–2002
| Calcium | Magnesium | Zinc | Selenium | |||||
|---|---|---|---|---|---|---|---|---|
| U-Cd tertiles (μg/g Cr) | Adjusted Mean (SE) | Mean difference (95% CI) | Adjusted Mean (SE) | Mean difference (95% CI) | Adjusted Mean (SE) | Mean difference (95% CI) | Adjusted Mean (SE) | Mean difference (95% CI) |
| Low micronutrient intake (mg/day)1 | ||||||||
| ≤0.21 | 86.2 ± 0.6 | 0.0 (Reference) | 86.1±0.7 | 0.0 (Reference) | 86.33 ± 0.63 | 0.0 (Reference) | 85.65 ± 0.63 | 0.0 (Reference) |
| 0.22–0.41 | 85.7 ± 0.7 | −0.26 (−2.2, 1.68) | 86.0 ± 0.7 | 0.1 (−1.87, 2.07) | 84.94 ± 0.68 | 1.4 (−3.28, 0.47) | 85.6 ± 0.66 | −0.03 (−1.9, 1.8) |
| ≥0.42 | 84.8 ± 0.65 | −1.3 (−3.12, 0.51) | 84.9 ± 0.65 | −0.9 (−2.8, 0.96) | 84.89 ± 0.67 | −1.20 (−3.04, 0.64) | 84.9 ± 0.65 | −0.42 (−2.23, 1.40) |
| High micronutrient intake (mg/day)2 | ||||||||
| ≤0.21 | 86.9 ± 0.65 | 0.0 (Reference) | 87.0±0.6 | 0.0 (Reference) | 86.7 ± 0.64 | 0.0 (Reference) | 87.4 ± 0.64 | 0.0 (Reference) |
| 0.22–0.41 | 85.8 ± 0.64 | −1.2 (−3.1, 0.6) | 85.5 ± 0.6 | −1.6 (−3.3, 0.22) | 86.4 ± 0.65 | −0.37 (−2.2, 1.46) | 85.9 ± 0.67 | −1.42 (−3.3, 0.44) |
| ≥0.42 | 84.01 ± 0.74 | −2.5 (−4.5, −0.5)a | 83.9 ± 0.7 | −2.98 (−4.9, −1.04)b | 84.04 ± 0.70 | −2.63 (−4.5, −0.7)c | 83.9 ± 0.72 | −3.6 (−5.5, −1.60)d |
Adjusted for maternal age, race/ethnicity, parity, smoking, prenatal vitamin use family history of hypertension, GDM case, calories intake and physical activity for women with low micronutrients daily intake as follows: calcium (≤1100 mg, N=280); magnesium (≤276 mg, N=281); zinc (≤11 mg, N=259) and selenium (≤99 mg; N=286).
Adjusted for maternal age, race/ethnicity, parity, smoking, prenatal vitamin use, family history of hypertension, GDM case, calories intake and physical activity for women with micronutrient high intake: calcium (>1100 mg, N=269); magnesium (>276 mg, N=268); zinc (>11 mg, N=290) and selenium (>99 mg; N=263).
P-value= 0.013
P-value=0.003
P-value=0.008
P-value<0.001
Fig 2.
Associations between maternal third trimester mean arterial pressure (MAP) and U-Cd tertiles after stratification by micronutrients low/high daily intake. The bars represent the Mean ± SE mean arterial pressure after adjustment for age, race/ethnicity, parity, smoking, prenatal vitamin use, family history of hypertension, GDM case, calories intake and physical activity. *p<0.05, **p<0.01 and ***p<0.001. The P-values for the higher intake groups are as follows: calcium (p=0.013), magnesium (p=0.003), zinc (p=0.008) and selenium (p<0.001); T1, U-Cd ≤0.21 μg/g Cr; T2, U-Cd 0.22–0.41 μg/g Cr and T3, ≥0.42 μg/g Cr
Regarding trimester-specific MAP, micronutrients intake (Mg, Zn, Se) modified U-Cd and MAP associations in the second trimester and no effect modification was found in the first trimester of pregnancy (data not shown).
Further, we compared third trimester MAP in relation to U-Cd among ever smokers [(N=173) (former and current)] and never smokers (N=441). Third-trimester MAP decreased in the highest U-Cd tertile (−1.8 mmHg; 95% CI: −3.34, −0.33; p=0.017) compared with the lowest tertile (≥0.42 vs ≤0.21 μg/g creatinine) in never smokers. Contrary, no significant associations were found between third trimester-MAP and U-Cd for ever smokers (−1.96 mmHg; 95% CI: −4.4, 0.45; p=0.11). The interaction terms between U-Cd and smoking status were not significant.
Finally, we did not find a significant change in the effect estimates more than 10% in sensitivity analyses after the exclusion of women with: 1) high or low creatinine concentrations (<30 or ≥ 300 mg/dL) or 2) high U-Cd concentrations (>1.0 μg/g creatinine) or 3) missing values of blood pressure across pregnancy. Additionally, we compare the estimates in multivariate models including women with disease conditions prior to pregnancy such as chronic hypertensive cases, renal disease and diabetes finding no significant changes in the magnitude or direction of U-Cd and BP associations.
Discussion
This cohort study of pregnant women shows that U-Cd is inversely associated third trimester MAP, particularly in normal weight women (pre-pregnancy BMI ≤ 25 kg/m2) and women with high dietary intake of micronutrients (calcium, magnesium, zinc and selenium). Women who developed preeclampsia during pregnancy did not present higher U-Cd concentrations compared with those who stayed normotensive.
To the best of our knowledge, no previous study has examined possible associations of trimester-specific blood pressure and U-Cd during pregnancy. Our results are in line with some studies [34, 9], but not all [35] reporting BP decrease in relation to higher levels of U-Cd among no pregnant adults. For instance, Kurihara et al. [34] found lower risk of hypertension associated with U-Cd in men (OR=0.62; mean U-Cd 1.8 μg/g Cr) and women (OR=0.67; mean U-Cd 2.4 μg/g Cr) after adjustment by age, BMI, smoking, alcohol intake and serum chemistry. Contrary, Tellez-Plaza et al. [35] using the 1999–2004 National Health and Nutrition Examination Survey (NHANES) reported no-significant associations between SBP or DBP with U-Cd in multivariate models adjusted for age, sex, race/ethnicity, education, smoking status, cotinine, alcohol intake, among other confounders. A meta-analysis suggested lower hypertension risk (OR=0.65; 95% IC: 0.45–0.94) associated with higher U-Cd concentrations [9].
Pregnancy is known as a susceptible period to Cd toxicity due to low iron concentrations and Cd mobilization from liver to kidney, among other factors [36]. Specifically, at third trimester of pregnancy, blood concentrations of some metals increase because of increases in bone turnover and/or increased in gastrointestinal absorption [37]. Interestingly, U-Cd was inversely associated with blood pressure at third trimester of pregnancy. Late pregnancy might be a susceptible window to Cd toxicity because of a reduction of maternal metallothionein (MT) (MT-1 and MT-2 isoforms) [38, 39]. Maternal rat liver concentrations of MT mRNA decrease 5-fold in late gestation compared to expression values earlier in pregnancy [39]. MT decrease in late pregnancy also corresponds to a time of rising MT mRNA in fetal liver and increased transfer of Zn across the placenta [39]. MT has been proposed as playing an important role in the homeostasis of essential metals and in the detoxification of heavy metals [40]. Future studies are needed to clarify the influence of maternal MT variation during pregnancy on the Cd-blood pressure association.
Possible biological mechanisms for the inverse relationship between Cd and blood pressure are suggested through animal models. Sutoo and Akiyama showed that Cd reduces BP and inhibits sympathetic nerve activity through dopamine increase via calcium-calmodulin in rats [41]. Cd might be also associated with low blood pressure through estrogenic effects. Previous evidence shows that cadmium mimics the in vivo effects of estradiol in target organs in animal studies [42] and estradiol has been associated with blood pressure decrease in women [43]. In clear contrast with our results, previous studies showed endothelial dysfunction and oxidative stress associated with Cd exposure [44] possible linked to blood pressure increase [45].
At molecular level Cd competes with calcium, selenium and magnesium and low intake of these micronutrients has been associated with higher risk of preeclampsia [15,16,17,21]. We are aware of only 1 prior study that examined micronutrients concentrations in relation to PE risk associated with Cd. Laine et al. suggested a protective role of selenium status in PE risk associated with Cd in a cohort of pregnant women from across the south-east US. Women with higher placental concentrations of selenium had lower PE risk associated with Cd (OR=0.98; CI 95%: 0.5–1.9) compared with those with lower placental selenium levels (OR=2.0; 95% CI: 1.1–3.5) [19]. Contrary to Laine, we found blood pressure decrease associated with U-Cd (≥0.42 vs. ≤0.21 μg/g Cr) in those women with high Se dietary intake (≥99 mg/day) compared with low Se intake group (<99 mg/day). Obvious differences exist between these two studies and the effect of micronutrients on Cd-blood pressure associations need to be further investigated. In our study setting, U-Cd concentrations increased in those women with higher dietary intake of zinc and non-heme iron. Food sources of non-heme iron include egg yolks and foods of plant origin [46] while seafood (oyster, crab and lobster) is a source of both Zn [47] and Cd [1,10]. It is reasonable to think that seafood might be one of the sources of Cd exposure in our study setting, among other food sources. A previous study conducted in Seattle, WA –among women with no reported history of smoking- showed increased U-Cd concentrations associated with regular intake of eggs, hot cereals, organ meats, tofu, vegetable soups, leafy greens, and yams [48].
On the other hand, our results did not show a statistically significant association between U-Cd and smoking status. Our finding might be explained at least in part due to smoking habits during pregnancy tend to be less common and diet might be the main source of Cd exposure [49]. The magnitude of association between U-Cd and maternal smoking status may be underestimated, in part because of biased reporting of cigarette smoking in pregnancy. Inferences concerning the relation between maternal smoking and her exposure to environmental tobacco smoke may be enhanced in studies that directly measure maternal urine cotinine. By so doing we may improve the accuracy of classifying study subjects according to their tobacco smoke exposure status during pregnancy.
Regarding BMI, our results showed an inverse association between U-Cd and blood pressure in normal weight women but not in overweight/obese women. Liu and coworkers observed a negative association between BMI and blood Cd during pregnancy [50]. Friedman observed in Ukrainian children a significant association between low BMI and elevated blood Cd [51]. Animal research has shown that protein-caloric malnutrition can increase Cd absorption [52]. Accordingly, our results showed increased U-Cd concentration with lower BMI. The possibility that Cd might accumulate in fat tissue and be less available to exert its effects should be addressed in future studies.
Finally, our findings did not support a positive association between incident preeclampsia and U-Cd. U-Cd concentrations were not different among women who developed preeclampsia as compared with those who remained normotensive throughout pregnancy (0.32 vs. 0.39 μg/g creatinine, respectively). Consistent with our results, Cd concentrations assessed in mother whole blood (0.54 vs. 0.50 μg/L) or umbilical cord blood (0.34 vs. 0.35 μg/L) were not significantly different in preeclamptic women compared the control group [53]. In the EDEN Cohort Study comparable B-Cd concentrations were found in the pregnancy induced hypertension (PIH) (0.8 μg/L) group and normotensives pregnant women (0.7 μg/L) [54]. However, other investigators have observed associations of PE/PIH with elevated serum Cd [18]. For instance, Kolusari in Turkey found significantly higher Cd serum concentrations in the preeclamptic women compared with normotensive pregnant women (0.033 vs. 0.029 μg/dL, respectively) [18]. Variations in these findings may be in part due to differences in biological samples used (blood vs. urine) for Cd determination, sample size, sensitivity of the techniques employed for Cd determination, study populations, study design, and varying degrees of control for confounding factors. On the other hand, animal studies have shown features of preeclampsia including hypertension and proteinuria [55].
The present study has a number of strengths and limitations. We used a large well-characterized cohort of pregnant women to complete our research. Structured interviews, medical record abstraction, and a semi-quantitative FFQ provided rich covariate data. Urinary metals were assessed using a robust, well-validated, and accurate method (ICP-MS). Further, our study and its findings provide new information to address a knowledge gap in the literature. There are some limitations of using creatinine to adjust for urine dilution [56]. Although U-Cd was assessed in early pregnancy, previous studies have showed that urinary Cd concentrations remain constant over the course of pregnancy [57, 58]. The inverse associations between U-Cd and MAP cannot be extended to the general population due to effect modification of micronutrients and pre-pregnancy BMI. On the other hand, given that Cd is a well-established nephrotoxicant even at low concentrations of chronic exposure [33], underestimation of the potential effects of Cd on blood pressure and hypertension is a concern. Methallothionein concentrations, which could explain part of the variability of the association of cadmium with blood pressure end points, were not determined in our study.
This study represents the first exploration of relationships among trimester-specific blood pressure and U-Cd considering the effect of dietary intake of micronutrients. Future studies should take into account diet when assessing the health effects of Cd in pregnant women.
Supplementary Material
Acknowledgments
This research was supported by awards (R01HD-32562 and K01HL103174) from the National Institutes of Health (NIH). Dr. Osorio-Yáñez has been financially supported by Consejo Nacional de Ciencia y Tecnología (CONACYT-México; 238130). The authors thank the staff of the Center for Perinatal Studies for their skillful technical assistance.
Footnotes
Competing financial interests
The authors have no conflicts of interest to declare.
References
- 1.ATSDR (Agency for Toxic Substances and Disease Registry) (2008) Public Health Statement for Cadmium. http://www.atsdr.cdc.gov/phs.asp?id=46&tid=15. Accessed 1 May 2015
- 2.Egan SK, Tao SS, Pennington JA, Bolger PM (2002) US Food and Drug Administratiońs Total Diet Study: intake of nutritional and toxic elements, 1991–96. Food Addit Contam 19: 103–125 [DOI] [PubMed] [Google Scholar]
- 3.Egan SK, Bolger PM, Carrington CD (2007) Update of US FDÁs Total Diet Study food list and diets. J Exposure Sci Environ Epidemiol 17: 573–582 [DOI] [PubMed] [Google Scholar]
- 4.Satarug S, Garrett SH, Sens MA, Sens DA (2010) Cadmium, environmental exposure, and health outcomes. Environ Health Perspect 118:182–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tellez-Plaza M, Jones MR, Dominguez-Lucas A, Guallar E, Navas-Acien A (2013) Cadmium exposure and clinical cardiovascular disease: A systematic review. Curr Atheroscler Rep 15:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagerberg B, Bergström G, Borén J, Barregard L (2013) Cadmium exposure, intercellular adhesion molecule-1 and peripheral artery disease: a cohort and experimental study. BMJ Open 3:e002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng W, He X, Chen M, Deng S, Qiu G, Li X (2015) Urinary metals and heart rate variability: a cross-sectional study of urban adults in Wuhan, China. Environ Health Perspect 123: 217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams SV, Newcomb PA (2014) Cadmium blood and urine concentrations as measures of exposure: NHANES 1999–2010. J Expo Sci Environ Epidemiol 24:163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher CM, Meliker JR (2010) Blood and urine cadmium, blood pressure, and hypertension: A systematic review and meta-analysis. Environ Health Perspect 118:1676–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Järup L, Berglund M, Elinder CG, Nordberg G, Vahter M (1998) Health effects of cadmium exposure--a review of the literature and a risk estimate. Scand J Work Env Hea 24:1–51 [PubMed] [Google Scholar]
- 11.Johnston JE, Valentiner E, Maxson P, Miranda ML, Fry RC (2014) Maternal cadmium levels during pregnancy associated with lower birth weight in infants in a north carolina cohort. PloS One 9:e109661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romano ME, Enquobahrie DA, Simpson CD, Checkoway H, Williams MA (2015) A case-cohort study of cadmium body burden and gestational diabetes mellitus in american women. Environ Health Perspect 123:993–998 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Hall ME, George EM, Granger JP (2011) The heart during pregnancy. Rev Esp Cardio 64:1045–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson ML, Ananth CV, Jaddoe VW, Miller RS, Williams MA (2014) The association of maternal adult weight trajectory with preeclampsia and gestational diabetes mellitus. Paediatr Perinat Epidemiol 28:287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts JM, Balk JL, Bodnar LM, Belizan JM, Bergel E, Martinez A (2003) Nutrient involvement in preeclampsia. J Nutr 133:1684S–1692S [DOI] [PubMed] [Google Scholar]
- 16.Rayman MP, Bath SC, Westaway J, Williams P, Mao J, Vanderlelie JJ, Perkins AV, Redman CW (2015) Selenium status in UK pregnant women and its relationship with hypertensive conditions of pregnancy. Br J Nutr 9:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoenaker DA, Soedamah-Muthu SS, Mishra GD (2014) The association between dietary factors and gestational hypertension and pre-eclampsia: A systematic review and meta-analysis of observational studies. BMC Med 12:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolusari A, Kurdoglu M, Yildizhan R, Adali E, Edirne T, Cebi A, Demir H, Yoruk IH (2008) Catalase activity, serum trace element and heavy metal concentrations, and vitamin a, d and e levels in pre-eclampsia. J Int Med Res 36:1335–1341 [DOI] [PubMed] [Google Scholar]
- 19.Laine JE, Ray P, Bodnar W, Cable PH, Boggess K, Offenbacher S, Fry RC (2015) Placental Cadmium Levels Are Associated with Increased Preeclampsia Risk. Plos One 10:e0139341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohta H, Ichikawa M, Seki Y (2002) Effects of cadmium intake on bone metabolism of mothers during pregnancy and lactation. Tohoku J Exp Med 196:33–42 [DOI] [PubMed] [Google Scholar]
- 21.Goyer RA (1995) Nutrition and metal toxicity. Am J Clin Nutr 61:646S–650S [DOI] [PubMed] [Google Scholar]
- 22.Qiu C, Zhang C, Gelaye B, Enquobahrie DA, Frederick IO, Williams MA (2011) Gestational diabetes mellitus in relation to maternal dietary heme iron and nonheme iron intake. Diabetes Care 34:1564–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T (1999) Measurement characteristics of the women’s health initiative food frequency questionnaire. Ann Epidemiol 9:178–187 [DOI] [PubMed] [Google Scholar]
- 24.Heitland P, Koster HD (2004) Fast, simple and reliable routine determination of 23 elements in urine by ICP-MS. J Anal At Spectrom 19:1552–1558 [Google Scholar]
- 25.Darovic GO (2002) Haemodynamic Monitoring: Invasive and non-invasive Clinical Application. Saunders, Philadelphia [Google Scholar]
- 26.Magriples U, Boynton MH, Kershaw TS, Duffany KO, Rising SS, Ickovics JR (2013) Blood pressure changes during pregnancy: impact of race, body mass index, and weight gain. Am J Perinatol 5:415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Report of the national high blood pressure education program working group on high blood pressure in pregnancy (2000) Am J Obstet Gynecol 183:S1–S22 [PubMed] [Google Scholar]
- 28.American Diabetes Association, ADA (2004) Gestational diabetes mellitus. Diabetes Care 27:S88–S90 [DOI] [PubMed] [Google Scholar]
- 29.Kippler M, Goessler W, Nermell B, Ekstrom EC, Lonnerdal B, El Arifeen S, Vahter M (2009) Factors influencing intestinal cadmium uptake in pregnant bangladeshi women--a prospective cohort study. Environ Res 109:914–921 [DOI] [PubMed] [Google Scholar]
- 30.ATSDR (2012) Toxicological Profile of Cadmium. http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=48&tid=15. Accessed 30 January 2015 [PubMed]
- 31.Mund M, Louwen F, Klingelhoefer D, Gerber A (2013) Smoking and pregnancy--a review on the first major environmental risk factor of the unborn. Int J Environ Res Public Health 10:6485–6499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.International Programme on Chemical Safety, World Health Organization (1996) Biologial monitoring of chemical exposure in the workplace: guidelines. Geneva, Switzerland: World Health Organization [Google Scholar]
- 33.Akesson A, Lundh T, Vahter M, Bjellerup P, Lidfeldt J, Nerbrand C, Samsioe G, Strömberg U, Skerfving S (2005) Tubular and glomerular kidney effects in swedish women with low environmental cadmium exposure. Environ Health Perspect 113:1627–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurihara I, Kobayashi E, Suwazono Y, Uetani M, Inaba T, Oishiz M, Kido T, Nakagawa H, Nogawa K (2004) Association between exposure to cadmium and blood pressure in japanese peoples. Arch Environ Health 59:711–716 [DOI] [PubMed] [Google Scholar]
- 35.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E (2008) Cadmium exposure and hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES). Environ Health Perspect 116:51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishijo M, Satarug S, Honda R, Tsuritani I, Aoshima K (2004) The gender differences in health effects of environmental cadmium exposure and potential mechanisms. Mol Cell Biochem 255:87–92 [DOI] [PubMed] [Google Scholar]
- 37.Hertz-Picciotto I, Schramm M, Watt-Morse M, Chantala K, Anderson J, Osterloh J (2000) Patterns and determinants of blood lead during pregnancy. Am J Epidemiol 152:829–837 [DOI] [PubMed] [Google Scholar]
- 38.Chisolm JC, Handorf CR (1987) Increased absorption of and sensitivity to cadmium during late pregnancy: Is there a relationship between markedly decreased maternal cadmium binding protein (metallothionein) and pregnancy-induced hypertension? Med Hypotheses 24:347–351 [DOI] [PubMed] [Google Scholar]
- 39.Andersen RD, Piletz JE, Birren BW, Herschman HR (1983) Levels of metallothionein messenger rna in foetal, neonatal and maternal rat liver. Eur J Biochem 131:497–500 [DOI] [PubMed] [Google Scholar]
- 40.Klassen CD, Liu J, Diwan BA (2009) Metallothionein Protection of Cadmium Toxicity. Toxicol Appl Pharmacol 238:215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutoo D, Akiyama K (2000) Effect of cadmium or magnesium on calcium-dependent central function that reduces blood pressure. Arch Toxicol 74:1–4 [DOI] [PubMed] [Google Scholar]
- 42.Byrne C, Divekar SD, Storchan GB, Parodi DA, Martin MB (2009) Cadmium--a metallohormone?. Toxicol Appl Pharmacol 238:266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomczy R, Paluch K, Galuszka-Bednarczyk A, Milewicz T, Janeczko J, Klocek M (2015) Changes in blood pressure and heart rate by an increase in serum estradiol in women undergoing controlled ovarian hyperstimulation.Przegl Lek 4:174–177 [PubMed] [Google Scholar]
- 44.Almenara CC, Broseghini-Filho GB, Vescovi MV, Angeli JK, Faria Tde O, Stefanon I, Vassallo DV, Pahilha AS (2013) Chronic cadmium treatment promotes oxidative stress and endothelial damage in isolated rat aorta. PloS One 8: e68418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Houston MC (2007) The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, and myocardial infarction. Altern Ther Health Med 13: S128–133. [PubMed] [Google Scholar]
- 46.Aggett PJ (2012) Iron: In: Erdman J (ed) Present Knowledge in Nutrition, 10th edn Wiley, Washington, pp 506–520 [Google Scholar]
- 47.U.S. Department of Agriculture, Agricultural Research Service (2011) USDA National Nutrient Database for Standard Reference, Release 24. Nutrient Data Laboratory; http://www.ars.usda.gov/ba/bhnrc/ndl. Accessed 12 December 2015 [Google Scholar]
- 48.Adams SV, Newcomb PA, Shafer MM, Atkinson C, Bowles EJ, Newton KM, Lampe JW (2011) Source of Cadmium Exposure Among Healthy Premenopausal Women. Sci Total Environ 409:1632–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Järup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208 [DOI] [PubMed] [Google Scholar]
- 50.Liu K, Gu P, Chen W, Shi J, Shi C, Xia L (2013) Effect of pregnancy on the levels of blood cadmium and lead: Analysis of 2006–2011 nanjing maternity and child health care hospital survey data. Iran J Public Health 42:691–699 [PMC free article] [PubMed] [Google Scholar]
- 51.Friedman LS, Lukyanova EM, Kundiev YI, Shkiryak-Nizhnyk ZA, Chislovska NV, Mucha A, Zvinchuk AV, Oliynyk I, Hryhorczuk D (2006) Anthropometric, environmental, and dietary predictors of elevated blood cadmium levels in ukrainian children: Ukraine elspac group. Environ Res 102:83–89 [DOI] [PubMed] [Google Scholar]
- 52.Fox MR, Jacobs RM, Jones AO, Fry BE ,Jr (1979) Effects of nutritional factors on metabolism of dietary cadmium at levels similar to those of man. Environ Health Perspect 28:107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vigeh M, Yokoyama K, Ramezanzadeh F, Dahaghin M, Sakai T, Morita Y, Kitamura F, Sato H, Kobayashi Y (2006) Lead and other trace metals in preeclampsia: A case-control study in Tehran, Iran. Environ Res 100:268–275 [DOI] [PubMed] [Google Scholar]
- 54.Yazbeck C, Thiebaugeorges O, Moreau T, Goua V, Debotte G, Sahuquillo J, Forhan A, Foliguet B, Magnin G, Slama R, Charles MA, Huel G (2009) Maternal blood lead levels and the risk of pregnancy-induced hypertension: The eden cohort study. Environ Health Perspect 117:1526–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang F, Zhang Q, Zhang X, Luo S, Ye D, Guo Y, Chen S, Huang Y (2014) Preeclampsia induced by cadmium in rats is related to abnormal local glucocorticoid synthesis in placenta. Reprod Biol Endocrin 12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL (2005) Urinary creatinine concentrations in the U.S. Population: Implications for urinary biologic monitoring measurements. Environ Health Perspect 113:192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suarez-Ortegon MF, Mosquera M, Caicedo DM, De Plata CA, Mendez F (2013) Nutrients intake as determinants of blood lead and cadmium levels in colombian pregnant women. Am J Hum Biol 25:344–350 [DOI] [PubMed] [Google Scholar]
- 58.Hernandez M, Schuhmacher M, Fernandez JD, Domingo JL, Llobet JM (1996) Urinary cadmium levels during pregnancy and postpartum. A longitudinal study. Biol Trace Elem Res 53: 205–212 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.