Abstract
Protein disulfide isomerase (PDI) plays an important role in fibrin generation in vivo, but the underlying mechanism remains largely unknown. In this study, using thrombin generation assay (TGA), we investigated whether PDI contributes to tissue factor (TF)-mediated thrombin generation. Human peripheral blood mononuclear cells (PBMCs) were treated with 100 ng/ml lipopolysaccharide (LPS), the expression of TF on cell surface was analyzed by flow cytometry. After incubation with an inhibitory anti-TF antibody, recombinant PDI protein or a PDI inhibitor PACMA31, LPS-stimulated human PBMCs were incubated with human plasma, and thrombin generation was assessed by Ceveron Alpha TGA and a fluorescent thrombin substrate. Bone marrow mononuclear cells isolated from PDI-knockout and with-type mice were stimulated by LPS, followed by measurement of thrombin generation. LPS stimulation increased expression of TF on PBMCs, and thrombin generation. Inhibitory anti-TF antibody almost completely suppressed thrombin generation of LPS-stimulated PBMCs, suggesting that thrombin generation was TF-dependent. Recombinant PDI protein increased thrombin generation, while PACMA31 attenuated thrombin generation. Compared with control cells, PDI-deficient marrow mononuclear cells had less capacity in thrombin generation. Taken together, these data suggest that PDI enhances TF-dependent thrombin generation.
Keywords: Protein disulfide isomerase, Tissue factor, Thrombin generation
Introduction
Protein disulfide isomerase (PDI) is an enzyme in the endoplasmic reticulum (ER) in eukaryotes that catalyzes the formation and isomerization of disulfide bonds within proteins as they fold[1–2]. This allows proteins to more quickly find the correct arrangement of disulfide bonds, thus facilitating protein folding. Recently, an increasing body of evidence demonstrates that PDI is released from platelets and endothelial cells to the cell surface, and that extracellular PDI plays an important role in thrombosis[3–6]. However, the underlying mechanism remain largely unknown. Previous studies have shown that anti-PDI antibody RL90 inhibited the activity of tissue factor[6], suggesting that PDI might contribute to coagulation activation. Because the specificity of RL-90 has been questioned, the role of PDI in coagulation requires further investigation.
Thrombin generation assay (TGA) is a quantitative method evaluating dynamic conversion of prothrombin to thrombin (resulting from the action of the procoagulant driver) and decay (resulting from the action of the anticoagulant driver), thus assessing the balance between the two processes. Coagulation of the test plasma is activated by small amounts of tissue factor and phospholipids, and the reaction of thrombin generation is continuously monitored by means of a thrombin-specific fluorogenic substrate. Among the parameters derived from the thrombin-generation curve, the most important is the endogenous thrombin potential, defined as the net amount of thrombin. Historically, thrombin generation has been investigated by means of the basic tests of coagulation, prothrombin time (PT) and activated partial thromboplastin time (APTT). TGA has several advantages over these traditional coagulation tests [7–9]. First, the measurement of PT and APTT is dependent on fibrinogen, including concentration and activity; However, the TGA is not affected by fibrinogen and can detect the coagulation activity even after fibrin network has formed. Second, in the measurement of PT and APTT, plasma tends to clot after 5% of the entire thrombin potential is generated. This means that 95% of the generated thrombin is not accounted for by these tests. Third, TGA offers multiple parameters reflecting the whole process of blood coagulation including lag time, the velocity of thrombin generation, peak of thrombin generation and endogenous thrombin potential. Therefore, in this study, we determine the role of PDI in thrombin generation using recombinant PDI protein, specific PDI inhibitor PACMA31 and PDI-knockout cells.
Materials and Methods
Materials
PE-labeled anti-TF antibody was purchased form BD Bioscience Inc. Blocking monoclonal anti-TF antibody 4501 was from American Diagnostica Inc. Propynoic Anti-ERp57 antibody (ab10287) from Abcam, Anti-ERp72 antibody (D70D12, #5033) from Cell Signal. Anti-PDI antibody (H160, sc-20132) and anti-β-actin antibody from Abcam. Acid Carbamoyl Methyl Amide-31 (PACMA31) was bought from Merck Millipore. Calf intestinal alkaline phosphatase (CIAP) and DNase were purchased from New England Biolabs. RPMI 1640 medium and fetal bovine serum (FBS) were purchased from Invitrogen. Lipopolysaccharide (LPS), Polyinosinic:polycytidylic acid [poly(I:C)], Histopaque-1077, Histopaque-1083 and other reagents were obtained from Sigma-Aldrich unless otherwise specified.
Mice
Mx1-Cre/PDIfl/fl mice were generated by mating Mx1-Cre mice (The Jackson Laboratory) with PDIfl/fl mice, as previously described[5]. PDI gene deletion was induced by intraperitoneal injection of 15 mg/kg poly(I:C) for 2 weeks every other day. Two weeks after the last injection, marrow mononuclear cells were prepared from mice. Experiments with mice were performed in accordance with institutional guidelines and with the approval of the Institutional Animal Care and Use Committees at Soochow University.
Isolation of mouse marrow mononuclear cells
After Mx1-cre/PDIfl/fl mice and littermate control mice were euthanized, bone marrow mononuclear cells (BMMCs) were isolated from femur and tibia. In brief, BMMCs were flushed by inserting a 20-gauge needle attached to the 10 mL syringe into 6 mm cell culture dish in PBS. The single cell suspension was filtered through a 70-mm strainer. The filtered cell suspension was centrifuged for 10 min at 250g[10–11]. The pellet was resuspended in 3 ml of PBS, layered over 3 ml of Histopaque-1083 in 15 ml centrifuge tube. After centrifugation for 30min at 400g, the cells were removed from interface, and washed three times in PBS. Cells were cultured in complete RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 1 mM L-glutamine, and 1% penicillin-streptomycin at 37°C in a humidified atmosphere with 5% CO2.
Isolation of human PBMC
Human blood was collected using sodium citrate as an anti-coagulant from healthy donors after informed consent was obtained. Mononuclear cells were prepared by density gradient centrifugation, as described previously[12]. Briefly, fresh citrated human whole blood was diluted with equal volume of PBS, overlayered onto Histopaque-1077 at a volume ratio of 1:1. After centrifugation at 400g for 30 min, the mononuclear cells were collected and washed twice in PBS.
Flow cytometric analysis of TF expression on cell surface
Washed PBMCs (1 × 106/mL) were blocked with 2% BSA-PBS for 5 mins, then incubated with PE-conjugated control IgG, or PE-conjugated tissue factor for 10min. After washed twice with PBS, cells were fixed with 1% paraformaldehyde in PBS analyzed by flow cytometry analysis (FACS Calibur, BD).
Preparation of platelet-free plasma
Human and mouse blood was collected into 3.2% sodium citrate (9:1) and centrifuged twice at 2000g for 10min, each time the supernatant plasma was collected. The plasma was mixed and divided into several 1.5ml centrifuge tube, stored at −80°C. About 2 hours before TGA experiment, the plasma was moved to 4°C refrigerator, or placed on ice until the plasma was thawed completely. The plasma was obtained by a sequential centrifugation at 1000 g for 1 min and at 21,100 g for 30 min at 4°C. The collected plasma was used in thrombin generation measurement.
Thrombin generation assay
Thrombin generation initiated by mononuclear cells was performed as previously described[9]. Briefly, 6×104 mononuclear cells in 80 μl of 1640 medium were added into a TGA cuvette, 100ng/ml LPS or equal volume of PBS was added in and incubated for 4 hours at 37°C [12–14], followed by addition of 40μl of plasma. Thrombin generation was initiated by automatic dispensation of 30μl of fluorescent thrombin substrate containing 100 mM Ca2+, and assessed in real time by Ceveron® Alpha TGA (Technoclone, Vienna, Austria). In some experiments, after stimulated by LPS, cells were incubated with 50 μg/ml inhibitory anti-tissue factor antibody 4501, 100μg/ml PDI-wt recombinant protein, or various concentration of PACMA31[15], before addition of plasma and thrombin substrate. The change of fluorescent signal was transformed into thrombin generation curve in real time by the Ceveron® Alpha TGA automatically.
Preparation of recombinant wild type PDI protein
Wild-type PDI (PDI-wt) cDNA was cloned into pTriEX-4 Neo vector with an N-terminal histidine tag. BL21 cell transformed within PDI-wt recombinant plasmid was induced by 0.5μM IPTG for 4 hours at 37°C. PDI-wt recombinant protein in BL21 cell lysate was purified using Ni Sepharose High Performance column (GE Healthcare)[16–18].
SDS-PAGE and western blotting
Cells were lysed by NP40 lysis buffer for 30 min on ice, followed by addition of SDS-PAGE loading buffer and heating at 95°C for 10 minutes. After the samples were separated by electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore), the membrane was blocked with 5% non-fat dry milk in blocking buffer. After extensive washing, the immunoblots were incubated for 2 h with the primary antibodies. After incubation with anti-mouse IgG (LI-COR Bioscience) or IRDye 680-conjugated goat anti-rabbit IgG (LI-COR Bioscience), antibody binding was visualized with Odyssey Infrared Imaging System (Li-Cor Biosciences).
Statistical analysis
The results are expressed as the means ± SEM of at least three experiments. Statistical analyses were performed on GraphPad Prism v5.0 (GraphPad software Inc., San Diego, CA, USA). One-way analysis of variance followed by Tukey’s test (for multiple groups) or Student’s t-test (for comparisons between two groups) was used. A p value less than 0.05 was considered statistically significant. Unless stated otherwise, the data shown are from a single experiment that is representative of at least three separate experiments.
Results and Discussion
LPS-stimulated human PBMC express tissue factor with enhanced thrombin generation
LPS upregulates expression of tissue factor of cells. After LPS stimulation for 4 hours, human PBMCs expressed higher levels of tissue factor on the cell membrane shown by flow cytometry (Fig. 1A). As analyzed by TGA, TF-bearing PBMC possessed stronger thrombin generation than non-stimulated PBMC (Fig. 1B and 1C). To determine the contribution of TF to procoagulant activity of LPS-stimulated PBMC, the cells were pretreated with 50 μg/ml inhibitory anti-TF antibody 4501 before the addition of plasma and the thrombin substrate. As shown in Fig. 1 (B and C), the anti-TF antibody completed inhibited thrombin generation, suggesting that thrombin generation by LPS-stimulated PBMC is TF-dependent.
Figure 1. LPS-stimulated human PBMCs expresses TF and initiates thrombin generation.
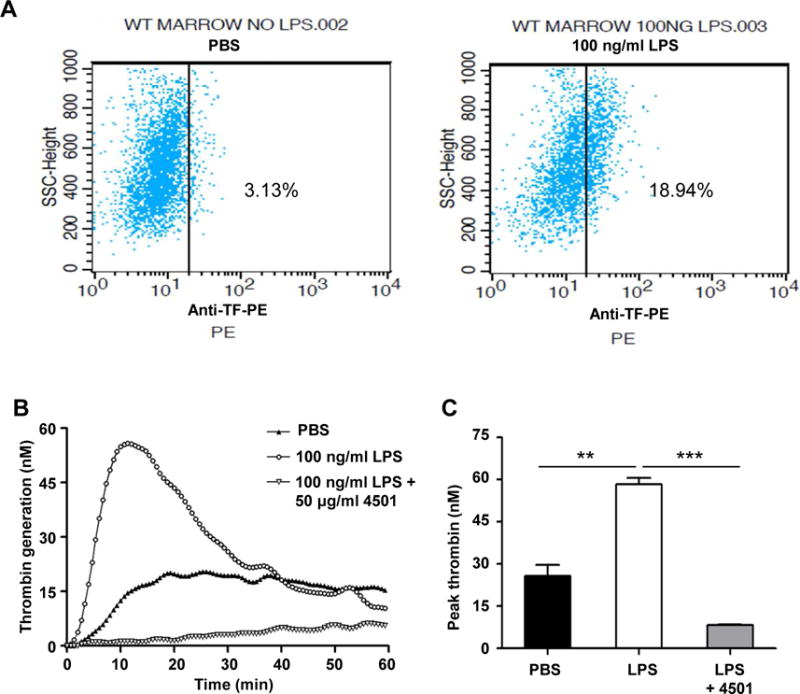
Human PBMCs were treated with PBS or 100 ng/ml LPS at 37°C for 4 hours, the expression of TF on PBMCs were analyzed by flow cytometry using PE-labeled anti-TF antibody (A). Thrombin generation initiated by PBMCs was performed as described in the Materials and Methods. PBMCs were treated with PBS, 100 ng/ml LPS, or 100 ng/ml LPS plus 50 μg/ml anti-TF antibody (4501) (n=3). Representative trace (B) and peak thrombin generation are shown (C). **P < 0.01, ***P < 0.001, t test.
PDI contributes to thrombin generation by LPS-stimulated PBMCs
To determine the role of PDI in TF-dependent thrombin generation, PBMCs were incubated with 100 μg/ml of recombinant PDI-wt protein. As shown by TGA, PDI-wt significantly increased thrombin generation (Fig. 2A). Moreover, the PDI inhibitor PACMA31 inhibited thrombin generation of LPS-stimulated PBMCs in a concentration-dependent fashion (Fig. 2B). The above results imply that PDI enhances TF-dependent thrombin generation.
Figure 2. Thrombin generation by LPS-stimulated PBMCs is potentiated by recombinant PDI protein and inhibited by the PDI inhibitor PACMA31.
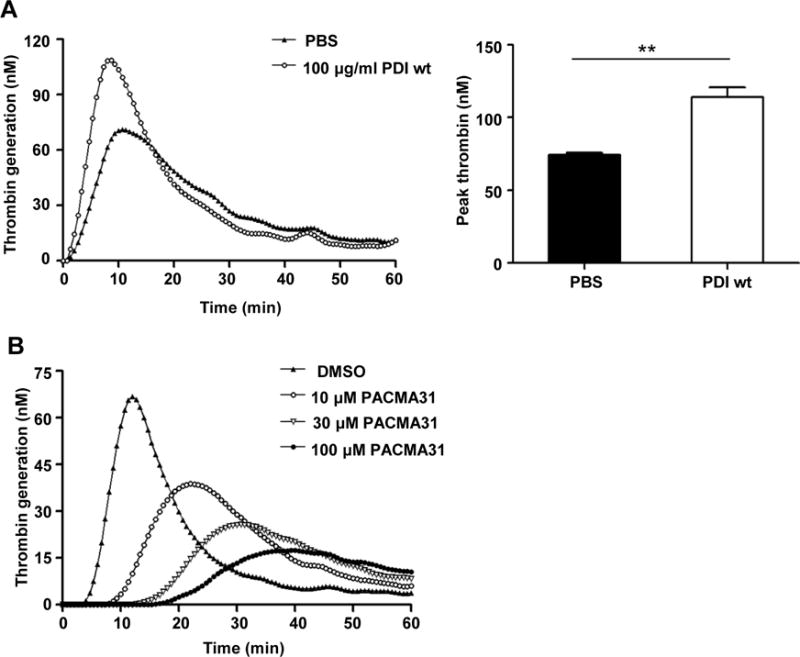
Human PBMCs were treated with 100 ng/ml of LPS, followed by incubation with PBS or 100 μg/ml of recombinant wild-type PDI protein (PDI-wt) at 37°C for 4 hours (A). Thrombin generation was measured. Representative trace (left panel) and peak thrombin generation (right panel) are shown. **P < 0.01, t test. (B) Human PBMCs were treated with 100 ng/ml of LPS, followed by incubation with DMSO or PACMA31 at the indicated concentration. Representative trace of thrombin generation are shown.
PDI deficiency inhibits thrombin generation of mouse bone marrow mononuclear cells
To further determine the role of PDI on TF-dependent thrombin generation, PDI-deficient bone marrow mononuclear cells were isolated from inducible PDI-knockout mice as we previously described[15]. Mx1-Cre/PDIfl/fl mice were generated by mating Mx1-Cre mice with PDIfl/fl mice. After treatment with poly(I:C) for 2 weeks, bone marrow mononuclear cells were isolated, and the absence of PDI protein expression was confirmed by western blotting, in spite of normal level of other disulfide isomerases such as ERp57 and ERp72. (Fig. 3A and 3B). As detected by TGA, when WT and PDI-deficient cells were incubated with 100 ng/ml LPS for 4 hours, bone marrow mononuclear cells from Mx1-Cre/PDIfl/fl mice showed a decrease in thrombin generation compared with WT wild-type cells (Fig. 3A), demonstrating that PDI plays a specific role in potentiation of TF-dependent thrombin generation.
Figure 3. PDI deficient marrow mononuclear cells show a decrease in thrombin generation.
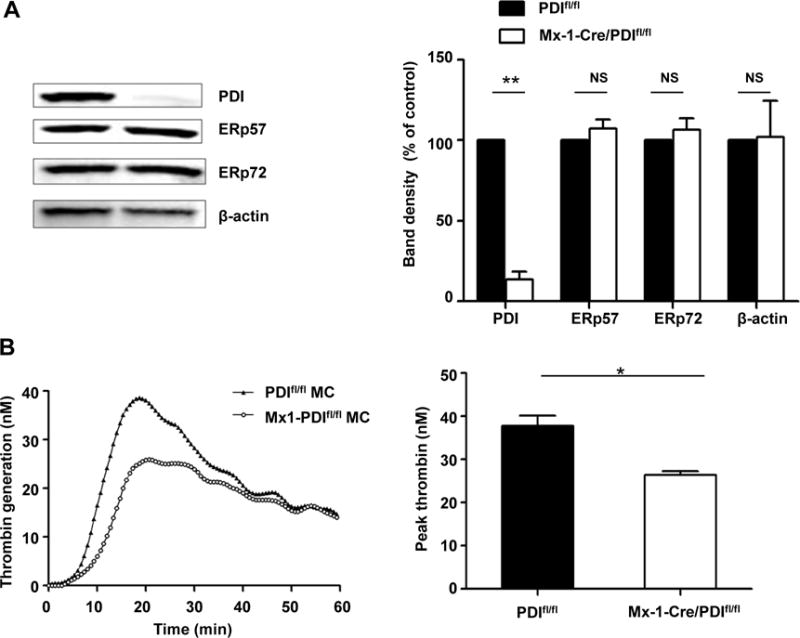
(A) PDI and other disulfide isomerases ERp57 and ERp72 in bone marrow mononuclear cells from WT and Mx1-cre/PDIfl/fl mice was detected by immunoblotting. β-actin served as a loading control. The blots (left panel) and densitometric analysis (right panel) are shown (n=3). **P < 0.01, t test. (B) Bone marrow mononuclear cells from WT PDIfl/fl mice and Mx1-cre/PDIfl/fl mice were treated with LPS, followed by TGA analysis (n=3). Representative trace (left panel) and peak thrombin generation (right panel) are shown. *P < 0.05, t test.
The above results provide evidence showing that PDI is positive regulator of TF-dependent on thrombin generation. It has been noted that PDI contributes to thrombosis and haemostasis[3, 5–6, 19–20]. PDI-knockout mice have a prolonged bleeding time[5]. In a laser-induced thrombosis model of the cremaster muscle arterioles, both Mx1-Cre/PDIfl/fl mice and mice expressing an inactive mutation of the C-terminal active site showed a decrease in fibrin deposition[5]. In a rat inferior vena cava ligation model, PDI expression has been detected in the venous thrombi PDI, suggesting its participation in venous thrombosis[21]. However, the mechanism by which PDI regulates coagulation remains elusive. Tissue factor plays a critical role in hemostasis and thrombosis, however, uncertainty remains about the role of PDI in the modulation of tissue factor activity there is lack of strong evidence showing that PDI enhances coagulation via modulation of tissue factor [6, 22–26]. In this study, using PDI recombinant protein, PDI inhibitor and PDI-deficient cell models, we provide evidence that PDI mediates tissue factor thrombin generation. Thus, PDI may serve as a novel therapeutic target for anti-coagulant treatment[27–28].
Acknowledgments
This work was supported by grants from the National Natural Scientific Foundation (81770138, 81670133, 91539122) and the NIH grant R01HL118526.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions: F.C., Z.Z and J.Z. performed research, analyzed data. Y.L. contributed critical reagent. D.W.E. and Y.W. designed research, interpreted the data. F.C. D.W.E. and Y.W. wrote the paper.
Disclosure of Conflicts of Interest: All the authors declare no competing financial interests.
References
- 1.Essex DW. Redox control of platelet function. Antioxidants & redox signaling. 2009;11:1191–1225. doi: 10.1089/ars.2008.2322. [DOI] [PubMed] [Google Scholar]
- 2.Appenzeller-Herzog C, Ellgaard L. The human PDI family: versatility packed into a single fold. Biochim Biophys Acta. 2008;1783:535–548. doi: 10.1016/j.bbamcr.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. The Journal of clinical investigation. 2008;118:1123–1131. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim K, Hahm E, Li J, Holbrook LM, Sasikumar P, Stanley RG, Ushio-Fukai M, Gibbins JM, Cho J. Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood. 2013;122:1052–1061. doi: 10.1182/blood-2013-03-492504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J, Wu Y, Wang L, Rauova L, Hayes VM, Poncz M, Essex DW. The C-terminal CGHC motif of protein disulfide isomerase supports thrombosis. The Journal of clinical investigation. 2015;125:4391–4406. doi: 10.1172/JCI80319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. The Journal of clinical investigation. 2008;118:1110–1122. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dargaud Y, Trzeciak MC, Bordet JC, Ninet J, Negrier C. Use of calibrated automated thrombinography +/− thrombomodulin to recognise the prothrombotic phenotype. Thrombosis and haemostasis. 2006;96:562–567. [PubMed] [Google Scholar]
- 8.Dargaud Y, Luddington R, Gray E, Lecompte T, Siegemund T, Baglin T, Hogwood J, Regnault V, Siegemund A, Negrier C. Standardisation of thrombin generation test–which reference plasma for TGT? An international multicentre study. Thrombosis research. 2010;125:353–356. doi: 10.1016/j.thromres.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Langer F, Spath B, Fischer C, Stolz M, Ayuk FA, Kroger N, Bokemeyer C, Ruf W. Rapid activation of monocyte tissue factor by antithymocyte globulin is dependent on complement and protein disulfide isomerase. Blood. 2013;121:2324–2335. doi: 10.1182/blood-2012-10-460493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 11.Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noel D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 12.Ferro D, Basili S, Alessandri C, Cara D, Violi F. Inhibition of tissue-factor-mediated thrombin generation by simvastatin. Atherosclerosis. 2000;149:111–116. doi: 10.1016/s0021-9150(99)00291-9. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Ding G, Liu W, Wang L, Lu Y, Cao H, Zheng J. Lipopolysaccharide could be internalized into human peripheral blood mononuclear cells and elicit TNF-alpha release, but not via the pathway of toll-like receptor 4 on the cell surface. Cell Mol Immunol. 2004;1:373–377. [PubMed] [Google Scholar]
- 14.Khajuria A, Houston DS. Induction of monocyte tissue factor expression by homocysteine: a possible mechanism for thrombosis. Blood. 2000;96:966–972. [PubMed] [Google Scholar]
- 15.Xu S, Butkevich AN, Yamada R, Zhou Y, Debnath B, Duncan R, Zandi E, Petasis NA, Neamati N. Discovery of an orally active small-molecule irreversible inhibitor of protein disulfide isomerase for ovarian cancer treatment. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16348–16353. doi: 10.1073/pnas.1205226109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Ahmad SS, Zhou J, Wang L, Cully MP, Essex DW. The disulfide isomerase ERp57 mediates platelet aggregation, hemostasis, and thrombosis. Blood. 2012;119:1737–1746. doi: 10.1182/blood-2011-06-360685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Wu Y, Zhou J, Ahmad SS, Mutus B, Garbi N, Hammerling G, Liu J, Essex DW. Platelet-derived ERp57 mediates platelet incorporation into a growing thrombus by regulation of the alphaIIbbeta3 integrin. Blood. 2013;122:3642–3650. doi: 10.1182/blood-2013-06-506691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, Wu Y, Wang L, Rauova L, Hayes VM, Poncz M, Essex DW. The disulfide isomerase ERp57 is required for fibrin deposition in vivo. Journal of thrombosis and haemostasis: JTH. 2014;12:1890–1897. doi: 10.1111/jth.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho J, Kennedy DR, Lin L, Huang M, Merrill-Skoloff G, Furie BC, Furie B. Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of beta3 integrins. Blood. 2012;120:647–655. doi: 10.1182/blood-2011-08-372532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manukyan D, von Bruehl ML, Massberg S, Engelmann B. Protein disulfide isomerase as a trigger for tissue factor-dependent fibrin generation. Thrombosis research. 2008;122(Suppl 1):S19–22. doi: 10.1016/S0049-3848(08)70013-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, May L, Liao P, Gross PL, Weitz JI. Inferior vena cava ligation rapidly induces tissue factor expression and venous thrombosis in rats. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:863–869. doi: 10.1161/ATVBAHA.109.185678. [DOI] [PubMed] [Google Scholar]
- 22.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13932–13937. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. The Journal of biological chemistry. 2007;282:25416–25424. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- 24.Raturi A, Ruf W. Effect of protein disulfide isomerase chaperone activity inhibition on tissue factor activity. Journal of thrombosis and haemostasis: JTH. 2010;8:1863–1865. doi: 10.1111/j.1538-7836.2010.03918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruf W, Versteeg HH. Tissue factor mutated at the allosteric Cys186-Cys209 disulfide bond is severely impaired in decrypted procoagulant activity. Blood. 2010;116:500–501. doi: 10.1182/blood-2010-04-281287. author reply 502-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kothari H, Nayak RC, Rao LV, Pendurthi UR. Cystine 186-cystine 209 disulfide bond is not essential for the procoagulant activity of tissue factor or for its de-encryption. Blood. 2010;115:4273–4283. doi: 10.1182/blood-2009-09-241356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flaumenhaft R. Protein disulfide isomerase as an antithrombotic target. Trends in cardiovascular medicine. 2013;23:264–268. doi: 10.1016/j.tcm.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flaumenhaft R, Furie B, Zwicker JI. Therapeutic implications of protein disulfide isomerase inhibition in thrombotic disease. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:16–23. doi: 10.1161/ATVBAHA.114.303410. [DOI] [PMC free article] [PubMed] [Google Scholar]


