Abstract
Background
Breast cancer is one of the most malignant tumors worldwide. The natural flavonoid diosmetin has been reported to exhibit various pharmacological activities, including anti-cancer effects. This study aimed to investigate the anti-breast cancer effects of diosmetin on MDA-MB-231 cells and to explore the underlying molecular mechanisms of cell apoptosis.
Material/Methods
The MDA-MB-231 cells were incubated with diosmetin for 24 h. Then, cell viability and lactate dehydrogenase (LDH) leakage were detected using CCK-8 and LDH assay kits, respectively. Inverted fluorescence microscopy and flow cytometry were used to measure the mitochondrial membrane potential (MMP) and intracellular reactive oxygen species (ROS). Cell apoptosis and cell cycle were determined by flow cytometry. The expressions of apoptosis and cell cycle-related genes were determined by Western blotting and qRT-PCR.
Results
The results revealed that diosmetin exerts significant cytotoxic effects on MDA-MB-231 cells, as indicated by decreased cell viability, increased intracellular ROS accumulation and LDH release, as well as cell cycle arrest in G0/G1 phase, inducing mitochondrial dysfunction and apoptosis. Moreover, diosmetin treatment significantly downregulated the expression levels of Bcl-2 and Cyclin D1, and upregulated that of p53, Bax, caspase 3, cleaved caspase 9, and cleaved caspase 3.
Conclusions
These findings demonstrate that diosmetin has anti-proliferative and pro-apoptotic activities against MDA-MB-231 cells via cell cycle arrest and the mitochondria-mediated intrinsic apoptotic pathway. Our results extend the understanding of the anti-tumor mechanism of diosmetin and suggest that it may be of use as an active natural agent for the prevention or treatment of human breast cancer.
MeSH Keywords: Apoptosis, Breast Neoplasms, Cell Cycle Checkpoints, Reactive Oxygen Species
Background
Breast cancer, the most common malignancy, is a heterogeneous disease and the major cause of cancer death in females throughout the world. Breast cancer can be classified into several subtypes based on gene expression profiling [1]. Furthermore, triple-negative breast cancer (TNBC) is one of the most malignant subtypes without a progesterone receptor, estrogen receptor, and human epidermal growth factor receptor 2. Although many cancers initially respond to chemotherapy, TNBC cells may have acquired or intrinsic resistance to current therapies, and the high toxicity and adverse effects of chemotherapeutics are major obstacles in breast cancer treatment. Therefore, discovery and development of new alternative drugs are urgently needed to expand overall survival and improve quality of life of TNBC patients.
Herbal medicines have been used to treat cancer patients for many centuries. Many natural phytochemicals from herbal medicines have shown anti-cancer potential, and they are suggested for use as complementary or alternative anti-tumor agents [2]. These natural products exhibit anti-tumor activity through various mechanisms, such as inhibiting cell proliferation via cell cycle arrest and triggering apoptosis through high expression of pro-apoptotic proteins [3,4].
Diosmetin (3′,5,7-trihydroxy-4′-methoxyflavone), a natural flavonoid, has been isolated from some citrus fruits and medicinal herbs. Recently, we confirmed that diosmetin displayed cytoprotective effects on oxidative-damaged L02 cells via the Nrf2-ARE signaling pathway [5]. Previous pharmacological analyses have demonstrated that diosmetin exhibits anti-tumor activity against various cancer cell lines, such as human prostate cancer, hepatocellular carcinoma cell line HepG2 [6,7], SK-HEP-1 and MHcc97H HCC [8], lung cancer radio-resistant cell line A549/IR [9], human colon cancer cell Colon205 [10], and human breast cancer cell lines MCF-7 [11] and MDA-MB 468 [12]. However, so far, few studies have focused on the anti-proliferation and pro-apoptotic activities of diosmetin on breast cancer cells.
In this study, we aimed to demonstrate the chemopreventive and therapeutic effects of diosmetin on the TNBC cell line MDA-MB-231, and to elucidate the potential molecular mechanisms underpinning this process.
Material and Methods
Chemicals and reagents
Diosmetin was purchased from Zelang Medical Biotech Co. (Nanjing, China). The stock solution was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich; Merck KGaA), and working solutions were freshly diluted in the culture media prior to experimentation. DMSO concentrations did not exceed 0.1%. Rhodamine 123(Rh123) was purchased from Sigma-Aldrich.
Cell culture
The human breast cancer cell line MDA-MB-231 was purchased from the Cell Bank of Shanghai Institute for Biological Sciences (Shanghai, China). The cells were maintained at 37°C in DMEM (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) (HyClone, USA) and 1% penicillin-streptomycin in a humidified atmosphere containing 5% CO2.
Cell viability and lactate dehydrogenase (LDH) leakage assays
Cells (5×103 cells/well) were seeded in 96-well plates, then incubated with 5, 10, 20, 30, 40, 50, 60, and 70 μM diosmetin for 24 h, and the cells treated with DMSO alone served as the control. Cell viability was determined with Cell Counting kit-8 (CCK-8; Dojindo, Kumamoto, Japan) colorimetric assay using a microplate reader (Bio-Tek, USA). The LDH concentration was detected using an LDH assay kit according to the manufacturer’s protocol (Jiancheng, Nanjing, China).
Detection of cell cycle and cell apoptosis
Cells (2×105 cells/well) were seeded in 6-well plates, then treated with diosmetin (0, 10, 30, and 50 μM) for 24 h at 37°C. Cells were fixed with 70% ethanol at −20°C overnight. The cells were then stained with propidium iodide (50 μg/mL) after treatment with RNaseA (100 μg/mL). The DNA content of 10 000 cells was measured by a BD Accuri C6 flow cytometry (BD Biosciences, CA).
The proportions of apoptotic cells were measured using an Annexin V-FITC Apoptosis Kit (Beyotime, Shanghai, China). In brief, cells were seeded in 6-well plates at a density of 2×105 cells/well overnight, and treated with diosmetin for 24 h. Subsequently, the floating and adherent cells were centrifuged at 1000 rpm for 5 min, and incubated with Annexin V-FITC and PI in the dark for 25 min at room temperature. Samples were then analyzed immediately using a BD Accuri C6 flow cytometer.
Measurement of mitochondrial membrane potential (MMP) and intracellular reactive oxygen species (ROS)
The alteration of cellular MMP was evaluated using Rhodamine 123 (Rh123), and the generation of ROS was detected using 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA; Beyotime, Shanghai, China). The cells of 2×105 cells/well were planted in 6-well plates and cultured overnight, exposed to diosmetin (0, 10, 30, and 50 μM) at 37°C for 24 h, washed, and finally incubated with Rh123 (1 μM) or DCFH-DA (10 μM) in the dark for 30 min at 37°C. Then, fluorescence intensities of 1×104 cells were analyzed immediately by a BD Accuri C6 flow cytometer using BD Accuri™ C6 1.0.264.21 software (BD Biosciences, CA).
The cells (5×104 cells/well) were plated in 24-well plates, and then treated as described above. After incubation, the cells were stained by Rh123 (1μM) for 30 min or DCFH-DA (10 μM) for 20 min at 37°C in the dark and photographed using a fluorescence microscope (Olympus, Tokyo, Japan).
Quantitative real-time PCR (qRT-PCR)
Total RNAs of all samples were extracted by TRIzol reagent (Invitrogen, MA), and were reverse-transcribed into cDNA using an RT reagent kit (Takara, Otsu, Japan). Quantitative PCR was performed using SYBR Premix Ex Taq II (Takara, Otsu, Japan), and GAPDH was used as an internal control to normalize the relative gene expression levels. The primers used for qRT-PCR are listed in Table 1.
Table 1.
Primer sequences used.
| Gene | Primer sequences (5′-3′) | Length (bp) |
|---|---|---|
| p53 | F: CTCTCCCACCAACATCCACT | 178 |
| R: ACGTCCACCACCATTTGAAC | ||
| Caspase-3 | F: CGTGTATTGTGTCCATGCTCAC | 271 |
| R: CCATCATTGACAGTTACTTGCTCC | ||
| Bax | F: GACGAACTGGACAGTAACATG | 230 |
| R: AGGCACCCAGGGTGATGCAA | ||
| Bcl-2 | F: GTGGAGGAGCTCTTCAGGGA | 304 |
| R: AGGCACCCAGGGTGATGCAA | ||
| Cyclin D1 | F: GGAGAACAAACAGATCATCC | 491 |
| R: GAATGAAGCTTTCCCTTCTC | ||
| GAPDH | F: ACGGATTTGGTCGTATTGGG | 230 |
| R: TGATTTTGGAGGGATCTCGC |
Western blot analysis
Cells from treatment and control groups were lysed in RIPA buffer (Beyotime, Shanghai, China). The BCA assay was used to determine the protein concentrations of all samples. Forty μg/lane of proteins were separated on 10% SDS-PAGE and transferred onto PVDF membranes (Millipore, MA). The membranes were incubated with primary monoclonal antibody anti-caspase 3 (1: 1000; CST, #14220), anti-cleaved caspase 3 (1: 1000; CST, #9661), anti-cleaved caspase 9 (1: 1000; CST, #52873), anti-p53 (1: 1000; CST, #2527), anti-CyclinD1 (1: 10 000; Abcam, ab134175), anti-Bax (1: 2000; Abcam, ab182733), anti-Bcl-2 (1: 1000; Abcam, ab32124), or β-actin (1: 5000; Affinity, T0022;) overnight at 4°C. The membranes were then rinsed 3 times and incubated with HRP-conjugated secondary antibodies (1: 6000) at room temperature for 1 h. The respective proteins were detected using enhanced chemiluminescence (Bio-Rad ChemiDoc XRS; CA).
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software (SPSS, Inc., Chicago, IL). Data are expressed as mean ± SD of 3 independent experiments. Statistical significance was analyzed using one-way analysis of variance (ANOVA) and the least significant difference test. A difference was considered to be significant at P≤0.05.
Results
Diosmetin induces cell proliferation inhibition, LDH release, and apoptosis in MDA-MB-231 cells
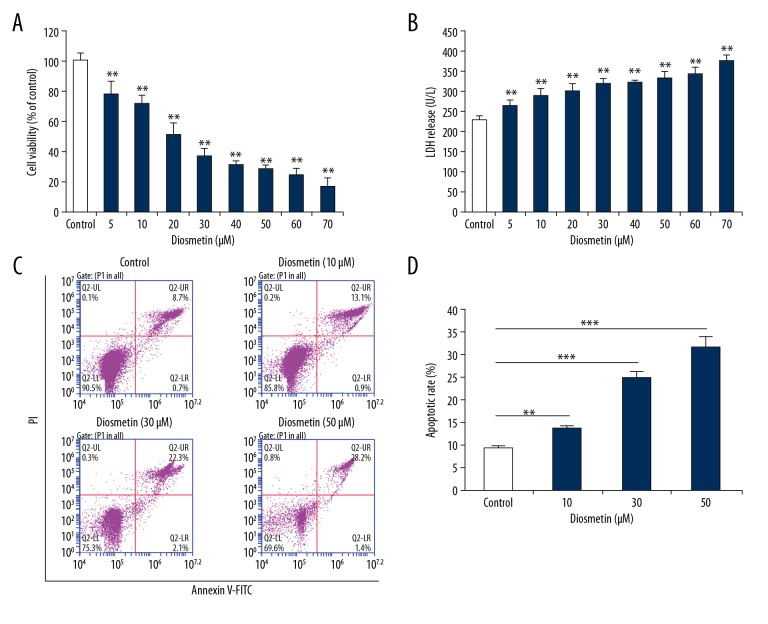
Diosmetin significantly reduced the cell viability of MDA-MB-231 cells in a concentration-dependent manner (P<0.01) (Figure 1A). The LDH release levels were increased significantly compared with the control group (P<0.01) in a concentration-dependent manner (Figure 1B). These results of cell viability and LDH release assays indicated that diosmetin exerted cytotoxic effects on the MDA-MB-231 cells; therefore, 3 concentrations of diosmetin – 10 μM, 30 μM, and 50 μM – were selected for further analysis.
Figure 1.
Diosmetin induced cytotoxicity and apoptosis in MDA-MB-231 cells. (A) Cell viabilities were detected by Cell Counting kit-8 assay. (B) LDH levels in the culture medium were measured with an LDH assay kit. (C) Apoptotic rates were assessed using Annexin V-FITC/PI by flow cytometry. (D) Quantitative analysis of apoptotic rates. All data are expressed as mean ±SD of 3 independent experiments. ** P<0.01 and *** P<0.001 compared to the control group.
To determine whether decreased proliferation and increased LDH release were associated with apoptosis, the apoptotic effects of diosmetin on MDA-MB-231 cells were investigated. As shown in Figure 1C and 1D, diosmetin noticeably reduced the number of surviving cells and significantly increased the percentage of apoptotic and dead cells as compared with the control group (P< 0.01 or P<0.001).
Diosmetin causes mitochondrial dysfunction
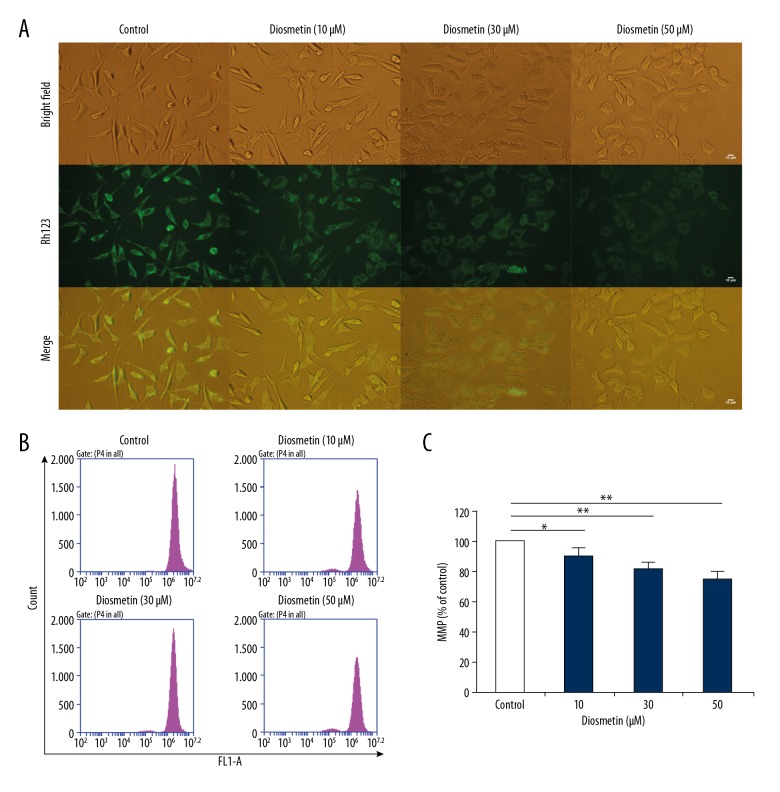
Mitochondrial function plays a key role during cell apoptosis. MMP change mostly occurs in the early phase of apoptosis caused by drugs. In this study, as presented in Figure 2B and 2C, the loss of MMP after treatment with diosmetin occurred in a concentration-dependent manner (P<0.05 or P<0.01). The fluorescence intensity of Rh123 also decreased in the dose-dependent manner (Figure 2A), indicating that diosmetin caused the loss of MMP in MDA-MB-231 cells.
Figure 2.
Diosmetin caused mitochondrial membrane potential (MMP) loss in MDA-MB-231 cells. (A) Representative bright-field and Rh123 fluorescence photomicrographs were observed under an inverted fluorescence microscope. (B) MMP was determined with Rh123 by flow cytometry. (C) Quantitative analysis of percentage of MMP loss. The results are expressed as mean ±SD of 3 independent experiments. * P<0.05 and ** P<0.01compared to the control group.
Diosmetin provokes ROS accumulation in MDA-MB-231 cells
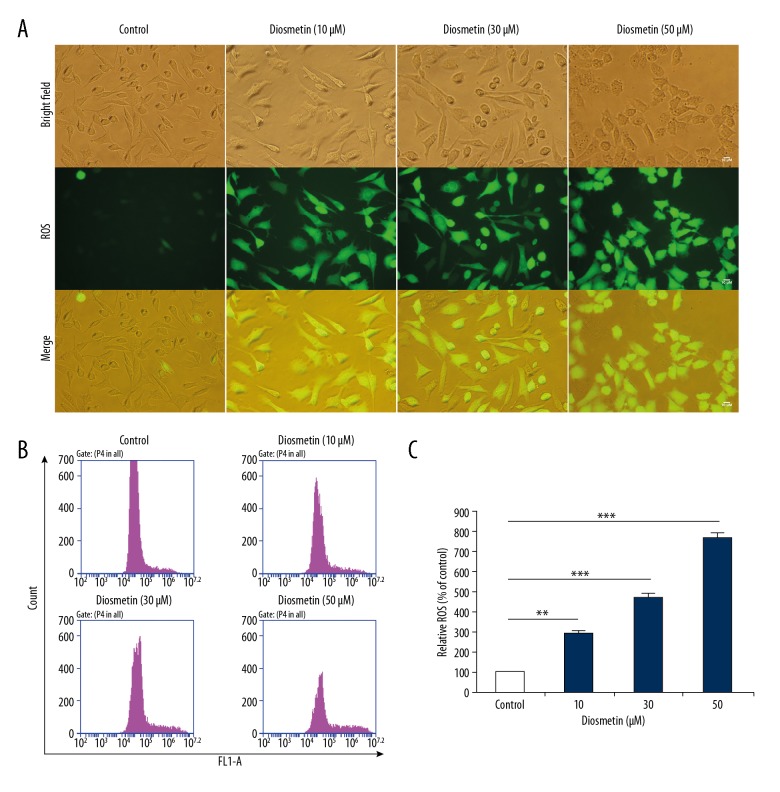
Intracellular ROS production is reported to be related to various stresses, and can affect the fate of cancer cells, including apoptosis and cell cycle arrest. As shown in Figure 3B and 3C, the intracellular ROS levels were significantly increased in MDA-MB-231 cells after treatment with different concentrations of diosmetin for 24 h (P<0.001), and the levels of ROS treated with 10, 30, and 50 μM diosmetin were approximately 2.89, 4.74, and 7.74 times higher, respectively, than that of the control group.
Figure 3.
Diosmetin induced reactive oxygen species (ROS) accumulation in MDA-MB-231 cells. (A) Representative bright-field and DCFH-DA fluorescence photomicrographs were observed under an inverted fluorescence microscope. (B) Intracellular ROS levels were measured with DCFH-DA by flow cytometry. (C) Quantitative analysis of fold change of ROS production. Data are expressed as mean ±SD of 3 independent experiments. ** P<0.01 and *** P<0.001 compared to the control group.
To directly evaluate the accumulation of ROS, DCFH-DA-labeled cells were observed under a fluorescence microscope (Figure 3A). As expected, the fluorescence intensity of ROS was enhanced with increased diosmetin concentration. These observations further supported the result that treatment with diosmetin provoked the significantly increased ROS generation in MDA-MB-231 cells.
Diosmetin induces G0/G1 phase arrest in MDA-MB-231 cells
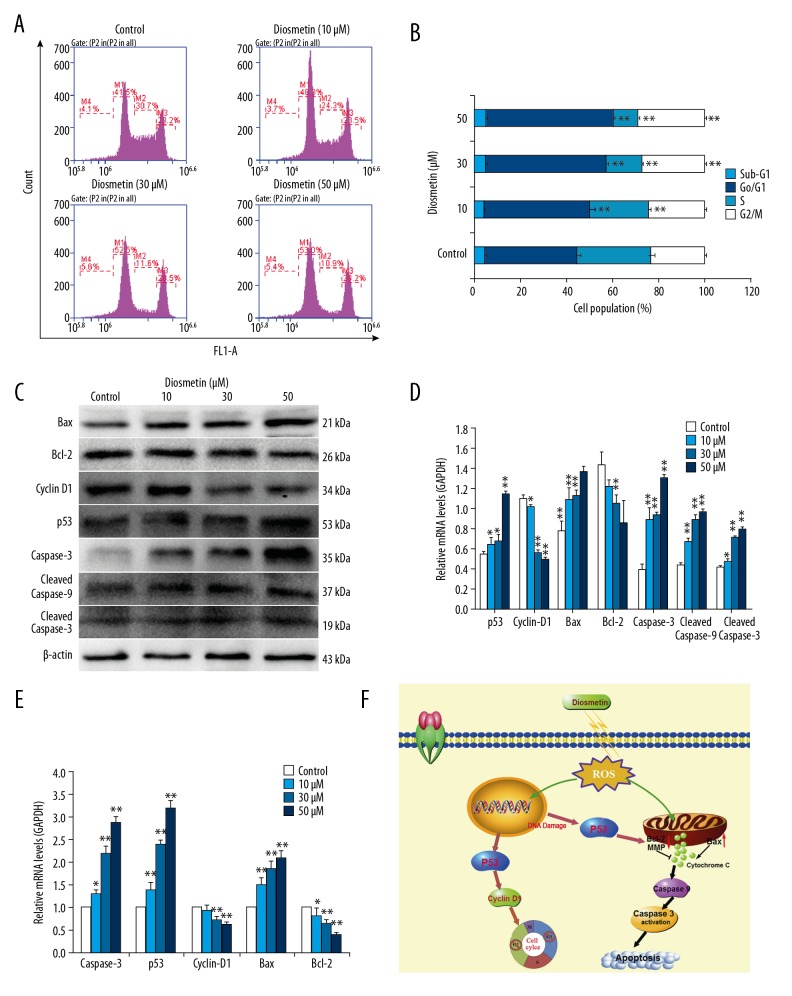
Cell cycle arrest is a common cytotoxic mechanism of anti-cancer drugs. As shown in Figure 4A and 4B, the cell cycle of MDA-MB-231 cells was also affected after diosmetin treatment for 24 h. The G0/G1 population was significantly increased (P<0.01) in a concentration-dependent manner, from 39.9% in the control cells to 46.0%, 52.1%, and 55.2% in the 10, 30, and 50 μM diosmetin-treated cells, respectively. However, the S phase proportion significantly decreased (P<0.01), from 32.2% in the untreated cells to 25.4%, 15.6%, and 10.6% in the 10, 30, and 50 μM diosmetin-treated cells, respectively. Compared with the control cells, the G2/M phase cells treated with 30 and 50 μM diosmetin also increased significantly (P<0.01), which might indicate that some S phase cells entered into the G2/M phase. Thus, the cell cycle of MDA-MB-231 cells was arrested at G0/G1 phase induced by diosmetin.
Figure 4.
Effects of diosmetin on cell cycle progression, mRNA and protein expression levels of cell cycle- and apoptosis-related genes in MDA-MB-231 cells. (A) Cell cycle distribution was analyzed with PI-staining by flow cytometry. (B) The data indicated the percentage of cells in different phases of the cell cycle. (C) Relative protein expression levels of cell cycle- and apoptosis-related genes were detected by Western blotting. (D) Scanning densitometry was used for semi-quantitative analysis of Western blotting. (E) Relative mRNA expression levels of cell cycle- and apoptosis-related genes were analyzed by qRT-PCR. (F) A schematic diagram of diosmetin mechanism of action for apoptosis. * P<0.05 and ** P<0.01 compared to the control group.
Diosmetin regulates the expression of apoptosis- and cell cycle-related genes
The expressions of apoptosis- and cell cycle-related genes were all affected by the various concentrations of diosmetin, as detected by qRT-PCR and Western blotting analysis. As shown in Figures 4C–4E, upon treatment with increasing concentration of diosmetin, there was a significant rise in the mRNA and protein expression of p53, Bax, and caspase 3 in MDA-MB-231 cells. Furthermore, diosmetin also induced the cleavage of caspase 9 and caspase 3 in MDA-MB-231 cells. The expression levels of cleaved caspase 9 and cleaved caspase 3 increased markedly in a concentration-dependent manner (P<0.05 or P<0.01). In contrast, the expression of Bcl-2 and Cyclin D1 was dose-dependently decreased compared to untreated cells. This also indicated that down-regulation of Cyclin D1 resulted in G0/G1 arrest in MDA-MB-231 cells. The Bax/Bcl-2 mRNA expression ratio increased approximately 1.85-, 2.88-, and 5.29-fold at concentration of 10, 30, and 50 μM of diosmetin, respectively, and the Bax/Bcl-2 protein expression ratio increased by approximately 1.66-, 1.99-, and 2.95-fold, respectively. The increased Bax/Bcl-2 ratio indicated that diosmetin promoted MDA-MB-231 cell apoptosis via the mitochondria-mediated intrinsic pathway.
Discussion
Breast cancer, especially TNBC, is one of the most malignant tumors in women throughout the world. There has been growing interest in searching for natural compounds as cancer prophylactic and therapeutic agents. Diosmetin, a flavonoid found in citrus fruits and some medicinal herbs, has been found to display a range of anti-cancer actions, such as inhibiting cancer cell proliferation, metastasis, and metabolism, and inducing apoptosis. Two previous studies demonstrated that diosmetin possesses cytostatic effects against MCF-7 and MDA-MB-468 cell lines [11,12]. In the present study, we focused on unraveling the chemopreventive and therapeutic action of diosmetin and deciphered its potential molecular targets in the MDA-MB-231 cell line. The results showed that diosmetin was active against MDA-MB-231 cells by decreasing cell viability, increasing LDH release, and inducing mitochondrial dysfunction, apoptosis, and G0/G1 phase arrest. Moreover, the anti-proliferation and pro-apoptotic effects of diosmetin were associated with the levels of ROS, cell cycle, and expressions of apoptosis-related genes. To the best of our knowledge, this is the first study to demonstrate that diosmetin has anti-proliferation and pro-apoptotic activities on MDA-MB-231 cells through ROS accumulation, cell cycle arrest, and activation of the intrinsic mitochondrial apoptotic pathway.
Multiple intracellular signal transduction pathways involve the inhibition of oncogenesis, resulting in cell cycle arrest and then inducing cell apoptosis [13]. It is well known that p53 plays important roles in a variety of regulatory mechanisms via the transcriptional activation of target genes, such as p21 and cyclins, to induce cell cycle arrest [14,15]. Cyclin D1 is a critical regulator of the cyclin family essential for blocking the cell cycle at the G1/S checkpoint [16,17]. Our study showed that diosmetin could block the growth of MDA-MB-231 cells by inducing the cell cycle arrest at G0/G1 phase. Furthermore, treatment with diosmetin significantly up-regulated the mRNA and protein levels of p53 and down-regulated the expression of cyclin D1. Therefore, p53 and Cyclin D1 may be the regulators of cell cycle arrest in diosmetin-treated MDA-MB-231 cells.
Apoptosis, a process of programmed cell death, plays a crucial role in homeostasis and can be induced and regulated by many physiological conditions. Dysregulation of apoptosis is considered as an important cancer hallmark [18], so apoptosis induction should be the most important potential defense against cancer. Many studies have revealed that a variety of gene products can promote or block apoptosis [19,20]. In addition to p53, the intrinsic apoptotic pathway is regulated by the Bcl-2 family proteins, especially the ratio of pro-apoptotic protein Bax and anti-apoptotic protein Bcl-2 [21]. The results of this study demonstrate that treatment with diosmetin can significantly increase the Bax/Bcl-2 ratio, and the increased ratio of Bax/Bcl-2 promotes mitochondrial dysfunction, release of some apoptotic factors, and caspase 9 activation. Then, active caspase 9 cleaves the downstream apoptosis effector caspase 3 [22]. Our study showed that diosmetin could increase the expression of p53, Bax/Bcl-2 ratio, cleaved caspase 9, and cleaved caspase 3. Furthermore, mitochondrial membrane potential (MMP) sharply decreased in a concentration-dependent manner after diosmetin treatment, indicating that diosmetin depolarized mitochondria in MDA-MB-231 cells. Mitochondrial membrane depolarization can result in the release of mitochondrial pro-apoptotic factors and activation of caspase, ultimately triggering cell apoptosis [23,24]. These mitochondrial apoptotic events indicate that diosmetin can induce apoptosis of MDA-MB-231 cells through the mitochondria-mediated apoptotic pathway.
ROS are recognized as important signaling molecules in the cellular signal transduction pathway, and excessive ROS production may trigger cell damage and play critical roles in inducing both cell cycle progression and apoptosis [25,26], suggesting an anti-cancer effect. As expected, diosmetin significantly increased ROS generation in MDA-MB-231 cells. Increased accumulation of ROS can induce cell cycle arrest, especially in the G0/G1 phase, and result in cells apoptosis [26–28]. ROS-mediated oxidative stress is also linked to the loss of MMP [29]. Our findings are consistent with these previous studies, and suggest that the cytotoxic effects of diosmetin occur through ROS-mediated modulation of the cell cycle and apoptosis.
The present study demonstrates that diosmetin can suppress the proliferation and induce apoptosis of MDA-MB-231 cells via the ROS- and p53-mediated Bax/Bcl-2 signaling pathway, and induced G0/G1 phase arrest through p53-and cyclin D1-mediated mechanisms, as illustrated in Figure 4F. These findings provide a theoretical foundation for understanding the mechanism of the tumor-inhibitory action of diosmetin, and suggest that it may be used as an active natural agent for the prevention and treatment of human breast cancer.
Conclusions
This study is the first to demonstrate that diosmetin has anti-proliferative and pro-apoptotic activities against MDA-MB-231 cells through modulating cell cycle arrest and the mitochondria-mediated intrinsic apoptotic pathway. Therefore, our results extend the understanding of the anti-tumor mechanism of diosmetin and suggest that it could be used as an active natural agent for the prevention and treatment of human breast cancer.
Footnotes
Source of support: This work was financially supported by the National Natural Science Foundation of China (grant no. 81771381 and 31800996), the Natural Science Foundation of the Higher Education Institutions of Anhui Province (grant no. KJ2017A215), and the Undergraduate Innovative Training Program of Anhui Province (grant no. 201710367080)
Conflict of interest
None.
References
- 1.Lewinska A, Adamczyk-Grochala J, Kwasniewicz E, et al. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol Lett. 2016;265:117–30. doi: 10.1016/j.toxlet.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Darwiche N, Elbanna S, Galimuhtasib H. Cell cycle modulatory and apoptotic effects of plant-derived anticancer drugs in clinical use or development. Expert Opin Drug Discov. 2007;2(3):361–79. doi: 10.1517/17460441.2.3.361. [DOI] [PubMed] [Google Scholar]
- 3.Galimuhtasib H, Hmadi R, Kareh M, et al. Cell death mechanisms of plant-derived anticancer drugs: beyond apoptosis. Apoptosis. 2015;20(12):1531–62. doi: 10.1007/s10495-015-1169-2. [DOI] [PubMed] [Google Scholar]
- 4.Wang R, Yang L, Li S, et al. Quercetin inhibits breast cancer stem cells via downregulation of aldehyde dehydrogenase 1A1 (ALDH1A1), chemokine receptor type 4 (CXCR4), mucin 1 (MUC1), and epithelial cell adhesion molecule (EpCAM) Med Sci Monit. 2018;24:412–20. doi: 10.12659/MSM.908022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C, Liao Y, Wang S, et al. Cytoprotective effects of diosmetin against hydrogen peroxide-induced L02 cell oxidative damage via activation of the Nrf2-ARE signaling pathway. Mol Med Rep. 2018;17(5):7331–38. doi: 10.3892/mmr.2018.8750. [DOI] [PubMed] [Google Scholar]
- 6.Androutsopoulos VP, Spandidos DA. The flavonoids diosmetin and luteolin exert synergistic cytostatic effects in human hepatoma HepG2 cells via CYP1A-catalyzed metabolism, activation of JNK and ERK and P53/P21 up-regulation. J Nutr Biochem. 2013;24(2):496–504. doi: 10.1016/j.jnutbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Liu B, Zhang M, Liu S, et al. Diosmetin, a potential p53 activator, performs anticancer effect by regulating cell cycling and cell proliferation in HepG2 cells. Protein Pept Lett. 2017;24(5):413–18. doi: 10.2174/0929866524666170223094634. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Wen X, Liu B, et al. Diosmetin inhibits the metastasis of hepatocellular carcinoma cells by downregulating the expression levels of MMP-2 and MMP-9. Mol Med Rep. 2016;13(3):2401–8. doi: 10.3892/mmr.2016.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Z, Yan Y, Xiao L, et al. Radiosensitizing effect of diosmetin on radioresistant lung cancer cells via Akt signaling pathway. PLoS One. 2017;12(4):e0175977. doi: 10.1371/journal.pone.0175977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie YY, Yuan D, Yang JY, et al. Cytotoxic activity of flavonoids from the flowers of Chrysanthemum morifolium on human colon cancer Colon205 cells. J Asian Nat Prod Res. 2009;11(9):771–78. doi: 10.1080/10286020903128470. [DOI] [PubMed] [Google Scholar]
- 11.Androutsopoulos V, Wilsher N, Arroo RRJ, Potter GA. Bioactivation of the phytoestrogen diosmetin by CYP1 cytochromes P450. Cancer Lett. 2009;274(1):54–60. doi: 10.1016/j.canlet.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 12.Androutsopoulos VP, Mahale S, Arroo RR, Potter G. Anticancer effects of the flavonoid diosmetin on cell cycle progression and proliferation of MDA-MB 468 breast cancer cells due to CYP1 activation. Oncol Rep. 2009;21:1525–28. doi: 10.3892/or_00000384. [DOI] [PubMed] [Google Scholar]
- 13.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411(6835):342–48. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 14.Shen Y, White E. p53-dependent apoptosis pathways. Adv Cancer Res. 2001;82(8):55–84. doi: 10.1016/s0065-230x(01)82002-9. [DOI] [PubMed] [Google Scholar]
- 15.Hu D, Su C, Jiang M, et al. Fenofibrate inhibited pancreatic cancer cells proliferation via activation of p53 mediated by upregulation of LncRNA MEG3. Biochem Biophys Res Commun. 2016;471(2):290–95. doi: 10.1016/j.bbrc.2016.01.169. [DOI] [PubMed] [Google Scholar]
- 16.Wikman H, Kettunen E. Regulation of the G1/S phase of the cell cycle and alterations in the RB pathway in human lung cancer. Expert Rev Anticancer Ther. 2006;6(4):515–30. doi: 10.1586/14737140.6.4.515. [DOI] [PubMed] [Google Scholar]
- 17.Liao K, Li J, Wang Z. Dihydroartemisinin inhibits cell proliferation via AKT/GSK3β/cyclinD1 pathway and induces apoptosis in A549 lung cancer cells. Int J Clin Exp Pathol. 2014;7(12):8684–91. [PMC free article] [PubMed] [Google Scholar]
- 18.Abbott RG, Forrest S, Pienta KJ. Simulating the hallmarks of cancer. Artif Life. 2006;12(4):617–34. doi: 10.1162/artl.2006.12.4.617. [DOI] [PubMed] [Google Scholar]
- 19.Meng C, Song L, Wang J, et al. Propofol induces proliferation partially via downregulation of p53 protein and promotes migration via activation of the Nrf2 pathway in human breast cancer cell line MDA-MB-231. Oncol Rep. 2017;37:841–48. doi: 10.3892/or.2016.5332. [DOI] [PubMed] [Google Scholar]
- 20.Lv W, Su B, Li Y, et al. KIAA0101 inhibition suppresses cell proliferation and cell cycle progression by promoting the interaction between p53 and Sp1 in breast cancer. Biochem Biophys Res Commun. 2018;503(2):600–6. doi: 10.1016/j.bbrc.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 21.Chresta CM, Masters JRW, Hickman JA. Hypersensitivity of human testicular tumors to etoposide-induced apoptosis is associated with functional p53 and a high Bax: Bcl-2 ratio. Cancer Res. 1996;56(8):1834–41. [PubMed] [Google Scholar]
- 22.Vaculova A, Zhivotovsky B. Caspases: Determination of their activities in apoptotic cells. Methods Enzymol. 2008;442:157–81. doi: 10.1016/S0076-6879(08)01408-0. [DOI] [PubMed] [Google Scholar]
- 23.Daugas E, Nochy D, Ravagnan L, et al. Apoptosis-inducing factor (AIF): A ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett. 2000;476(3):118–23. doi: 10.1016/s0014-5793(00)01731-2. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Xu H, Zhao X. Baicalin inhibits human cervical cancer cells by suppressing protein kinase C/signal transducer and activator of transcription (PKC/STAT3) signaling pathway. Med Sci Monit. 2018;24:1955–61. doi: 10.12659/MSM.909640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang R, Ma L, Weng D, et al. Gallic acid induces apoptosis and enhances the anticancer effects of cisplatin in human small cell lung cancer H446 cell line via the ROS-dependent mitochondrial apoptotic pathway. Oncol Rep. 2016;35(5):3075–83. doi: 10.3892/or.2016.4690. [DOI] [PubMed] [Google Scholar]
- 26.Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337(35):1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 27.Visconti R, Monica RD, Grieco D. Cell cycle checkpoint in cancer: A therapeutically targetable double-edged sword. J Exp Clin Cancer Res. 2016;35(1):153–60. doi: 10.1186/s13046-016-0433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang SL, Li BL, Li W, et al. The effects of ludartin on cell proliferation, cell migration, cell cycle arrest and apoptosis are associated with upregulation of p21WAF1 in Saos-2 osteosarcoma cells in vitro. Med Sci Monit. 2018;24:4926–33. doi: 10.12659/MSM.909193. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Skała E, Kowalczyk T, Toma M, et al. Induction of apoptosis in human glioma cell lines of various grades through the ROS-mediated mitochondrial pathway and caspase activation by Rhaponticum carthamoides transformed root extract. Mol Cell Biochem. 2018;445(1):89–97. doi: 10.1007/s11010-017-3254-z. [DOI] [PubMed] [Google Scholar]