Abstract
With the current carbapenem-resistant organism crisis, conventional approaches to optimizing pharmacokinetic-pharmacodynamic parameters are frequently inadequate, and traditional salvage agents (eg, colistin, tigecycline, etc) confer high toxicity and/or have low efficacy. However, several β-lactam agents with activity against carbapenem-resistant organisms were approved recently by the US Food and Drug Administration, and more are anticipated to be approved in the near future. The primary goal of this review is to assist infectious disease practitioners with preferentially selecting 1 agent over another when treating patients infected with a carbapenem-resistant organism. However, resistance to some of these antibiotics has already developed. Antibiotic stewardship programs can ensure that they are reserved for situations in which other options are lacking and are paramount for the survival of these agents.
Keywords: aztreonam-avibactam, cefiderocol, ceftazidime-avibactam, ceftolozane-tazobactam, imipenem-cilastatin–relebactam, meropenem-vaborbactam
Several β-lactam agents with activity against carbapenem-resistant organisms have been approved by the US Food and Drug Administration, and more are in the pipeline. The goal of this review is to assist infectious disease practitioners with understanding when to preferentially select 1 agent over another when treating patients infected with a carbapenem-resistant organism.
Carbapenem-resistant Gram-negative organisms continue to pose a serious clinical threat, and few treatment options are available [1]. However, a number of β-lactam antibiotics with activity against these organisms are currently in or recently completed phase III studies in the United States. As clinical trials in adults continue, studies investigating dosage and infusion strategies for optimizing pharmacokinetics and pharmacodynamics in children are being explored or have begun for all of these agents.
In this article, we provide a brief overview of mechanisms of carbapenem resistance followed by a discussion of recently approved β-lactam agents (ie, ceftazidime-avibactam, ceftolozane-tazobactam, and meropenem-vaborbactam) and agents that, at the time this review was prepared, have not yet obtained US Food and Drug Administration (FDA) approval (eg, aztreonam-avibactam, cefiderocol, and imipenem-cilastatin–relebactam [referenced herein as imipenem-relebactam]) along with their role in the treatment of infections caused by carbapenem-resistant Enterobacteriaceae (CRE), carbapenem-resistant Pseudomonas aeruginosa, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia. The primary goal of this review is to assist infectious disease practitioners in preferentially selecting 1 agent over another when treating patients infected with a carbapenem-resistant organism. Information on pharmacokinetics and pharmacodynamics will generally not be addressed. Because ongoing studies are being conducted for all of these agents, particularly those not yet approved by the FDA, we anticipate that our understanding of the role of these antibiotics will continue to evolve over the next several months to years.
OVERVIEW OF MECHANISMS OF CARBAPENEM RESISTANCE
Carbapenem resistance occurs as a consequence of a number of heterogenous mechanisms [2]. Carbapenemase enzymes, which hydrolyze the β-lactam ring of carbapenem antibiotics, are common to Enterobacteriaceae and A baumannii. Carbapenemase producers account for slightly less than 50% of CRE strains in the United States; approximately 95% of carbapenemases are Klebsiella pneumoniae carbapenemases (KPCs), and the remainder belong to the New Delhi metallo-β-lactamases (NDMs) or oxacillinase-48-like (OXA-48-like) carbapenemase group [3, 4]. KPCs and OXA-48-like carbapenemases are serine carbapenemases, and NDMs, along with Verona integron-encoded metallo-β-lactamases (VIMs) and imipenemases (IMPs), are common metallo-β-lactamase (MBL) carbapenemases, named as such because they require the presence of zinc at their active site to function [5]. The remainder of carbapenem resistance in Enterobacteriaceae is generally caused by the production of extended-spectrum β-lactamases (ESBLs) and/or AmpC β-lactamases (AmpCs), in combination with reduced porin expression (eg, Ompk35 mutation, Ompk36 mutation, etc) [6] or overexpression of efflux pumps (eg, the AcrAB–TolC efflux pump) [7].
The mechanisms of carbapenem resistance in glucose-nonfermenting organisms differ according to the organism. Carbapenem-resistant P aeruginosa strains generally evolve because of an interplay of multiple complex mechanisms, including mutations in OprD porins, hyperproduction of AmpCs, upregulation of efflux pumps, and mutations in penicillin-binding proteins [8]. Carbapenemases are an infrequent mechanism behind carbapenem-resistant P aeruginosa in the United States [8] and are found more commonly in other regions of the world such as Europe, Asia, and Latin America; VIM carbapenemases are responsible for approximately 11% of carbapenem-resistant P aeruginosa infections in Europe [9], 12% of overall P aeruginosa infections (regardless of carbapenem susceptibility) from Asia [10], and up to 19% of carbapenem-resistant infections in Latin America [11]. Carbapenem resistance in A baumannii strains in both the United States and abroad is generally the result of the production of class D carbapenemases, with OXA-23-like, OXA-40-like, OXA-58-like, and OXA-143-like carbapenemases commonly implicated [8]. S maltophilia has a chromosomally mediated MBL, L1 β-lactamase, that renders this organism intrinsically resistant to carbapenems [12]. A number of phenotypic and genotypic tests are available to clinical microbiology laboratories for identifying carbapenemase production by Gram-negative organisms and the specific carbapenemase(s) produced [13, 14]. Because the newer β-lactams exhibit unique profiles in their activity against some carbapenemases but not others, we believe that the role of the clinical microbiology laboratory in identifying both the presence of a carbapenemase as well as the specific carbapenemase gene is becoming increasingly important for guiding effective treatment decisions.
AZTREONAM-AVIBACTAM
Spectrum of Activity
Aztreonam is known for its ability to withstand hydrolysis by MBL carbapenemases. Aztreonam, however, is generally susceptible to hydrolysis by serine β-lactamases, including ESBLs, AmpCs, KPCs, and OXA-48-like carbapenemases, which is concerning because plasmids that contain MBL genes generally also harbor genes that encode several of these other β-lactamases [5]. Avibactam is a β-lactamase inhibitor that is not susceptible to hydrolysis by ESBLs, AmpCs, KPCs, or OXA-48-like carbapenemases and therefore overcomes the shortcomings of aztreonam [15, 16]. Together, the combination of aztreonam and avibactam provides broad coverage against a wide range of β-lactamase–producing Enterobacteriaceae (Figure 1). More specifically, in a large surveillance study that included clinical isolates from both the United States and abroad, the minimum inhibitory concentrations required to inhibit the growth of 90% of organisms (MIC90) for aztreonam-avibactam against KPC producers (n = 102), MBL producers (n = 59), and OXA-48-like producers (n = 57) were ≤0.50 µg/mL for all of these carbapenemase-producing Enterobacteriaceae [17]. A separate international collection of isolates yielded similar results [18]. MBL producers can be particularly challenging to treat given the limited number of agents with activity against them. Aztreonam-avibactam has been found to be 8- to 32-fold more potent than meropenem against MBL-producing Enterobacteriaceae [9]. Furthermore, in vitro data have suggested that aztreonam-avibactam is also effective against isolates that simultaneously produce both serine and MBL carbapenemases [19].
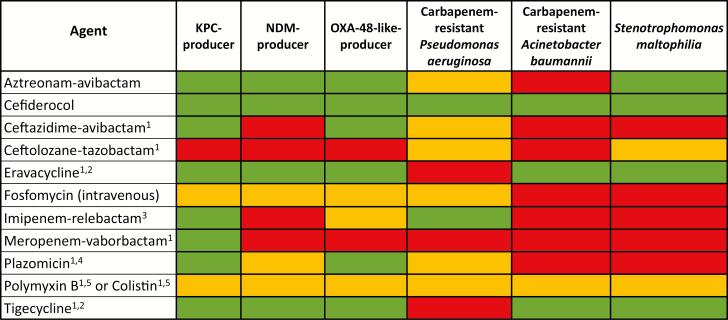
Figure 1.
Select antibiotics with activity against carbapenem-resistant organisms. Green, susceptibility anticipated to be >80%; yellow, susceptibility anticipated to be 30% to 80%; red, intrinsic resistance or susceptibility anticipated to be <30%. 1, US Food and Drug Administration–approved agent; 2, synthetic tetracycline derivative; 3, imipenem-cilastatin–relebactam; 4, synthetic aminoglycoside; 5, polymyxin class. Abbreviations: KPC, Klebsiella pneumoniae carbapenemase; NDM, New Delhi metallo-β-lactamase.
The activity of aztreonam-avibactam against P aeruginosa is less reliable [9, 18]. In a collection of 11 842 international clinical isolates of P aeruginosa, the MIC90 of aztreonam-avibactam was 32 µg/mL regardless of whether the organisms were or were not producing MBLs [9]. The poor activity of aztreonam-avibactam against P aeruginosa highlights the multiple complex resistance mechanisms likely to be concurrently present in this organism. Because MBL production is intrinsic to S maltophilia, aztreonam-avibactam will generally provide coverage against this organism [20]. In contrast, the addition of avibactam to aztreonam is unlikely to restore susceptibility to aztreonam-resistant A baumannii [18].
Clinical Data
A phase II prospective nonrandomized study in which 36 hospitalized adults with complicated intra-abdominal infections (cIAIs) treated with aztreonam-avibactam was recently completed (ClinicalTrials.gov identifier NCT02655419); the results are still pending. Because the main objective of this study was to understand the safety and tolerability of aztreonam-avibactam, targeted enrollment of patients infected with CRE was not undertaken. A phase III randomized controlled trial (RCT) is currently enrolling adults with a serious Gram-negative infection, including those with cIAIs, hospital-acquired pneumonia (HAP), or ventilator-associated pneumonia (VAP); these participants are being randomly assigned to receive aztreonam-avibactam with or without metronidazole or meropenem with or without colistin (ClinicalTrials.gov identifier NCT03329092). A subgroup analysis to evaluate patients infected with CRE is planned. An additional phase III RCT will focus specifically on serious infections caused by MBL-producing organisms and compare aztreonam-avibactam versus best available therapy (ClinicalTrials.gov identifier NCT03580044).
Potential Role
Overall, familiarity with both aztreonam and avibactam in children and adults makes aztreonam-avibactam an attractive treatment option. Furthermore, it can be administered safely to patients with CRE infections and severe penicillin allergies, when the use of other β-lactams should be avoided. In vitro data have suggested that aztreonam-avibactam has reliable activity against carbapenemase-producing Enterobacteriaceae and is indifferent to the type of carbapenemase produced (Figure 1). Furthermore, this agent is anticipated to provide coverage against S maltophilia, when first-line agents such as trimethoprim-sulfamethoxazole or ceftazidime are not active or when drug allergies preclude their use.
CEFIDEROCOL
Mechanism of Action
The innate immune system minimizes available free iron in response to bacterial infections (“nutritional immunity”), because iron is an essential cation for bacterial growth [21]. Most iron is bound to hemoglobin, myoglobin, or iron-binding proteins [21]. In response to reduced host availability of iron, bacteria upregulate the production of siderophores, which are high-affinity iron-chelating compounds that scavenge for available free iron [21].
Cefiderocol is an injectable siderophore cephalosporin. It binds to iron via a catechol moiety, using a “Trojan horse” approach to gain entry into bacteria by capitalizing on available active iron-transport systems [21, 22]. Once across the outer membrane, cefiderocol dissociates from the iron molecule and binds to penicillin-binding proteins, which disrupts cell-wall synthesis [22]. This unique mechanism of cell entry ensures transport into bacterial cells even in the presence of porin channel loss and overexpression of efflux pumps [22]. Furthermore, the structure of cefiderocol ensures that it is highly stable against hydrolysis from both serine carbapenemases and MBLs [23]. Experimental data have suggested that a deficiency of the iron transporter PiuA in P aeruginosa or CirA and Fiu in Escherichia coli causes a 16-fold increase in cefiderocol MICs, indicating that these iron transporters contribute to the permeation of cefiderocol across the outer membrane [24].
Spectrum of Activity
Cefiderocol confers activity against a broad range of highly drug-resistant Gram-negative organisms (Figure 1). In iron-depleted cation-adjusted Mueller–Hinton broth preparations, the antibacterial activity of cefiderocol remained favorable against 753 clinical isolates of multidrug-resistant Gram-negative organisms, including both serine carbapenemase and MBL-producing Enterobacteriaceae, P aeruginosa, and A baumannii [25]. Only colistin and tigecycline had comparable activity against many of these isolates (recognizing tigecycline’s notable gap of pseudomonal coverage). However, cefiderocol has a more appealing pharmacokinetic-pharmacodynamic profile over either of these agents. In addition, cefiderocol has potent in vitro activity against S maltophilia and members of the Burkholderia cepacia complex [24, 26].
Clinical Data
In a multicenter double-blind RCT that included 371 adults with complicated urinary tract infections (cUTIs), cefiderocol met the noninferiority composite clinical and microbiological end points when compared with imipenem-cilastatin; 73% and 55% of patients in the cefiderocol and imipenem-cilastatin groups, respectively, achieved this end point (ClinicalTrials.gov identifier NCT02321800) [27]. Patients infected with a carbapenem-resistant organism represented less than 3% of the study population. Emergence of resistance to cefiderocol was not investigated. Phase III studies evaluating the role of cefiderocol, administered as an extended 3-hour infusion are current underway; one compared with best available therapy for the treatment of carbapenem-resistant pathogens from a variety of sources, and another compared with meropenem for nosocomial pneumonia (ClinicalTrials.gov identifiers NCT02714595 and NCT03032380).
Although cefiderocol seems to be an exciting addition to the antibiotic armamentarium, it is important to proceed with caution, because unknowns remain in the use of siderophore antibiotics. The experience with MB-1, a siderophore monobactam conjugate, serves as a cautionary tale [28]. In vitro data against a cohort of P aeruginosa isolates seemed promising. However, in a neutropenic mouse thigh model, variable efficacy of MB-1 against P aeruginosa isolates was observed, and the correlation between in vitro MB-1 MICs and the corresponding level of MB-1 efficacy in vivo was limited [28]. Investigators hypothesized that increases in endogenously produced P aeruginosa siderophores downregulated other siderophore receptors, including those used by MB-1 [28]. It is fortunate that cefiderocol differs from MB-1 structurally, and potent activity of cefiderocol has been observed in a murine neutropenic thigh model [29].
Potential Role
Cefiderocol circumvents common resistance mechanisms. Moreover, cross-resistance to cefiderocol is anticipated to be low because of its unique mechanism of action. For patients at high risk of infection from extremely drug-resistant organisms, this agent offers broad empiric Gram-negative coverage. All other agents discussed in this review fail to provide comprehensive coverage against CRE (regardless of the specific mechanism of resistance), carbapenem-resistant P aeruginosa, carbapenem-resistant A baumannii, and S maltophilia. The fact that clinical experience with siderophore antibiotics is still in its nascency is an important concern. The adverse event profile will not be well understood until more clinical data are available.
CEFTAZIDIME-AVIBACTAM
Spectrum of Activity
Avibactam is a synthetic β-lactamase inhibitor that binds reversibly to β-lactamases [30] and, unlike older inhibitors (eg, tazobactam, sulbactam, clavulanate), has activity against carbapenemases. Various in vitro studies have found the activity of ceftazidime-avibactam against KPC-producing organisms to consistently be well over 95% [31–34]. However, resistance has been observed in KPC-2– and KPC-3–producing isolates and is generally caused by decreased porin expression [35–44]. Perhaps of even greater concern is that resistance has emerged during exposure to ceftazidime-avibactam therapy, most frequently because of an amino acid substitution within or proximal to the omega loop of the KPC enzyme [40, 43, 45–47]. Study results have suggested that the emergence of resistance during ceftazidime-avibactam therapy occurs approximately 10% of the time [43, 46]. Interestingly, some of the mutations that confer resistance to ceftazidime-avibactam can reduce the carbapenemase activity of KPC-3, resulting in lower carbapenem MICs and restoring the susceptibility of these isolates to carbapenems [43, 46, 48]. However, this restored carbapenem activity is generally not sustainable [49].
Avibactam is able to reinstate the activity of ceftazidime against isolates that produce OXA-48-like enzymes [15, 50] (Figure 1). Although OXA-48-like enzymes only weakly hydrolyze ceftazidime, they generally exist in an environment in which multiple other β-lactamases are present. Ceftazidime-avibactam also provides enhanced activity against carbapenem-resistant P aeruginosa. In various studies, ceftazidime-avibactam was active against 67% to 88% of meropenem-nonsusceptible P aeruginosa isolates [33, 51]. The addition of avibactam to ceftazidime does not improve its activity against carbapenem-resistant A baumannii or S maltophilia. However, when ceftazidime-avibactam is used in combination with aztreonam, an inhibitor of MBLs, activity against S maltophilia can be restored [20, 52].
Clinical Data
Ceftazidime-avibactam received approval from the FDA in February 2015 for the treatment of cUTIs and for the treatment of cIAIs when used in combination with metronidazole [53] (Table 1). A phase I study to evaluate pharmacokinetics, safety, and tolerability of ceftazidime-avibactam in healthy 3-month to <18-year-olds [54] and a phase II study comparing ceftazidime-avibactam with metronidazole versus meropenem in children with cIAIs in the same age range informed the dosing recommendations outlined in Table 1 (ClinicalTrials.gov identifiers NCT01893346 and NCT02475733).
Table 1.
Dosing Under Evaluation for Novel β-Lactam Agents That Target Carbapenem-Resistant Gram-Negative Organisms
| Novel β-Lactam | Dose (Assuming Normal Renal Function) | Data Informing Dosing | |
|---|---|---|---|
| Adult | Pediatrica | ||
| Aztreonam-avibactam | Aztreonam 6500 mg with avibactam 2167 mg (loading dose, extended loading dose and maintenance dose) by IV infusion on day 1, followed by a total daily dose of aztreonam 6000 mg with avibactam 2000 mg | TBD | Adult dosing informed by an ongoing phase III study (ClinicalTrials.gov identifier NCT03329092) for the treatment of serious infections, including those caused by a metallo-β-lactamase producing bacterium; dosing here differs from that in a completed phase II study that evaluated aztreonam-avibactam for generally susceptible IAI (ClinicalTrials.gov identifier NCT02655419) |
| Cefiderocol | Cefiderocol 2000 mg IV q8h infused over 3 hours | TBD | Adult dosing informed by an ongoing phase III study (ClinicalTrials.gov identifier NCT02714595) of carbapenem-resistant infections, which is the same dose used in an ongoing phase III pneumonia study (ClinicalTrials.gov identifier NCT03032380). A completed phase II UTI study used the same adult dosing shown in column 2 but as 1-hour infusions (ClinicalTrials.gov identifier NCT02321800). |
| Ceftazidime-avibactam | Ceftazidime 2000 mg with avibactam 500 mg IV q8h infused over 2 hours | 3 months to <6 months: ceftazidime 40 mg/kg per dose with avibactam 10 mg/kg per dose IV q8h infused over 2 hours; 6 months to <18 years of age: ceftazidime 50 mg/kg per dose with avibactam 12.5 mg/kg per dose IV q8h infused over 2 hours | Adult dosing is the FDA-approved dose; pediatric dosing informed by a completed phase I study (ClinicalTrials.gov identifier NCT01893346) and completed phase II IAI study (ClinicalTrials.gov identifier NCT02475733) of children aged 3 months to <18 years |
| Ceftolozane-tazobactam | UTI and IAI: ceftolozane 1000 mg with tazobactam 500 mg IV q8h; pneumonia: ceftolozane 2000 mg with tazobactam 1000 mg IV q8h | >32 weeks gestational age and >/=7 days postnatal age to <18 years old, for UTI and IAI: ceftolozane 20 mg/kg per dose with tazobactam 10 mg/kg per dose IV q8h; for pneumonia: ceftolozane 40 mg/kg per dose with tazobactam 20 mg/kg per dose IV q8h | Adult dosing for UTI and IAI is the FDA-approved dose; adult pneumonia dosing here was informed by a completed phase III pneumonia study in adults (ClinicalTrials.gov identifier NCT02070757); because epithelial lining fluid concentrations of ceftolozane are approximately 50% of serum concentrations, dosages were doubled in the pneumonia study; pediatric dosing for ceftolozane-tazobactam to treat pneumonia was extrapolated from the adult data; pediatric UTI and IAI dosing here is being investigated in an ongoing pediatric phase II UTI study (ClinicalTrials.gov identifier NCT03230838) and ongoing pediatric phase II IAI study (ClinicalTrials.gov identifier NCT03217136) |
| Imipenem-cilastatin–relebactam | Imipenem 500 mg with cilastatin 500 mg with relebactam 250 mg IV q6h | 1 month to <18 years old: imipenem 15 mg/kg per dose with cilastatin 15 mg/kg per dose with relebactam 7.5 mg/kg per dose IV q6h | Adult dosing informed by a completed phase III study of carbapenem-resistant infections in adults (ClinicalTrials.gov identifier NCT02452047); pediatric dosing is being investigated in an ongoing phase I study (ClinicalTrials.gov identifier NCT03230916). Based on experience with dosing imipenem-cilastatin in infants and children, these doses can be considered for infants 1 month of age and older until additional data become available. |
| Meropenem-vaborbactam | Meropenem 2000 mg with vaborbactam 2000 mg IV q8h, infused over 3 hours | 1 month to <18 years old: meropenem 40 mg/kg per dose with vaborbactam 40 mg/kg per dose IV q8h, infused over 3 hours | Adult dosing is the FDA-approved dose; pediatric dosing is being investigated in an ongoing phase I study (ClinicalTrials.gov identifier NCT02687906). Based on experience with dosing meropenem in infants and children, these doses can be considered for infants 1 month of age and older until additional data become available. |
Abbreviations: FDA, US Food and Drug Administration; IAI, intra-abdominal infection; IV, intravenous; q6h, every 6 hours; q8h, every 8 hours; TBD, to be determined; UTI, urinary tract infection.
aPediatric dose should not exceed the adult dose for the specified indication.
Phase II clinical trials in adults consisted of a study that compared ceftazidime-avibactam and imipenem-cilastatin for cUTIs [55] and a study that compared ceftazidime-avibactam (with metronidazole) to meropenem for cIAIs [56]. Both studies met the predetermined clinical and microbiological noninferiority end points. Phase III trials in adults include a study that compared ceftazidime-avibactam to doripenem for the treatment of cUTIs [57] and a study that compared ceftazidime-avibactam (with metronidazole) to meropenem for cIAIs [58]; again, both studies met the clinical and microbiological noninferiority end points. CRE infections were not evaluated for any of the aforementioned phase II or III studies. A subsequent phase III study compared ceftazidime-avibactam versus best available therapy for patients with cUTIs or cIAIs caused by a ceftazidime-resistant Enterobacteriaceae or P aeruginosa in a randomized open-label trial [59]; 97% of those in the best-available-therapy group received a carbapenem. Clinical outcomes in the treatment groups were similar [59]. Finally another phase III study found ceftazidime-avibactam to be noninferior to meropenem for the treatment of adults with HAP or VAP [60].
Postmarketing surveillance from observational studies that include patients with infections caused by carbapenem-resistant organisms is becoming increasingly available. These studies are a welcome addition to the literature because they investigate the performance of ceftazidime-avibactam in clinical scenarios in which the agent is likely to be prescribed. Shields et al [61] evaluated the outcomes of patients with KPC-producing bloodstream infections and compared ceftazidime-avibactam (n = 13), carbapenems plus aminoglycosides (n = 25), carbapenems plus colistin (n = 30), and a variety of other combinations (n = 41). Patients who received ceftazidime-avibactam had significantly better clinical outcomes and survival compared to patients who received any of the other regimens. Moreover, the risk of acute kidney injury was lower with ceftazidime-avibactam than with other combinations. A separate observational study that compared 137 patients receiving either ceftazidime-avibactam or colistin-based regimens for CRE infections from a variety of sources found ceftazidime-avibactam to be superior to colistin for the 30-day all-cause mortality outcome [62].
Potential Role
Ceftazidime-avibactam remains an excellent choice for treating infections caused by non–MBL-producing CRE. However, while the availability of aztreonam-avibactam is pending, ceftazidime-avibactam can be used in combination with aztreonam to treat infections caused by an MBL-producing organism. While ceftolozane-tazobactam remains the primary consideration out of existing commercially available agents for carbapenem-resistant P aeruginosa infections, it is reasonable to test ceftazidime-avibactam against such organisms. In 1 cohort of patients, most of whom had carbapenem-resistant P aeruginosa infections, 62% of the isolates were susceptible to ceftazidime-avibactam, whereas 73% were susceptible to ceftolozane-tazobactam [63]. Among ceftolozane-tazobactam–resistant isolates, 9% were susceptible to ceftazidime-avibactam, whereas 36% of the ceftazidime-avibactam–resistant isolates were susceptible to ceftolozane-tazobactam [63]. The biggest concern with ceftazidime-avibactam is the reports of emergence of resistance in KPC-producing organisms that seem remarkably consistent across preclinical and postmarketing studies, which raises concerns about the continued effectiveness of this agent once it is prescribed with increasing frequency.
CEFTOLOZANE-TAZOBACTAM
Spectrum of Activity
Ceftolozane-tazobactam has the most potent antipseudomonal activity compared with other commercially available β-lactam–β-lactamase inhibitor combinations. Farrell et al [64] evaluated ceftolozane-tazobactam activity against 1019 P aeruginosa isolates from the United States and Europe. Ceftolozane-tazobactam was active against 78% of the carbapenem-resistant P aeruginosa isolates. Activity of ceftolozane-tazobactam against P aeruginosa strains varies between the United States and Europe, partly because of the higher prevalence of VIM-producing P aeruginosa strains in Europe. Activities were 93% and 57%, respectively, against US and European extensively drug-resistant P aeruginosa isolates. In an evaluation of 720 meropenem-nonsusceptible P aeruginosa isolates from European hospitals, 58% were susceptible to ceftolozane-tazobactam [65]. In a separate cohort of 42 US carbapenem-resistant P aeruginosa isolates, ceftolozane-tazobactam remained active against 95% of isolates; whereas, ceftazidime-avibactam remained active against 71% of the same isolates [66].
The activity of ceftolozane-tazobactam against P aeruginosa isolates from patients with cystic fibrosis, which frequently display a mucoid phenotype, is less reliable. Although some investigations have reported activity against a high percentage of P aeruginosa isolates recovered from the lungs of patients with cystic fibrosis [67, 68], when specifically evaluated against extensively drug-resistant P aeruginosa isolates from patients with cystic fibrosis, ceftolozane-tazobactam was active against 30% to 54% of isolates [69, 70]. Ceftolozane-tazobactam susceptibility of S maltophilia isolates from patients with cystic fibrosis has been reported to range from 0% to 85%, using the P aeruginosa breakpoint [67, 69, 71] (Figure 1). Ceftolozane-tazobactam has limited to no activity against CRE, MBL-producing P aeruginosa, or carbapenem-resistant A baumannii [72]. The emergence of resistance in P aeruginosa during ceftolozane-tazobactam therapy has been reported. In 1 study, 14% of adults with multidrug-resistant P aeruginosa infection experienced emergence of ceftolozane-tazobactam resistance during therapy [73], attributed mainly to de novo mutations that affect AmpC expression.
Clinical Data
Ceftolozane-tazobactam was approved in December 2014 by the FDA for the treatment of cUTIs and cIAIs in patients aged ≥18 years [74]. Phase II RCTs are currently underway to investigate the safety and efficacy of ceftolozane-tazobactam versus meropenem in children with cUTIs (ClinicalTrials.gov identifier NCT03230838) and (in combination with metronidazole) cIAIs (ClinicalTrials.gov identifier NCT03217136).
Available phase II and III clinical trial data indicate that ceftolozane-tazobactam is safe and effective compared to commonly prescribed agents for both cUTIs [75, 76] and cIAIs [77, 78]. These studies were unable to provide data on its role for the treatment of carbapenem-resistant organisms. A phase III clinical study of ceftolozane-tazobactam versus meropenem for adult patients with VAP was recently completed, and preliminary reports [79] indicate that ceftolozane-tazobactam met the pre-specified non-inferiority clinical end points of mortality and clinical cure (ClinicalTrials.gov identifier NCT02070757).
Potential Role
Ceftolozane-tazobactam is a reasonable consideration for patients infected with carbapenem-resistant P aeruginosa, with greater activity observed for isolates from patients without cystic fibrosis, compared to patients with cystic fibrosis. The fact that a significant proportion of P aeruginosa isolates resistant to all other antipseudomonal β-lactams exhibit elevated ceftolozane-tazobactam MIC values is concerning, particularly as little progress in the development of new anti-infectives targeting P aeruginosa has been made.
IMIPENEM-CILASTATIN–RELEBACTAM
Spectrum of Activity
Relebactam is a novel β-lactamase inhibitor that is structurally related to avibactam [80, 81]. Similar to avibactam, it provides potent activity against KPC producers [82, 83]. In a collection of clinical isolates from New York City, imipenem was active against 9% of 111 KPC-producing K pneumoniae isolates, whereas imipenem-relebactam was active against 97% of these isolates [82]. Imipenem-relebactam does not have activity against MBL carbapenemases, regardless of whether they are produced by Enterobacteriaceae or P aeruginosa [84]. Available data indicate that its activity against OXA-48-like carbapenemases is poor, and more in vitro testing to evaluate its specific role against OXA-48-like carbapenemases is needed [84]. Given the similarities between avibactam and relebactam, the limited to no restoration of activity against OXA-48-like producers is intriguing. In an evaluation of 27 imipenem-nonsusceptible K pneumoniae isolates, imipenem-relebactam restored activity against 74% of the isolates [85]. The 7 isolates to which both imipenem and imipenem-relebactam were inactive produced either VIM-type, OXA-48-like, or the class A GES-type carbapenemases [85].
In addition to activity against KPCs, imipenem-relebactam has excellent activity against carbapenem-resistant P aeruginosa [82, 83, 86, 87]. The enhanced activity of imipenem-relebactam compared to that of imipenem is largely a result of the ability of relebactam to inhibit the imipenem-hydrolyzing AmpC enzymes commonly produced by this species [84]. Moreover, imipenem is a poor substrate for efflux pumps common to P aeruginosa, which makes it an attractive option for conjugating to relebactam [84]. In the previously described cohort of New York City isolates, imipenem-relebactam was active against 92% of imipenem-resistant P aeruginosa isolates [82]. In a separate investigation, relebactam restored imipenem susceptibility for 81% of 251 imipenem-nonsusceptible P aeruginosa isolates [85]. It is notable that imipenem-relebactam does not seem to have enhanced activity against A baumannii isolates that are resistant to imipenem [82, 85] and does not have activity against S maltophilia [82, 84].
Clinical Data
A phase I study is currently underway to investigate the pharmacokinetics, safety, and tolerability of single doses of imipenem-relebactam for children less than 18 years of age with confirmed or suspected Gram-negative infections (ClinicalTrials.gov identifier NCT03230916) (Table 1). Phase II studies evaluated the performance of imipenem-relebactam versus that of imipenem against cUTIs (ClinicalTrials.gov identifier NCT01505634) [88] and, separately, cIAIs (ClinicalTrials.gov identifier NCT01506271) [89] in adults. Both studies included predominantly imipenem-susceptible isolates, and outcomes were similar between the comparators. The phase II cIAI study included 34 patients with imipenem-resistant infections, all of whom had favorable outcomes [89]. A phase III study compared imipenem-relebactam versus imipenem in combination with colistin for HAP/VAP, cIAIs, and cUTIs caused by imipenem-resistant organisms (ClinicalTrials.gov identifier NCT02452047). Preliminary results demonstrated 28-day clinical responses of 71% and 40% in the imipenem-relebactam and imipenem-colistin arms, respectively, and all-cause mortality of 10% in the imipenem-relebactam group and 30% in the imipenem-colistin group [90]. A phase III study is enrolling patients with HAP and VAP comparing imipenem-relebactam versus piperacillin-tazobactam (ClinicalTrials.gov identifier NCT02493764).
Potential Role
Our long-standing experience with β-lactams in general, and imipenem specifically, makes imipenem-relebactam an appealing option. KPC-producing and carbapenem-resistant P aeruginosa strains—particularly carbapenem-resistant P aeruginosa—pose the most likely threat among Gram-negative pathogens for hospitals of all sizes in the United States [91], and imipenem-relebactam restores activity against both of them. This spectrum of activity is an attractive feature of imipenem-relebactam compared to those of other β-lactam–β-lactamase inhibitor combinations that are currently available on the US market (Figure 1).
MEROPENEM-VABORBACTAM
Spectrum of Activity
Meropenem-vaborbactam consists of an injectable synthetic carbapenem and a boronic acid β-lactamase inhibitor [92]. In a collection of 991 KPC-producing Enterobacteriaceae isolates, vaborbactam restored meropenem activity against 99% of the isolates [93]. In an evaluation of 133 KPC-producing clinical isolates between 2013 and 2014 from patients in New York City, meropenem-vaborbactam had activity against 99% of KPC-producing K pneumoniae isolates [94]. Meropenem-vaborbactam MICs remain elevated against MBL- or OXA-48-like–producing Enterobacteriaceae. In addition, this combination has lower activity against Enterobacteriaceae that exhibit decreased porin expression (Ompk35, Ompk36, Ompk37) [94–97] and/or elevated expression of the AcrAB–TolC efflux system [96, 97]. Resistance to meropenem-vaborbactam seems rare and less frequent than resistance to ceftazidime-avibactam, but this may change with increased clinical use [97–99]. On the basis of preliminary reports [93], cross-resistance between meropenem-vaborbactam and ceftazidime-avibactam is anticipated to occur for approximately 20% of isolates. Vaborbactam does not enhance the activity of meropenem against meropenem-resistant P aeruginosa or meropenem-resistant A baumannii [94, 100] (Table 1).
Clinical Data
A phase I study to evaluate single-dose pharmacokinetics, safety, and tolerability of meropenem-vaborbactam in children is currently underway (ClinicalTrials.gov identifier NCT02687906) (Table 1). Because of the completion of clinical trials for a similar agent (biapenem-RPX7009), evaluation of meropenem-vaborbactam proceeded directly to phase III studies in adults. In a phase III cUTI trial that included patients treated with meropenem-vaborbactam or piperacillin-tazobactam, clinical success rates were similar between the groups (ClinicalTrials.gov identifier NCT02166476) [101]. Meropenem-vaborbactam was subsequently approved by the FDA for the treatment of patients aged 18 years or older with cUTI caused by Enterobacteriaceae [102]. A subsequent phase III study evaluated meropenem-vaborbactam in 77 adults with cUTIs, cIAIs, HAP/VAP, or bacteremia caused by a CRE (ClinicalTrials.gov identifier NCT02168946) [103]. Better clinical outcomes were observed with meropenem-vaborbactam than with best available therapy; clinical cure at the end of therapy was achieved in 66% of patients in the meropenem-vaborbactam group and 33% in the best available therapy group, and 28-day mortality rates were 16% and 33% in the meropenem-vaborbactam and best available therapy arms, respectively. Finally, results from a phase III study performed to compare meropenem-vaborbactam and piperacillin-tazobactam for patients with nosocomial pneumonia is under evaluation (ClinicalTrials.gov identifier NCT03006679).
Potential Role
Although meropenem-vaborbactam has excellent activity against KPC-producing isolates, its activity against other carbapenemase-producing Enterobacteriaceae is negligible. However, because resistance to ceftazidime-avibactam, the only other β-lactam–β-lactamase inhibitor combination currently available to treat KPC-producing Enterobacteriaceae infections, is emerging, meropenem-vaborbactam provides a valuable treatment option for organisms that produce KPCs. Head-to-head comparisons of clinical outcomes of meropenem-vaborbactam and ceftazidime-avibactam are not currently available. Although as of August 2018 approval is limited to adults, pediatric dosing for meropenem-vaborbactam is currently being studied (Table 1).
CONCLUSION
With the current crisis of carbapenem-resistant organisms, conventional approaches to optimizing pharmacokinetic-pharmacodynamic parameters such as extended-infusion carbapenem administration are frequently inadequate. Similarly, traditional salvage agents, including polymyxins and tigecycline, confer high toxicity and have low efficacy. However, several β-lactam agents with activity against carbapenem-resistant organisms were approved recently, and more are anticipated to be approved in the near future. Administration of the newer β-lactams as monotherapy versus combination therapy (ie, addition of aminoglycoside, polymyxin, etc) has not been evaluated rigorously. Phase III studies that have evaluated their use for the treatment of carbapenem-resistant infections (ClinicalTrials.gov identifiers NCT02452047 and NCT02168946) and observational studies published since FDA approval of ceftazidime-avibactam, ceftolozane-tazobactam, and meropenem-vaborbactam have overwhelmingly reported favorable outcomes when these agents are used as monotherapy [61, 62, 99, 104, 105]. The general consensus is that when a strain is susceptible, these agents can be used without the routine addition of a second agent, even for invasive infections. It is unfortunate that resistance to some of these newer agents is already being observed. Antibiotic stewardship programs are paramount to the survival of these agents, because they can ensure that these agents are reserved for situations in which other, more commonly used antibiotics are inactive.
Notes
Financial support. This work was supported by funding from National Institutes of Health grant K23-AI127935 awarded to P. D. T. P. D. T. has received investigator-initiated research funding from Merck, with all work and funding completed in June 2018, and unrelated to this work.
Potential conflicts of interest. Both authors: No reported conflicts of interest. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bonomo RA, Burd EM, Conly J, et al. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis 2018; 66:1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 2007; 20:440–58, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012–2013. JAMA 2015; 314:1479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tamma PD, Goodman KE, Harris AD, et al. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis 2017; 64:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Logan LK, Bonomo RA. Metallo-β-lactamase (MBL)-producing Enterobacteriaceae in United States children. Open Forum Infect Dis 2016; 3:ofw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsai YK, Fung CP, Lin JC, et al. Klebsiella pneumoniae outer membrane porins OmpK35 and OmpK36 play roles in both antimicrobial resistance and virulence. Antimicrob Agents Chemother 2011; 55:1485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Du D, Wang Z, James NR, et al. Structure of the AcrAB-TolC multidrug efflux pump. Nature 2014; 509:512–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gniadek TJ, Carroll KC, Simner PJ. Carbapenem-resistant non-glucose-fermenting Gram-negative bacilli: the missing piece to the puzzle. J Clin Microbiol 2016; 54:1700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karlowsky JA, Kazmierczak KM, de Jonge BLM, et al. In vitro activity of aztreonam-avibactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated by clinical laboratories in 40 countries from 2012 to 2015. Antimicrob Agents Chemother 2017; 61:e00472-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karlowsky JA, Kazmierczak KM, Bouchillon SK, et al. In vitro activity of ceftazidime-avibactam against clinical isolates of Enterobacteriaceae and Pseudomonas aeruginosa collected in Asia-Pacific countries: results from the INFORM global surveillance program, 2012 to 2015. Antimicrob Agents Chemother 2018; 62:e02569-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Escandón-Vargas K, Reyes S, Gutiérrez S, Villegas MV. The epidemiology of carbapenemases in Latin America and the Caribbean. Expert Rev Anti Infect Ther 2017; 15:277–97. [DOI] [PubMed] [Google Scholar]
- 12. Chang YT, Lin CY, Chen YH, Hsueh PR. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol 2015; 6:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tamma PD, Simner PJ.Phenotypic detection of carbapenemase-producing organisms from clinical isolates. J Clin Microbiol 2018; 56:e01140–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rood IGH, Li Q. Review: molecular detection of extended spectrum-β-lactamase- and carbapenemase-producing Enterobacteriaceae in a clinical setting. Diagn Microbiol Infect Dis 2017; 89:245–50. [DOI] [PubMed] [Google Scholar]
- 15. Livermore DM, Mushtaq S, Warner M, et al. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2011; 55:390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lahiri SD, Johnstone MR, Ross PL, et al. Avibactam and class C β-lactamases: mechanism of inhibition, conservation of the binding pocket, and implications for resistance. Antimicrob Agents Chemother 2014; 58:5704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sader HS, Mendes RE, Pfaller MA, et al. Antimicrobial activities of aztreonam-avibactam and comparator agents against contemporary (2016) clinical Enterobacteriaceae isolates. Antimicrob Agents Chemother 2018; 62:e01856-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Biedenbach DJ, Kazmierczak K, Bouchillon SK, et al. In vitro activity of aztreonam-avibactam against a global collection of Gram-negative pathogens from 2012 and 2013. Antimicrob Agents Chemother 2015; 59:4239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chew KL, Tay MKL, Cheng B, et al. Aztreonam-avibactam combination restores susceptibility of aztreonam in dual-carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2018; 62:e00414-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mojica MF, Papp-Wallace KM, Taracila MA, et al. Avibactam restores the susceptibility of clinical isolates of Stenotrophomonas maltophilia to aztreonam. Antimicrob Agents Chemother 2017; 61:e00777-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ito A, Nishikawa T, Matsumoto S, et al. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2016; 60:7396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tillotson GS. Trojan horse antibiotics—a novel way to circumvent Gram-negative bacterial resistance? Infect Dis (Auckl) 2016; 9:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ito-Horiyama T, Ishii Y, Ito A, et al. Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob Agents Chemother 2016; 60:4384–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito A, Sato T, Ota M, et al. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrob Agents Chemother 2018; 62:e01454-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dobias J, Dénervaud-Tendon V, Poirel L, Nordmann P. Activity of the novel siderophore cephalosporin cefiderocol against multidrug-resistant Gram-negative pathogens. Eur J Clin Microbiol Infect Dis 2017; 36:2319–27. [DOI] [PubMed] [Google Scholar]
- 26. Hackel MA, Tsuji M, Yamano Y, et al. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of Gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother 2018; 62:e01968-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Portsmouth S, van Veenhuyzen D, Echols R, et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2018; 18:1319–28. [DOI] [PubMed] [Google Scholar]
- 28. Tomaras AP, Crandon JL, McPherson CJ, et al. Adaptation-based resistance to siderophore-conjugated antibacterial agents by Pseudomonas aeruginosa. Antimicrob Agents Chemother 2013; 57:4197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ghazi IM, Monogue ML, Tsuji M, Nicolau DP. Pharmacodynamics of cefiderocol, a novel siderophore cephalosporin, in a Pseudomonas aeruginosa neutropenic murine thigh model. Int J Antimicrob Agents 2018; 51:206–12. [DOI] [PubMed] [Google Scholar]
- 30. Zhanel GG, Lawson CD, Adam H, et al. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Drugs 2013; 73:159–77. [DOI] [PubMed] [Google Scholar]
- 31. Castanheira M, Mills JC, Costello SE, et al. Ceftazidime-avibactam activity tested against Enterobacteriaceae isolates from U.S. hospitals (2011 to 2013) and characterization of β-lactamase-producing strains. Antimicrob Agents Chemother 2015; 59:3509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sader HS, Castanheira M, Flamm RK. Antimicrobial activity of ceftazidime-avibactam against Gram-negative bacteria isolated from patients hospitalized with pneumonia in U.S. Medical Centers, 2011 to 2015. Antimicrob Agents Chemother 2017; 61:e02083-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sader HS, Castanheira M, Farrell DJ, et al. Ceftazidime-avibactam activity when tested against ceftazidime-nonsusceptible Citrobacter spp., Enterobacter spp., Serratia marcescens, and Pseudomonas aeruginosa from Unites States medical centers (2011-2014). Diagn Microbiol Infect Dis 2015; 83:389–94. [DOI] [PubMed] [Google Scholar]
- 34. Castanheira M, Mendes RE, Sader HS. Low frequency of ceftazidime-avibactam resistance among Enterobacteriaceae isolates carrying blaKPC collected in U.S. Hospitals from 2012 to 2015. Antimicrob Agents Chemother 2017; 61:e02369-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Satlin MJ, Chen L, Patel G, et al. Multicenter clinical and molecular epidemiological analysis of bacteremia due to carbapenem-resistant Enterobacteriaceae (CRE) in the CRE epicenter of the United States. Antimicrob Agents Chemother 2017; 61:e02349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haidar G, Clancy CJ, Chen L, et al. Identifying spectra of activity and therapeutic niches for ceftazidime-avibactam and imipenem-relebactam against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 2017; 61:e00642-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen Z, Ding B, Ye M, et al. High ceftazidime hydrolysis activity and porin OmpK35 deficiency contribute to the decreased susceptibility to ceftazidime/avibactam in KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother 2017; 72:1930–6. [DOI] [PubMed] [Google Scholar]
- 38. Humphries RM, Hemarajata P. Resistance to ceftazidime-avibactam in Klebsiella pneumoniae due to porin mutations and the increased expression of KPC-3. Antimicrob Agents Chemother 2017; 61:e00537-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Humphries RM, Yang S, Hemarajata P, et al. First report of ceftazidime-avibactam resistance in a KPC-3-expressing Klebsiella pneumoniae isolate. Antimicrob Agents Chemother 2015; 59:6605–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giddins MJ, Macesic N, Annavajhala MK, et al. Successive emergence of ceftazidime-avibactam resistance through distinct genomic adaptations in blaKPC-2-harboring Klebsiella pneumoniae sequence type 307 isolates. Antimicrob Agents Chemother 2018; 62:e02101-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Castanheira M, Arends SJR, Davis AP, et al. Analyses of a ceftazidime-avibactam-resistant Citrobacter freundii isolate carrying blaKPC-2 reveals a heterogenous population and reversible genotype. mSphere 2018; 3:e00408-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gaibani P, Campoli C, Lewis RE, et al. In vivo evolution of resistant subpopulations of KPC-producing Klebsiella pneumoniae during ceftazidime/avibactam treatment. J Antimicrob Chemother 2018; 73:1525–9. [DOI] [PubMed] [Google Scholar]
- 43. Shields RK, Potoski BA, Haidar G, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 2016; 63:1615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shields RK, Nguyen MH, Press EG, et al. Emergence of ceftazidime-avibactam resistance and restoration of carbapenem susceptibility in Klebsiella pneumoniae carbapenemase-producing K pneumoniae: a case report and review of literature. Open Forum Infect Dis 2017; 4:ofx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Winkler ML, Papp-Wallace KM, Bonomo RA. Activity of ceftazidime/avibactam against isogenic strains of Escherichia coli containing KPC and SHV β-lactamases with single amino acid substitutions in the Ω-loop. J Antimicrob Chemother 2015; 70:2279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shields RK, Chen L, Cheng S, et al. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 2017; 61:e02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lomovskaya O, Castanheira M, Vazquea J, et al. Assessment of MIC increases with meropenem-vaborbactam and ceftazidime-avibactam in Tango II (a Phase 3 study of the treatment of CRE infections) [Abstract 1874]. In: ID Week 2017, San Diego. [Google Scholar]
- 48. Haidar G, Clancy CJ, Shields RK, et al. Mutations in blaKPC-3 that confer ceftazidime-avibactam resistance encode novel KPC-3 variants that function as extended-spectrum beta-lactamases. Antimicrob Agents Chemother 2017; 61:e02534-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shields RK, Nguyen MH, Press EG, et al. In vitro selection of meropenem resistance among ceftazidime-avibactam-resistant, meropenem-susceptible Klebsiella pneumoniae isolates with variant KPC-3 carbapenemases. Antimicrob Agents Chemother 2017; 61:e00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aktaş Z, Kayacan C, Oncul O. In vitro activity of avibactam (NXL104) in combination with β-lactams against Gram-negative bacteria, including OXA-48 β-lactamase-producing Klebsiella pneumoniae. Int J Antimicrob Agents 2012; 39:86–9. [DOI] [PubMed] [Google Scholar]
- 51. Alatoom A, Elsayed H, Lawlor K, et al. Comparison of antimicrobial activity between ceftolozane-tazobactam and ceftazidime-avibactam against multidrug-resistant isolates of Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Int J Infect Dis 2017; 62:39–43. [DOI] [PubMed] [Google Scholar]
- 52. Mojica MF, Ouellette CP, Leber A, et al. Successful treatment of bloodstream infection due to metallo-β-lactamase-producing Stenotrophomonas maltophilia in a renal transplant patient. Antimicrob Agents Chemother 2016; 60:5130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Allergan. Ceftazidime/avibactam package insert Available at: https://www.allergan.com/assets/pdf/avycaz_pi. Accessed August 30, 2018.
- 54. Bradley JS, Armstrong J, Arrieta A, et al. Phase I study assessing the pharmacokinetic profile, safety, and tolerability of a single dose of ceftazidime-avibactam in hospitalized pediatric patients. Antimicrob Agents Chemother 2016; 60:6252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vazquez JA, González Patzán LD, Stricklin D, et al. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin 2012; 28:1921–31. [DOI] [PubMed] [Google Scholar]
- 56. Lucasti C, Popescu I, Ramesh MK, et al. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, phase II trial. J Antimicrob Chemother 2013; 68:1183–92. [DOI] [PubMed] [Google Scholar]
- 57. Wagenlehner FM, Sobel JD, Newell P, et al. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis 2016; 63:754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mazuski JE, Gasink LB, Armstrong J, et al. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 2016; 62:1380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carmeli Y, Armstrong J, Laud PJ, et al. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis 2016; 16:661–73. [DOI] [PubMed] [Google Scholar]
- 60. Torres A, Zhong N, Pachl J, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis 2018; 18:285–95. [DOI] [PubMed] [Google Scholar]
- 61. Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 2017; 61:e00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Duin D, Lok JJ, Earley M, et al. ; Antibacterial Resistance Leadership Group Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 2018; 66:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Humphries RM, Hindler JA, Wong-Beringer A, Miller SA. Activity of ceftolozane-tazobactam and ceftazidime-avibactam against beta-lactam-resistant Pseudomonas aeruginosa isolates. Antimicrob Agents Chemother 2017; 61:e01858-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Farrell DJ, Sader HS, Flamm RK, Jones RN. Ceftolozane/tazobactam activity tested against Gram-negative bacterial isolates from hospitalised patients with pneumonia in US and European medical centres (2012). Int J Antimicrob Agents 2014; 43:533–9. [DOI] [PubMed] [Google Scholar]
- 65. Sader HS, Farrell DJ, Castanheira M, et al. Antimicrobial activity of ceftolozane/tazobactam tested against Pseudomonas aeruginosa and Enterobacteriaceae with various resistance patterns isolated in European hospitals (2011-12). J Antimicrob Chemother 2014; 69:2713–22. [DOI] [PubMed] [Google Scholar]
- 66. Wi YM, Greenwood-Quaintance KE, Schuetz AN, et al. Activity of ceftolozane-tazobactam against carbapenem-resistant, non-carbapenemase-producing Pseudomonas aeruginosa and associated resistance mechanisms. Antimicrob Agents Chemother 2018; 62:e01970-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Forrester JB, Steed LL, Santevecchi BA, et al. In vitro activity of ceftolozane/tazobactam vs nonfermenting, Gram-negative cystic fibrosis isolates. Open Forum Infect Dis 2018; 5:ofy158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kuti JL, Pettit RS, Neu N, et al. Microbiological activity of ceftolozane/tazobactam, ceftazidime, meropenem, and piperacillin/tazobactam against Pseudomonas aeruginosa isolated from children with cystic fibrosis. Diagn Microbiol Infect Dis 2015; 83:53–5. [DOI] [PubMed] [Google Scholar]
- 69. Grohs P, Taieb G, Morand P, et al. In vitro activity of ceftolozane-tazobactam against multidrug-resistant nonfermenting Gram-negative bacilli isolated from patients with cystic fibrosis. Antimicrob Agents Chemother 2017; 61:e02688-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Finklea JD, Hollaway R, Lowe K, et al. Ceftolozane/tazobactam sensitivity patterns in Pseudomonas aeruginosa isolates recovered from sputum of cystic fibrosis patients. Diagn Microbiol Infect Dis 2018; 92:75–7. [DOI] [PubMed] [Google Scholar]
- 71. Gramegna A, Millar BC, Blasi F, et al. In vitro antimicrobial activity of ceftolozane/tazobactam against Pseudomonas aeruginosa and other non-fermenting Gram-negative bacteria in adults with cystic fibrosis. J Glob Antimicrob Resist 2018; 14:224–7. [DOI] [PubMed] [Google Scholar]
- 72. Zhanel GG, Chung P, Adam H, et al. Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant Gram-negative bacilli. Drugs 2014; 74:31–51. [DOI] [PubMed] [Google Scholar]
- 73. Haidar G, Philips NJ, Shields RK, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis 2017; 65:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Merck. Ceftolozane-tazobactam package insert Available at: https://www.merck.com/product/usa/pi_circulars/z/zerbaxa/zerbaxa_pi.pdf. Accessed January 28, 2019.
- 75. Wagenlehner FM, Umeh O, Steenbergen J, et al. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, phase 3 trial (ASPECT-cUTI). Lancet 2015; 385:1949–56. [DOI] [PubMed] [Google Scholar]
- 76. Umeh O, Cebrik D, Friedland I, et al. A double-blind, randomized, phase 2 study to compare the safety and efficacy of intravenous CXA-101 (CXA) and intravenous ceftazidime (CTZ) in complicated urinary tract infections (cUTI). In: 50th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, 2010. [Google Scholar]
- 77. Solomkin J, Hershberger E, Miller B, et al. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis 2015; 60:1462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lucasti C, Hershberger E, Miller B, et al. Multicenter, double-blind, randomized, phase II trial to assess the safety and efficacy of ceftolozane-tazobactam plus metronidazole compared with meropenem in adult patients with complicated intra-abdominal infections. Antimicrob Agents Chemother 2014; 58:5350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Merck’s ZERBAXA (ceftolozane and tazobactam) met primary endpoints of non-inferiority compared to meropenem in pivotal phase 3 study of adult patients with hospital-acquired bacterial pneumonia or ventilator-associated bacterial pneumonia Available at: https://www.businesswire.com/news/home/20180911005151/en/Merck’s-ZERBAXA®-ceftolozane-tazobactam-Met-Primary-Endpoints. Accessed January 28, 2019.
- 80. Olsen I. New promising β-lactamase inhibitors for clinical use. Eur J Clin Microbiol Infect Dis 2015; 34:1303–8. [DOI] [PubMed] [Google Scholar]
- 81. Zhanel GG, Lawrence CK, Adam H, et al. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-β-lactamase inhibitor combinations. Drugs 2018; 78:65–98. [DOI] [PubMed] [Google Scholar]
- 82. Lapuebla A, Abdallah M, Olafisoye O, et al. Activity of imipenem with relebactam against Gram-negative pathogens from New York City. Antimicrob Agents Chemother 2015; 59:5029–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hirsch EB, Ledesma KR, Chang KT, et al. In vitro activity of MK-7655, a novel β-lactamase inhibitor, in combination with imipenem against carbapenem-resistant Gram-negative bacteria. Antimicrob Agents Chemother 2012; 56:3753–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Livermore DM, Warner M, Mushtaq S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother 2013; 68:2286–90. [DOI] [PubMed] [Google Scholar]
- 85. Lob SH, Hackel MA, Kazmierczak KM, et al. In vitro activity of imipenem-relebactam against Gram-negative ESKAPE pathogens isolated by clinical laboratories in the United States in 2015 (results from the SMART Global Surveillance Program). Antimicrob Agents Chemother 2017; 61:e02209-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Karlowsky JA, Lob SH, Young K, et al. Activity of imipenem-relebactam against Pseudomonas aeruginosa with antimicrobial-resistant phenotypes from seven global regions—SMART 2015–2016. J Glob Antimicrob Resist 2018; 15:140–7. [DOI] [PubMed] [Google Scholar]
- 87. Barnes MD, Bethel CR, Alsop J, et al. Inactivation of the Pseudomonas-derived cephalosporinase-3 (PDC-3) by relebactam. Antimicrob Agents Chemother 2018; 62:e02406-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sims M, Mariyanovski V, McLeroth P, et al. Prospective, randomized, double-blind, phase 2 dose-ranging study comparing efficacy and safety of imipenem/cilastatin plus relebactam with imipenem/cilastatin alone in patients with complicated urinary tract infections. J Antimicrob Chemother 2017; 72:2616–26. [DOI] [PubMed] [Google Scholar]
- 89. Lucasti C, Vasile L, Sandesc D, et al. Phase 2, dose-ranging study of relebactam with imipenem-cilastatin in subjects with complicated intra-abdominal infection. Antimicrob Agents Chemother 2016; 60:6234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Abstract 427. RESTORE-IMI 1: A multicenter, double-blind, comparator-controlled trial comparing the efficacy and safety of imipenem/relebactam versus colistin plus imipenem in patients with imipenem-non-susceptible bacterial infections. In: 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain, April 21–24, 2018. [Google Scholar]
- 91. Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect Control Hosp Epidemiol 2016; 37:1288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hecker SJ, Reddy KR, Totrov M, et al. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs class A serine carbapenemases. J Med Chem 2015; 58:3682–92. [DOI] [PubMed] [Google Scholar]
- 93. Hackel MA, Lomovskaya O, Dudley MN, et al. In vitro activity of meropenem-vaborbactam against clinical isolates of KPC-positive Enterobacteriaceae. Antimicrob Agents Chemother 2018; 62:e01904-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lapuebla A, Abdallah M, Olafisoye O, et al. Activity of meropenem combined with RPX7009, a novel β-lactamase inhibitor, against Gram-negative clinical isolates in New York City. Antimicrob Agents Chemother 2015; 59:4856–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lomovskaya O, Sun D, Rubio-Aparicio D, et al. Vaborbactam: spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob Agents Chemother 2017; 61:e01443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Castanheira M, Rhomberg PR, Flamm RK, Jones RN. Effect of the β-lactamase inhibitor vaborbactam combined with meropenem against serine carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2016; 60:5454–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sun D, Rubio-Aparicio D, Nelson K, et al. Meropenem-vaborbactam resistance selection, resistance prevention, and molecular mechanisms in mutants of KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2017; 61:e01694-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sabet M, Tarazi Z, Rubio-Aparicio D, et al. Activity of simulated human dosage regimens of meropenem and vaborbactam against carbapenem-resistant Enterobacteriaceae in an in vitro hollow-fiber model. Antimicrob Agents Chemother 2018; 62:e01969-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Athans V, Neuner EA, Hassouna H, et al. Meropenem-vaborbactam as salvage therapy for ceftazidime-avibactam-resistant Klebsiella pneumoniae bacteremia and abscess in a liver transplant recipient. Antimicrob Agents Chemother 2019; 63:e01551-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pogue JM, Bonomo RA, Kaye KS. Ceftazidime/avibactam, meropenem/vaborbactam or both? Clinical and formulary considerations. Clin Infect Dis 2018, in press. [DOI] [PubMed] [Google Scholar]
- 101. Kaye KS, Bhowmick T, Metallidis S, et al. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA 2018; 319:788–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Facta Farmaceutici, S.p.A. Meropenem-vaborbactam package insert Available at: http://www.vabomere.com/media/pdf/vabomere-us-prescribing-information.pdf. Accessed January 28, 2019.
- 103. Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther 2018; 7:439–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. King M, Heil E, Kuriakose S, et al. Multicenter study of outcomes with ceftazidime-avibactam in patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother 2017; 61:e00449-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gallagher JC, Satlin MJ, Elabor A, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: a multicenter study. Open Forum Infect Dis 2018; 5:ofy280. [DOI] [PMC free article] [PubMed] [Google Scholar]