Abstract
The present study aimed to investigate the antispasmodic effect of higenamine on cold-induced cutaneous vasoconstriction and the underlying molecular mechanisms. A cold-induced cutaneous vasoconstriction rat model was established and different doses of higenamine were delivered by intravenous injection. The changes of cutaneous regional blood flow (RBF) between groups were analyzed. In vitro, the proliferation of human dermal microvascular endothelial cells was measured by MTT. The NO concentration was detected by a nitrate reductase assay. Flow cytometry was applied to measure reactive oxygen species (ROS) levels. The protein expression levels were detected by western blotting. The results demonstrated that in the model group, RBF declined compared with the normal control group, but was reversed by treatment with higenamine. The expression of endothelial nitric oxide synthase (eNOS), phosphorylated (p)-eNOS, protein kinase B (Akt1), p-Akt1, AMP-activated protein kinase (AMPK) α1 and p-AMPKα1 was upregulated by hypothermic treatment but was reversed by higenamine treatment. Treatment with higenamine significantly reduced the level of intracellular α2C-adrenoreceptor (AR) compared with the hypothermia group (P<0.05). Furthermore, the expression of twinfilin-1 (PTK9) was downregulated in the higenamine and positive control groups compared with the hypothermia group (P<0.05). Compared with the hypothermia group, the levels of ROS and α2C-AR (intracellular & membrane) were decreased in higenamine and the positive control group (P<0.05 and P<0.01, respectively). This study, to the best of our knowledge, is the first to assess the effects of higenamine on cold-induced vasoconstriction in vivo and its molecular mechanisms on the PI3K/Akt, AMPK/eNOS/nitric oxide, ROS/α2C-AR and PTK9 signaling pathways under hypothermia conditions. Higenamine may be a good therapeutic option for Raynaud's phenomenon (RP) and cold-induced vasoconstriction.
Keywords: higenamine, vasoconstriction, vasodilation, regional blood flow, human dermal microvascular endothelial cells, signaling pathways
Introduction
Raynaud's phenomenon (RP) is a disease caused by an excessive cold-induced vasoconstriction of skin arterioles with an incidence of ~10% of the general population (1–7). Patients affected by RP suffer from vasospastic attacks, ulcerations and puffiness at the level of fingers, nose, toes, and nipples (7–9). Although attempts have been made to elucidate and treat RP, definitive or specific treatment options for this illness are limited and efforts to uncover efficient medications for this disease needs to be undertaken.
Higenamine is a chemical compound extracted from the roots of aconite. As a traditional Chinese herb, aconite has been used solely or in combination with other medicines for the treatment of patients suffering from heart failure in oriental Asia for millenaries (10–13). Phase III clinical trials have conveyed the potential of higenamine-based pharmacological stress agents in coronary artery diseases (14). Numerous studies have also reported a multitude of candidate pharmacological properties and a wide range of medical uses of higenamine (14–18). Specifically, Higenamine can inhibits apoptosis and protects gastric smooth muscle cell from death in an in vivo model of diabetic gastroparesis by promoting the β2-adrenoreceptor (AR)/phosphatidylionositol 3 kinase (PI3K)/protein kinase B (Akt) pathway (10). Higenamine was found to equally protect against ischemia/reperfusion associated cardiac injury and cardiac cell apoptosis via upregulating the β2-AR/PI3K/Akt signaling pathway (19). In addition, higenamine can also protect against arthritis by regulating PI3K/Akt/nuclear factor erythroid 2-related factor 3 (Nrf2) and heme oxygenase-1 (HO-1) (20), and decreases intestinal ischemia-reperfusion injury in vivo by regulating the Nrf2/HO-1/high mobility group protein B1 signaling pathway (21). Furthermore, it has been reported that the combination of higenamine with gingerol inhibits doxorubicin-induced oxidative stress and apoptotic cell death by promoting PI3K/Akt signaling in cardiomyocytes (13). However, although higenamine is effective against the circulatory system, whether it is efficient in counteracting cold-induced vasoconstriction or plays a role in clinically-induced hypothermia and the molecular mechanisms underlying these effects are still unknown.
The present study was designed to investigate the effects of higenamine on cold-induced vasodilation in vivo and elucidate the underlying molecular mechanisms. The skin blood flow was measured to assess the effect of higenamine on cold-induced vasoconstriction in vivo model, for the first time to the best of our knowledge. The effect of higenamine on PI3K/Akt and AMP-activated protein kinase (AMPK)α1/endothelial nitric oxide synthase (eNOS)/nitric oxide (NO) was also explored as well as reactive oxygen species (ROS)/α2C-AR and twinfilin-1 (PTK9) signaling transduction pathways to assess the effect of higenamine in hypothermic human dermal microvascular endothelial cells (HDMECs) in vitro for the first time. The present study provided extensive research evidence on the antispasmodic effect and mechanism of higenamine on cold-induced skin vasoconstriction. This will lay a foundation for the modernization of traditional Chinese medicine and the data exhibited has scientific significance and clinical value.
Materials and methods
In vivo studies
Grouping
A total of 48 female adult Wistar rats (12 weeks old; 200–250 g), obtained from the Guangdong Medical Laboratory Animal Centre (Foshan, China), were housed at 22±2°C and 40–60% humidity with light/dark cycle of 12-h. The rats had free access to food and water. Rats were randomly divided into six groups (8 rats in each group): Low dose higenamine group, medium dose higenamine group, high dose higenamine group, positive drug control group (prostaglandin E1; cat. no. 745-65-3; Sigma-Aldrich; Merck KGaA), model control group and normal control group.
Reagent preparation
Higenamine hydrochloride decoction: 1 mg of higenamine hydrochloride standard (cat. no. 11041-94-4; Sigma-Aldrich; Merck KGaA) was dissolved in 30 ml of physiological saline solution to obtain a decoction at a concentration of 33 µg/ml.
Dosage
According to published studies the experimental doses of different higenamine subgroups were determined as follows: The low dose higenamine group (18 µg·kg−1), the medium dose higenamine group (36 µg·kg−1) and high dose higenamine group (72 µg·kg−1) (14,22,23). The dose of prostaglandin E1 (5 µg·kg−1) for the positive drug control group was determined based on the clinical dosage. Rats in the model control group were injected with 0.1 ml physiological saline per rat.
Drug administration
Drug administration was performed by tail vein injection at room temperature (24°C) based on the above-mentioned dosages. Briefly, 75% ethanol cotton was used to swab the tail. Then, the tail was pulled with the left hand and the drugs were injected with the right hand after exposing the left and right lateral veins of the tail. During the intravenous administration, one rat died of a misplaced body position in the rat fixator, which corresponds to a mortality rate of 2.2%.
Modeling
After drug administration, a cold-induced rat skin vasoconstriction model was established in vivo. Briefly, rats were loaded into the rat fixator at room temperature 24°C and the rat tail was exposed outside the fixator. After adjusting the rat fixator and maintaining the rat body in a relatively fixed position, the rats were placed in a more comfortable state. The tails of rats (except for the normal control group) were placed in a 10°C water bath for 5 min to establish a cold-induced rat skin vasoconstriction in vivo model. The tails of rats in the model control group and administration group were placed in a 10°C water bath for 5 min after drug administration. The regional blood flow (RBF) of caudal arterial cortex was measured at 5 min in a 24°C, 10°C water bath and then at 5 min in a 10°C water bath. The first two measurements were used to compare the caudal RBF differences between the normal control group and the model group. The latter two measurements were used to compare the caudal RBF differences between the administration groups and the model control group.
RBF measurement
The rats were placed in a rat fixator and the tails were exposed outside the fixator. The rat fixator was slid forward and backward to adjust the stopper. In this position, the rat body was maintained in a relatively fixed position, placing the rat in a more comfortable state. The RBF of the rats' caudal arterial cortex was measured by using MoorDRT4 laser-Doppler flowmetry (LDF), the laser probe was placed in the rat caudal arterial cortex and RBF was monitored in real time at room temperature (24°C). After establishing the cold-induced skin vasoconstriction rat model, the laser probe was placed in the rat tail caudal arterial cortex and the RBF of rat caudal arterial cortex was monitored in real-time at 10°C using LDF. The RBF measurement was done before and after higenamine administration.
In vitro studies
Cell culture
HDMECs were purchased from ScienCell Research Laboratories, Inc. HDMECs were cultured in endothelial medium supplemented with 5% fetal bovine serum and 1% endothelial cell growth supplement (both ScienCell Research Laboratories, Inc.) and placed in an incubator containing 5% CO2 at 37°C in saturated humidity conditions.
MTT assay for cell proliferation
HDMECs were divided into 6 groups according to different concentrations (0, 1, 2, 4, 10 and 20 µmol/l) of higenamine. The HDMECs were collected at logarithmic phase and the cell suspension was adjusted to 1×105/ml with sterile PBS. Then, 100 µl suspension was added to each well (four replicates for each treatment). The culture was performed in an incubator at 37°C containing 5% CO2 overnight. Next, cells were treated with different concentrations of higenamine and incubated at 37°C in 5% CO2 incubator for 30 min. Subsequently, the culture conditions were switched to 5% CO2 in an incubator at 28°C and incubated for 30, 60, 90 and 120 min. The MTT assay was used to measure cell proliferation. Briefly, 20 µl MTT solution was added to each well and followed by incubation for an additional 4 h. Then, the culture was stopped and 150 µl of dimethyl sulfoxide (DMSO) was added to each well with shaking for 10 min at a low-speed shaker to fully dissolve the crystals. The absorbance (optical density; OD) of each well was measured by a microplate reader at a measurement wavelength of 490 nm and used to calculate the cell proliferation rate.
NO measurement
After calculating the optimal dosing concentration and optimal administration time, HDMECs were divided into 4 groups: Hypothermic higenamine group (higenamine, 20 µmol/l), hypothermic positive control group (prostaglandin E1, 100 ng/ml), hypothermic group and normal temperature group. The corresponding drugs were added to cells in the 4 groups and incubated at 37°C for 30 min. Next the medium was refreshed and cells in the hypothermia higenamine group (higenamine, 20 µmol/l), the hypothermia positive control group (prostaglandin E1, 100 ng/ml) and the hypothermia group were incubated at 28°C for 120 min while the normal temperature group was incubated at 37°C for 120 min. The concentration of NO in the supernatant of each group was determined using a nitrate reductase assay kit according to the manufacturer's protocol. The content of NO was calculated using the following formula: NO (µmol/l) = [(measured OD value-blank OD value)/(standard OD value-blank OD value)] × standard product concentration (20 µmol/l) × dilution factor).
ROS measurement
HDMECs were digested with trypsin to prepare a single cell supernatant and resuspended with 0.5–1 ml ice cold PBS. Next, the probe solution [2,7-dichlorodihydrofluorescein diacetate (DCFH-DA); cat. no. 4091-99-0; Sigma-Aldrich; Merck KGaA] was added directly to the cell suspension to reach a final concentration of 10 µmol/l followed by incubation for 20 min at 37°C. Subsequently, the cells were washed with fresh serum-free medium to wash away DCFH-DA so that DCFH-DA could not enter the cells. The fluorescence intensity (the wavelength of excitation and emission were 488 and 525 nm, respectively) was detected by flow cytometry (BD FACSAria™ III) and the data analysis was performed using BD CellQuest™ Pro Software (version 5.1; both BD Biosciences). Cells should be divided into two subpopulations: ROS-negative cells with very low fluorescence intensity and ROS-positive cells with strong green fluorescence. It is worth noting that fluorescence intensity is positively correlated with the intracellular ROS level and can be used to reflect the level of intracellular ROS (24).
Western blotting
HDMECs were lysed in radioimmunoprecipitation assay lysis buffer (Roche Diagnostics) according to the standard protocol. Protein concentrations were detected using the Micro bicinchoninic acid protein assay kit (Youdi Biotechnology Co., Ltd.). Total cell lysate (50 µg) was loaded into each lane and separated by 12% SDS-PAGE, followed by transfer to PVDF membrane (EMD Millipore). The membranes were blocked with 3% bovine serum albumin (cat. no. PRO-422; ProSpec-Tany TechnoGene Ltd.) in TBS-Tween-20 for 1 h at room temperature. Primary antibodies including eNOS (cat. no. 32027; 1:500), p-eNOS (cat. no. 9570), Akt1 (cat. no. 75692), p-Akt1 (cat. no. 12178), AMPKα1 (cat. no. 5832), p-AMPKα1 (cat. no. 2537), α2C-AR (cat. no. ab151618), PTK9 (cat. no. NBP2-37456; all 1:500) and GAPDH (cat. no. 2118; 1:1,000) were added and incubated overnight at 4°C. Subsequently, after washing with TBS-Tween-20 three times (×5 min), membranes were incubated with the horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary antibody (cat. no. 4030-05; 1:1,000; SouthernBiotech) for 1 h at room temperature. Immunoblot detection and visualization were performed using enhanced chemiluminescence western blotting detection reagents (SuperSignal™ West Pico PLUS Chemiluminescent Substrate; cat. no. 34577; Thermo Fisher Scientific, Inc.). Immunoblotting was performed with target antibodies and protein bands were scanned and quantified using a ChemiDoc image analysis system (Bio-Rad Laboratories, Inc.). ImageJ software (version 1.46; National Institute of Health) was used for densitometry analysis. Except for the PTK9 antibody, which was bought from Novus Biologicals, LLC and the α2C-AR, which was bought from Abcam, all primary antibodies were obtained from the Cell Signaling Technology, Inc.
Statistics
All experiments were performed in triplicate. Experimental data was expressed as the mean ± standard deviation. All data were statistically analyzed using GraphPad Prism 6.0 (GraphPad Software). One-way analysis of variance (ANOVA) or two-way ANOVA were used to compare multiple sets of means where appropriate. After homogeneity of variance test, the variance was used together with Dunnett test for pairwise comparisons. The Wilcoxon rank sum test was used to compare non-normally distributed data sets in non-parametric tests. Mann-Whitney U method was used to test the significance of the differences between the groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Higenamine exerts antispasmodic effects on cold-induced cutaneous vasoconstriction in rats
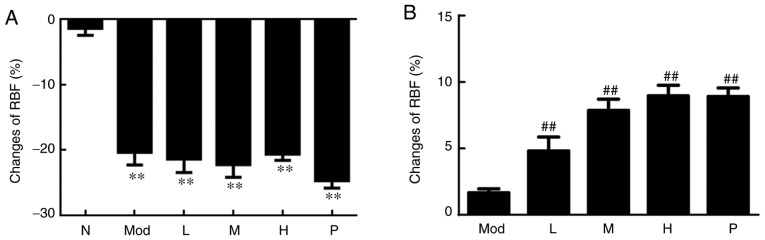
After modeling, as presented in Fig. 1A, the cortical RBF of the rat caudal artery region in the five groups was significantly decreased compared with the normal control group (P<0.01), indicating that the experimental model was successful. After drug administration, the changes of cortical RBF in the rat caudal artery cortex in the medium, high dose and the positive control groups (prostaglandin E1) were compared with the model group. It was observed that the medium and high doses of higenamine and the positive control group (prostaglandin E1) had significantly elevated cortical RBF of the rat caudal artery (P<0.01; Fig. 1B). These results indicated that higenamine improves cold-induced vasoconstriction.
Figure 1.
Effect of higenamine on RBF in cold-induced vasoconstriction rat model. (A) RBF changes in rat caudal arterial cortical region after modeling. Apart from rats in the normal control group, rats in the other groups were subjected to modelling and RBF in each group was measured without drug administration. (B) RBF changes in the rat caudal arterial cortical region after dosing. Statistical analysis was performed using the one-way analysis of variance followed by Dunnett's test analysis. **P<0.01 vs. the normal control group. ##P<0.01 vs. Mod. RBF, relative blood flow; Mod, the model group; N, the normal control group; L, the low dose higenamine group (18 µg·kg−1); M, the medium dose higenamine group (36 µg·kg−1); H, the high dose higenamine Group (72 µg·kg−1): P, the positive drug control group.
Effect of higenamine on PI3K/Akt and AMPK/eNOS/NO signaling pathways in hypothermic HDMECs
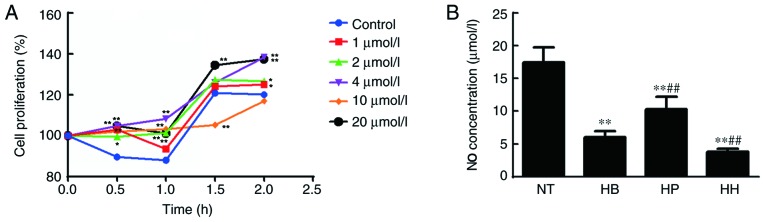
The MTT assay was used to detect the effect of higenamine on the proliferation of hypothermic HDMECs. Compared with the control group, the results shown in Fig. 2A indicated that 1, 2, 4 and 20 µmol/l of higenamine significantly increased cell proliferation at the time point of 0.5 h compared with the control group (P<0.01, except for 2 µmol/l at P<0.05). At 1 h, similar results were obtained (P<0.01). Moreover, at 1.5 h, 20 µmol/l of higenamine significantly increased while 10 µmol/l of higenamine significantly inhibited cell proliferation compared with the control group (P<0.01). At 2 h, 1, 2 (P<0.05), 4 and 20 µmol/l (P<0.01) of higenamine significantly increased cell proliferation ompared with the control group. The treatment concentration of 20 µmol/l and the incubation time of 120 min were the best concentration and incubation time, respectively. Therefore, these conditions were used for subsequent studies.
Figure 2.
Effect of higenamine on the proliferation of cold-treated HDMECs and NO production in vivo. (A) The proliferation rate of hypothermia HDMEC stimulated by higenamine were detected by MTT assay. *P<0.05, **P<0.01 vs. the control group. (B) NO concentration in the supernatant of HDMECs. Statistical analysis was performed using the one-way analysis of variance followed by Dunnett's test analysis. **P<0.01 vs. the NT group. ##P<0.01 vs. the HB group. HH, hypothermia+higenamine; HP, hypothermia+Positive control drug; HB, hypothermia; NT, Normal temperature; NO, nitric oxide; HDMECs, human dermal microvascular endothelial cells.
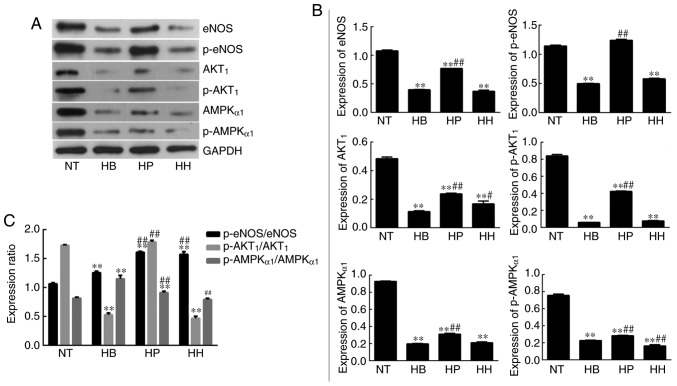
The concentration of NO in the supernatant of HDMEC cultures was detected by the nitrate reductase method. The results (Fig. 2B) demonstrated that the NO concentration in the hypothermia group was significantly decreased compared with the normal temperature group (P<0.05) but was not reversed by treatment with higenamine. This suggested that the production of NO in hypothermic HDMECs was suppressed and that cold may impair the production and function of NO in HDMECs. No statistically significant difference in NO concentration was found between the higenamine group and hypothermia group, indicating that higenamine could not reverse the NO inhibition caused by hypothermia in HDMECs. Compared with the hypothermia group, the NO concentration in the positive control group was significantly increased (P<0.01), suggesting that prostaglandin E1 moderated the inhibition of NO production in hypothermic HDMECs. Western blotting analysis demonstrated that the expression levels of eNOS, p-eNOS, Akt1, p-Akt1, AMPKα1 and p-AMPKα1 were significantly downregulated in the hypothermia group compared with the normal temperature group (P<0.01; Fig. 3). This indicated that hypothermia inhibits the PI3K/Akt and AMPK/eNOS signaling pathways in HDMECs. Compared with the hypothermia group, the expression of Akt1 was significantly increased (P<0.01) by treatment with higenamine. This suggested that higenamine stimulates the PI3K/Akt signaling pathway in hypothermic HDMECs. Compared with the hypothermia group, p-Akt1, eNOS, p-eNOS, AMPKα1, p-AMPKα1 were downregulated by higenamine (P<0.05), suggesting that higenamine inhibits the AMPK/eNOS signaling pathway in hypothermic HDMECs.
Figure 3.
Effect of higenamine on PI3K/Akt and AMPK/eNOS/NO signaling pathways in hypothermic human dermal microvascular endothelial cells. (A) Representative band images obtained from western blot analysis. (B) Densitometry analysis. (C) Graph demonstrating the relative expression ratio of phosphorylated over unphosphorylated proteins. Statistical analysis was performed using the one-way analysis of variance followed by Dunnett's test analysis. **P<0.01 vs. the NT group. #P<0.05 and ##P<0.01 vs. the HB group. HH, hypothermia+higenamine; HP, hypothermia+Positive control drug; HB, hypothermia; NT, Normal temperature; eNOS, endothelial nitric oxide synthase; AMPK, AMP-activated protein kinase; PI3K, phosphatidylinositol 3 kinase; p-Akt, phosphorylated protein kinase B; NO, nitric oxide.
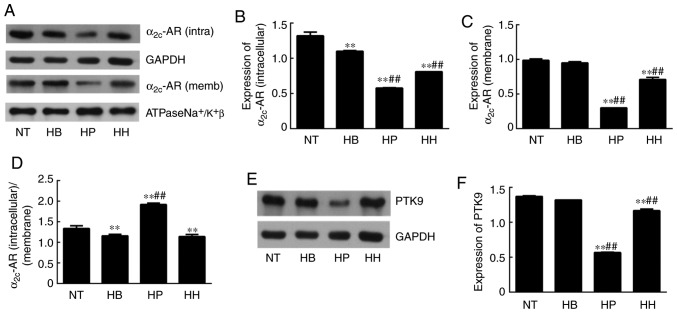
Effect of higenamine on ROS/α2C-AR and PTK9 signaling pathways in hypothermic HDMECs
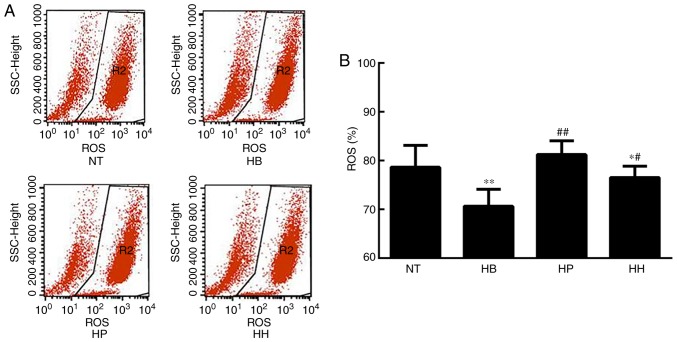
ROS analysis demonstrated that the level of ROS was significantly elevated in the hypothermic group compared with the normal temperature group (P<0.01; Fig. 4). Since ROS is an indicator of cellular oxidative stress, it was suggested that hypothermia induced oxidative stress in HDMECs. Compared with the hypothermic group, ROS levels were suppressed by treatment with higenamine and the positive control drug, suggesting that higenamine as well as prostaglandin E1 inhibited the accumulation of ROS (Fig. 4). This result suggested that higenamine prevented oxidative stress. To further elucidate the molecular mechanisms involved, western blotting analysis was performed and the results demonstrated that compared with the normal temperature group, the expression of α2C-AR (intracellular) was significantly downregulated in the positive control group (P<0.01), but the expression of α2C-AR (membrane) was significantly increased in the hypothermia group (P<0.05; Fig. 5) while the expression of α2C-AR (membrane) was downregulated by the positive control drug (P<0.01). Compared with the hypothermia group, the ratio of α2C-AR (intracellular)/α2C-AR (membrane) was significantly decreased in higenamine group (P<0.01). The ratio of α2C-AR (intracellular)/α2C-AR (membrane) was significantly decreased, suggesting that α2C-AR in hypothermic HDMECs translocated intracellularly to the membrane. This suggested that hypothermia may induce α2C-AR translocation, which transfers intracellularly to transmembrane and enhances the contraction of vascular smooth muscle. Compared with the normal temperature group, the expression of PTK9 was upregulated in the hypothermia group (P<0.05) and downregulated in the higenamine group (P<0.05). This implied that the expression of PTK9 was upregulated in cold-induced HDMECs but reversed by treatment with higenamine. As PTK9 is associated with actin binding, it was postulated that the activity of actin in cold-treated HDMECs was increased and that the contraction of smooth muscle was enhanced by hypothermic treatment. Compared with the hypothermia group, the expression of α2C-AR (intracellular or membrane) was significantly downregulated in the higenamine and the positive control group (P<0.05). The ratio of α2C-AR (intracellular)/α2C-AR (membrane) ratio significantly increased in the two groups (P<0.01). This indicated that higenamine and prostaglandin E1 can suppress the expression of intracellular and membrane α2C-AR, and prevent the translocation of α2C-AR from the cytoplasm to the membrane. Overall, compared with the normal temperature group, the expression of ROS/α2C-AR and PTK9 was increased in the hypothermia group (Fig. 5F), indicating that hypothermia can induce an oxidative stress response in the HDMECs, and initiate the translocation of the original α2C-AR from the cytoplasm to the membrane. Compared with the hypothermia group, higenamine treatment inhibited the activation of ROS/α2C-AR and PTK9 signaling pathways in the hypothermic HDMECs, suggesting that higenamine may inhibit the effect of cold-induced vasoconstriction.
Figure 4.
Effect of higenamine on ROS in hypothermic human dermal microvascular endothelial cells. (A) Representative band images obtained from flow cytometry analysis. (B) Fluorescence intensity from each group. Statistical analysis was performed using the one-way analysis of variance followed by Dunnett's test analysis. *P<0.05 and **P<0.01 vs. the NT group. #P<0.05 and ##P<0.01 vs. the HB group. HH, hypothermia+higenamine; HP, hypothermia+Positive control drug; HB, hypothermia; NT, Normal temperature; ROS, reactive oxygen species.
Figure 5.
Effect of higenamine on α2C-AR and PTK9 signaling pathways in hypothermic human dermal microvascular endothelial cells. (A) Representative band images obtained from western blot analysis of the expression of intracellular and membrane α2C-AR. Densitometry analysis of (B) intracellular and (C) membrane α2C-AR. (D) Evaluation of the α2C-AR (intracellular)/α2C-AR (membrane) ratio. (E) Western blot analysis and (F) densitometry of PTK9 expression. Statistical analysis was performed using the one-way analysis of variance followed by Dunnett's test analysis. **P<0.01 vs. the NT group. ##P<0.01 vs. the HB group. HH, hypothermia+higenamine; HP, hypothermia+Positive control drug; HB, hypothermia; NT, Normal temperature; AR, adrenoreceptor; PTK9, twinfilin-1.
Discussion
Previously, pharmacological studies have demonstrated that higenamine has a vasodilating effect (14,19,23), but its underlying mechanisms are not fully understood. This study was aimed to investigate the antispasmodic effect of higenamine on cold-induced vasoconstriction and the underlying mechanisms. An in vivo model of cold-induced cutaneous vasoconstriction in rats was established. The results indicated that cold-decreased the RBF of rat caudal arterial cortex, but the medium and high doses of higenamine reversed these effects. Although certain studies have reported the vasodilatory effect of higenamine, these previous studies have only focused on isolated aortas (23,25) and there have been no reports on medial arteries and arterioles. In the present study, it was demonstrated that establishing a cold-induced rat skin vasoconstriction in vivo model reduced the RBF of the rat caudal arterial cortex. Studies have demonstrated that in an animal model of cold blood stasis syndrome, microcirculatory flow decreases the diameter of the microvasculature and the flow velocity and flow rate would be impaired and suppressed (26–28). The RBF reduction in the cold-induced cutaneous vasoconstriction model is similar to that in the cold blood stasis syndrome model.
Higenamine can be found in a variety of medicinal herbs such as Aconite, Asarum, Galangal and Citrus aurantium. However, whether its vasodilation effect is related to cold has not been reported so far. In the present study, increasing doses of higenamine gradually increased the change in RBF, indicating that the effect of higenamine on cold-induced skin vasoconstriction in rats may be positively correlated with the dose. The in vivo studies of cardiovascular pathophysiology in the past mainly focused on the positive inotropic effects in the heart and cardiac electrophysiological effects of higenamine. The primary method is to measure the blood pressure and heart rate of the model (29,30). These studies also suggested the effect of higenamine on the cardiac pump function and electrophysiology.
Vascular endothelial cells (VECs) play an important role in regulating vascular homeostasis. The main functions of VECs are as follows: Endothelial cell barrier, secretion of multiple vasoactive substances and regulation of vascular tone (31). VECs secrete active substances that promote vasodilation and vasoconstriction (32). Whether the vasodilatory effect of higenamine on cold-induced vasoconstriction is endothelium-dependent is a major problem to be discussed. Few studies have been conducted on the effects of cold on microvascular endothelial cells. The HDMECs were used for the investigation of the mechanisms of cold-induced vasoconstriction. Previous studies indicated that the PI3K/Akt/mTOR signaling pathway and the AMPK/eNOS/NO signaling pathway play a key role in metabolism and is associated with vasodilation and increased blood flow (33–36). Akt/PKB phosphorylates eNOS at serine-1177 (Ser1177) and mediates the non-genetic rapid activation of eNOS and the AMPK-mediated signaling pathway also phosphorylates eNOS at the serine-1177 (Ser1177) site, causing vasodilation (37). However, it is rarely reported whether this signaling pathway is associated with cold-induced VED. This study found that the expression of PI3K/Akt and AMPK/eNOS/NO signaling pathways were downregulated in hypothermic HDMECs, demonstrating that cold can downregulate these signaling pathways in vascular endothelial cells. RP is mainly induced by cold and the pathogenesis of RP is not yet fully understood. RP is associated with excessive contraction of the terminal artery and previous studies have suggested that VED is the main pathological manifestation of RP (38–40). However, the mechanism of VED in RP is not fully understood. Downregulation of PI3K/Akt and AMPK/eNOS/NO signaling pathways in hypothermic HDMECs suggests that the pathogenesis of cold induced VED in RP may be related to the downregulation of PI3K/Akt and AMPK/eNOS/NO signaling pathways.
Studies have demonstrated that in the model of heart failure induced by doxorubicin, higenamine exerts antioxidative stress, cell apoptosis and protects cardiomyocytes by activating the PI3K/Akt signaling pathway (13,19,41). The results of the present study demonstrated that in the hypothermia group, the expression of Akt1 was increased while the expression of p-Akt1 was downregulated in the higenamine group. The results of the present study suggested that higenamine may improve the inhibition of PI3K/Akt signaling pathway in HDMECs at low temperature and the vasodilating effect of higenamine may have an endothelium-dependent effects.
The AMPK/eNOS/NO signaling pathway plays an important regulatory role in energy metabolism. NO is a vasodilating factor produced by endothelial cells, which mainly regulates vascular tone. In this study, it was demonstrated that the NO concentration and the expression levels of eNOS, p-NOS, AMPKα1, and p-AMPKα1 in the hypothermia positive control group (prostaglandin E1) were upregulated compared with the hypothermia group. This suggested that prostaglandin E1 can ameliorate the inhibition of the AMPK/eNOS/NO signaling pathway in hypothermic HDMECs and promote NO production in endothelial cells. In contrast, the NO concentration and the expression of eNOS, p-NOS, AMPKα1 and p-AMPKα1 in the hypothermia higenamine group was downregulated compared with the hypothermia group. This suggested that higenamine exacerbates the inhibition of the AMPK/eNOS/NO signaling pathway in HDMECs induced by hypothermia but inhibits NO production in endothelial cells. The PI3K/Akt and AMPK/eNOS/NO signaling pathways have significant regulatory functions in energy metabolism. Higenamine had an effect on the inhibition of the PI3K/Akt signaling pathway and NO production in cold-induced endothelial cells, but had no effect on the inhibition of AMPK/eNOS/NO signaling pathway. Therefore, the endothelium-dependent vasodilation effect of higenamine remains to be further studied.
Cold-induced vasoconstriction is associated with oxidative stress. Studies have found that cold induces the production and activation of ROS in endothelial cells, and promotes inflammatory responses in vivo (42). ROS levels are indicators of cellular oxidative stress. Under oxidative stress conditions, ROS production is increased and leads to subsequent changes in membrane lipids, proteins, and nucleic acids. Oxidative damage to these biomolecules is associated with aging and various pathological events, including atherosclerosis, tumorigenesis, ischemia-reperfusion injury and neurodegenerative diseases. In this experiment, it was demonstrated that the ROS level in the hypothermia group was increased compared with the normal temperature group. Compared with the hypothermia group, ROS level was decreased in the higenamine and positive control groups. Studies have found that cold causes a rapid increase of ROS in skin vascular smooth muscle cells (VSMCs), activates the Rho/Rho-kinase signaling pathway, leading to translocation of smooth muscle cell α2C-AR from the trans-Golgi apparatus to the extracellular membrane, therefore promoting distal vasoconstriction (43–45). Cold-induced vasoconstriction was also found to be associated with the production of ROS in the mitochondria through redox signaling in VSMCs, which activates RhoA/Rho kinase signaling and causes the original intracellular stationary α2C-ARs to migrate to the cell surface, resulting in contraction of vascular smooth muscle (46–48). In addition, it was reported that hypothermic stimulation can increase PTK activity in patients with RP and promote PTK phosphorylation. Furspan et al (49) found that increased PTK phosphorylation in RP patients mediates vasoconstriction caused by cold stimulation. In the present study, ROS/α2C-AR and PTK9 signaling pathways in HDMECs were investigated. Compared with the normal temperature group, the expression of α2C-AR (membrane) was upregulated in the hypothermia group and the ratio of α2C-AR (intracellular)/α2C-AR (the membrane) was decreased in the higenamine and the hypothermia groups, suggesting that the membrane α2C-AR production of HDMEC was increased and the ratio of α2C-AR (intracellular)/α2C-AR (cell membrane) was decreased. This data demonstrated that α2C-AR was translocated from the cytoplasm to the membrane in the hypothermic HDMECs, indicating that cold induced the α2C-AR translocation and enhanced the vascular smooth muscle contraction. Compared with the normal temperature group, the expression of PTK9 was downregulated in the hypothermia group, suggesting that cold-induced upregulation of PTK9 expression in HDMECs was associated with actin binding. ROS/α2C-AR and PTK9 signaling pathways, oxidative stress, α2C-AR translocation, and smooth muscle contraction were induced by hypothermia in HDMECs. The study found that the expression of α2C-AR (membrane) in the hypothermia higenamine group was decreased compared with normal temperature group. Compared with the hypothermia group, the expression of α2C-AR (intracellular) in the hypothermia higenamine group was also decreased. The ratio of α2C-AR (intracellular)/α2C-AR (membrane) was increased in the higenamine group, suggesting that the expression of intracellular and membrane α2C-AR was inhibited and the translocation of α2C-AR from the cytoplasm to the membrane was prevented. These observations suggested that higenamine has an inhibitory effect on the activation of ROS/α2C-AR and PTK9 signaling pathways in cold-induced HDMECs and inhibits cold-induced vasoconstriction.
Studies indicate that clinical-induction of hypothermia can improve the neurological function of unconscious subjects following unexpected cardiac arrest by preserving heart and brain stability (50–53). Recently, it has been reported that early application of mild hypothermia therapy not only improves the neurological outcome, but also increases the survival probability of patients postdischarge (54). The present study's findings indicated that higenamine and its regulated pathways could play a significant role in the mild hypothermia therapy. The analysis of temperature from a range of mild hypothermia therapies is of great significance in clinical application and is also important for in-depth elucidation in further studies. The present study has some limitations that need to be addressed in future studies. Firstly, the results of the present study demonstrated that the vasodilating effect of higenamine may not be related to the AMPK/eNOS/NO signaling pathway, which requires an in-depth investigation. Secondly, studies have demonstrated that higenamine can downregulate the expression of iNOS mRNA induced by lipopolysaccharide and has anti-inflammatory effects (55–57). In this study, it was found that the expression of eNOS/NO was downregulated by higenamine in hypothermic HDEMCs. Therefore, whether there is an inhibitory effect of higenamine on different types of NOS requires further study. Finally, as an emerging drug, the research on the dosage and pharmacokinetics of higenamine in vitro and in vivo are encouraged.
Higenamine may reverse the inhibition of the PI3K/Akt signaling pathway in hypothermic HDMECs, however it may not have regulatory roles in the inhibition of the AMPK/eNOS/NO signaling pathway. The ROS/α2C-AR and PTK9 signaling pathways were upregulated in the hypothermic HDMECs. Cold can induce the oxidative stress in the vascular smooth muscle and strengthen the contraction function. Higenamine inhibits the activation of ROS/α2C-AR and PTK9 signaling pathways in hypothermic HDMECs and may play an important role in inhibiting the oxidative stress in cold-induced vasoconstriction. The results of the present study will boost the modernization of traditional Chinese medicine and has clinical and scientific relevance.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science Foundation of China (grant no. 81874404).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
GP Zhao designed the study. JH Guan, HM Lin, MJ Xie, MN Huang, D Zhang, SS Ma and WY Bian carried out experiments. HM Lin, JH Guan and MJ Xie analyzed data. JH Guan, HM Lin and GP Zhao interpreted results of experiments. JH Guan, HM Lin, MJ Xie, MN Huang and D Zhang prepared the figures. HM Lin and JH Guan wrote the manuscript. All authors contributed equally. All authors read and approved the final manuscript.
Ethics approval
Animal studies were performed in accordance with the declaration of Helsinki and approved by the institutional Ethics Committee of the School of traditional Chinese medicine, Jinan University, Guangzhou (China).
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.al-Awami M, Schillinger M, Minar E. Vasospasm of the scrotum-a manifestation of Raynaud's phenomenon? VASA. 2004;33:87–88. doi: 10.1024/0301-1526.33.2.87. [DOI] [PubMed] [Google Scholar]
- 2.Barr WG, Fahey PJ. Reduction of pulmonary capillary blood volume following cold exposure in patients with Raynaud's phenomenon. Chest. 1988;94:1195–1199. doi: 10.1378/chest.94.6.1195. [DOI] [PubMed] [Google Scholar]
- 3.Belch JJ, Land D, Park RH, McKillop JH, MacKenzie JF. Decreased oesophageal blood flow in patients with Raynaud's phenomenon. Br J Rheumatol. 1988;27:426–430. doi: 10.1093/rheumatology/27.6.426. [DOI] [PubMed] [Google Scholar]
- 4.Brouwer RM, Wenting GJ, Schalekamp MA. Acute effects and mechanism of action of ketanserin in patients with primary Raynaud's phenomenon. J Cardiovasc Pharmacol. 1990;15:868–876. doi: 10.1097/00005344-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Brown S. Diagnosis and management of patients with Raynaud's phenomenon. Nurs Stand. 2012;26:41–46. doi: 10.7748/ns2012.07.26.46.41.c9214. [DOI] [PubMed] [Google Scholar]
- 6.Engelhart M, Seibold JR. The effect of local temperature versus sympathetic tone on digital perfusion in Raynaud's phenomenon. Angiology. 1990;41:715–723. doi: 10.1177/000331979004100906. [DOI] [PubMed] [Google Scholar]
- 7.Fardoun MM, Nassif J, Issa K, Baydoun E, Eid AH. Raynaud's Phenomenon: A brief review of the underlying mechanisms. Front Pharmacol. 2016;7:438. doi: 10.3389/fphar.2016.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes M, Herrick AL. Raynaud's phenomenon. Best Pract Res Clin Rheumatol. 2016;30:112–132. doi: 10.1016/j.berh.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Kuryliszyn-Moskal A, Kita J, Hryniewicz A. Raynaud's phenomenon: New aspects of pathogenesis and the role of nailfold videocapillaroscopy. Reumatologia. 2015;53:87–93. doi: 10.5114/reum.2015.51508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An X, Long C, Deng X, Tang A, Xie J, Chen L, Wang Z. Higenamine inhibits apoptosis and maintains survival of gastric smooth muscle cells in diabetic gastroparesis rat model via activating the beta2-AR/PI3K/AKT pathway. Biomed Pharmacother. 2017;95:1710–1717. doi: 10.1016/j.biopha.2017.08.112. [DOI] [PubMed] [Google Scholar]
- 11.Bai G, Yang Y, Shi Q, Liu Z, Zhang Q, Zhu YY. Identification of higenamine in Radix Aconiti Lateralis Preparata as a beta2-adrenergic receptor agonist1. Acta Pharmacol Sin. 2008;29:1187–1194. doi: 10.1111/j.1745-7254.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- 12.Bao YX, Yu GR, Xu JM, Xu YQ, Bian YT, Zheng DS. Effect of acute higenamine administration on bradyarrhythmias and HIS bundle. A clinical study of 14 cases and animal experiment on dogs. Chin Med J (Engl) 1982;95:781–784. [PubMed] [Google Scholar]
- 13.Chen YL, Zhuang XD, Xu ZW, Lu LH, Guo HL, Wu WK, Liao XX. Higenamine combined with [6]-gingerol suppresses doxorubicin-triggered oxidative stress and apoptosis in cardiomyocytes via upregulation of PI3K/Akt pathway. Evid Based Complement Alternat Med. 2013;2013:970490. doi: 10.1155/2013/970490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang N, Lian Z, Peng X, Li Z, Zhu H. Applications of Higenamine in pharmacology and medicine. J Ethnopharmacol. 2017;196:242–252. doi: 10.1016/j.jep.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Stajić A, Anđelković M, Dikić N, Rašić J, Vukašinović-Vesić M, Ivanović D, Jančić-Stojanović B. Determination of higenamine in dietary supplements by UHPLC/MS/MS method. J Pharm Biomed Anal. 2017;146:48–52. doi: 10.1016/j.jpba.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Zhang N, Qu K, Wang M, Yin Q, Wang W, Xue L, Fu H, Zhu H, Li Z. Identification of higenamine as a novel alpha1-adrenergic receptor antagonist. Phytother Res. 2019;33:708–717. doi: 10.1002/ptr.6261. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Geng J, Jiang M, Li C, Han Y, Jiang J. The cardiac electrophysiology effects of higenamine in guinea pig heart. Biomed Pharmacother. 2019;109:2348–2356. doi: 10.1016/j.biopha.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Cohen PA, Travis JC, Keizers PHJ, Boyer FE, Venhuis BJ. The stimulant higenamine in weight loss and sports supplements. Clin Toxicol (Phila) 2019;57:125–130. doi: 10.1080/15563650.2018.1497171. [DOI] [PubMed] [Google Scholar]
- 19.Wu MP, Zhang YS, Zhou QM, Xiong J, Dong YR, Yan C. Higenamine protects ischemia/reperfusion induced cardiac injury and myocyte apoptosis through activation of β2-AR/PI3K/AKT signaling pathway. Pharmacol Res. 2016;104:115–123. doi: 10.1016/j.phrs.2015.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan W, Chen J, Wu Y, Zhang Y, Xu Y. Protective effect of higenamine ameliorates collagen-induced arthritis through heme oxygenase-1 and PI3K/Akt/Nrf-2 signaling pathways. Exp Ther Med. 2016;12:3107–3112. doi: 10.3892/etm.2016.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, Zhu C, Wang G, Xu R, Zhu Y. Higenamine regulates Nrf2-HO-1-Hmgb1 axis and attenuates intestinal ischemia-reperfusion injury in mice. Inflamm Res. 2015;64:395–403. doi: 10.1007/s00011-015-0817-x. [DOI] [PubMed] [Google Scholar]
- 22.Lee YS, Kang YJ, Kim HJ, Park MK, Seo HG, Lee JH, Yun-Choi HS, Chang KC. Higenamine reduces apoptotic cell death by induction of heme oxygenase-1 in rat myocardial ischemia-reperfusion injury. Apoptosis. 2006;11:1091–1100. doi: 10.1007/s10495-006-7110-y. [DOI] [PubMed] [Google Scholar]
- 23.Chang KC, Chong WS, Lee IJ. Different pharmacological characteristics of structurally similar benzylisoquinoline analogs, papaverine, higenamine, and GS 389, on isolated rat aorta and heart. Can J Physiol Pharmacol. 1994;72:327–334. doi: 10.1139/y94-049. [DOI] [PubMed] [Google Scholar]
- 24.Luo D, Xu Z, Hu X, Zhang F, Bian H, Li N, Wang Q, Lu Y, Zheng Q, Gu J. URI prevents potassium dichromate-induced oxidative stress and cell death in gastric cancer cells. Am J Transl Res. 2016;8:5399–5409. [PMC free article] [PubMed] [Google Scholar]
- 25.Chang KC, Lim JK, Park CW. Pharmacological evaluation of GS-389, a novel tetrahydroisoquinoline analog related to higenamine, on vascular smooth muscle. Life Sci. 1992;51:67–74. doi: 10.1016/0024-3205(92)90220-J. [DOI] [PubMed] [Google Scholar]
- 26.Hao EW, Deng JG, Du ZC, Yan K, Zheng ZW, Wang Q, Huang LZ, Bao CH, Deng XQ, Lu XY, Tang ZL. Experimental study on two-way application of traditional Chinese medicines capable of promoting blood circulation and removing blood stasis with neutral property in cold and hot blood stasis syndrome I. Zhongguo Zhong Yao Za Zhi. 2012;37:3302–3306. (In Chinese) [PubMed] [Google Scholar]
- 27.Ryan TJ, Copeman PW. Microvascular pattern and blood stasis in skin disease. Br J Dermatol. 1969;81:563–573. doi: 10.1111/j.1365-2133.1969.tb16039.x. [DOI] [PubMed] [Google Scholar]
- 28.Ning SY, Jiang BP, Xu L, Fang TH, Wu MH. Effect of Liangxuehuayu Recipe on hemorheology in rats with blood stasis syndrome. Asian Pac J Trop Med. 2012;5:935–938. doi: 10.1016/S1995-7645(12)60177-1. [DOI] [PubMed] [Google Scholar]
- 29.Kimura I, Makino M, Takamura Y, Islam MA, Kimura M. Positive chronotropic and inotropic effects of higenamine and its enhancing action on the aconitine-induced tachyarrhythmia in isolated murine atria. Jpn J Pharmacol. 1994;66:75–80. doi: 10.1254/jjp.66.75. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Liu X, Tao Z, Shi R, Zhang X, Yao Z, Liu Y, Zhu K, Chen B. Effects of higeramine on hemodynamics and its tolerability and safety, an experimental study. Zhonghua Yi Xue Za Zhi. 2002;82:352–355. (In Chinese) [PubMed] [Google Scholar]
- 31.Michiels C. Endothelial cell functions. J Cell Physiol. 2003;196:430–443. doi: 10.1002/jcp.10333. [DOI] [PubMed] [Google Scholar]
- 32.Brandes RP, Schmitz-Winnenthal FH, Félétou M, Gödecke A, Huang PL, Vanhoutte PM, Fleming I, Busse R. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc Natl Acad Sci USA. 2000;97:9747–9752. doi: 10.1073/pnas.97.17.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang PC, Ng YF, Ho S, Gyda M, Chan SW. Resveratrol and cardiovascular health-promising therapeutic or hopeless illusion? Pharmacol Res. 2014;90:88–115. doi: 10.1016/j.phrs.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Fu J, Han Y, Wang J, Liu Y, Zheng S, Zhou L, Jose PA, Zeng C. Irisin lowers blood pressure by improvement of endothelial dysfunction via AMPK-Akt-eNOS-NO pathway in the spontaneously hypertensive rat. J Am Heart Assoc. 2016;5:e003433. doi: 10.1161/JAHA.116.003433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M, Hara Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun. 2011;408:339–343. doi: 10.1016/j.bbrc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 36.Fujimura N, Jitsuiki D, Maruhashi T, Mikami S, Iwamoto Y, Kajikawa M, Chayama K, Kihara Y, Noma K, Goto C, Higashi Y. Geranylgeranylacetone, heat shock protein 90/AMP-activated protein kinase/endothelial nitric oxide synthase/nitric oxide pathway, and endothelial function in humans. Arterioscler Thromb Vasc Biol. 2012;32:153–160. doi: 10.1161/ATVBAHA.111.237263. [DOI] [PubMed] [Google Scholar]
- 37.Thors B, Halldórsson H, Thorgeirsson G. Thrombin and histamine stimulate endothelial nitric-oxide synthase phosphorylation at Ser1177 via an AMPK mediated pathway independent of PI3K-Akt. FEBS Lett. 2004;573:175–180. doi: 10.1016/j.febslet.2004.07.078. [DOI] [PubMed] [Google Scholar]
- 38.Liu W, Liu JY, Yin ZY, Long CL, Wang H. The characteristics of vascular endothelial injuries induced by extreme environmental factors. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2013;29:494–500. [PubMed] [Google Scholar]
- 39.Turton EPL, Kent PJ, Kester RC. VASCULAR REVIEW: The aetiology of Raynaud's phenomenon. Cardiovasc Surgery. 1998;6:431–440. doi: 10.1016/S0967-2109(98)00054-4. [DOI] [PubMed] [Google Scholar]
- 40.Matucci-Cerinic M, Kahaleh B, Wigley FM. Review: Evidence that systemic sclerosis is a vascular disease. Arthritis Rheum. 2013;65:1953–1962. doi: 10.1002/art.37988. [DOI] [PubMed] [Google Scholar]
- 41.Cao Y, Ruan Y, Shen T, Huang X, Li M, Yu W, Zhu Y, Man Y, Wang S, Li J. Astragalus polysaccharide suppresses doxorubicin-induced cardiotoxicity by regulating the PI3k/Akt and p38MAPK pathways. Oxid Med Cell Longev. 2014;2014:674219. doi: 10.1155/2014/674219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Awad EM, Khan SY, Sokolikova B, Brunner PM, Olcaydu D, Wojta J, Breuss JM, Uhrin P. Cold induces reactive oxygen species production and activation of the NF-kappa B response in endothelial cells and inflammation in vivo. J Thromb Haemost. 2013;11:1716–1726. doi: 10.1111/jth.12357. [DOI] [PubMed] [Google Scholar]
- 43.Maeng J, Sheverdin V, Shin H, Ha I, Bae SS, Yang-Yen HF, Lee K. Up-regulation of Rhoa/Rho kinase pathway by translationally controlled tumor protein in vascular smooth muscle cells. Int J Mol Sci. 2014;15:10365–10376. doi: 10.3390/ijms150610365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeyaraj SC, Unger NT, Eid AH, Mitra S, Paul El-Dahdah N, Quilliam LA, Flavahan NA, Chotani MA. Cyclic AMP-Rap1A signaling activates RhoA to induce α(2c)-adrenoceptor translocation to the cell surface of microvascular smooth muscle cells. Am J Physiol Cell Physiol. 2012;303:C499–C511. doi: 10.1152/ajpcell.00461.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: Role of alpha2C-adrenoceptor translocation. Circ Res. 2004;94:1367–1374. doi: 10.1161/01.RES.0000128407.45014.58. [DOI] [PubMed] [Google Scholar]
- 46.Eid AH, Chotani MA, Mitra S, Miller TJ, Flavahan NA. Cyclic AMP acts through Rap1 and JNK signaling to increase expression of cutaneous smooth muscle alpha2C-adrenoceptors. Am J Physiol Heart Circ Physiol. 2008;295:H266–H272. doi: 10.1152/ajpheart.00084.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Honda M, Suzuki M, Nakayama K, Ishikawa T. Role of alpha2C-adrenoceptors in the reduction of skin blood flow induced by local cooling in mice. Br J Pharmacol. 2007;152:91–100. doi: 10.1038/sj.bjp.0707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jantschak F, Popp AM, Hofmann RA, Villalón CM, Centurión D, Pertz HH. Postjunctional α2C-adrenoceptors mediate vasoconstriction in rat tail artery: Influence of precontraction and temperature on vasoreactivity. Naunyn Schmiedebergs Arch Pharmacol. 2010;382:487–497. doi: 10.1007/s00210-010-0564-z. [DOI] [PubMed] [Google Scholar]
- 49.Furspan PB, Chatterjee S, Mayes MD, Freedman RR. Cooling-induced contraction and protein tyrosine kinase activity of isolated arterioles in secondary Raynaud's phenomenon. Rheumatology (Oxford) 2005;44:488–494. doi: 10.1093/rheumatology/keh517. [DOI] [PubMed] [Google Scholar]
- 50.Leonov Y, Sterz F, Safar P, Radovsky A, Oku K, Tisherman S, Stezoski SW. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab. 1990;10:57–70. doi: 10.1038/jcbfm.1990.8. [DOI] [PubMed] [Google Scholar]
- 51.Arbour RB. Brain death: Assessment, controversy, and confounding factors. Critical Care Nurse. 2013;33:27–46. doi: 10.4037/ccn2013215. [DOI] [PubMed] [Google Scholar]
- 52.Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: Effects of posttraumatic hypothermia. J Neurochem. 1995;65:1704–1711. doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- 53.Geocadin RG, Koenig MA, Jia X, Stevens RD, Peberdy MA. Management of brain injury after resuscitation from cardiac arrest. Neurol Clin. 2008;26(ix):487–506. doi: 10.1016/j.ncl.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bradley SM, Liu W, McNally B, Vellano K, Henry TD, Mooney MR, Burke MN, Brilakis ES, Grunwald GK, Adhaduk M, et al. Temporal trends in the use of therapeutic hypothermia for out-of-hospital cardiac arrest. JAMA Netw Open. 2018;1:e184511. doi: 10.1001/jamanetworkopen.2018.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee HY, Lee JS, Kim EJ, Han JW, Lee HW, Kang YJ, Chang KC. Inhibition of lipopolysaccharide-induced inducible nitric oxide (iNOS) mRNA expression and nitric oxide production by higenamine in murine peritoneal macrophages. Arch Pharm Res. 1999;22:55–59. doi: 10.1007/BF02976436. [DOI] [PubMed] [Google Scholar]
- 56.Kang YJ, Lee YS, Lee GW, Lee DH, Ryu JC, Yun-Choi HS, Chang KC. Inhibition of activation of nuclear factor kappaB is responsible for inhibition of inducible nitric oxide synthase expression by higenamine, an active component of aconite root. J Pharmacol Exp Ther. 1999;291:314–320. [PubMed] [Google Scholar]
- 57.Park JE, Kang YJ, Park MK, Lee YS, Kim HJ, Seo HG, Lee JH, Hye Sook YC, Shin JS, Lee HW, et al. Enantiomers of higenamine inhibit LPS-induced iNOS in a macrophage cell line and improve the survival of mice with experimental endotoxemia. Int Immunopharmacol. 2006;6:226–233. doi: 10.1016/j.intimp.2005.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.