Abstract
BACKGROUND
Endometriosis is a chronic gynaecological disorder that affects 2–10% of women of reproductive age. The aetiology of endometriosis is largely under-explored, yet abnormalities in the immune system have been suggested to explain the origin of ectopic endometrial tissues, and an association between endometriosis and autoimmune diseases has been proposed. Evaluation of current evidence investigating the association between endometriosis and autoimmune diseases from population-based studies will facilitate our understanding of the causes and consequences of endometriosis and provide a reference for better healthcare practices population-wide.
OBJECTIVE AND RATIONALE
The aim of this study was to systematically review the literature on population-based studies investigating an association between endometriosis and autoimmune diseases and to conduct a meta-analysis of combinable results to investigate the extent and robustness of evidence.
SEARCH METHODS
Four electronic databases were searched (MEDLINE, Embase, Web of Science, and CINAHL) from each database inception date until 7 April 2018. Search terms included a combination of database-specific controlled vocabulary terms and free-text terms relating to ‘endometriosis’ and ‘autoimmune diseases’. Study inclusion criteria focused on peer-reviewed published articles that reported an association between endometriosis and autoimmune diseases, excluding case reports/series, review papers, meta-analyses, organizational guidelines, editorial letters, expert opinions, and conference abstracts. Quality assessment of included studies was performed based on GRADE criteria. Key information of eligible studies was abstracted into a standard form. Meta-analysis was performed for autoimmune diseases with combinable study results from at least three studies investigating an association with endometriosis. For cross-sectional studies and case–control studies, raw data from each study were documented to calculate a Mantel–Haenszel odds ratio with 95% CIs. For cohort studies, an inverse variance probability weighted model was used to pool study results to calculate a rate ratio (a hazard ratio or a standardized incidence rate) with 95% CIs.
OUTCOMES
A total of 26 published population-based cross-sectional, case–control, and cohort studies that investigated the association between endometriosis and autoimmune diseases met all eligible criteria and were included in the review. The studies quantified an association between endometriosis and several autoimmune diseases, including systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS), rheumatoid arthritis (RA), autoimmune thyroid disorder, coeliac disease (CLD), multiple sclerosis (MS), inflammatory bowel disease (IBD), and Addison’s disease. However, the quality of the evidence was generally poor due to the high risk of bias in the majority of the chosen study designs and statistical analyses. Only 5 of the 26 studies could provide high-quality evidence, and among these, 4 supported a statistically significant association between endometriosis and at least 1 autoimmune disease: SLE, SS, RA, CLD, MS, or IBD.
WIDER IMPLICATIONS
The observed associations between endometriosis and autoimmune diseases suggest that clinicians need to be aware of the potential coexistence of endometriosis and autoimmune diseases when either is diagnosed. Scientists interested in research studies on endometriosis or autoimmune diseases should consider the likelihood of comorbidity when studying these two types of health conditions. Well-designed large prospective cohort studies with confounding control and mediation quantification, as well as genetic and biological studies, are needed to generate further insights into whether endometriosis is a risk factor for, or a consequence of, autoimmune diseases, and whether these two types of disorders share pathophysiological mechanisms even if they arise independently. Such insights may offer opportunities for the development of novel non-hormonal medications such as immuno-modulators or repurposing of existing immunomodulatory therapies for endometriosis.
Keywords: endometriosis, autoimmune diseases, population study, systemic lupus erythematosus, Sjögren’s syndrome, multiple sclerosis, rheumatoid arthritis, thyroid disorder, celiac disease, inflammatory bowel disease
Introduction
Endometriosis is a chronic gynaecological disorder characterized by the presence of endometrial-like tissue outside the uterus (Farquhar, 2007). Symptoms of endometriosis often include dysmenorrhea (pain with periods), non-menstrual pelvic pain, and infertility (Farquhar, 2007; Ballard et al., 2008). Epidemiological studies suggest an estimated prevalence of 2–10% in women within the reproductive age range overall (Eskenazi and Warner, 1997) and a prevalence as high as 20–30% in women suffering from infertility (Prescott et al., 2016). The gold standard of diagnosis is laparoscopy, which is an invasive method and thus only indicated in women with relevant symptoms. The prevalence of asymptomatic endometriosis therefore remains unknown. The main treatment methods of endometriosis include surgical removal of ectopic tissue and/or hormonal treatment to suppress ovarian function with adverse side effects (Zondervan et al., 2018). The aetiology of endometriosis is complex and under-explored, yet abnormalities in the immune system of women with endometriosis have been consistently demonstrated and may reflect the chronic inflammatory response to the presence of ectopic endometrium. In addition, these abnormalities may imply that the pre-disease milieu was supportive for adherence, survival, and maintenance of these ectopic endometrial tissues (Barrier, 2010; Eisenberg et al., 2012; de Barros et al., 2017; Izumi et al., 2018). Thus, a hypothesis was formed that women affected by endometriosis have an immunity-associated disorder, and such an association between endometriosis and autoimmune diseases has been proposed by several studies (Sinaii et al., 2002; Eaton et al., 2007; Eisenberg et al., 2012; Kvaskoff et al., 2014).
Robust quantification of the association between endometriosis and autoimmune diseases could facilitate understanding of the causes and consequences of both disorders, especially on the question of whether endometriosis is associated with immunological disorders. Also, considering the current medication for endometriosis is mainly hormonal treatment with considerable side effects due to interference with oestrogen levels, such evidence could help to provide reference for better healthcare practices through the discovery of novel drug targets, such as immuno-modulators, and development of new diagnostic tools for endometriosis. Therefore, we conducted a systematic review of published studies that investigated the association between endometriosis and autoimmune diseases, by applying methods that rank the quality of evidence and by summarizing to inform scientific discovery and clinical practice.
Methods
Search strategy
This systematic review was registered and accepted for inclusion in PROSPERO (Shigesi et al., 2017) in December 2017 (PROSPERO ID Number: CRD42017084175).
A protocol-driven systematic search for articles reporting on an association between endometriosis and autoimmune disease was conducted using the MEDLINE (via OVID), Embase (via OVID), Web of Science Core Collection (Indexes = SCI-Expanded, SSCI, ESCI, CPCI-S, CPCI-SSH), and CINAHL (via EbscoHost) databases. Each database was searched from the database inception date until 7 April 2018. Search terms included a combination of database-specific controlled vocabulary terms and free-text terms relating to endometriosis (e.g. ‘endometrio*’) and autoimmune diseases (e.g. ‘autoimmune diseas*’, ‘autoimmune response’, ‘autoimmunit*’, ‘autoantibod*’). To capture other potentially relevant articles, we also combined search terms representing endometriosis with search terms for 23 autoimmune diseases (i.e. Addison’s disease, autoimmune hepatitis, antiphospholipid syndrome, alopecia areata, coeliac disease (CLD), Crohn’s disease (CD), dermatitis herpetiformis, Graves’ disease, Hashimoto’s autoimmune thyroiditis, immune thrombocytopenic purpura, Kawasaki disease, multiple sclerosis (MS), narcolepsy, pernicious anemia, primary biliary cirrhosis, rheumatoid arthritis (RA), rheumatic fever, scleroderma, Sjögren’s syndrome (SS), systemic lupus erythematosus (SLE), temporal arteritis, type 1 diabetes, ulcerative colitis (UC), vitiligo) that were reported to be most prevalent (i.e. with a worldwide prevalence rate ≥10 per 100 000 based on a review (Hayter and Cook, 2012) that included a comprehensive list of autoimmune diseases). To be as comprehensive as possible, the search was not restricted to any study types. An example search strategy for the MEDLINE database is described in Supplementary Material, Table I.
Inclusion criteria and study selection
Study inclusion criteria comprised peer-reviewed publications with population-based studies that reported an association between endometriosis and any type of autoimmune diseases. Case reports and case series were excluded as their sampling from among, and thus representation of, the larger populations is unknown. Review papers, meta-analysis, organizational guidelines, editorial letters, and expert opinions were excluded in order to avoid duplication and erroneous weighting towards more frequently cited or discussed publications. Conference abstracts were also excluded as their full study reports were not obtainable to be assessed and their scientific rigor had not been peer-reviewed. Moreover, only studies published in English language journals were included.
All articles identified by searching the four databases were imported into Mendeley for screening. There were two rounds of screening for study inclusion. In the first round, two reviewers (N.S. and Q.F.) performed screening separately by reviewing titles, abstracts, and key terms for relevance of both endometriosis and autoimmune diseases. In the second round of screening, full texts of the articles identified during the initial screening were retrieved and read in detail to assess for eligibility. Possible discordance during study selections were discussed with a third reviewer (N.R.) to reach a consensus.
Data extraction
Data from the included studies were extracted into a standard form, detailing the author(s), publication year, country, time of study conduct, study design, ascertainment method of diseases and control or comparison group selection, number of study participants, reported risk estimates of endometriosis in association with autoimmune diseases, any adjustment for confounding factors in generating the effect estimates, and any measures taken in addressing temporality for diagnosis of endometriosis and autoimmune diseases. When effect estimates for association between endometriosis and autoimmune diseases were not listed in the original article but enough information was available, their effect estimates were calculated by Review Manager software Version 5.3. Also, where the listed effect estimates in the original article were presented with enough information to be recalculated, such effect estimates were calculated again by RevMan to check for accuracy of the original report.
Quality assessment
Quality assessment was performed based on GRADE criteria (Guyatt et al., 2011) and carried out separately by two reviewers independently (N.S. and M.K.). Guided by the Cochrane group (Ryan and Hill, 2016), the quality of evidence was assessed for each study outcome of interest (any reported autoimmune diseases in association with endometriosis by at least three studies) based on study design and factors that could reduce the quality of evidence (risk of bias, inadequacy or inaccuracy of statistical analyses, inconsistency of results, indirectness, imprecision, publication bias) as well as factors that could increase the quality of evidence (population size, number of outcome events, adjustment for potential confounders, temporality of diagnosis). Any discordance during the assessment of the quality of evidence was discussed with a third reviewer (C.B.) to reach consensus.
Data synthesis and meta-analysis
Meta-analyses were performed for each autoimmune disease reported to be associated with endometriosis by at least three studies and were also stratified by study design (cross-sectional, case–control, or cohort studies) for each outcome. For cross-sectional and case–control studies, raw data from each study were extracted to calculate a Mantel–Haenszel odds ratio (OR) with 95% CIs (hence, the ORs displayed in the meta-analysis figures and in the study characteristics tables differ slightly). For cohort studies, an inverse variance probability weighted model was used to pool study results to calculate a rate ratio (RR) with 95% CIs. As almost all of the cohort studies had adjusted for potential confounding variables (albeit not consistent covariates among them), pooling raw data was not possible. Statistical heterogeneity among studies for each outcome was assessed by inspecting the study-specific magnitudes of effect and the heterogeneity (I2) statistics. The I2 statistics for heterogeneity in meta-analysis was interpreted based on the GRADE approach recommended by Cochrane group (Schünemann et al., 2013). In meta-analyses of multiple studies for a specific outcome, a fixed-effect estimate was calculated if I2 heterogeneity = 0%; however, if I2 heterogeneity ≥0% in the meta-analysis, a random-effect estimate and a fixed-effect estimate were both calculated for sensitivity analysis.
Forest plots were constructed to present risk estimates and meta-analysis results for each autoimmune disease reported by at least three studies to be associated with endometriosis.
In meta-analyses of cohort studies, hazard ratio (HR) and standardized incidence ratio (SIR) were treated as equal to the rate ratio or relative risk.
In addition, for study results that did not explicitly specify the type of autoimmune disease but instead used broad terms such as ‘rheumatic disease’, ‘arthritis’, ‘colitis’, or ‘autoimmune diseases’, their study results and risk estimates were presented in the data extraction characteristics form, but they were not included in the meta-analysis or forest plots.
A sensitivity analysis was performed for the meta-analyses by taking out each study one at a time from the meta-analysis and recalculating the pooled effect size and I2 to assess for the influence of each study on the pooled effect size, if there were at least three studies included in the meta-analysis.
Results
Study selection
The initial search retrieved 653 results from MEDLINE, 1543 from Embase, 49 from CINAHL, and 732 from Web of Science Core Collection. All articles identified in the four databases were imported into Mendeley for screening. After removing duplicates, there were 2070 papers uploaded to Mendeley for first-stage screening. After the first-stage screening by reviewing titles, abstracts, and key terms related to endometriosis or autoimmune diseases, 1948 publications were excluded for not addressing both endometriosis and autoimmune diseases and 122 articles were identified to be potentially relevant and then assessed for eligibility. After the first round, 96 articles were excluded for not investigating an association between endometriosis and autoimmune diseases at a population-level (n = 77) or for being a review article or meta-analysis (n = 8), a conference abstract (n = 10), an expert opinion (n = 1), or a case report (n = 1). Finally, 26 articles met all the inclusion criteria and were included in this review, 14 of which were included for quantitative synthesis for having calculable risk estimates (Fig. 1).
Figure 1.
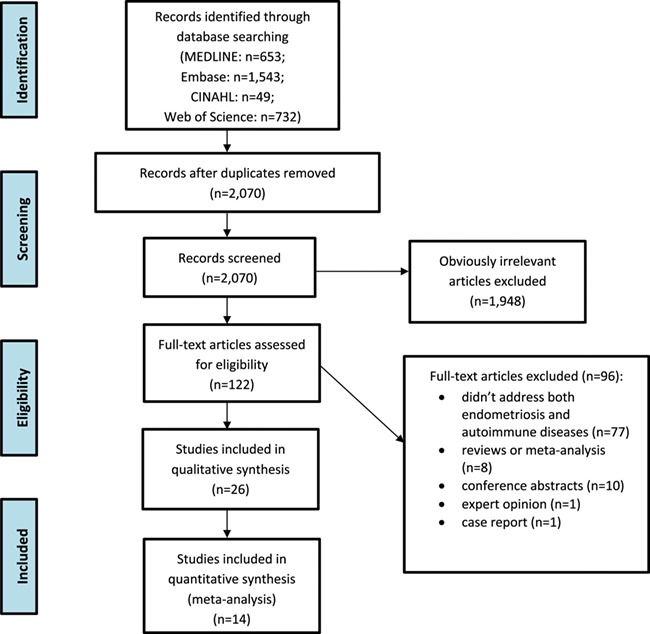
Flow chart for study inclusion and exclusion process.
Study characteristics
Detailed description of key characteristics for the 26 included studies is shown in Tables I–IV. Briefly, 8 (30.8%) studies were implemented in the population in North America, 12 (46.2%) were in Europe, 3 (11.5%) were in South America, and 3 were (11.5%) in Asia (Table I). With regard to study design, 6 (37.5%) studies were cross-sectional, 12 (46.2%) were case–control studies, and 6 (37.5%) were cohort studies (including 4 prospective cohort studies and 2 retrospective cohort studies), while 2 (7.7%) studies did not generate risk estimates for an association between endometriosis and autoimmune diseases, but instead compared clinical manifestations and serum autoantibody test results for endometriosis and SLE (Pasoto et al., 2005) or treatment methods and serum autoantibody test results for endometriosis and inflammatory bowel disease (IBD) (Lee et al., 2016).
Table I.
Characteristics of included studies (part 1).
| Citation | Country | Study period | Study design | Endometriosis ascertainment and number |
|---|---|---|---|---|
| Grimes et al. (1985) | USA | 1973–1982 | Case–control study | Hospital diagnosis (n = 12) |
| Lamb and Nichols (1986) | USA | Not specified | Case–control study | Self-reports (n = 43) |
| Smith et al. (1993) | USA | 1988–1989 | Case–control study | Hospital diagnosis with hysterectomy (n = 22) |
| Sinaii et al. (2002) | USA, Canada | 1998 | Cross-sectional study | Self-reports of laparoscopy/laparotomy (n = 3680) |
| Merlino et al. (2003) | USA | 1986–1997 | Prospective cohort study; 11 years follow-up | Self-reports (n = 155) |
| Poppe and Velkeniers (2003) | Belgium | 1999–2000 | Case–control study | Hospital diagnosis (n = 21) |
| Haga et al. (2005) | Norway | Not specified | Case–control study | Self-reports (n = 7) |
| Pasoto et al. (2005) | Brazil | Not specified | Cross-sectional study | Laparoscopy with histological analysis (n = 45 including 13 stage I/II and 32 stage III/IV) |
| Petta et al. (2007) | Brazil | 2005–2006 | Case–control study (reported as cross-sectional study) | Surgical and histological diagnosis (n = 148) |
| Eaton et al. (2007) | Denmark | 2001 | Cross-sectional study | Hospital diagnosis (prevalence: 4.91 per 1000 population) |
| Matorras et al. (2007) | Spain | 1990–2004 | Case–control study | Surgical with histological diagnosis (n = 342) |
| Aguiar et al. (2009) | Brazil | 2000–2003 | Case–control study | Laparoscopy with histological diagnosis ≤6 months before blood collection (n = 120) |
| Gemmill et al. (2010) | USA | 1998 | Cross-sectional study | Self-reports of surgical diagnosis (n = 4331) |
| Stephansson et al. (2011) | Sweden | 1969–2008 | Retrospective cohort study; 5 years follow-up | Hospital diagnosis (n = 517) |
| Nielsen et al. (2011) | Denmark | 1977–2007 | Retrospective cohort study; 1 year follow-up | Hospital diagnosis (n = 37 661) |
| Jess et al. (2012) | Denmark | 1977–2007 | Retrospective cohort study; 13 years follow-up | Hospital diagnosis (n = 37 661) |
| Santoro et al. (2014) | Italy | 2012 | Case–control study | Laparoscopy with histological diagnosis (n = 223) |
| Caserta et al. (2016) | Italy | 2009–2013 | Case–control study | Hospital diagnosis (n = 304) |
| Harris et al. (2016a) | USA | 1989–2011 | Prospective cohort study; 22 years follow-up | Self-reports of laparoscopy with hospital records checked in part of the participants (n = 6434) |
| Harris et al. (2016b) | Sweden | 1964–2011 | Case–control study | Hospital diagnosis (n = 371) |
| Yuk et al. (2016) | Korea | 2009–2011 | Cross-sectional study | Hospital diagnosis (n = 5615) |
| Huang et al. (2016) | Taiwan | 2000–2010 | Cross-sectional study (rheumatoid disease) | Hospital diagnosis (n = 27 973) |
| Lee et al. (2016) | USA | Not specified | Case–control study | Hospital diagnosis (n = 51) |
| Brouwer et al. (2017) | Netherlands | 2002–2010 | Cross-sectional study | Hospital diagnosis (n = 4) and self-reports (n = 1) |
| Wu et al. (2017) | Taiwan | 2001–2006 | Case–control study (SS) | Hospital diagnosis (n = 9191) |
| de Silva et al. (2018) | Denmark | 1994–2015 | Case–control study (IBD) | Hospital diagnosis (n = 297 in IBD cohort vs. n = 18 708 in IBD-free cohort) |
SS, Sjögren’s syndrome; IBD, inflammatory bowel disease.
Table IV.
Characteristics of included studies (part 4).
| Citation | Risk factors adjusted | Temporality |
|---|---|---|
| Grimes et al. (1985) | Age, race, parity; same hospital, same study period | Investigated risk of newly diagnosed SLE in women with and without ENDO hix |
| Lamb and Nichols (1986) | — | Investigated risk of ENDO in women with and without RA hix |
| Smith et al. (1993) | — | Investigated risk of ENDO in women with and without SLE/AD hix |
| Sinaii et al. (2002) | — | Prevalence study of AD (SLE, MS, RA, SS, thyroid disorder) in women with ENDO vs. general women population |
| Merlino et al. (2003) | Age, smoking status, age at last pregnancy, drug to stop lactation, age at menopause, polycystic ovary syndrome, HRT | Prospective cohort study for incident RA occurred after 1987 with ENDO exposure Ø |
| Poppe and Velkeniers (2003) | Control matched on age | Investigated risk of ENDO in women with and without having thyroid auto-immunity |
| Haga et al. (2005) | Control matched on age, region | Investigated risk of SS in women with and without ENDO hix |
| Pasoto et al. (2005) | — | Clinical manifestations/serological tests for women with ENDO vs. SLE vs. healthy controls; women diagnosed with ENDO by laparoscopy after SLE diagnosis was identified as ENDO cases |
| Petta et al. (2007) | — | Investigated risk of ENDO in women with and without thyroid disorder |
| Eaton et al. (2007) | Age, sex | Cross-sectional study of ADs prevalence (ENDO listed as an AD) |
| Matorras et al. (2007) | — | Investigated risk of SLE, SS in women with and without ENDO hix and risk of ENDO in women with and without AD hix |
| Aguiar et al. (2009) | Control from same city | Investigated risk of ENDO in women with and without CLD hix |
| Gemmill et al. (2010) | — | Prevalence study of Addison’s disease in women with ENDO hix vs. general women population |
| Stephansson et al. (2011) | Control matched on age, county, period | Prospective cohort study for incident ENDO in women with and without CLD hix Ø; excluded cases with ENDO diagnosis before CLD diagnosis |
| Nielsen et al. (2011) | Control matched on age, period | Prospective cohort study for incident SLE, SS, MS in women with and without ENDO hix vs. general women population; women diagnosed with AD before ENDO diagnosis was excluded Ø |
| Jess et al. (2012) | Control matched on age, period | Prospective cohort study for incident IBD in women with and without ENDO hix Ø |
| Santoro et al. (2014) | — | Investigated risk of ENDO in women with and without CLD hix |
| Caserta et al. (2016) | — | Investigated risk of ENDO in women with and without AD hix |
| Harris et al. (2016a) | Age at menarche, parity, menstrual cycle length, BMI, physical activity, smoking, OCP, ethnicity, infertility and analgesic use. For RA plus parity, duration of breast feeding. | Prospective cohort study for incident SLE, RA in women with and without ENDO hix Ø |
| Harris et al. (2016b) | Control matched on age, county | Investigated risk of SLE in women with and without ENDO hix; selected SLE diagnosis after ENDO diagnosis |
| Yuk et al. (2016) | Control matched on age, sampling year, sampling weight | Prevalence study of ATD in women with and without ENDO hix |
| Huang et al. (2016) | Matched controls (not specified) | Investigated risk of ENDO in women with and without rheumatoid disease hix |
| Lee et al. (2016) | Control matched on age | Clinical manifestation, prognosis, and treatment comparing women with IBD and ENDO vs. women with IBD but without ENDO |
| Brouwer et al. (2017) | — | Prevalence study of ENDO in women with RA vs. general women |
| Wu et al. (2017) | Case–control study for risk of ENDO in relation to SS hix SS | |
| de Silva et al. (2018) | Investigated risk of IBD at pregnancies in women with and without ENDO hix |
AD, autoimmune disease; ATD, autoimmune thyroid disorder; ENDO, endometriosis; OCP, oral contraceptive pill; SLE, systemic lupus erythematosus; SS, Sjögren’s syndrome; RA, rheumatoid arthritis; CLD, coeliac disease; MS, multiple sclerosis; IBD, inflammatory bowel disease; HRT, hormone replacement therapy.
Ø Denotes studies that addressed disease diagnosis time during data analysis.
For the reported autoimmune diseases, one group (Eaton et al., 2007) conducted a nationwide cross-sectional study in Denmark estimating the prevalence and pairwise comparison of 31 autoimmune diseases (endometriosis was also listed as one of the autoimmune diseases under study). Furthermore, nine (34.6%) studies reported on SLE, six (23.1%) on SS, five (19.2%) on RA, four (15.4%) on autoimmune thyroid disorders (ATDs), four (15.4%) on IBD, four (15.4%) on CLD, three (11.5%) on MS, one on Addison’s disease (Gemmill et al., 2010), and one reported on rheumatic disease (Huang et al., 2016).
Ascertainment of cases and controls
For ascertainment of endometriosis, 5 studies were based on self-reported data, 1 was based on self-reported data validated by hospital records in some of the study participants, and 20 were based on hospital records, among which 6 studies further confirmed endometriosis diagnosed by surgery and/or histology (Table I). There were four studies which categorized endometriosis diagnosis by the Revised American Society for Reproductive Medicine classification of endometriosis (Anon 1997) into Stage I/II vs. Stage III/IV endometriosis. For ascertainment of autoimmune diseases, 6 studies were based on self-reported data and 2 were based on self-reported data validated by hospital records in some of the study participants, while for 18 studies, the disease ascertainment were based on hospital records (Table II).
Table II.
Characteristics of included studies (part 2).
| Citation | Autoimmune disease ascertainment and number | Control selection and number |
|---|---|---|
| Grimes et al. (1985) | Hospital diagnosis: SLE (n = 109) | SLE-free women from the same hospital excluding those admitted for obstetric/gynaecologic conditions (n = 109) |
| Lamb and Nichols (1986) | Self-reports: RA (n = 1) | Friend control: self-reported ENDO-free (n = 43) |
| Smith et al. (1993) | Hospital diagnosis: hysterectomy: SLE (n = 2); AD other than SLE (n = 12); any AD (n = 14) | Women diagnosed with fibroids by hysterectomy (n = 185) |
| Sinaii et al. (2002) | Self-reports of hospital diagnosis: SLE (n = 31); MS (n = 19); RA (n = 68); SS (n = 23) | Literature statistics: US general female population |
| Merlino et al. (2003) | Physician diagnosis: RA (n = 158) | Women without RA (n = 31 178) |
| Poppe and Velkeniers (2003) | Serological test: TPO positive (n = 6) | Randomly selected, age-matched parous women (n = 100) |
| Haga et al. (2005) | Hospital diagnosis: SS (n = 47) | Healthy control matched on age, region (n = 142) |
| Pasoto et al. (2005) | Hospital diagnosis: SLE (n = 15) | Healthy women from the same clinical centre underwent laparoscopy (n = 21) |
| Petta et al. (2007) | Self-reports (hospital diagnosis validated partly) ATD (n = 38) | Women without ENDO from a family planning clinic (n = 158) |
| Eaton et al. (2007) | National registry, hospital diagnosis | National registry: Danish general population |
| Matorras et al. (2007) | Hospital diagnosis: SLE (n = 120), SS (n = 22) | Asymptomatic women attending gynaecology clinic in the same hospital as the cases (n = 501) |
| Aguiar et al. (2009) | Blood test: CLD (n = 17); biopsy: CLD (n = 13) | Healthy female donated blood in the same city (n = 1500) |
| Gemmill et al. (2010) | Self-reports: Addison’s disease (n = 10) | Literature statistics: US general female population |
| Stephansson et al. (2011) | CLD biopsy (n = 11 097) | National registry: matched on age, county, calendar period (n = 54 992) |
| Nielsen et al. (2011) | Hospital diagnosis in ENDO cohort; SLE (n = 54), SS (n = 86), MS (n = 130) | National registry: Danish general female population (age-/period-specific incident rate) |
| Jess et al. (2012) | Hospital diagnosis in ENDO cohort; UC (n = 228), CD (n = 92), IBD (n = 320) | National registry: Danish general female population (age-/period-specific incidence rate) |
| Santoro et al. (2014) | Serological and histological diagnosis: CLD (n = 7); self-reports of ADs (n = 37) | Female nurses with no known ENDO (n = 246) |
| Caserta et al. (2016) | Self-reports (?weren’t mention): AD (n = 41) | Female patient from the same hospital with no known ENDO or immune dysfunction (n = 318) |
| Harris et al. (2016a) | Self-reports confirmed by physician diagnosis: SLE (n = 103), RA (n = 390) | Female nurses responded to the survey with no known ENDO (n = 108 019) |
| Harris et al. (2016b) | Hospital diagnosis: SLE (n = 2834) | National registry: female controls matched on birth year, sex, county (n = 14 164) |
| Yuk et al. (2016) | Hospital diagnosis: Graves’s disease (n = 105); ATD (n = 159) | National registry: general female population matched on age, sampling year, sampling weight (n = 22 460) |
| Huang et al. (2016) | Hospital diagnosis: rheumatic disease (n = 2891) | Nationwide registry: general female population (n = 27 937) |
| Lee et al. (2016) | Hospital diagnosis: CD (n = 82), UC (n = 71) | Women with IBD but without ENDO (n = 102) matched on age, IBD type |
| Brouwer et al. (2017) | Hospital diagnosis: RA (n = 178) | Literature statistics: Dutch general females |
| Wu et al. (2017) | Hospital diagnosis: SS (n = 301) | Nationwide registry: female controls matched on age, income, geographic location, first diagnosis year of ENDO (n = 27 573) |
| de Silva et al. (2018) | Hospital diagnosis: UC (n = 7548), CD (n = 6731), IBD (n = 14 279) | Nationwide registry: women without IBD at conception (n = 1 832 732) |
AD, autoimmune disease; ATD, autoimmune thyroid disorder; ENDO, endometriosis; SS, Sjögren’s syndrome; RA, rheumatoid arthritis; TPO, thyroid peroxidase antibody; CLD, coeliac disease; MS, multiple sclerosis; IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis.
Two (7.7%) studies used prevalence statistics from the general female population identified from the literature as comparison group. Two (7.7%) studies used age- and period-specific incident rates identified from national statistics as risk estimates for comparison group, while eight (30.7%) studies identified female controls from nationwide registers. Seven (26.9%) studies selected controls and cases from the same region/or hospital. Lamb and Nichols (1986) used friend controls of recruited endometriosis patients. Lee et al. (2016) compared women diagnosed with both endometriosis and IBD and compared them to women with IBD but without endometriosis matched by age and IBD type. The other five (19.2%) studies recruited women who attended obstetric/gynaecological clinics or a family planning unit.
Of all the included studies, seven (26.9%) took measures to address the temporal sequence for diagnosis of endometriosis and autoimmune diseases while six of the studies looked at incidence of autoimmune diseases after diagnosis of endometriosis, and one study looked at incidental endometriosis after diagnosis of autoimmune diseases (Table IV). With regard to sample size, 5 (19.2%) studies had small sample size (≤100), 10 (38.5%) had a moderate sample size (100–1000), and 11 (42.3%) had a large sample size (≥1000). Among the 11 studies that had a large sample size (≥1000), 4 studies reported a large effect (OR/RR/SIR/HR ≥ 2) for the association between endometriosis and autoimmune diseases. As for adjustments of confounding factors or stratum-specific evaluation of effect modifiers or interactions in estimating risks, 3 (11.5%) studies included comprehensive adjustment for factors such as age, residence location, hormonal factors, or other potential confounding health conditions, 12 (46.2%) studies had made some adjustments of confounding factors such as age, sampling year, location of residence, and the other 11 (42.3%) studies did not adjust for any confounding factors (Table IV).
Quality of evidence
Assessment of the risk of bias and quality of the evidence of included studies was conducted based on GRADE criteria (Guyatt et al., 2011) (Tables V and VI). Quality assessment was carried out for each study outcome (a specific autoimmune disease reported to be in association with endometriosis) if the outcome was reported by at least three studies. The quality of the evidence for each reported autoimmune disease generally received a score of ‘LOW’ or ‘VERY LOW’ by GRADE criteria (Guyatt et al., 2011) due to potential risk of bias in their study design such as self-reported outcomes, cross-sectional study design, small sample size, unrepresentativeness of the control group or whole study population, limited or no adjustment of confounding factors, and reporting no CIs for risk estimates (Table V). Only 5 of the 26 studies under review (Merlino et al., 2003; Stephansson et al., 2011; Nielsen et al., 2011; Jess et al., 2012; Harris et al., 2016a) could provide high-quality evidence, and 4 of the studies (Stephansson et al., 2011; Nielsen et al., 2011; Jess et al., 2012; Harris et al., 2016a) supported a significant association between endometriosis and SLE, SS, RA, CLD, MS, and IBD.
Table V.
Quality assessment for each study outcome under review (part 1).
| Study outcome | No. of studies | GRADE score | Study design | Risk of bias | Inconsistency | |||
|---|---|---|---|---|---|---|---|---|
| Score | Comment | Score | Comment | Score | Comment | |||
| ENDO and SLE | Eight | LOW | 2 | Low, non-RCT | −1 | Four studies with high risk of bias (self-reported outcomes, small sample size, unrepresentativeness of the control group or whole study population) | 0 | All studies showed a positive association |
| ENDO and SS | Six | VERY LOW | 2 | Low, non-RCT | −1 | Three studies with high risk of bias (self-reported outcomes, small sample size, unrepresentativeness of the control group or whole study population) | 0 | All studies showed a positive association |
| ENDO and RA | Five | LOW | 2 | Low, non-RCT | 0 | Three studies with high risk of bias (self-reported outcomes, small sample size, unrepresentativeness of the control group or whole study population) | 0 | All studies showed a positive association |
| ENDO and ATD | Four | VERY LOW | 2 | Low, non-RCT | 0 | Two studies with higher risk of bias (self-reported outcomes, small sample size, unrepresentativeness of the control group) | 0 | All studies showed a positive association |
| ENDO and CLD | Four | VERY LOW | 2 | Low, non-RCT | −1 | Two studies with high risk of bias (self-reported outcomes, small sample size, unrepresentativeness of the control group) | 0 | All studies showed a positive association |
| ENDO and MS | Three | VERY LOW | 2 | Low, non-RCT | −1 | One study with high risk of bias (self-reported outcomes, small sample size, unrepresentativeness of the control group) | 0 | All studies showed a positive association |
| ENDO and IBD | Four | VERY LOW | 2 | Low, non-RCT | −1 | One study with high risk of bias (small sample size, unrepresentativeness of the control group) | 0 | All studies showed a positive association |
ATD, autoimmune thyroid disorder; CLD, celiac disease; ENDO, endometriosis; IBD, inflammatory bowel disease; MS, multiple sclerosis; RA, rheumatoid arthritis; SLE, systemic lupus evythmatosus; SS, Sjögren’s syndrome.
Table VI.
Quality assessment for each study outcome under review (part 2).
| Study outcome | Indirectness | Imprecision | Publication bias | Uplifting factors | ||||
|---|---|---|---|---|---|---|---|---|
| Score | Comment | Score | Comment | Score | Comment | Score | Comment | |
| ENDO and SLE | 0 | One study explored indirect association | −1 | Four studies with wide CIs or didn’t report a CI | 0 | — | 2 | Three studies reported large effect; two other studies with good adjustment for confounders and large sample size |
| ENDO and SS | 0 | All studies explored the association directly | −1 | Four studies with wide CIs or didn’t report a CI | 0 | — | 2 | Three studies with higher degree of quality: large sample size, adequate disease ascertainment, ability to approach temporality, including one study with good adjustment for potential confounders |
| ENDO and RA | 0 | All studies explored the association directly | −1 | Three studies with wide CIs or didn’t report a CI | 0 | — | 2 | One study reported large effect; two other studies with good adjustment for confounders and large sample size, adequate disease ascertainment, with one study with ability to approach temporality |
| ENDO and ATD | 0 | All studies explored the association directly | −2 | Three studies with wide CIs or didn’t report a CI | 0 | — | 0 | — |
| ENDO and CLD | 0 | All studies explored the association directly | −2 | Three studies with wide CIs or didn’t report a CI | 0 | — | 1 | One study with higher degree of quality: large sample size, adequate disease ascertainment, ability to approach temporality |
| ENDO and MS | 0 | All studies explored the association directly | −2 | Two studies with wide CIs or didn’t report a CI | 0 | — | 1 | One study with higher degree of quality: large sample size, adequate disease ascertainment, ability to approach temporality, good adjustment for potential confounders |
| ENDO and IBD | −1 | One study explored an indirect association | −1 | One study didn’t report a CI | 0 | — | 1 | One study with higher degree of quality: large sample size, adequate disease ascertainment, ability to approach temporality |
ATD, autoimmune thyroid disorder; CI, confidence interval; CLD, celiac disease; ENDO, endometriosis; IBD, inflammatory bowel disease; MS, multiple sclerosis; RA, rheumatoid arthritis; SLE, systemic lupus evythmatosus; SS, Sjögren’s syndrome.
Data synthesis and meta-analysis
Of the 26 studies in this systematic review, Eaton et al. (2007) conducted a nationwide cross-sectional study in Denmark using hospital record data to estimate the prevalence and pairwise comparison of 31 autoimmune diseases (endometriosis was also listed as an autoimmune disease). In this study, women with endometriosis were found to have a greater risk of 28 autoimmune diseases including SLE, CLD, and MS in comparison to the general Danish female population; however, the associated CIs of ORs were not reported in the article, hence providing limited evidence. The following paragraphs discuss the other epidemiological studies excluding Eaton et al. (2007), which reported on an association between endometriosis and autoimmune diseases (Table III).
Table III.
Characteristics of included studies (part 3).
| Citation | Risk estimates (in original reports) | Risk estimates (calculated/with correction) |
|---|---|---|
| Grimes et al. (1985) | SLE: OR = 2.0 (0.6–6.8) | |
| Lamb and Nichols (1986) | RA: RR = 1.00 | RA: OR = 3.07 (0.12, 77.50) |
| Smith et al. (1993) | SLE prevalence was too low to generate ORs | SLE prevalence: 9.09% vs. 0% in women with ENDO vs. fibroids; ADs other than SLE: OR = 0.75 (0.09, 6.13); any AIDs: OR = 2.50 (0.64, 9.75) |
| Sinaii et al. (2002) | SLE: POR = 20.7 (14.3–29.9)*; MS: POR = 7.1 (4.4–11.3)*; RA: POR = 1.5 (1.2–1.9)*; SS: POR = 23.9 (15.5–36.5)* | |
| Merlino et al. (2003) | Multivariate-adjusted; RA: RR = 1.59 (0.82–3.08) all women; RA: RR = 1.48 (0.71-3.07) ever pregnant women | |
| Poppe and Velkeniers (2003) | TPO-Abs positive: RR = 3.57 (1.09–11.8)* | OR = 4.60 (1.40, 15.13) |
| Haga et al. (2005) | — | OR = 4.31 (0.93, 20.01) |
| Pasoto et al. (2005) | Musculoskeletal manifestations, mucocutaneous, manifestations and antibodies | |
| Petta et al. (2007) | ATD: OR = 0.52 (0.25–1.06) | ATD: OR = 0.51 (0.25–1.04) |
| Eaton et al. (2007) | Pairwise ORs of ENDO with type 1 diabetes: 1.0; CLD: 1.6; pernicious anemia: 1.1; purpura: 1.9; MS: 1.3; Gulian Barre syndrome: 1.3; CD: 1.5; UC: 1.4; primary biliary cirrhosis: 2.0; psoriasis: 1.3; vitiligo: 2.2; scleroderma: 1.5; SLE: 1.6; SS: 1.6 | |
| Matorras et al. (2007) | OR for SLE in women with ENDO history: 0.37 (0.09–1.59); OR for ENDO in SLE history: 2.9 (0.27–32.57); OR for SS in ENDO history: 2.17 (0.48–9.90) | ENDO risk in SLE vs. non-SLE: OR = 2.94 (0.27–32.56); SS risk in ENDO vs. non-ENDO: OR = 2.18 (0.48, 9.91) |
| Aguiar et al. (2009) | Positive serology indicative for CLD: OR = 5.4 (1.8–15.5)*; Biopsy-confirmed CLD: OR = 3.8 (1.0–14.1)* | Positive serology indicative for CLD: OR = 5.39 (1.87, 15.57); Biopsy-confirmed CLD: OR = 3.82 (1.04–14.07) |
| Gemmill et al. (2010) | Addison’s disease: prevalence: 2.31 per 1000 population in women with ENDO vs. 0.09 per 1000 population in general women population | |
| Stephansson et al. (2011) | ENDO risk in women with CLD: HR = 1.39 (1.14–1.70)* | |
| Nielsen et al. (2011) | SLE: SIR = 1.6 (1.2–2.1)*; SS: SIR = 1.6 (1.3–2.0)*; MS: SIR = 1.2 (1.05–1.5)* | |
| Jess et al. (2012) | UC: SIR = 1.5 (1.3–1.7)*; CD: SIR = 1.6 (1.3–2.0)*; IBD: SIR = 1.5 (1.4–1.7)* | |
| Santoro et al. (2014) | CLD: OR = 2.80 (0.54, 14.57); any AD: OR = 1.33 (0.68, 2.60) | |
| Caserta et al. (2016) | AD: OR = 0.90 (0.48, 1.96) | |
| Harris et al. (2016a) | SLE: HR = 2.03 (1.17–3.51)*; RA: HR = 1.41 (1.05–1.89)* | |
| Harris et al. (2016b) | SLE: OR = 1.39 (1.09–1.78)* | |
| Yuk et al. (2016) | Graves’ disease: OR = 2.52 (1.30–4.88)*; autoimmune hypothyroidism: OR = 1.61 (0.88–2.94) | All ATD: OR = 1.45 (1.10, 1.91)* |
| Huang et al. (2016) | Rheumatic disease: OR = 1.37 (1.28, 1.47) | |
| Lee et al. (2016) | Comparison of IBD disease phenotype, the use of immuno-modulators, antiTNF agents, combination therapy, and the need for IBD-related surgery | |
| Brouwer et al. (2017) | RA: OR = 1.82 (0.47, 7.02) [control using Thonneau 1991 reference] | |
| Wu et al. (2017) | SS: OR = 1.71 (1.35, 2.17)* | |
| de Silva et al. (2018) | UC: OR = 1.97 (1.67, 2.31)* ; CD: OR = 2.16 (1.84, 2.55)*; IBD: OR = 2.06 (1.83, 2.31)* |
AD, autoimmune disease; ATD, autoimmune thyroid disorder; ENDO, endometriosis; SLE, systemic lupus erythematosus; SS, Sjögren’s syndrome; RA, rheumatoid arthritis; TPO, thyroid peroxidase antibody; CLD, coeliac disease; MS, multiple sclerosis; IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis.
*Denotes P < 0.05 that indicates there were significant differences between comparison groups.
SLE
Sinaii et al. (2002) reported, in a cross-sectional study of 3680 US women, a significantly higher prevalence of SLE in women with endometriosis in comparison to the general US female population (prevalence OR = 20.7 (95% CI: 14.3–29.9), P < 0.001). This exceptionally high risk estimate is probably accounted by the self-reported nature of disease ascertainment from the study participants who were probably very conscious of the health impact of endometriosis as they were all recruited from an endometriosis patient association. Smith et al. (1993) conducted a small case–control study in 22 women with endometriosis vs. 185 control women with uterine fibroids and found that SLE prevalence was higher in women with endometriosis (SLE prevalence: 9.09% vs. 0% in women with endometriosis vs. fibroids). However, it should be noted that women with endometriosis have been found to be at a significantly increased risk of uterine fibroids (Gallagher et al., 2018), and considering the small sample size of this study, this study was heavily biased. Meta-analysis of three case–control studies (Grimes et al., 1985; Matorras et al., 2007; Harris et al., 2016b) and two cohort studies (Nielsen et al., 2011; Harris et al., 2016a) has shown a significantly greater risk of SLE in women with endometriosis compared to control or comparison women (OR for case–control studies: 1.36 (95% CI: 1.07–1.73), P = 0.010, I2 = 49%; RR for cohort studies: 1.74 (95% CI: 1.10–2.77), P = 0.020, I2 = 0%) (Fig. 2a). Among these five studies in the meta-analysis, two case–control studies (Grimes et al., 1985; Matorras et al., 2007) had a high risk of bias in their study design and a small sample size; the other single case–control study and the two cohort studies (Nielsen et al., 2011; Harris et al., 2016a,b) were of high quality because of their large size and appropriate adjustment for confounders. When restricting to these three studies of high quality, their results were consistent in demonstrating a statistically significant association between SLE and endometriosis with low heterogeneity in magnitude of effect. Finally, Pasoto et al. (2005) compared musculoskeletal and serum immunologic abnormalities in a cross-sectional study comparing three groups of women with endometriosis vs. SLE vs. healthy controls recruited from a hospital in Brazil, but did not find any noticeable similarities among these women, possibly because of the indirect study approach and small sample size.
Figure 2.
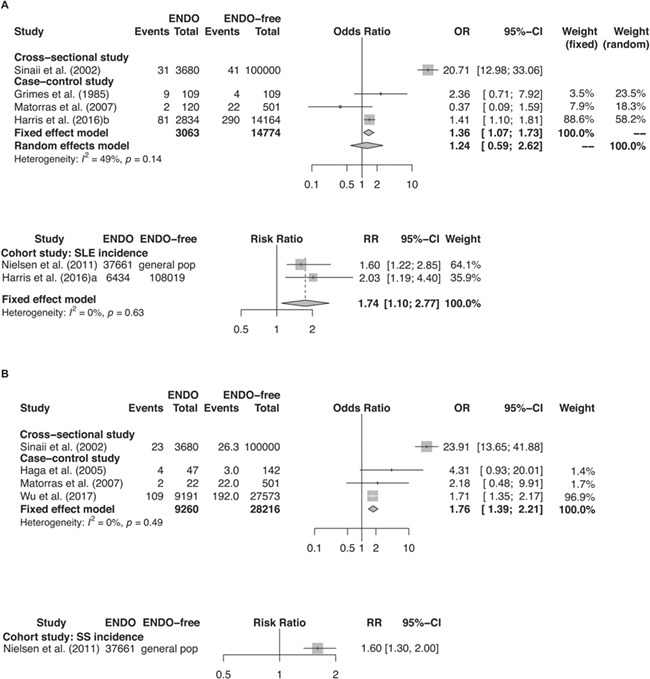
Forest plots of studies. (A) studies of ENDO and SLE (i.e. event); (B) studies of ENDO and SS (i.e. event).
Figure 2.
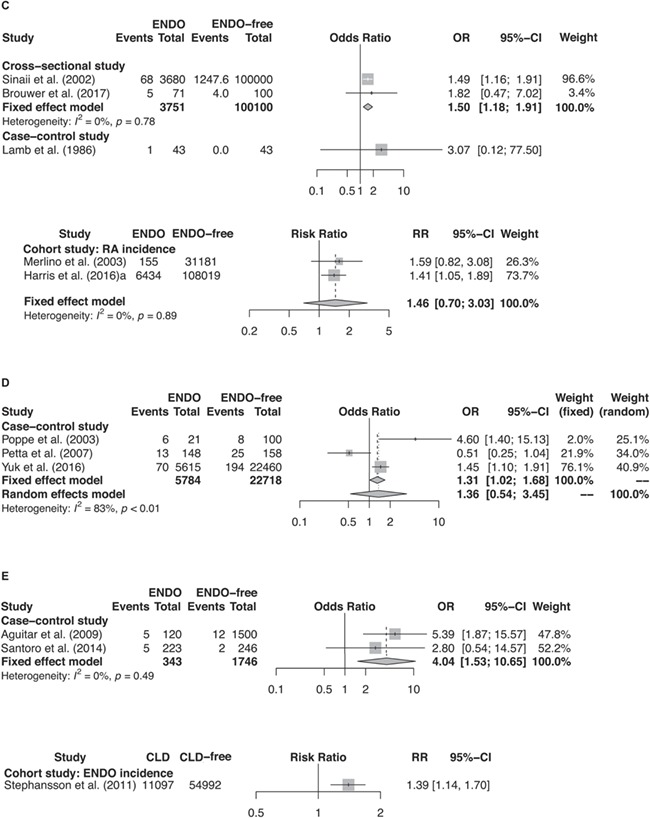
Continued: Forest plots of studies. (C) studies of ENDO and RA (i.e. event); (D) studies of ENDO and ATD (i.e. event); (E) studies of ENDO and CLD (i.e. event).
SS
Sinaii et al. (2002) reported, in a cross-sectional study of US women, a greater risk of SS in women with endometriosis in comparison to the general US female population (prevalence OR = 23.9 (95% CI: 15.5–36.5), P < 0.001); however, as discussed above, there was a high risk of bias in their study design. Meta-analysis of three case–control studies (Haga et al., 2005; Matorras et al., 2007; Wu et al., 2017) conducted in Norway, Spain, and Taiwan revealed a 76% greater odds of SS in association with endometriosis (OR = 1.76 (95% CI: 1.39–2.21), P < 0.001, I2 = 0%) (Fig. 2b). This greater odds of SS in association with endometriosis was also observed in the previously mentioned retrospective cohort study (Nielsen et al., 2011) that followed up 37 661 Danish women through hospital systems (SIR = 1.6 (95% CI: 1.3–2.0), P < 0.001).
RA
Meta-analysis of two cross-sectional studies by Sinaii et al. (2002) and by Brouwer et al. (2017) reported a significantly increased RA risk in women with endometriosis in comparison to the general female population (OR = 1.50 (95% CI: 1.18–1.91), P = 0.002, I2 = 0%) (Fig. 2c). Yet the poor quality for these two studies should be noted. However, in a case–control study (Lamb and Nichols, 1986), the greater odds for endometriosis in women with and without RA history was largely insignificant with a wide CI (OR = 3.07 (95% CI: 0.12, 77.50), P = 0.46) probably due to its small sample size (43 vs. 43 women with and without endometriosis history). Meta-analysis of two prospective cohort studies (Merlino et al., 2003; Harris et al., 2016a) also suggested a greater rate of RA among women with endometriosis, but this estimate was not statistically significant (RR = 1.46 (95% CI: 0.70–3.03), P = 0.31, I2 = 0%) (Fig. 2c) possibly due to the small sample size and wide CIs in the study conducted by Merlino et al. (2003) as the risk estimate in the study by Harris et al. (2016a) was significant.
ATD
Meta-analysis of three case–control studies (Poppe and Velkeniers, 2003; Petta et al., 2007; Yuk et al., 2016) showed an increased risk of ATD in women with endometriosis, but this risk estimate was not significant with a wide CI and a high I2 for heterogeneity (OR = 1.36 (95% CI: 0.54, 3.45), P = 0.52, I2 = 83%) (Fig. 2d). The quality of evidence provided by the meta-analysis was low, since two of the studies (Poppe and Velkeniers, 2003; Petta et al., 2007) had a higher risk of bias in their study design for having a small sample size and unrepresentative control selection, while the other study (Yuk et al., 2016) was implemented with large study population (a cross-sectional study in 5615 Korean women with endometriosis history) but were with no adjustment for confounders and thus there was a high risk for bias.
CLD
A greater odds of CLD was reported to be associated with endometriosis in two case–control studies (OR by meta-analysis: 4.04 (95% CI: 1.53–10.65), P = 0.005, I2 = 0% (Aguiar et al., 2009; Santoro et al., 2014) (Fig. 2e). However, these two studies had small sample sizes and wide CIs in risk estimates. Yet in a large retrospective cohort study by Stephansson et al. (2011) that followed up 11 097 Swedish women who had CLD biopsy for endometriosis incidence in comparison to healthy female controls (Stephansson et al., 2011), endometriosis was found to be significantly associated with CLD (HR = 1.39 (95% CI: 1.14–1.70), P < 0.001).
MS
For MS and endometriosis, the cross-sectional study by Sinaii et al. (2002) reported a 7-fold greater prevalence for MS in women with endometriosis in comparison to the general female population (prevalence OR = 7.1 (95% CI: 4.4–11.3), P < 0.001). However, the issue of selective study population and wide CIs in the study by Sinaii et al. (2002) has been discussed. Nevertheless, in the retrospective cohort study by Nielsen et al. (2011), a 20% greater risk for MS was observed in 37 661 Danish women with hospital-diagnosed endometriosis in comparison to general female controls (SIR = 1.2 (95% CI: 1.1–1.5), P < 0.001).
IBD
De Silva et al. (2018) conducted a case–control study in 14 279 Danish women with IBD at time of conception and 1 832 732 women without IBD at conception based on hospital records during 1994–2015 and found an increased risk of pregnant women with IBD in women with endometriosis history (UC: OR = 1.97 (95% CI: 1.67–2.31); CD: OR = 2.16 (95% CI: 1.84–2.55); IBD: OR = 2.06 (95% CI: 1.83–2.31), P < 0.001). Also, an earlier cohort study (Jess et al., 2012) during 1977–2007 using hospital records in Danish women reported a significantly increased incidence of IBD in women with endometriosis history in comparison with women without known endometriosis (UC: SIR = 1.5 (95% CI: 1.3–1.7); CD: SIR = 1.6 (95% CI: 1.3–2.0); IBD: SIR = 1.5 (95% CI: 1.4–1.7), P < 0.001). The two studies in Danish women using hospital records during two different periods provided higher quality evidence for an association between endometriosis and IBD due to their large sample size, hospital-based disease ascertainment, and the ability to approach temporality between IBD and endometriosis. Lee et al. (2016) studied endometriosis and IBD with an indirect approach by comparing the IBD phenotype, prognosis, treatments among women with IBD and with or without co-morbid endometriosis, and no significant difference between two groups were found except a slightly higher rate of bowel strictures in women with both IBD and endometriosis. However, this indirect approach by Lee et al. (2016) and their small sample size (51 women with IBD and endometriosis vs. 102 women with IBD alone) should be noted.
Other autoimmune diseases
In addition, Gemmill et al. (2010) reported, in a cross-sectional study, that the prevalence of Addison’s disease in US women with endometriosis was higher than the general US females (2.31 per 1000 population vs. 0.09 per 1000 population), but these prevalence estimates were too low to generate meaningful risk estimates for the studied association. Huang et al. (2016) in their retrospective cohort study following up 55 000 Taiwanese women through hospital systems described an increased risk of rheumatic diseases in women with endometriosis (OR = 1.37 (95% CI: 1.28–1.47), P < 0.001) compared to the general female population. Although rheumatic diseases were often recognized as autoimmune diseases such as SLE, SS, and RA, they did not specify which kind of rheumatic diseases were studied.
Sensitivity analysis
A sensitivity analysis for each meta-analysis with at least three studies was conducted. Within each meta-analysis, each study was taken out respectively to assess for its influence on the overall risk estimates.
The high heterogeneity in the meta-analysis for studies on SLE risk or SS risk in women with and without endometriosis history is accounted by the study by Matorras et al. (2007). In the meta-analysis of three case–control studies (Poppe and Velkeniers, 2003; Petta et al., 2007; Yuk et al., 2016) for endometriosis risk in women with and without ATD, each study seemed to account for the high heterogeneity (I2 = 83%).
For meta-analysis with an I2 = 0% (which indicates there is no evidence of heterogeneity), a fixed-effect model was used for meta-analysis. As the random-effect estimates and fixed-effect estimates would provide different meta-analysis results because of giving different weight to each studies when I2 > 0% with evidence of heterogeneity, a switch between random-effect estimate and fixed-effect estimate for each meta-analysis was performed to assess for small-study effects, since the included studies in the random-effects meta-analysis, especially small studies, would be weighted relatively more equally than a fixed-effect analysis in the presence of heterogeneity indicated by I2 more than 0%.
A switch from a fixed-effect model to a random-effect model for the meta-analysis of three case–control studies for SLE risk in women with and without endometriosis history, the pooled OR became largely insignificant (OR = 1.24, 95% CI: 0.59–2.62, P = 0.59) with more weighing towards smaller studies. For the meta-analysis of three case–control studies for endometriosis risk in women with ATD history in comparison to those without, a switch from a random-effect model to a fixed-effect model to weight down for smaller studies, the pooled OR became significant with a P-value equals to 0.04 (OR = 1.31, 95% CI: 1.02–1.68).
Discussion
In this systematic review of 26 population-based cross-sectional, cohort, and case–control studies, endometriosis was suggested to be associated with a range of autoimmune diseases including SLE, SS, RA, ATD, CLD, MS, IBD, and Addison’s disease, but the quality of the evidence for each reported autoimmune disease in association with endometriosis generally received a score of ‘LOW’ or ‘VERY LOW’ by GRADE criteria (Guyatt et al., 2011) due to potential risk of bias in their study design. Only 4 of the 26 studies under review (Stephansson et al., 2011; Nielsen et al., 2011; Jess et al., 2012; Harris et al., 2016a) could provide high-quality evidence to support for a significant association between endometriosis and SLE, SS, RA, CLD, MS, and IBD.
While there are a number of review articles on the (auto)immunity of endometriosis, these articles mostly discuss the biological or immunological aspects of endometriosis. This systematic review, however, is the first review amalgamating the evidence from available population-based studies with a focus on the association between endometriosis and autoimmune diseases. Our review benefits from a comprehensive search strategy including 23 most common autoimmune diseases (i.e. with a prevalence rate ≥10 per 100 000 people worldwide identified by Hayter and Cook (2012)) occurring with endometriosis. As a result, we were able to identify 18 additional population-based studies compared to 8 studies previously included in a review investigating of risk of chronic disease in endometriosis patients (Kvaskoff et al., 2014). Moreover, the current review captures several large-scale and well-designed studies with six studies reporting a significant association between endometriosis and autoimmune diseases. Furthermore, meta-analyses were conducted to better evaluate the overall evidence for association of endometriosis and autoimmune disease risks. The meta-analyses were designed carefully with strict subgroup analysis of study results from cross-sectional, case–control, or cohort studies. Sensitivity analyses were conducted to examine robustness of the meta-analysis results.
However, some limitations in this review or in the included studies should be noted. The GRADE score for quality assessment for each study outcome were either ‘LOW’ or ‘VERY LOW’. Many of the included studies were small in size and had limited number of endometriosis and/or autoimmune cases, presenting a high possibility to generate spurious associations. Also, because of the limited statistical power of studies with small samples size included in a meta-analysis, a random-effect estimate would weigh more towards the small studies, thus neutralizing a significant association found in large and well-conducted studies.
Another issue is the difficulty in establishing the temporality between endometriosis and autoimmune diseases, that is, to determine the order of disease development and presentation, and a potential causal relationship or whether they share the same pathophysiology. In cross-sectional and case–control studies, a temporal relationship between these two conditions was impossible to address because the design of the incorporated studies did not include time of diagnosis. However, prospective cohort studies that follow up people with certain exposure(s) prior to occurrence of the health outcome of interest could provide better evidence on temporality. Moreover, there is an estimated average delay of 7 years between onset of endometriosis-associated symptoms and its diagnosis (Nnoaham et al., 2011). In addition, some autoimmune diseases such as CLD could remain undetected for years, thus posing a greater challenge in defining the accurate time of disease onset in order to establish the temporal sequence between the two.
In addition, the cross-sectional and case–control studies might be biased because of suboptimal control selection. For example, Lamb and Nichols (1986) recruited friends of endometriosis patients as endometriosis-free controls. Friend controls might have biased the association under study since friends are more likely to share similar lifestyles or come from the same socio-economic status that could have influenced their health outcomes and likelihood of valid diagnoses. Smith et al. (1993) recruited women from hospital records diagnosed with fibroids confirmed by hysterectomy as controls for women with endometriosis but fibroids and endometriosis are correlated diseases (Zondervan et al., 2018). Other studies recruited women who attended obstetrical/gynaecological clinics or family planning units as endometriosis-free controls, but since endometriosis is a gynaecological disorder, if these women have sought help for obstetric or gynaecological reasons, there exists a risk that their health conditions might be potentially associated with endometriosis.
In order to generate the best evidence, we recommend future population-based studies to further investigate the association between endometriosis and autoimmune diseases by adopting a prospective cohort study approach with long follow-up time, large study populations, strict disease ascertainment based on hospital diagnosis records (preferably surgical diagnosis for endometriosis and biomarker tests for certain autoimmune disease), carefully selected disease-free controls, and good adjustment for potential confounding factors. Genome-wide association study for endometriosis and autoimmune diseases may help to establish a potential causal relationship between the two.
Possible explanations for the observed association between endometriosis and autoimmune diseases
While the aetiology of endometriosis is largely unknown, the most widely accepted hypothesis for the pathogenesis of endometriosis is the retrograde menstruation theory proposing the pelvic dissemination of endometrial cells through the uterine tubes (Sampson, 1927). However, as retrograde menstruation is present in almost all women (Halme et al., 1984), and only a proportion of women develop endometriosis, other causes including aberrations in the immune system of endometriosis patients have been proposed (Zondervan et al., 2018), preventing clearance of ectopic endometrial cells and allowing for their implantation, survival, and maintenance outside the uterus (Christodoulakos et al., 2007).
Many studies have investigated the (auto)immunological pathology of endometriosis; however, there is little understanding of shared biological pathways between endometriosis and autoimmune diseases that would explain an increased comorbidity. Studies have shown an escape of cells from immune surveillance by analysing peritoneal environment in endometriosis (Selam et al., 2002). Abnormalities in almost all types of immune cells have been identified, including increased levels of peritoneal neutrophils and macrophages, reduced cytotoxic function of natural killer cells, and aberrant numbers of T and B lymphocytes that aid endometriotic cell growth, maintenance, invasion, and angiogenesis (Izumi et al., 2018). Moreover, there is evidence of increased levels of a range of autoantibodies in women with endometriosis, spurring enthusiasm among researchers to investigate their potential role as biomarkers for endometriosis. A recent series of Cochrane reviews on blood biomarkers and urinary biomarkers for endometriosis found that although several antibodies have been indicated to be increased in women with endometriosis, only anti-endometrial antibodies and interleukin-6 were found to be useful for detecting endometriosis but their accuracy still cannot replace the standard surgical diagnosis (Liu et al., 2015; Nisenblat et al., 2016), supporting earlier systematic reviews (May et al., 2010, 2011) and clinical guidelines (Dunselman et al., 2014).
As endometriosis is a highly heritable disease, a number of candidate gene studies of endometriosis have been conducted to investigate the association between autoimmune-related genes and endometriosis (Bianco et al., 2012). Among these studies, genes such as PTPN22 that have been well established to be associated with RA have also been reported to be associated with endometriosis in comparison to endometriosis-free controls. Also, HLA alleles that are most commonly associated with autoimmune diseases have also been reported to be associated with endometriosis. However, these studies have generally included small sample sizes, poorly defined ethnicity and limited genotyping of loci. Importantly, these autoimmune-related genes were not reported in robust, large-scale genome-wide association studies of endometriosis (Sapkota et al., 2017).
Finally, while endometriosis is an oestrogen-dependent disorder, hormonal factors have also been found to play an important role to increase the activity or severity of autoimmune diseases. For example, a study of 16 patients with relapsing–remitting MS found that assisted reproduction technology for infertility treatment using gonadotropin-releasing hormone agonists and recombinant FSH was associated with a 7-fold increase in risk of MS exacerbation (Correale et al., 2012).
Conclusions and clinical implications
Evidence from our systematic review of observational population-based studies suggests an increased risk of comorbidity of autoimmune diseases including SLE, SS, RA, ATD, CLD, MS, IBD, and Addison’s disease. However, although there were a few high-quality, well-designed studies with large sample size, many of the included studies in this review were of low quality and had limitations. It is important to note that the evidence did not allow for accurate estimation of increased risk and have no clinical diagnostic utility. As most studies of endometriosis include women of reproductive age, longer follow-up studies are needed to ascertain the true risk of autoimmune diseases that may occur after menopause. Larger follow-up studies would also help understand whether endometriosis is a risk factor or a consequence of autoimmune diseases or if these two types of disorders share pathological mechanism and pathways resulting in their co-occurrence.
The observational evidence also does not exclude the possibility that selection and diagnostic biases in the studies underlie some of the associations observe, nor was there any evidence that the observed associations are biologically underpinned. Further studies are needed to explore the latter. Genetic and biological and studies of immunological dysfunctions in the context of endometriosis may improve our understanding of the pathogenesis of both conditions, and if shared pathogenic origins are observed, contribute to the identification of novel therapeutic and diagnostic targets.
Supplementary Material
Acknowledgements
The authors are thankful to Prof. Rafael Perera (Nuffield Department of Primary Care Health Sciences, University of Oxford) for his advice on meta-analysis.
Authors’ roles
N.S. was involved in all parts of the study: design, acquisition of data, analysis, interpretation, drafting of paper, and final approval. M.K. was involved in information extraction, quality assessment, advice on meta-analysis plots, drafting of the paper, and final approval. S.K. identified the search terms, developed all of the search strategies, conducted the search on each database to find relevant studies, reviewed the literature search methods section of the manuscript and was involved in the drafting of the paper, and final approval. Q.F. was involved in two rounds of screening to include eligible studies for review, drafting of the paper, and final approval. H.F. was involved in study design, interpretation of data, drafting of the paper, and final approval. J.C.K. was involved in study design, interpretation, drafting of the paper, and final approval. S.M. was involved in study design, interpretation, drafting of the manuscript, and final approval. N.R. was involved in study design, advice on meta-analysis methods, interpretation, review of the manuscript, and final approval. K.Z. was responsible for conception, study design, analysis methods, interpretation, drafting of manuscript, final approval, and obtaining funding. C.B. was responsible for study conception, study design, information extraction and quality assessment, review of the manuscript, final approval, and obtaining funding.
Funding
Wellbeing of Women (RG2031); Wellcome Trust Investigator Award (204969/Z/16/Z to J.C.K.); Innovative Medicines Initiative Joint Undertaking (ULTRA-DD; 115766 to H.F.).
Conflict of interest
K.Z. and C.B. report grants from Bayer AG, AbbVie Inc., Volition Rx, MDNA Life Sciences, and Roche Diagnostics Inc., and non-financial scientific collaboration with Population Diagnostics Ltd, outside the submitted work. N.S., M.K., S.K., Q.F., H.F., J.K., S.M., and N.R. have no relevant conflicts of interest to this published work.
References
- Aguiar FM, Melo SB, Galvão LC, Rosa-e-Silva JC, Ferriani RA. Serological testing for celiac disease in women with endometriosis. A pilot study. Clin Exp Obstet Gynecol 2009;36:23–25. [PubMed] [Google Scholar]
- Anon Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997;67:817–821. [DOI] [PubMed] [Google Scholar]
- Ballard KD, Seaman HE, De Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case–control study—part 1. BJOG 2008;115:1382–1391. [DOI] [PubMed] [Google Scholar]
- Barrier BF. Immunology of endometriosis. Clin Obstet Gynecol 2010;53:397–402. [DOI] [PubMed] [Google Scholar]
- Bianco B, Andre GM, Vilarino FL, Peluso C, Mafra FA, Christofolini DM, Barbosa CP. The possible role of genetic variants in autoimmune-related genes in the development of endometriosis. Hum Immunol 2012;73:306–315. [DOI] [PubMed] [Google Scholar]
- Brouwer J, Fleurbaaij R, Hazes JM, Dolhain RJ, Laven JS. Subfertility in women with rheumatoid arthritis and the outcome of fertility assessments. Arthritis Care Res (Hoboken) 2017;69:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta D, Mallozzi M, Pulcinelli FM, Mossa B, Moscarini M. Endometriosis allergic or autoimmune disease: pathogenetic aspects--a case control study. Clin Exp Obstet Gynecol 2016;43:354–357. [PubMed] [Google Scholar]
- Christodoulakos G, Augoulea A, Lambrinoudaki I, Sioulas V, Creatsas G. Pathogenesis of endometriosis: the role of defective ‘immunosurveillance’. Eur J Contracept Reprod Health Care 2007;12:194–202. [DOI] [PubMed] [Google Scholar]
- Correale J, Farez MF, Ysrraelit MC. Increase in multiple sclerosis activity after assisted reproduction technology. Ann Neurol 2012;72:682–694. [DOI] [PubMed] [Google Scholar]
- Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, Heikinheimo O, Horne AW, Kiesel L, Nap A et al. . ESHRE guideline: management of women with endometriosis. Hum Reprod 2014;29:400–412. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Rose NR, Kalaydjian A, Pedersen MG, Mortensen PB. Epidemiology of autoimmune diseases in Denmark. J Autoimmun 2007;29:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg VH, Zolti M, Soriano D. Is there an association between autoimmunity and endometriosis? Autoimmun Rev 2012;11:806–814. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am 1997;24:235–258. [DOI] [PubMed] [Google Scholar]
- Farquhar C. Endometriosis. BMJ 2007;334:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher CS, Makinen N, Harris HR, Uimari O, Cook JP, Shigesi N, Rahmioglu N, Ferreira T, Velez-Edwards DR, Edwards TL et al. . Genome-wide association analysis identifies 27 novel loci associated with uterine leiomyomata revealing common genetic origins with endometriosis. 2018. bioRxiv doi: 10.1101/324905. [DOI]
- Gemmill JA, Stratton P, Cleary SD, Ballweg ML, Sinaii N. Cancers, infections, and endocrine diseases in women with endometriosis. Fertil Steril 2010;94:1627–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes DA, LeBolt SA, Grimes KR, Wingo PA. Systemic lupus erythematosus and reproductive function: a case–control study. Am J Obstet Gynecol 1985;153:179–186. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380–382. [DOI] [PubMed] [Google Scholar]
- Haga HJ, Gram Gjesdal C, Irgens LM, Östensen M. Reproduction and gynaecological manifestations in women with primary Sjögren’s syndrome: a case–control study. Scand J Rheumatol 2005;34:45–48. [DOI] [PubMed] [Google Scholar]
- Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol 1984;64:151–154. [PubMed] [Google Scholar]
- Harris HR, Costenbader KH, Mu F, Kvaskoff M, Malspeis S, Karlson EW, Missmer SA. Endometriosis and the risks of systemic lupus erythematosus and rheumatoid arthritis in the Nurses Health Study II. Ann Rheum Dis 2016a;75:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HR, Simard JF, Arkema EV. Endometriosis and systemic lupus erythematosus: a population-based case–control study. Lupus 2016b;25:1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayter SM, Cook MC. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun Rev 2012;11:754–765. [DOI] [PubMed] [Google Scholar]
- Huang BS, Chang WH, Wang KC, Huang N, Guo CY, Chou YJ, Huang HY, Chen TJ, Lee WL, Wang PH. Endometriosis might be inversely associated with developing chronic kidney disease: a population-based cohort study in Taiwan. Int J Mol Sci 2016;17:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Barros IB, Malvezzi H, Gueuvoghlanian-Silva BY, Piccinato CA, Rizzo LV, Podgaec S. What do we know about regulatory T cells and endometriosis? A systematic review. J Reprod Immunol 2017;120:48–55. [DOI] [PubMed] [Google Scholar]
- Izumi G, Koga K, Takamura M, Makabe T, Satake E, Takeuchi A, Taguchi A, Urata Y, Fujii T, Osuga Y. Involvement of immune cells in the pathogenesis of endometriosis. J Obstet Gynaecol Res 2018;44:191–198. [DOI] [PubMed] [Google Scholar]
- Jess T, Frisch M, Jørgensen KT, Pedersen BV, Nielsen NM. Increased risk of inflammatory bowel disease in women with endometriosis: a nationwide Danish cohort study. Gut 2012;61:1279–1283. [DOI] [PubMed] [Google Scholar]
- Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L, Missmer SA. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update 2014;21:500–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb K, Nichols TR. Endometriosis: a comparison of associated disease histories. Am J Prev Med 1986;2:324–329. [PubMed] [Google Scholar]
- Lee KK, Jharap B, Maser EA, Colombel JF. Impact of concomitant endometriosis on phenotype and natural history of inflammatory bowel disease. Inflamm Bowel Dis 2016;22:159–163. [DOI] [PubMed] [Google Scholar]
- Liu E, Nisenblat V, Farquhar C, Fraser I, Bossuyt PM, Johnson N, Hull ML. Urinary biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2015;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matorras R, Ocerin I, Unamuno M, Nieto A, Peiro E, Burgos J, Exposito A. Prevalence of endometriosis in women with systemic lupus erythematosus and Sjögren’s syndrome. Lupus 2007;16:736–740. [DOI] [PubMed] [Google Scholar]
- May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update 2010;16:651–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update 2011;17:637–653. [DOI] [PubMed] [Google Scholar]
- Merlino LA, Cerhan JR, Criswell LA, Mikuls TR, Saag KG. Estrogen and other female reproductive risk factors are not strongly associated with the development of rheumatoid arthritis in elderly women. Semin Arthritis Rheum 2003;33:72–82. [DOI] [PubMed] [Google Scholar]
- Nielsen NM, Jørgensen KT, Pedersen BV, Rostgaard K, Frisch M. The co-occurrence of endometriosis with multiple sclerosis, systemic lupus erythematosus and Sjögren syndrome. Hum Reprod 2011;26:1555–1559. [DOI] [PubMed] [Google Scholar]
- Nisenblat V, Bossuyt PM, Shaikh R, Farquhar C, Jordan V, Scheffers CS, Mol BW, Johnson N, Hull ML. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnoaham KE, Hummelshoj L, Webster P, d’Hooghe T, de Cicco Nardone F, de Cicco Nardone C, Jenkinson C, Kennedy SH, Zondervan KT, Study WE. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011;96:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva PS, Hansen HH, Wehberg S, Friedman S, Nørgård BM. Risk of ectopic pregnancy in women with inflammatory bowel disease: a 22-year nationwide cohort study. Clin Gastroenterol Hepatol 2018;16:83–89. [DOI] [PubMed] [Google Scholar]
- Pasoto SG, Abrao MS, Viana VS, Bueno C, Leon EP, Bonfa E. Endometriosis and systemic lupus erythematosus: a comparative evaluation of clinical manifestations and serological autoimmune phenomena. Am J Reprod Immunol 2005;53:85–93. [DOI] [PubMed] [Google Scholar]
- Petta CA, Arruda MS, Zantut-Wittmann DE, Benetti-Pinto CL. Thyroid autoimmunity and thyroid dysfunction in women with endometriosis. Hum Reprod 2007;22:2693–2697. [DOI] [PubMed] [Google Scholar]
- Poppe K, Velkeniers B. Thyroid disorders in infertile women. Ann Endocrinol 2003;64:45–50. [PubMed] [Google Scholar]
- Prescott J, Farland LV, Tobias DK, Gaskins AJ, Spiegelman D, Chavarro JE, Rich-Edwards JW, Barbieri RL, Missmer SA. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum Reprod 2016;31:1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan R, Hill S. How to GRADE the quality of the evidence. Cochrane Consumers and Communication Group, Version 3.0.2016. http://cccrg.cochrane.org/author-resources.
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927;14:422–469. [Google Scholar]
- Santoro L, Campo S, D’Onofrio F, Gallo A, Covino M, Campo V, Palombini G, Santoliquido A, Gasbarrini G, Montalto M. Looking for celiac disease in Italian women with endometriosis: a case control study. Biomed Res Int 2014;236821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota Y, Steinthorsdottir V, Morris AP, Fassbender A, Rahmioglu N, De, Vivo I, Buring JE, Zhang F, Edwards TL, Jones S et al. . Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun 2017;8:15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations: Updated. The GRADE Working Group, 2013. [Google Scholar]
- Selam B, Kayisli UA, Garcia-Velasco JA, Arici A. Extracellular matrix-dependent regulation of Fas ligand expression in human endometrial stromal cells. Biol Reprod 2002;66:1–5. [DOI] [PubMed] [Google Scholar]
- Shigesi N, Rahmioglu N, Kvaskoff M, Feng Q, Fang H, Knight J, Missmer S, Becker C, Zondervan K. A systematic review of the association between endometriosis and autoimmune diseases. PROSPERO: International prospective register of systematic reviews. 2017. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=84175. [DOI] [PMC free article] [PubMed]
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod 2002;17:2715–2724. [DOI] [PubMed] [Google Scholar]
- Smith S, Howell R, Scott L. Is endometriosis associated with systemic lupus erythematosus? Int J Fertil 1993;38:343–346. [Google Scholar]
- Stephansson O, Falconer H, Ludvigsson JF. Risk of endometriosis in 11,000 women with celiac disease. Hum Reprod 2011;26:2896–2901. [DOI] [PubMed] [Google Scholar]
- Wu CC, Chung SD, Lin HC. Endometriosis increased the risk of bladder pain syndrome/interstitial cystitis: a population-based study. Neurourol Urodyn 2017;37:1413–1418. [DOI] [PubMed] [Google Scholar]
- Yuk JS, Park EJ, Seo YS, Kim HJ, Kwon SY, Park WI. Graves disease is associated with endometriosis: a 3-year population-based cross-sectional study. Medicine (Baltimore) 2016;95:2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers 2018;4:9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


