An involvement of the immune system in the pathophysiology of schizophrenia is a current topic of intensive investigation. As summarized by Marques et al. in this issue of Psychological Medicine, preliminary evidence comes from several lines of research, including genetic and epidemiological data as well as observations of increases of pro-inflammatory markers in blood and cerebrospinal fluid (Marques et al., 2018). However, as the authors note, in order to confirm the presence of a dysfunctional immune system in the brain, more direct methods are needed. The most established approach to examine brain immune function in vivo is to use positron emission tomography (PET) and radioligands that target the glial cell marker 18 kDa translocator protein (TSPO). There are at present 12 published studies that have applied this technique in psychosis patients, with seemingly inconclusive and even contradictory results (Van Berckel et al., 2008; Banati and Hickie, 2009; Doorduin et al., 2009; Bloomfield et al., 2015; Kenk et al., 2015; Coughlin et al., 2016; Van Der Doef et al., 2016; Holmes et al., 2016; Collste et al., 2017; Di Biase et al., 2017; Hafizi et al., 2017; Ottoy et al., 2018). However, all of these studies have employed small sample sizes (patient groups have ranged from N = 7 to N = 19): a common problem in PET neuroimaging research resulting in a low statistical power to detect patient–control differences.
One approach to overcome this limitation is to synthesize data from multiple studies using meta-analysis, which yields an estimate of an overall effect size of patient–control differences using aggregate data from published papers. In the article by Marques et al., the results of such analyses are reported, leading the authors to the conclusion that brain TSPO levels are elevated in patients, based mainly on studies using the first generation TSPO radioligand (R)-[11C]PK11195. They further conclude that there is no patient–control difference when analysing studies using the second generation TSPO radioligands [11C]PBR28, [18F]FEPPA, [18F]PBR111 and [11C]DPA713. The overall result of higher TSPO levels is in contrast to a recently published multi-center individual-participant data meta-analysis (mega-analysis) co-authored by us, partly based on the same studies (Plavén-Sigray et al., 2018a). Below we highlight some caveats that should be considered when interpreting these divergent results.
(R)-[11C]PK11195 was developed in the early 1990s and has been used to study glial activation in a wide range of somatic, neurological and psychiatric disorders. However, concerns regarding the low signal to noise ratio of (R)-[11C]PK11195 have led to the development of a series of second generation TSPO radioligands during the last decade. For (R)-[11C]PK11195, two main factors that contribute to the low signal-to-noise are; (1) low brain uptake (Kreisl et al., 2010; Kobayashi et al., 2017) and (2) low specific-to-background binding ratio. In PET experiments where the specific binding is blocked using a cold compound, it is possible to determine the ratio between specific and non-displaceable (background) binding, referred to as non-displaceable binding potential (BPND) (Innis et al., 2007). For (R)-[11C]PK11195, BPND in healthy controls assessed in this way are in the range of 0.7–0.8, suggesting that non-displaceable binding (background signal) is proportionally larger than specific binding (target signal) (Kobayashi et al., 2017). This ratio is much lower than has been reported for the second-generation TSPO radioligands [11C]PBR28 (Owen et al., 2014; Plavén-Sigray et al., 2018c), [11C]DPA713 (Kobayashi et al., 2017) and the more recently developed [11C]ER176 (Ikawa et al., 2017). A consequence of lower biological signal is lower accuracy and reliability of the measurement (Jučaite et al., 2012).
In addition to the properties of the radioligand used, another factor that affects the signal-to-noise ratio of a PET outcome measure is the method of analysis. An important premise for quantification of TSPO binding is that this protein is expressed across the entire brain (Doble et al., 1987). This means that no region can serve as true reference for simplified quantification approaches where binding in a target region is expressed in relation to a region of non-target brain tissue. Instead, arterial blood sampling is necessary in order to model radioligand delivery to the brain (i.e. an arterial input function, AIF). Using this method, the gold standard outcome is considered to be the total distribution volume (VT), which is an estimate of radioligand binding in target tissue relative to the concentration of radioligand in plasma. In the initial two (R)-[11C]PK11195 studies on schizophrenia, arterial samples were collected and AIFs were established. However, instead of calculating VT, rate constants from the compartmental model was used to obtain two different types of BP: BPP (denoting specific binding over plasma) (Van Berckel et al., 2008) and BPND (Doorduin et al., 2009). In the remaining (R)-[11C]PK11195 studies in psychosis, no AIF was collected, and BPND was calculated using the simplified reference tissue model (SRTM). Reference time-activity curves were derived either from cerebellum as a ‘pseudo-reference’ region (Holmes et al., 2016; Di Biase et al., 2017) or using the supervised cluster analysis method (Van Der Doef et al., 2016). Using a test–retest dataset in healthy control subjects, we recently evaluated the reliability of different measures of (R)-[11C]PK11195 BPND, finding intraclass correlation coefficient values in the range of 0.3–0.5 (Plavén-Sigray et al., 2018b). This suggests that at least half of the variability in (R)-[11C]PK11195 BPND is due to measurement error. In the case of reference tissue methods, this is likely due to similar shape and magnitude of the time-activity curves in the target and reference input, yielding noisy BPND values close to zero (Plavén-Sigray et al., 2018b), an effect evident also in some of the patient studies (Holmes et al., 2016; Van Der Doef et al., 2016).
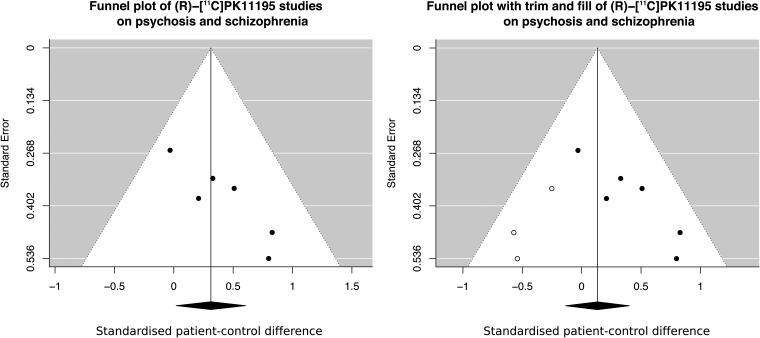
Low accuracy and reliability of a measurement leads to loss of sensitivity to detect true differences, as well as a higher risk for chance findings (Button et al., 2013; Matheson, 2018). When examining the funnel plot of the (R)-[11C]PK11195 meta-analysis carried out by Marques et al., there is a strong association between the magnitude of the patient–control difference and the measurement error of the included studies (r = 0.9, p = 0.015, Fig. 1). In other words, the larger the study, the smaller the reported effect size. This suggests that some (R)-[11C]PK11195 studies may have yielded inflated effect sizes, potentially due to a combination of using outcomes with low reliability, and small sample sizes. As reported by Marques et al., when correcting for this bias using the standard trim-and-fill method, the significant finding of elevated levels of TSPO in patients disappears. When the funnel plot displays such a shape, and the trim-and-fill correction negates an apparent effect, a general recommendation is that any non-corrected differences should be interpreted with strong caution (Duval and Tweedie, 2000; Rothstein et al., 2006).
Fig. 1.
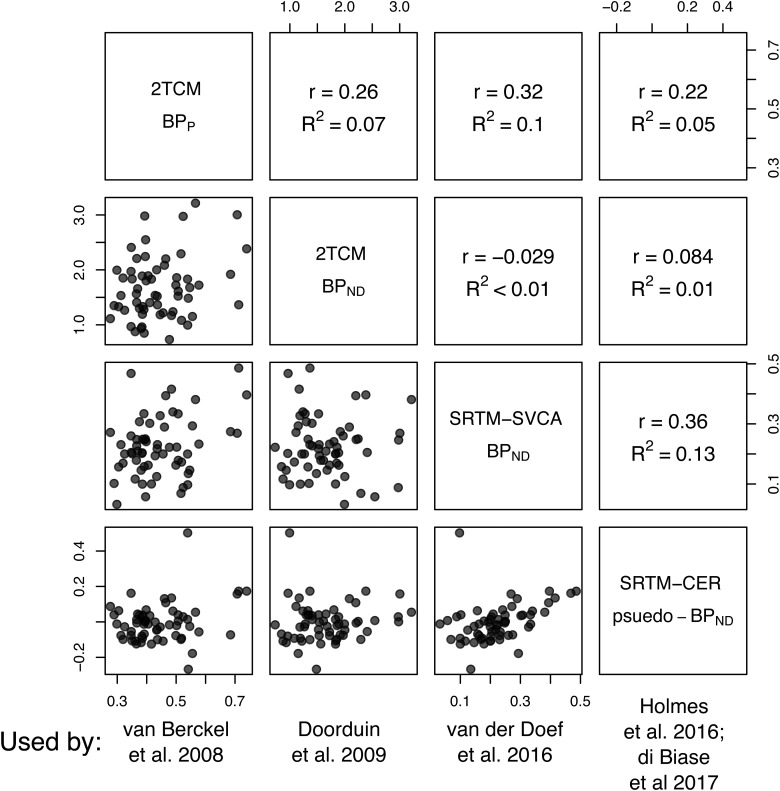
The different outcomes used by the studies included in the (R)-[11C]PK11195 meta-analysis by Marques et al. show little to no association with one another. This figure presents pooled data from 12 (R)-[11C]PK11195 examinations of healthy controls from a set of different regions (whole gray matter, thalamus, frontal cortex, hippocampus and striatum).
A further concern with the synthesis of the (R)-[11C]PK11195 data by Marques et al. is the mixing of different outcome measures of radioligand binding. Although some between-study variation (τ) is allowed, a pre-condition of a random-effect meta-analysis, as performed by Marques et al., is that all outcomes should reflect the same underlying population effect size (Higgins et al., 2009):
‘The effects may be (a) assumed different and unrelated, (b) assumed different but similar […]. In the first, each study is considered in isolation from the others and meta-analysis is ruled out as an option. In the second, a random-effects model may be assumed to reflect the similarity.’
- Higgins et al., 2009
In our test–retest paper (Plavén-Sigray et al., 2018b) we assessed whether the different (R)-[11C]PK11195 BP outcomes, included by Marques et al., are related to each other, such that criterion (b) above is fulfilled. We found low to negligible correlations between all outcomes (Fig. 2). Based on these results, it is unlikely that BPND or BPP derived from the use of an AIF (Van Berckel et al., 2008; Doorduin et al., 2009), pseudo-BPND calculated from the SRTM with cerebellum (Holmes et al., 2016; Di Biase et al., 2017) or BPND calculated using the supervised cluster analysis method (Van Der Doef et al., 2016) measure the same thing. Hence, it can be argued that apples and pears and perhaps even oranges are being entered into the same meta-analytical model, calling into question the interpretability of the resulting underlying effect size.
Fig. 2.
The magnitude of the effect size and the measurement error of the studies included in the (R)-[11C]PK11195 meta-analysis by Marques et al. (left figure) show a high degree of association (r = 0.9). Potential reasons for such a shape are publication bias or inflated effect sizes in studies with unreliable outcomes and small sample sizes, leading to an inflated overall effect size. When Marques et al. corrected for this bias, the difference between healthy controls and patients with psychosis or schizophrenia was no longer statistically significant (right figure).
To conclude, the low reliability and sensitivity of (R)-[11C]PK11195 outcomes used to examine TSPO in psychosis, caused by both radioligand characteristics and quantification methods, clearly limits the informational value of these studies. This is supported by the test–retest studies of (R)-[11C]PK11195 outcome measures, as well as the funnel-plot in the article by Marques et al. In addition, the lack of correlations between the different (R)-[11C]PK11195 outcome measures suggests that an important precondition of the meta-analysis model is violated. For these reasons, we do not believe that there is sufficient evidence to suggest an increase in TSPO levels in patients with psychosis or schizophrenia.
This conclusion is further supported by the second part of the meta-analysis by Marques et al. Here, the authors included data from studies employing second generation TSPO radioligands (Bloomfield et al., 2015; Kenk et al., 2015; Coughlin et al., 2016; Collste et al., 2017; Hafizi et al., 2017; Ottoy et al., 2018), showing no evidence in favor of higher TSPO levels in patients as compared to control subjects. We believe that this analysis has many strengths, such as (1) a higher proportion of specific signal in second-generation TSPO radioligands (Owen et al., 2014; Ikawa et al., 2017; Kobayashi et al., 2017; Plavén-Sigray et al., 2018c) as well as the use of a (2) homogeneous and (3) reliable outcome measure (VT) (Park et al., 2015; Collste et al., 2016; Ottoy et al., 2018). Moreover, we commend the authors decision not to include outcomes from these radioligands that are expressed ‘relative to tissue’ (such as distribution volume ratios), as the lack of a suitable ‘normalizing’ region makes such outcomes unreliable and prone to bias, at least in situations where there is no clear increase in the target region (Narendran and Frankle, 2016; Matheson et al., 2017). To summarize, we believe that the meta-analysis by Marques et al., including only second-generation TSPO radioligands, is both robust and of high evidential value.
The finding of no increase in VT in psychosis or schizophrenia is in line with the mega-analysis co-authored by us, as well as by some of the authors of the Marques et al. study (Plavén-Sigray et al., 2018a). In fact, in this multi-center collaboration on studies using second-generation TSPO radioligands, we not only found evidence against an increase in TSPO, but also showed strong evidence in favor of lower TSPO in patients. Since we had access to all individual data points, it was possible to control for potential co-founders such as sex, duration of illness, symptom severity and medication effects. This is something that cannot be done in a traditional meta-analysis based on summary statistics alone, and hence allows for more robust conclusions (Tudur Smith et al., 2016). It should however be noted that the recently published study by Ottoy et al., included by Marques et al., was not included in our analysis. This study did not find a group difference in VT, but did find a significant age v. patient–control interaction. More data from clinical studies employing second generation TSPO radioligands are likely yet to come, hopefully resolving the question on whether TSPO levels are lower, or unchanged in patients with psychosis or schizophrenia.
The lack of an increase, or perhaps even the presence of a decrease in TSPO in patients, at first sight appears to contradict results from other research suggesting a pro-inflammatory state in schizophrenia. However, a closer inspection of the literature reveals that the results may be reconcilable. Importantly, there is an ongoing discussion on the lack of specificity of TSPO as a pro-inflammatory marker that deserves to be highlighted. First, we know that TSPO is not specific for microglial activation. The protein is found in astrocytes (Lavisse et al., 2012; Toth et al., 2015; Notter et al., 2017) as well as in vascular cells (Veronese et al., 2017), and even neurons (Notter et al., 2018). Second, animal and in vitro human data has challenged the widely-held view of TSPO as an exclusively pro-inflammatory marker. In a mouse model of low-grade immune activation, TSPO was found to be decreased, despite elevated levels of classical pro-inflammatory markers such as interleukin (IL)-1β and IL-6 (Notter et al., 2017). In vitro assays of human immune cells have shown that TSPO does not increase upon stimulation with the pro-inflammatory agent lipopolysaccharide (Narayan et al., 2017), and might even show decreased levels (Owen et al., 2017).
To summarize, the notion that TSPO is a microglial activation marker that represents neuroinflammation is most likely an over-simplification. Hence, evidence against increased TSPO from PET studies should not be taken as evidence against a pro-inflammatory immune state in schizophrenia, and we therefore agree with Marques et al. that the discussion of increased microglia activity should be kept open. However, when it comes to finding a marker that can reliably be used to detect pro-inflammatory activation in patients with psychosis as a means of patient stratification and treatment monitoring, we suggest that the search should continue elsewhere. There are a wide range of potential targets and radioligands that can be explored (Narayanaswami et al., 2018), and we look forward to joint efforts in translating these from validation studies in experimental settings to application in patients, thus enabling PET to realize its full potential in supporting the development of new treatment approaches for schizophrenia.
Acknowledgements
We would like thank Granville J. Matheson for valuable input on the manuscript. S.C. is supported by the Swedish Research Council (Grant No. 523-2014-3467).
Author ORCIDs
S. Cervenka http://orcid.org/0000-0001-8103-6977, P. Plavén-Sigray https://orcid.org/0000-0001-5342-5641
Conflict of interest
SC has received grant support from AstraZeneca as a coinvestigator and has served as a speaker for Otsuka. PPS reports no conflict of interest.
References
- Banati R and Hickie IB (2009) Therapeutic signposts: using biomarkers to guide better treatment of schizophrenia and other psychotic disorders. Medical Journal of Australia 190, S26. [DOI] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, Bloomfield MAP, Bonoldi I, Kalk N and Turkheimer F (2015) Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [11C] PBR28 PET brain imaging study. American Journal of Psychiatry 173, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ and Munafò MR (2013) Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience 14, 365. [DOI] [PubMed] [Google Scholar]
- Collste K, Forsberg A, Varrone A, Amini N, Aeinehband S, Yakushev I, Halldin C, Farde L and Cervenka S (2016) Test–retest reproducibility of [11C]PBR28 binding to TSPO in healthy control subjects. European Journal of Nuclear Medicine and Molecular Imaging 43, 173–183. [DOI] [PubMed] [Google Scholar]
- Collste K, Plavén-Sigray P, Fatouros-Bergman H, Victorsson P, Schain M, Forsberg A, Amini N, Aeinehband S, Erhardt S, Halldin C, Flyckt L, Farde L and Cervenka S (2017) Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [11C]PBR28. Molecular Psychiatry 22, 850–856. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, Kim PK, Ford CN, Higgs C and Hayes LN (2016) In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [11C]DPA-713 PET and analysis of CSF and plasma. Translational Psychiatry 6, e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase MA, Zalesky A, O'keefe G, Laskaris L, Baune BT, Weickert CS, Olver J, McGorry PD, Amminger GP and Nelson B (2017) PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Nature Publishing Group Translational Psychiatry 7, e1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble A, Malgouris C, Daniel M, Daniel N, Imbault F, Basbaum A, Uzan A, Gueremy C and Le Fur G (1987) Labelling of peripheral-type benzodiazepine binding sites in human brain with [3H]PK 11195: anatomical and subcellular distribution. Elsevier Brain Research Bulletin 18, 49–61. [DOI] [PubMed] [Google Scholar]
- Doorduin J, De Vries EFJ, Willemsen ATM, De Groot JC, Dierckx RA and Klein HC (2009) Neuroinflammation in schizophrenia-related psychosis: a PET study. Journal of Nuclear Medicine 50, 1801–1807. [DOI] [PubMed] [Google Scholar]
- Duval S and Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Wiley Online Library Biometrics 56, 455–463. [DOI] [PubMed] [Google Scholar]
- Hafizi S, Tseng HH, Rao N, Selvanathan T, Kenk M, Bazinet RP, Suridjan I, Wilson AA, Meyer JH, Remington G, Houle S, Rusjan PM and Mizrahi R (2017) Imaging microglial activation in untreated first-episode psychosis: a PET study with [18F]FEPPA. American Journal of Psychiatry 174, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG and Spiegelhalter DJ (2009) A re-evaluation of random-effects meta-analysis. Wiley Online Library Journal of the Royal Statistical Society: Series A (Statistics in Society) 172, 137–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SE, Hinz R, Drake RJ, Gregory CJ, Conen S, Matthews JC, Anton-Rodriguez JM, Gerhard A and Talbot PS (2016) In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Molecular Psychiatry 21, 1672–1679. [DOI] [PubMed] [Google Scholar]
- Ikawa M, Lohith TG, Shrestha S, Telu S, Zoghbi SS and Castellano S (2017) 11C-ER176, a radioligand for 18-kDa translocator protein, has adequate sensitivity to robustly image all three affinity genotypes in human brain. Journal of Nuclear Medicine 58, 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF and Carson RE (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow & Metabolism 27, 1533–1539. [DOI] [PubMed] [Google Scholar]
- Jučaite A, Cselényi Z, Arvidsson A, Åhlberg G, Julin P, Varnäs K, Stenkrona P, Andersson J, Halldin C and Farde L (2012) Kinetic analysis and test–retest variability of the radioligand [11C](R)-PK11195 binding to TSPO in the human brain - a PET study in control subjects. EJNMMI Research 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G, Meyer JH, Wilson AA, Houle S and Mizrahi R (2015) Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. MPRC Schizophrenia Bulletin 41, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Jiang T, Telu S, Zoghbi SS, Gunn RN, Rabiner EA, Owen DR, Guo Q, Pike VW and Innis RB (2017) 11C-DPA-713 has much greater specific binding to translocator protein 18 kDa (TSPO) in human brain than 11C-(R)-PK11195. Journal of Cerebral Blood Flow & Metabolism 38, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Fujita M, Fujimura Y, Kimura N, Jenko KJ, Kannan P, Hong J, Morse CL, Zoghbi SS, Gladding RL, Jacobson S, Oh U, Pike VW and Innis RB (2010) Comparison of [(11)C]-(R)-PK 11195 and [(11)C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: implications for positron emission tomographic imaging of this inflammation biomarker. NeuroImage 49, 2924–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavisse S, Guillermier M, Hérard A-S, Petit F, Delahaye M, Van Camp N, Haim LB, Lebon V, Remy P, Dollé F, Ben Haim L, Lebon V, Remy P, Dollé F, Delzescaux T, Bonvento G, Hantraye P and Escartin C (2012) Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. The Journal of Neuroscience 32, 10809–10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques TR, Ashok AH, Pillinger T, Veronese M, Turkheimer FE, Dazzan P, Sommer IEC and Howes OD (2018) Neuroinflammation in schizophrenia: meta-analysis of in vivo microglial imaging studies. Psychological Medicine, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson GJ (2018) We need to talk about reliability: making better use of test retest studies for study design and interpretation. bioRxiv, 274894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson GJ, Plavén-Sigray P, Forsberg A, Varrone A, Farde L and Cervenka S (2017) Assessment of simplified ratio-based approaches for quantification of PET [11C]PBR28 data. EJNMMI Research 7, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan N, Mandhair H, Smyth E, Dakin SG, Kiriakidis S, Wells L, Owen D, Sabokbar A and Taylor P (2017) The macrophage marker translocator protein (TSPO) is down-regulated on pro-inflammatory ‘M1’ human macrophages. PLoS ONE 12, e0185767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanaswami V, Dahl K, Bernard-Gauthier V, Josephson L, Cumming P and Vasdev N (2018) Emerging PET radiotracers and targets for imaging of neuroinflammation in neurodegenerative diseases: outlook beyond TSPO. Molecular Imaging 17, 1536012118792317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R and Frankle WG (2016) Comment on analyses and conclusions of ‘microglial activity in people at ULTRA high risk of psychosis and in schizophrenia: an [11C] PBR28 pet brain imaging study’.American Journal of Psychiatry 173, 536–537. [DOI] [PubMed] [Google Scholar]
- Notter T, Coughlin JM, Gschwind T, Wang Y, Kassiou M, Vernon AC, Benke D and Pomper MG (2017) Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Nature Publishing Group, 1–12. [DOI] [PubMed]
- Notter T, Coughlin JM, Sawa A and Meyer U (2018) Reconceptualization of translocator protein as a biomarker of neuroinflammation in psychiatry. Molecular Psychiatry 23, 36. [DOI] [PubMed] [Google Scholar]
- Ottoy J, De Picker L, Verhaeghe J, Deleye S, Kosten L, Sabbe B, Coppens V, Timmers M, Van Nueten L and Ceyssens S (2018) [18F] PBR111 PET imaging in healthy controls and schizophrenia: test–retest reproducibility and quantification of neuroinflammation. Journal of Nuclear Medicine 59, 1267–1274. [DOI] [PubMed] [Google Scholar]
- Owen DR, Guo Q, Kalk NJ, Colasanti A, Kalogiannopoulou D, Dimber R, Lewis YL, Libri V, Barletta J and Ramada-Magalhaes J (2014) Determination of [11C]PBR28 binding potential in vivo: a first human TSPO blocking study. Journal of Cerebral Blood Flow & Metabolism 34, 989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Narayan N, Wells L, Healy L, Smyth E, Rabiner EA, Galloway D, Williams JB, Lehr J and Mandhair H (2017) Pro-inflammatory activation of primary microglia and macrophages increases 18 kDa translocator protein expression in rodents but not humans. Journal of Cerebral Blood Flow & Metabolism 37, 2679–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Gallezot J-D, Delgadillo A, Liu S, Planeta B, Lin S-F, O'Connor KC, Lim K, Lee J-Y and Chastre A (2015) 11C-PBR28 imaging in multiple sclerosis patients and healthy controls: test–retest reproducibility and focal visualization of active white matter areas.. European Journal of Nuclear Medicine and Molecular Imaging 42, 1081–1092. [DOI] [PubMed] [Google Scholar]
- Plavén-Sigray P, Matheson GJ, Collste K, Ashok AH, Coughlin JM, Howes OD, Mizrahi R, Pomper MG, Rusjan P and Veronese M (2018a) Positron emission tomography studies of the glial cell marker translocator protein in patients With psychosis: a meta-analysis using individual participant data. Biological Psychiatry 84, 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plavén-Sigray P, Matheson GJ, Cselényi Z, Jučaite A, Farde L and Cervenka S (2018b) Test–retest reliability and convergent validity of (R)-[11C] PK11195 outcome measures without arterial input function. EJNMMI Research 8, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plavén-Sigray P, Schain M, Zanderigo F, Rabiner I, Gunn R, Ogden T and Cervenka S (2018c) Accuracy and reliability of [11C] PBR28 specific binding estimated without the use of a reference region. NeuroImage 188, 102–110 [DOI] [PubMed] [Google Scholar]
- Rothstein HR, Sutton AJ and Borenstein M (2006) Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. West Sussex, England: John Wiley & Sons. [Google Scholar]
- Toth M, Little P, Arnberg F, Mulder J, Halldin C, Ha J and Holmin S (2015) Acute neuroinflammation in a clinically relevant focal cortical ischemic stroke model in rat: longitudinal positron emission tomography and immunofluorescent tracking. Brain Structure & Function 221, 1279–1290. [DOI] [PubMed] [Google Scholar]
- Tudur Smith C, Marcucci M, Nolan SJ, Iorio A, Sudell M, Riley R, Rovers MM and Williamson PR (2016) Individual participant data meta-analyses compared with meta-analyses based on aggregate data. The Cochrane Library 9, MR000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA and Kahn RS (2008) Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C] PK11195 positron emission tomography study. Biological Psychiatry 64, 820–822. [DOI] [PubMed] [Google Scholar]
- Van Der Doef TF, De Witte LD, Sutterland AL, Jobse E, Yaqub M, Boellaard R, De Haan L, Eriksson J, Lammertsma AA and Kahn RS (2016) In vivo (R)-[11C]PK11195 PET imaging of 18 kDa translocator protein in recent onset psychosis. NPJ Schizophrenia 2, 16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese M, Marques T, Bloomfield PS, Rizzo G, Singh N, Jones D, Agushi E, Mosses D, Bertoldo A, Howes O, Roncaroli F and Turkheimer FE (2017) Kinetic modelling of [11C]PBR28 for 18 kDa translocator protein PET data: a validation study of vascular modelling in the brain using XBD173 and tissue analysis. Journal of Cerebral Blood Flow and Metabolism 38, 1227–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]