Abstract
Santolina corsica Jord. & Fourr. Corsican-Sardinian is an endemism almost present all around Corsica; in Sardinia, it can only be found in Monte Albo (calcareous substratum and poor in nutrients). The aim of our study is to investigate the chemical composition of S. corsica essential oils from plants growing in three different stations located at different altitudes and evaluate the biological activity using anti-inflammatory, antioxidant and antimicrobial test. The composition of the essential oils was determined by gas chromatography and gas chromatography/mass spectrometry. The essential oils of the Sardinian-Corsican endemism S. corsica, growing in Monte Albo, showed a great variability, probably due to genetic characters different from the Corsican type. We found three different chemotypes: artemisia ketone-β-fellendrene; myrcene and β-fellandrene-myrcene. Standard microbiological assays demonstrated that the essential oils collected in the selected stations, compared with oil and compound with demonstrated antibacterial activity, don’t have any antibacterial activity. DPPH test carried out on the tree samples, compared with chatechin, demonstrated that the oils don’t have antioxidant activity.
Regarding anti-inflammatory activity the study demonstrated that the essential oils have a good anti-inflammatory activity on the bronchial tract.
The addition of essential oil make easy the exocytose and the histiocytes can expel the anthracotic pigment into the culture medium, purifying its cytoplasm and restoring its ability to phagocytize more material. With a higher concentration of granulocytes in the sample, the incubation of cells shows a non-specific inflammatory pattern in which the addition of the essential oils has a positive impact on the decrease of granulocytes.
More experiments are requested to confirm the data, but on the basis of these first results S. corsica essential oil showed potential activity against respiratory infections.
Keywords: Santolina corsica, Essential oil, Anti-inflammatory, Antibacterial and antioxidant activity
1. Introduction
Asteraceae, family of the flowering-plant order Asterales with more than 1.620 genera and 23.600 species of herbs, shrubs and trees distributed throughout the world, is one of the largest plant families (Kadereit and Jeffrey, 2007). Santolina belongs to this large family; it is the only genus in the Mediterranean region that can be found in a vast area including Italy and the Iberian Peninsula, France, Algeria, Morocco, Corsica, Sardinia and the Balearic Islands (Angiolini and Bacchetta, 2003).
Italy houses eight species (Arrigoni, 1982), among these, S. corsica and S. insularis are polyploide apoendemisms, with an area extended on the Sardinian-Corsican domain. S. corsica is a Sardinian-Corsican endemism located in three different sites of the French insula: it can be found at Cap Corse, around Corte and Fium'Orbo. It generally grows between 400 and 1000 m above sea level, preferably on limestone, but rarely also on granite and metamorphic (Gamisans and Jeanmonod, 1998). In Sardinia, its area is limited to a zone in the north-central part of the island called Monte Albo, a limestone massif 20 km long and 4 km wide. Its highest peak is 1127 m high; its slopes show deep defiles, canyons, hollows and caves. In Monte Albo, S. corsica normally grows on limestone but also on underlying granitic outcrops, between 500 and 1100 m above sea level (Arrigoni, 1982, Arrigoni, 2015).
There have been many publications about this particular genus and their central subjects are the composition of essential oils and the properties of some of its extracts (De Pascual et al., 1983, Lawrence, 1997, Poli et al., 1997, Flamini et al., 1999, Palá-Paúl et al., 1999, Garg et al., 2001, Palá-Paúl et al., 2001, Casado et al., 2001, Cherchi et al., 2001, Gnavi et al., 2010).
One of the most characteristic plants belonging to this specific genus is Santolina corsica Jordan & Fourreau. S. corsica grows in arid degraded environments. Its locus classicus is near Bastia in Corsica (Arrigoni, 1979). This recent polyploid (2n = 36) apoendemism is regarded by some authors as originating from the group of Santolinas of the Italian peninsula (Verlaque et al., 1995) and by others as originating from the Franco-Iberian group (Bechi et al., 1996). The vegetation types dominated by S. corsica have been described from central Corsica and North Sardinia and several authors attributed them to different phytosociological units, but still there are only few synecological studies on the species of Santolina genus (Marchi et al., 1980, Angiolini and De Dominicis, 2000).
In Sardinia, its use is common in ethnobotany and, in particular, its essence is used to produce soaps and perfumes and for its supposed tonic, vermifugal and emmenagogue properties. In the villages sited around Monte Albo, it was traditionally used in brews and as a fodder plant for horses suffering from strongyloidiasis (a parasitic disease caused by nematodes). Conversely, only few studies reported on the phytochemistry of this species. For S. corsica, from Corsica, some publications have been based on the chemistry of this species: a solvent extract from roots contains sesquiterpene hydrocarbons, triterpenes, furylthienylbutenynes and a spiroketalenol (Ferrari et al., 2005a); the composition of the essential oil of S. corsica was also reported (Ferrari et al., 2005b, Liu, 2008). The oil of Corsican origin contained artemisia ketone (20.0%), β-phellandrene (14.4%), myrcene (11.7%) and santolina triene (8.2%) as main components. The Corsican oil exhibited an appreciable antibacterial activity against Staphylococcus aureus and other bacteria (Rossi et al., 2007, Liu et al., 2007, Guinoiseau, 2010, Guinoiseau et al., 2010).
To the best of our knowledge, there has been only one publication on Santolina corsica, from Sardinia. As far as this publication is concerned, the first study on essential oils and their properties dates back to 1997 (Poli et al., 1997), in which two samples of essential oils from S. insularis (Marganai) and S. corsica (Monte Albo) were compared. This is only a preliminary work, where whole composition of essential oil is not completely defined. This work indicates that Sardinian S. corsica oil is dominated by camphor (18.5%), artemisia ketone (12.9%) and borneol (7.4%).
Taking into account the fact that this species in Sardinia was not further investigated; we evaluated to continue the research on both the composition of its essential oils and its biological activities. The aim of present study is to investigate the chemical composition of Santolina corsica essential oils from plants growing in different stations located at different altitudes and evaluate the biological activity using anti-inflammatory, antioxidant and antimicrobial experiments.
2. Materials and methods
2.1. Plant material and isolation of essential oil
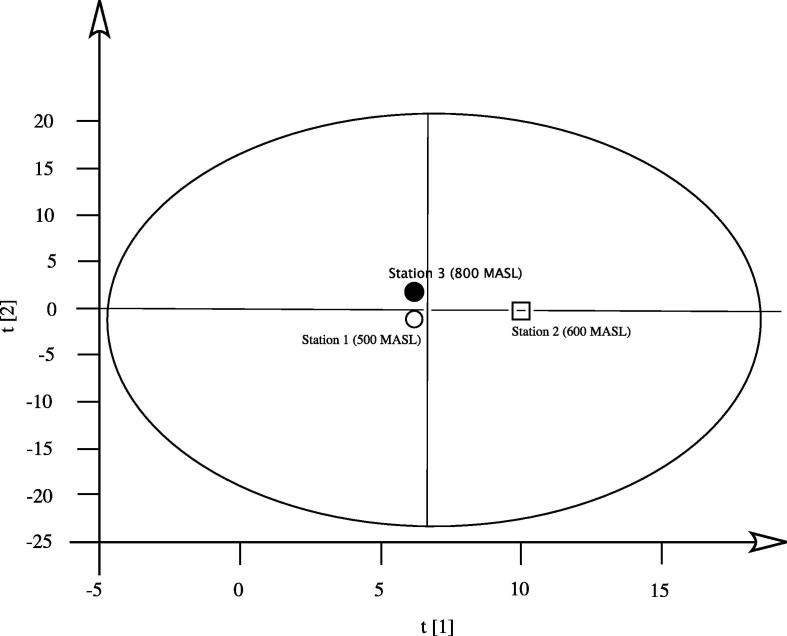
Santolina corsica Jord. & Fourr. was collected at flowering stage (July 2013) in three different stations in Monte Albo (40°29′34″N – 09°34′54″E). It is a calcareous massif located in the central Eastern part of Sardinia, near the city of Nuoro; it is characterized by a ridgeline long 13 km, with an average altitude higher than 1000 mt. We collected our samples at different altitudes: station 1 is located at 500 m above sea level, station 2 is situated at 600 m above sea level, presenting a very poor substrate and a limited land thickness; station 3 is at 800 m above sea level and presents a good thickness of soil and is very close to a watering place.
Dr Alessandro Ruggero identified the analysed plants. Voucher specimens have been deposited at the herbarium SASSA of the Department of Chemistry and Pharmacy, University of Sassari under a collective number (n° 732) for all S. corsica samples.
The plant material was submitted for 4 h to hydrodistillation using a Clevenger-type apparatus; the reached yield was between 0.28% and 0.31% (w/w). The oils were dried over anhydrous sodium sulphate and stored at −20 °C until analysed.
2.2. Oil analyses and quantification
GC (Gas chromatography) – Three replicates of each sample were analysed using a Hewlett-Packard Model 5890A GC equipped with a flame ionization detector and fitted with a 60 m × 0.25 mm, thickness 0.25 μm ZB-5 fused silica capillary column (Phenomenex). Injection port and detector temperature were 280 °C. The column temperature was programmed from 50 °C to 135 °C at 5 °C/min (1 min), 5 °C/min up 225 °C (5 min), 5 °C/min up 260 °C and held for 10 min.
Supplementary analyses were performed on a DB-Wax fused silica capillary column polyethylene glycol, 60 m × 0.25 mm, thickness 0.25 μm (Agilent) using an injector temperature of 250 °C and the following oven temperature: 40 °C for 4 min, followed by a ramp of 4 °C/min to 220 °C and held for 10 min.
The samples (0.1 μL each), generally analyzed without dilution (using 2,6-dimethylphenol as internal standard), were injected using a split/splitless automatic injector HP 7673 and using helium as carrier gas.
The data reported in Table 1 are the average of three GC injections, standard deviation is reported. The quantitation of each compound was expressed as absolute weight percentage using internal standard and response factors. The detector response factors (RFs) were determined for key components relative to 2,6-dimethylphenol and assigned to other components on the basis of functional group and/or structural similarity.
Table 1.
Chemical composition of essential oils from S. corsica collected in M. Albo (Sardinia) at different highs above sea level.
| Components | KI apolar |
KI polar |
(1) 500 m | (2) 600 m | (3) 800 m | IDa | References |
|---|---|---|---|---|---|---|---|
| 2-Methylhept-2-ene | 802 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | RI, MS | Orav et al., 1999 | |
| 3-Cyclohepten-1-one | 821 | 0.04 ± 0.01 | 0.02 ± 0.02 | 0.04 ± 0.01 | RI, MS | Sivadier et al., 2009 | |
| (Z)-3-hexen-1-ol | 858 | 1323 | 0.06 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.01 | RI, MS | Gancel et al., 2003 |
| Santolina triene | 909 | 1036 | 8.81 ± 0.02 | 4.51 ± 0.01 | 5.13 ± 0.02 | RI, MS | |
| β-Thujene | 920 | 1026 | 0.37 ± 0.01 | 0.05 ± 0.01 | 0.23 ± 0.03 | Std | |
| α-Pinene | 939 | 1025 | 0.6 ± 0.02 | 0.28 ± 0.01 | 0.47 ± 0.01 | Std | |
| Sabinene | 975 | 1122 | 3.52 ± 0.02 | 0.21 ± 0.01 | 5.77 ± 0.03 | Std | |
| β-Pinene | 976 | 1092 | 7.17 ± 0.03 | 1.19 ± 0.01 | 6.22 ± 0.02 | Std | |
| 6-Methyl-5-hepten-2-one | 986 | 1341 | nd | 0.22 ± 0.01 | nd | RI, MS | Lucero et al., 2006, Tatsuka et al., 1990 |
| Myrcene | 991 | 1153 | 5.74 ± 0.01 | 44.91 ± 0.04 | 9.57 ± 0.02 | Std | |
| Yomogi alcohol | 999 | 1395 | 1.68 ± 0.01 | 0.31 ± 0.01 | 0.88 ± 0.01 | RI, MS | |
| α-Phellandrene | 1003 | 1128 | 0.37 ± 0.01 | 5.21 ± 0.01 | 0.39 ± 0.01 | Std | |
| α-Terpinene | 1017 | 1177 | 0.79 ± 0.02 | 0.31 ± 0.01 | 0.74 ± 0.01 | Std | |
| p-Cymene | 1025 | 1268 | 0.41 ± 0.01 | 0.98 ± 0.02 | 0.53 ± 0.01 | Std | |
| Limonene | 1029 | 1196 | nd | 2.22 ± 0.02 | 0.19 ± 0.01 | Std | |
| β-Phellandrene | 1030 | 1209 | 20.97 ± 0.03 | 3.15 ± 0.02 | 26.74 ± 0.01 | Std | |
| 1,8-Cineole | 1031 | 1211 | 0.23 ± 0.01 | 0.54 ± 0.01 | 0.6 ± 0.01 | Std | |
| γ-Terpinene | 1060 | 1245 | 0.12 ± 0.01 | 0.59 ± 0.01 | 1.16 ± 0.01 | Std | |
| Artemisia ketone | 1062 | 1344 | 22.49 ± 0.03 | 0.08 ± 0.01 | 3.77 ± 0.02 | RI, MS | |
| Epi-isolyratol | 1062 | 1497 | 1.71 ± 0.01 | 1.43 ± 0.01 | RI, MS | ||
| Artemisia alcohol | 1084 | 1510 | 1.06 ± 0.01 | 0.06 ± 0.01 | 0.31 ± 0.01 | RI, MS | |
| Terpinolene | 1089 | 1162 | 1.42 ± 0.02 | 3.54 ± 0.01 | 1.92 ± 0.01 | Std | |
| Linalool | 1097 | 1550 | nd | 3.54 ± 0.02 | nd | Std | |
| n-Amyl isovalerate | 1107 | 1303 | 0.31 ± 0.01 | nd | 0.12 ± 0.01 | RI, MS | |
| 2-Methyl buthyl valerate | 1142 | nd | 0.04 ± 0.01 | nd | 0.05 ± 0.01 | RI, MS | Takeoka et al. (1990) |
| Trans-p-menth-2-en-1-ol | 1145 | 1584 | 0.9 ± 0.01 | 0.04 ± 0.01 | 0.49 ± 0.01 | RI, MS | |
| Sabinene hydrate isomer | 1148 | 1490 | 0.22 ± 0.01 | 0.03 ± 0.01 | 0.32 ± 0.01 | RI, MS | |
| Cis-chrysantemyl alcohol | 1163 | nd | 4.26 ± 0.01 | 6.76 ± 0.03 | 4.93 ± 0.01 | RI, MS | |
| Lavandulol | 1171 | 1674 | 0.03 ± 0.01 | 0.39 ± 0.02 | 0.11 ± 0.01 | RI, MS | |
| Terpinene-4-ol | 1177 | 1606 | 2.340.02± | 1.18 ± 0.01 | 4.23 ± 0.01 | Std | |
| p-Cymen-8-ol | 1183 | 1853 | nd | 0.32 ± 0.01 | nd | Std | |
| Cryptone | 1185 | 1660 | 0.27 ± 0.01 | nd | 0.33 ± 0.01 | RI, MS | |
| α-Terpineol | 1189 | 1701 | 0.24 ± 0.01 | 0.23 ± 0.01 | 0.69 ± 0.01 | Std | |
| Cis-piperitol | 1196 | 1712 | 0.11 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 | RI, MS | |
| Trans-piperitol | 1208 | 1710 | nd | nd | 0.17 ± 0.01 | RI, MS | |
| Thymyl methyl ether | 1235 | 1586 | nd | nd | 0.09 ± 0.01 | RI, MS | |
| Isobornyl formate | 1236 | 1574 | nd | 0.04 ± 0.01 | nd | RI, MS | |
| Piperitone | 1252 | 1729 | 0.02 ± 0.01 | nd | 0.17 ± 0.02 | RI, MS | |
| Phellandral | 1254 | 1723 | 0.12 ± 0.01 | nd | 0.15 ± 0.01 | RI, MS | |
| Thymol | 1290 | 2164 | 0.11 ± 0.01 | nd | 0.09 ± 0.01 | Std | |
| Lavandulyl acetate | 1290 | 1602 | nd | 0.06 ± 0.01 | nd | RI, MS | |
| Solanone | 1296 | 1756 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.12 ± 0.01 | RI, MS | |
| γ-Elemene | 1338 | 1639 | nd | 0.03 ± 0.01 | nd | Std | |
| Jasmone | 1398 | 1955 | nd | nd | 0.07 ± 0.01 | RI, MS | |
| Methyl eugenol | 1404 | 2006 | 0.09 ± 0.01 | 0.15 ± 0.03 | 0.19 ± 0.02 | RI, MS | |
| α-Gurjunene | 1410 | 1529 | nd | 0.05 ± 0.01 | 0.25 ± 0.01 | RI, MS | |
| (E)-β-caryophyllene | 1419 | 1599 | 0.07 ± 0.01 | 0.04 ± 0.01 | 0.15 ± 0.01 | Std | |
| ε-Muurolene | 1445 | 0.05 ± 0.02 | 0.04 ± 0.01 | nd | RI, MS | ||
| β-Santalene | 1447 | 1644 | 0.03 ± 0.01 | 0.11 ± 0.02 | nd | RI, MS | |
| Alloaromadendrene | 1460 | 1662 | nd | nd | 0.45 ± 0.02 | Std | |
| α-Curcumene | 1481 | 1798 | 1.45 ± 0.01 | 0.99 ± 0.01 | 2.46 ± 0.04 | Std | |
| γ-Curcumene | 1483 | 1692 | 0.69 ± 0.02 | 0.82 ± 0.01 | 1.15 ± 0.02 | Std | |
| D-Germacrene | 1485 | 1690 | 0.45 ± 0.01 | 0.36 ± 0.01 | nd | Std | |
| Epi-cubebol | 1494 | 1900 | 0.14 ± 0.01 | nd | 0.24 ± 0.01 | RI, MS | |
| Ledene | 1495 | 1679 | 0.09 ± 0.02 | 0.11 ± 0.02 | 0.13 ± 0.01 | RI, MS | |
| Bicyclogermacrene | 1500 | 1734 | 0.46 ± 0.02 | 0.81 ± 0.01 | 0.53 ± 0.01 | RI, MS | |
| β-Curcumene | 1500 | 1737 | 0.02 ± 0.01 | 0.06 ± 0.02 | 0.44 ± 0.01 | Std | |
| α-Muurolene | 1500 | 1734 | nd | 0.08 ± 0.01 | nd | Std | |
| Sesquicineole | 1521 | 0.03 ± 0.01 | nd | 0.04 ± 0.01 | RI, MS | ||
| δ-Cadinene | 1523 | 1755 | nd | 0.23 ± 0.01 | 0.34 ± 0.02 | Std | |
| Cis-nerolidol | 1533 | 2007 | 0.17 ± 0.01 | 0.39 ± 0.01 | 0.08 ± 0.01 | Std | |
| α-Calacorene | 1546 | 1921 | nd | 0.56 ± 0.05 | 0.21 ± 0.02 | RI, MS | |
| Trans-nerolidol | 1563 | 2036 | 0.22 ± 0.02 | nd | 0.43 ± 0.01 | Std | |
| Aromadendr-1-ene | 1569 | 1620 | nd | nd | 0.18 ± 0.02 | RI, MS | Weyerstahl et al (1998) |
| Spathulenol | 1578 | 1693 | 1.37 ± 0.01 | 1.14 ± 0.02 | 0.97 ± 0.01 | RI, MS | |
| Caryophyllene oxide | 1583 | 1986 | 0.67 ± 0.01 | 1.08 ± 0.02 | 0.98 ± 0.01 | RI, MS | |
| Isoaromadendrene epoxide | 1590 | 1807 | 1.52 ± 0.01 | 1.28 ± 0.03 | 1.45 ± 0.01 | RI, MS | |
| α-Bisabolol | 1689 | 2213 | 1.34 ± 0.01 | 1.75 ± 0.01 | 1.49 ± 0.02 | RI, MS | |
| β-Santalol | 1740 | 2391 | 0.67 ± 0.04 | 0.24 ± 0.01 | 0.32 ± 0.01 | RI, MS | Shellie et al. (2004) |
| 96.12 | 91.55 | 90.91 |
Data are the mean of three replicates ± SD. Not detected compounds were indicated as nd.
RI by comparison of retention index with those reported in literature.
Std by comparison of the retention time and mass spectrum of available authentic standards.
No-polar column ZB-5; polar column DB-Wax.
Identification methods: MS by comparison of the Mass spectrum with those of the computer mass libraries Adams, Nist 11 and by interpretation of the mass spectra fragmentations.
GC/MS (Gas chromatography/Mass): MS analyses were carried out with an Agilent Technologies model 7820A connected with a MS detector 5977E MSD (Agilent) and using the same conditions and column described above. The column was connected to the ion source of the mass spectrometer. Mass units were monitored from 10 to 900 at 70 eV. The identification of compounds was based on comparison of their retention times with those of authentic samples and/or by comparison of their mass spectra with those of published data (NIST, 2011, Adams, 2007) or on the interpretation of the EI-fragmentation of the molecules. Identified compound and their quantification are reported in Table 1.
2.3. Statistical analysis
Data were submitted to multivariate statistical evaluation. Prior to chemometric analysis, setting the total integral areas to 100 normalized the data and the generated ASCII file was imported into Microsoft EXCEL for the addition of labels. The matrix was imported into SIMCA-P software version 12.0, (Umetrics AB, Umeå, Sweden) for statistical analysis.
2.4. Anti-inflammatory activity
To study the anti-inflammatory activity of S. corsica essential oils we performed some experiments using samples of bronchial secrete coming from hospitalized patients suffering for respiratory diseases (bronchoalveolar lavage; BAL). To collect the samples was used the broncho alveolar lavage that is a medical procedure in which samples of sterile, isotonic solution are squirted through the distal airways and then collected to be analyzed (Danel et al., 1992, Stitt, 1927, Stitt, 1932, Reynolds, 2000, Henderson, 1994). This particular proceeding is based on a partially proved theory according to which cells and non-cellular components obtained from the lavage are similar in quantities and functions to those found in the alveolar pits or sockets and their characteristics are proportional to the general state of the inflammatory and immune system of the entire lower respiratory tract.
2.4.1. Cellular cultures
Stock solutions of the essential oils (were prepared by dissolving them in DMSO (0.1% w/vol.), solutions were then sterilized by filtration using sterile membrane filters (Sartorius, pore size 0.22 μm) and stored at −20 °C until use (The essential oil concentrations ranged was between 0 and 1 μg/mL).
Samples from patients affected by respiratory pathologies were incubated for 60 mins (at 37 °C in 5% CO2) in RPMI 1640 with 20% of bovine foetal serum enrichment and added with different essential oils concentrations. The samples are then washed in phosphate buffered saline (PBS) and prepared to be embedded in epoxide resin.
2.4.2. Samples inclusion
The preparation of samples includes four steps: Incubation; Fixation where molecular and macromolecular components of cellular architecture are “instantly” immobilized; Dehydration where water is removed from the samples with a solution of increasingly higher concentrations of ethanol; Inclusion where the dehydrating solution is gradually substituted with an inclusion compound (epoxide resin). Once the resin is hardened, the samples may be cut with an ultra-microtome in ultra-thin sections and then analysed using microscopy techniques. The structural analysis of biological samples is performed with optical microscope (MO), while the ultra-structural analysis is performed with an electron microscope (TEM). The semi-thin sections are stained with 1% of toluidine blue and then analysed using an optical microscope at a 20× magnification. The ultra-thin sections are stained with uranyl acetate and lead citrate and analysed using a Zeiss transmission electron microscope.
It is ethically substantial to clarify that these samples come from ordinary analysis and not from a specific investigation. We used a part of material that was designate to become waste because it was a residue of routine analyses and not a direct sampling for research.
2.5. Antibacterial and antimycotic activity
Stock solutions of the essential oils were prepared by dissolving them in PEG-200 in order to obtain a concentration of 100 mg/mL (10% w/vol.); solutions were then sterilized by filtration using sterile membrane filters (Sartorius, pore size 0.22 μm) and stored at -20 °C until use.
2.5.1. Microorganisms and media
The test organisms used in this study were as follows: Escherichia coli (ATCC 8739), Staphylococcus aureus (ATCC 6538), Pseudomonas aeruginosa (ATCC 9027), Candida albicans (ATCC 10231), (all purchased from Oxoid-Thermofisher Scientific, Rodano, Italy).
Mueller Hinton Agar (MHA), Mueller Hinton Broth (MHB), Sabouraud Liquid Medium (SLM), Sabouraud Dextrose Agar (SDA) and phosphate-buffered saline tablets (PBS, Dulbecco A, pH 7.3) were purchased from Oxoid-Thermofisher Scientific (Rodano, Italy). Culture media, PBS and other solutions were prepared using MilliQ water.
2.6. Antimicrobial activity of S. corsica essential oil
Antimicrobial activity of S. corsica essential oils was determined as Minimum Inhibitory Concentration (M.I.C.) through an agar macrodilution method (Barry, 1986). As positive controls (compounds known to have antimicrobial activity) we used chlorhexidine diacetate (Sigma, St. Louis, MO, USA), a broad-spectrum synthetic antimicrobial agent used primarily as a topical antiseptic and disinfectant, and a sample of essential oil of Thymus catharinae Camarda (formerly known as Thymus herba-barona Loisel), which exhibits a significant antimicrobial activity (Juliano et al., 2000).
Two-fold serial dilutions of essential oils in MHA or in SDA (for bacteria and Candida, respectively) were made in 5 mm-Petri dishes (total volume 10 mL) to have final concentrations of 2 mg/mL, 1 mg/mL, 0.5 mg/mL and 0.25 mg/mL. Plates with chlorhexidine diacetate were prepared with the same technique by using a mother solution 10 mg/ml in distilled water. The experiments were performed in triplicate. Control plates, containing only SDA, MHA, SDA + PEG-200 and MHA + PEG-200, were run simultaneously. The agar surface of the plates was then inoculated with 2 µL of a suspension containing about 1 × 104 microorganisms were inverted and incubated aerobically at 35 °C for 24 h. After incubation, plates were visually checked for microbial growth, and the MIC of the oil was defined as the lowest concentration at which no growth was observed. At the concentrations tested, PEG-200 had no inhibitory effect on microorganisms’ growth. Results are reported in Table 2.
Table 2.
Minimum inhibitory concentrations (M.I.C.s) of essential oils of S. corsica, essential oil of Thymus catharinae Camarda and chlorhexidine diacetate on different pathogens.
|
Escherichia coli ATCC 8739 |
Staphylococcus aureus ATCC 6538 |
Pseudomonas aeruginosa ATCC 9027 |
Candida albicans ATCC 10,231 |
|
|---|---|---|---|---|
| E.O. Santolina corsica | >2 mg/mL | 2 mg/mL | >4 mg/mL | 4 mg/mL |
| E.O. Thymus catharinae Camarda | 0.25 mg/mL | 0.046 mg/mL | 1 mg/mL | 0.25 mg/mL |
| Chlorhexidine diacetate | 1.96 μg/mL (15.6 μg/mL) | 0.90 μg/mL(3.9 μg/mL) | 21.9 μg/mL(125 μg/mL) | 7.8 μg/mL(7.8 μg/mL) |
2.7. Antioxidant activity of essential oils
The percentage of antioxidant activity (AA%) of each sample was assessed by DPPH free radical assay. The measurement of the DPPH radical scavenging activity was performed according to a methodology described by Brand-Williams et al. (1995). The samples were reacted with the stable DPPH radical in an ethanol solution. The reaction mixture consisted of 0.5 mL of sample, 3 mL of absolute ethanol and 0.3 mL of DPPH radical solution 0.5 mM in ethanol. When DPPH reacts with an antioxidant compound, which can donate hydrogen, it is reduced.
The changes in colour (from deep violet to light yellow) were read [Absorbance (Abs)] at 517 nm. The measurements were carried out every half minute from 0 to 15 min. The control solution was prepared by mixing ethanol (3.5 mL) and DPPH radical solution (0.3 mL). The scavenging activity percentage (AA%) was determined by the following formula: % Inhibition = [(Ab − Aa)/Ab] × 100. Where Ab = absorption of blank sample (t = 0) Aa = Mean of absorption after 5 min, until 15 min. Catechin has been used as reference substance.
3. Results and discussion
S. corsica Jord. & Fourr. in Sardinia it is only present in Monte Albo (calcareous substratum and poor in nutrients). Monte Albo has a generally hot and humid weather during summer and the temperatures rarely fall below zero during the coldest season. The temperature gradually decreases with altitude and in the plains of the mountainous areas. In the higher areas, the temperature can often drop to negative values and the snowpack persists sometimes for a longer time. Despite this, the yield of essential oils did not seem to suffer from it, in fact its yield reached an average level of 0.28%-0.31% (w/w) in the studied stations. Plant material was collected at flooring stage at three different altitudes. The essential oils were analysed by GC and GC/MS methodologies that allowed us to identify seventy-one fundamental compounds present in all samples; we were then able to identify a percentage of components between 96.12% and 90.91% (Table 1).
Among these constituents, twenty-three exceed 1% at least in one of the investigated stations. Our collecting stations were all at different altitudes: station 1 is located at 500 m above sea level, station 2 is situated at 600 m above sea level, presenting a very poor substrate and a limited land thickness; station 3 is at 800 m above sea level and presents a good thickness of soil and is very close to a watering place. The essential oils obtained from the plants coming from these stations are very different from each other.
As it is possible to observe in Table 1, the station at the lowest altitude (station 1) is characterized by a high content of β-phellandrene (20.97%) and artemisia ketone (22.49%). These two constituents together represent more than 42% of the total composition. More characterizing components of this station are: santolina triene (8.8%), β-pinene, myrcene (7.1 and 5.7% respectively) and chrysantemyl alcohol (4.2%).
Station 2 at 600 m of altitude presents a population of plants containing an essential oil characterized by the presence of 44.9% of myrcene and only little traces of artemisia ketone (0.08%).
Among the constituents in common with the previous station 1, we found that santolina triene, has a half concentration (about 4.5%), whereas cis-chysantemyl alcohol increases its concentration (6.7%). β-phellandrene, one of the major constituents in station 1, here reaches a concentration of 3.15% and decrease also β-pinene (1.2%). On the contrary, α-phellandrene in station 2 reach the concentration of 5.2% while in the other two stations has a very low concentration (lower than 0.4%).
In station 3 there is a different situation from the other two examined areas; in fact, the two major constituents are β-phellandrene with a 26.74% and myrcene (9.57%). Artemisia ketone reaches a concentration of 3.77% and santolina triene has a slightly higher concentration (5.1%) referred to station 2. β-pinene (6.2%) and cis chrysantemyl alcohol (4.9%) are very close to the concentration found in the oils of station 1.
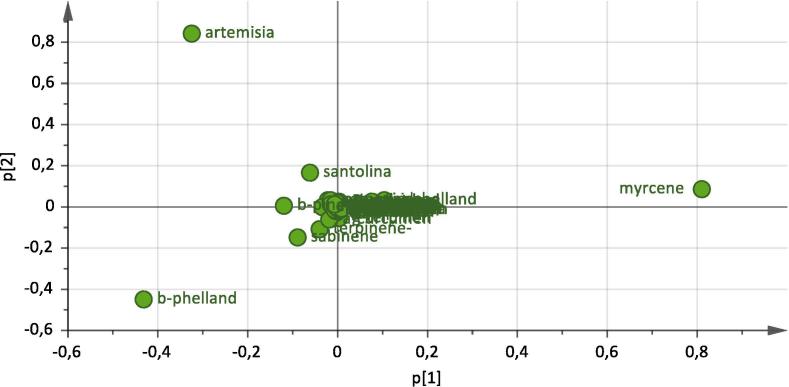
Referring to literature on S. corsica from Sardinia, we can only relate it to a publication (Poli et al., 1997), in which is reported a preliminary screening of essential oil composition of this species (in the paper is not reported the sampling altitude), this essential oil composition does not fit with those we analysed, our data could suggest a relationship of essential oil composition from altitude. In fact, statistical multivariate analysis (PCA) support this hypothesis (Fig. 1, Fig. 2):
Fig. 1.
PCA analysis: score plot.
Fig. 2.
PCA analysis: loading plot.
The score plot shows a clear separation among the three different altitudes of the stations and the loading plot show that the components responsible of this behaviour are artemisia ketone, myrcene and β-phellandrene (Fig. 2).
By combining these data, it seems that S. corsica in Sardinia presents different chemical profiles, even different from the one concerning of S. corsica of Corsica. This behavior could be also justified as reported in the work of Marchi et al., “1980″ where the authors (Marchi et al., 1980) compare the karyotype of S. corsica from Corsica (2n = 4x = 36 karyotype) to S. corsica from Monte Albo (karyotype of a tetraploid population). The karyotype of the individuals from Sardinia show satellites of sub terminal chromosomes in a distal position on the long arms instead of in the distal position on the short arms, as in other karyotypes examined in the species belonging to the same aggregate (S. chamaecyparissus). The tetraploid individuals from Sardinia show satellites and nucleoli, in varying quantities. This variability and the satellite position could mean that the karyotype of the Sardinian population shows an unsettled condition.
The karyotype structure of these tetraploids showed their stronger similarity to exaploids than to diploid species. These assertions could explain the nature of chemical compositions of the essential oils from the stations in Monte Albo.
The essential oils we analysed were then used in further biological tests. In our experiments, we took several different pathogens to control the antibacterial activity of essential oils; this activity was evaluated by M.I.C. and it is reported in Table 2.
We witnessed a low antibacterial activity in this sample of S. corsica on Staphylococcus aureus and Escherichia coli; no inhibiting activity was reported on Pseudomonas aeruginosa and Candida albicans. On the contrary, Corsican oil exhibited an appreciable antibacterial activity against Staphylococcus aureus and other bacteria (Rossi et al., 2007, Liu et al., 2007, Guinoiseau, 2010, Guinoiseau et al., 2010).
The essential oils coming from the three stations were also used to test the antioxidant activity using DPPH method. The relative absorbance of the essential oils of S. corsica were calculated in two different concentrations: 1.4 mg/mL and 5 mg/mL.
In Table 3 are reported percentages of the essential oil DPPH Scavenging activity. S. corsica essential oils, compared to catechin used as reference, did not show significant antioxidant activity.
Table 3.
S. corsica essential oils DPPH scavenging activity.
| 5 mg/mL essential oil DPPH scavenging activity | |
|---|---|
| S. corsica (1)* | 10.2% ± 0.03 |
| S. corsica (2)* | 7.5% ± 0.02 |
| S. corsica (3)* | 9.7% ± 0.04 |
| Catechin | |
| 1.4 mg/mL essential oil DPPH scavenging activity | |
| S. corsica (1)* | 7.3% ± 0.02 |
| S. corsica (2)* | 4.3% ± 0.08 |
| S. corsica (3)* | 3.4% ± 0.06 |
| Catechin (1 mg/mL)** | 81.2% ± 0.05 |
Note: number 1, 2 and 3 identified the different collecting altitudes: station 1 = 500 m above sea level; station 2600 m above sea level; station 3 = 800 m above sea level).
Catechin has been used as reference substance.
Due to the lack of investigation on anti-inflammatory activity of the essential oils of the Santolina genus, we decided to evaluate this aspect taking in consideration the bronchial tract, because it is frequently interested in inflammatory conditions caused by various elements such as flu, bronchitis, asthma. To carry out investigation on anti-inflammatory activity of the essential oils we used samples collected using the bronchoalveolar lavage, a medical procedure in which samples of sterile, isotonic solution are squirted through the distal airways and then collected to be analysed. Bronchoalveolar lavage (BAL) is a diagnostic procedure by which cells and other components from bronchial and alveolar spaces are obtained for various studies. One of the main advantages of this procedure is that it can be done as a day care procedure. The obtained material can give a definite diagnosis in conditions such as infections and malignancies (Sistla et al, 2014). Used bronchoalveolar lavages came from 15 cases. Age of patients ranged from 21 years to 93 years; 8 were males and 7 were females.
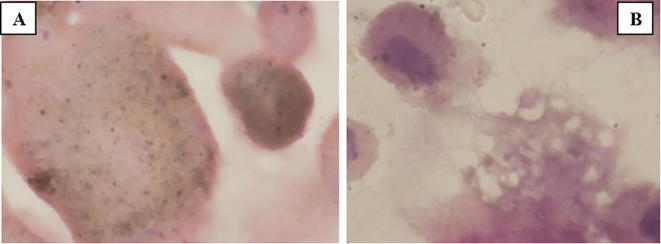
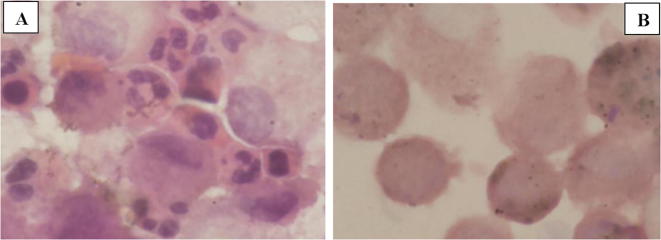
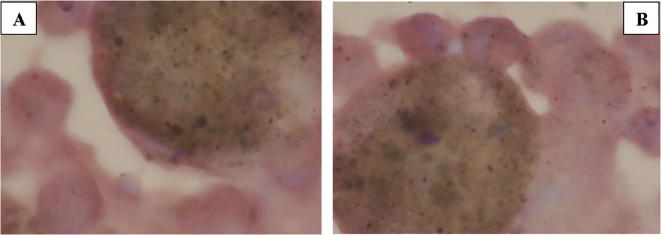
Results obtained are a first step towards the creation of an experimental protocol to serve as a reference model for further study. In fact the protocol, currently used for the in vitro cultures, was modified in our laboratory in order to appreciate the transformation of treated cells. Different timings and concentrations, along with their morphological assessments, were needed to define the experimental model under study. The following morphological data were obtained from the incubation of human cells, in particular from cells resulted from Bronchoalveolar Lavage, rich in macrophage-histiocytes, incubated in the essential oil of Santolina corsica during the two reference times (Fig. 3, Fig. 4, Fig. 5).
Fig. 3.
A: Cellular culture with contamination of hystiocytes filled with antracotic matter. B: Cellular culture plus S. corsica essential oil. The hystiocytes have expelled the antracotic pigment. Essential oils concentration: 1 μg/mL.
Fig. 4.
A: Non-specific inflammatory pattern, with high concentration of granulocytes B: Addition with essential oil: positive impact on the decrease of granulocytes. Essential oils concentration: 1 μg/mL.
Fig. 5.
A: Control BAL cells cultured in culture soil the results show a concentration of hystiocytes, both activated and rich in antracotic pigments before incubation; B: BAL cells cultured in culture soil the results show a concentration of hystiocytes, both activated and rich in antracotic pigments after incubation.
The image reported in Fig. 1A shows the cellular culture in a patient with a clear contamination of histiocytes filled with anthracotic matter. Due to the addition of essential oils of S. corsica (1 μg/mL), exocytose (see Fig. 1B) is therefore possible and the histiocytes can expel the anthracotic pigment into the culture medium, purifying its cytoplasm and restoring its ability to phagocytize more material. Some macrophages keep their foamy texture, which is directly connected to the activation of said cells.
The incubation of cells samples, with higher concentration of granulocytes (see Fig. 4A), shows a non-specific inflammatory pattern, in which the incubation of S. corsica essential oil (1 μg/mL), has a positive impact on the decrease of granulocytes (Fig. 4B).
In Fig. 5A are reported the control BAL cells cultured in culture medium before incubation; the results show a concentration of histiocytes, both activated and rich in anthracotic pigments. In Fig. 5B are reported the control BAL cells cultured in culture medium after incubation; the results show a concentration of histiocytes, both activated and rich in anthracotic pigments.
4. Conclusions
The compositions of the analysed essential oils are different as demonstrated with multivariate statistical analysis and they are also different from these reported for previously analyzed samples from Sardinia and Corsica. In particular is possible divide collected plants into two main chemotypes characterised by artemisia ketone and myrcene. This fact seems to be due to altitude and genetic situation were the satellite position keep the Sardinian population in an unsettled condition and the karyotype structure. The essential oils derived from our S. corsica samples have no antimicrobial activity and none antioxidant activity. As far as anti-inflammatory activity is concerned, the results show the clear ability of essential oils to interact with cells. Both chemotypes have shown a good activity against respiratory diseases with an inflammatory component. These results encourage further research towards more standardized parameters, with a cellular morphological and biochemical assessment and an immuno-histochemical analysis.
Conflict of interest
The authors declare no conflict of interest statement.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
We thank Mrs. Barbara Sechi for her skilled technical assistance; Dr Gaia Trebini for critical revision of English content
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Marzia Foddai, Email: mfoddai@uniss.it.
Mauro Marchetti, Email: mauro@ss.cnr.it.
Alessandro Ruggero, Email: alessandroruggero@tiscali.it.
Claudia Juliano, Email: julianoc@uniss.it.
Marianna Usai, Email: dsfusai@uniss.it.
References
- Adams, R.P., 2007. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry Allured Publ. Corp., Carol Stream, IL 60188, USA.
- Angiolini C., De Dominicis V. A synecological study of communities with Santolina corsica Jordan et Fourr. Acta Bot. Croat. 2000;59:383–401. [Google Scholar]
- Angiolini C., Bacchetta G. Analisi distributiva e studio fitosociologico delle comunità a Santolina insularis (Gennari ex Fiori) Arrigoni della Sardegna meridionale. Fitosociologia. 2003;40:109–127. [Google Scholar]
- Arrigoni P.V. Le genre Santolina L. en Italie. Webbia. 1979;34:257–264. [Google Scholar]
- Arrigoni P.V. Le piante endemiche della Sardegna: 99–100. Boll. Soc. Sarda Sci. Nat. 1982;21:338–348. [Google Scholar]
- Arrigoni P.V. Carlo Delfino Editore; Sassari: 2015. Flora dell’Isola di Sardegna. [Google Scholar]
- Barry A.L. Procedure for testing antimicrobial agents in agar media: theoretical considerations. In: Lorian V., editor. Antibiotics in Laboratory Medicine. 2nd ed. Lippincott Williams & Wilkins; Baltimore, USA: 1986. pp. 1–26. [Google Scholar]
- Bechi N., Garbari F., Miceli P. Indagini biosistematiche sulla flora apuana. VI contributo: risultati conseguiti e problemi aperti. Atti Soc. Tosc. Sci. Nat. Mem. Serie B. 1996;103:35–42. [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm.-Wiss. u.-Technol. 1995;28:25–30. [Google Scholar]
- Casado J.P., Martinez A., Navarro M.C., Utrilla P.M., Jimenez J. Multiple headspace extraction of volatiles from Santolina canescens Lagasca during its growth cycle. J. Ess. Oil Res. 2001;13:170–173. [Google Scholar]
- Cherchi G., Deidda D., De Gioannis B., Marongiu B., Pompei R., Porcedda S. Extraction of Santolina insularis essential oil by supercritical carbon dioxide: influence of some process parameters and biological activity. Flavour Fragr. J. 2001;16:35–43. [Google Scholar]
- De Pascual T.J., Vincente S., Gonzalez M.S., Bellido I.S. Nerolidol-5,8-oxides from the essential oil of Santolina oblongifolia. Phytochemistry. 1983;22:2235–2238. [Google Scholar]
- Ferrari B., Tomi F., Casanova J. a. Terpenes and acetylene derivatives from the roots of Santolina corsica (Asteraceae) Biochem. Syst. Ecol. 2005;33:445–449. [Google Scholar]
- Ferrari B., Tomi F., Richomme P., Casanova J. b. Two new irregular acyclic sesquiterpenes aldehydes from Santolina corsica essential oil. Magn. Reson. Chem. 2005;43:73–74. doi: 10.1002/mrc.1494. [DOI] [PubMed] [Google Scholar]
- Flamini G., Bertoli A., Taglioli V., Cioni P.L., Morelli I. Composition of the essential oil of Santolina ligustica. J. Ess. Oil Res. 1999;11:6–8. [Google Scholar]
- Gancel A.-L., Ollitrault P., Froelicher Y., Tomi F., Jacquemond C., Luro F., Brillouet J.-M. Leaf volatile compounds of seven citrus somatic tetraploid hybrids sharing willow leaf mandarin (Citrus deliciosa Ten.) as their common parent. J. Agric. Food Chem. 2003;51:6006–6013. doi: 10.1021/jf0345090. [DOI] [PubMed] [Google Scholar]
- Garg S.N., Gupta D., Mehta V.K., Kumar S. Volatile constituents of the essential oil of Santolina chamaecyparissus Linn. from the southern hills of India. J. Ess. Oil Res. 2001;13:234–235. [Google Scholar]
- Gnavi G., Bertea C.M., Usai M., Maffei M. Comparative characterization of Santolina insularis chemotypes by essential oil composition, 5S-rRNA-NTS sequencing and EcoRV RFLP-PCR. Phytochemistry. 2010;71:930–936. doi: 10.1016/j.phytochem.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Guinoiseau E. University of Corsica; Corte: 2010. Molécules antibactériennes issues d’huiles essentiellel: séparation, identification et mode d’action. PhD Thesys. [Google Scholar]
- Guinoiseau E., Luciani A., Rossi P.G., Quilichini Y., Ternengo S., Bradesi P., Berti L. Cellular effects induced by Inula graveolens and Santolina corsica essential oils on Staphylococcus aureus. EJCMID. 2010;29:873–879. doi: 10.1007/s10096-010-0943-x. [DOI] [PubMed] [Google Scholar]
- Henderson A.J. Bronchoalveolar lavage. Arch. Dis. Child. 1994;70:167–169. doi: 10.1136/adc.70.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamisans J., Jeanmonod D. Compléments au prodrome de la flore CorseConservatoire et Jardin Botaniques; Genève: 1998. Asteraceae-I; p. 203. [Google Scholar]
- Danel C., Israel-Biet D., Costabel U., Klech H. Therapeutic applications of bronchoalveolar lavage. Eur. Respir. J. 1992;5:1173–1175. [PubMed] [Google Scholar]
- Juliano C., Mattana A., Usai M. Composition and in vitro antimicrobial activity of the essential oil of Thymus herba-barona Loisel growing wild in Sardinia. J. Essent. Oil Res. 2000;12:516–522. [Google Scholar]
- Kadereit J.W., Jeffrey C. Asterales. Springer-VerlagBerlin; 2007. The families and genera of vascular plants. [Google Scholar]
- Lawrence B.M. Progress in essential oil research. Perfum. Flav. 1997;22:78–82. [Google Scholar]
- Liu K., Rossi P.G., Ferrari B., Berti L., Casanova J., Tomi F. Composition, irregular terpenoids, chemical variability and antibacterial activity of the essential oil from Santolina corsica Jordan et Fourr. Phytochemistry. 2007;68:1698–1705. doi: 10.1016/j.phytochem.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Liu K. Etude phytochimique de Clinipodium ascendens, Bupleurum fruticosum et Santolina corsica. PhD Thesys, Chimie. University of Corse; Corte: 2008. La RMN du carbone-13, outil d'analyse. [Google Scholar]
- Lucero M.E., Fredrickson E.L., Estell R.E., Morrison A.A., Richman D.B. Volatile composition of Gutierrezia sarothrae (Broom Snakeweed) as determined by steam distillation and solid phase microextraction. J. Essent. Oil Res. 2006;18:121–125. [Google Scholar]
- Marchi P., Capineri R., D'Amato G. The karyotype of Santolina corsica Jord. et Fourr. (Compositae) from near Bastia (Corsica) and other observations. Ann. Bot. 1980;38:1–13. [Google Scholar]
- NIST2011 Library of Mass Spectra, 2011. Agilent Technologies Co., Palo Alto, CA.
- Orav A., Kailas T., Muurisep M., Kann J. Composition of the oil from waste tires. 1. Fraction boiling at up to 160 °C. Proc. Estonian Acad. Sci. Chem. 1999;48:30–39. [Google Scholar]
- Palá-Paúl J., Perez-Alonso M.J., Velasco-Negueruela A., Ramos-Vázquez P., Gómez-Contreras F., Sanz J. Essential oil of Santolina rosmarinifolia L. ssp. rosmarinifolia: first isolation of capillene, a diacetylene derivative. Flavour Fragr. J. 1999;14:131–134. [Google Scholar]
- Palá-Paúl J., Perez-Alonso M.J., Velasco-Negueruela A., Palá-Paúl R., Sanz J., Conejero F. Seasonal variation in chemical constituents of Santolina rosmarinifolia L. ssp. rosmarinifolia. Biochem. Syst. Ecol. 2001;29:663–672. doi: 10.1016/s0305-1978(01)00032-1. [DOI] [PubMed] [Google Scholar]
- Poli F., Bonsignore L., Loy G., Sacchetti G., Ballero M. Comparison between the essential oils of Santolina insularis (Genn. ex Fiori) Arrigoni and Santolina corsica Jord. et Fourr. from the island of Sardinia (Italy) J. Ethnopharmacol. 1997;56:201–208. doi: 10.1016/s0378-8741(97)01528-6. [DOI] [PubMed] [Google Scholar]
- Reynolds H.Y. Use of bronchoalveolar lavage in humans-past necessity and future imperative. Lung. 2000;178:271–293. doi: 10.1007/s004080000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P.G., Berti L., Panighi J., Luciani A., Maury J., Muselli A. Antibacterial action of essential oils from Corsica. J. Essent. Oil Res. 2007;19:176–182. [Google Scholar]
- Shellie R., Marriott P., Morrison P. Comprehensive two-dimensional gas chromatography with flame ionization and time-of-flight mass spectrometry detection: qualitative and quantitative analysis of West Australian sandalwood oil. J. Chromatogr. Sci. 2004;42:417–422. doi: 10.1093/chromsci/42.8.417. [DOI] [PubMed] [Google Scholar]
- Sistla R., Tameem A., Sudheer P., Nallagonda R. Diagnostic utility of bronchoalveolar lavage. J. Cytol. 2014;31:136–138. doi: 10.4103/0970-9371.145636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivadier G., Ratel J., Engel E. Latency and persistence of diet volatile biomarkers in lamb fats. J. Agric. Food Chem. 2009;57:645–652. doi: 10.1021/jf802467q. [DOI] [PubMed] [Google Scholar]
- Stitt H.L. Bronchial aspiration and irrigation with a hypertonic saline solution. J. Med. 1927;5:112–117. [Google Scholar]
- Stitt H.L. Bull StLouis Medical Society; 1932. Bronchial lavage; pp. 246–249. [Google Scholar]
- Takeoka G.R., Flath R.A., Mon T.R., Teranishi R., Guentert M. Volatile Constituents of Apricot (Prunus armeniaca) J. Agric. Food Chem. 1990;38:471–477. [Google Scholar]
- Tatsuka K., Suekane S., Sakai Y., Sumitani H. Volatile constituents of kiwi fruit flowers: simultaneous distillation and extraction versus headspace sampling. J. Agric. Food Chem. 1990;38:2176–2180. [Google Scholar]
- Verlaque R., Contandriopoulos J., Aboucaya A. Cytotaxonomie et conservation de la flore insulaire: les espèces endémiques ou rares de Corse. Ecol. Medit. 1995;21:257–268. [Google Scholar]
- Weyerstahl P., Marschall H., Weirauch M., Thefeld, Surburg H. Constituents of commercial Labdanum oil. Flavour Fragr. J. 1998;13:295–318. [Google Scholar]