ABSTRACT
Exercise or work in hot environments increases susceptibility to exertional heat illnesses such as exertional heat stroke (EHS). EHS occurs when body heat gain exceeds body heat dissipation, resulting in rapid body heat storage and potentially life-threatening consequences. EHS poses a dangerous threat for athletes, agriculture workers, and military personnel, as they are often exposed to hot environmental conditions that restrict body heat loss or contribute to body heat gain. Currently, there is limited guidance on return to activity (RTA) after an episode of EHS. While examining biomarkers in the blood is thought to be beneficial for determining RTA, they are not sensitive or specific enough to be a final determining factor as organ damage may persist despite blood biomarkers returning to baseline levels. As such, additional assessment tests to more accurately determine RTA are desired. One method used for determining RTA is the heat tolerance test (HTT, 120 minutes treadmill walking; 40°C, 40% relative humidity). Unfortunately, the HTT provides even less information about EHS recovery since it offers no test sensitivity or specificity even after years of implementation. We provide an overview of the HTT and the controversy of this test with respect to assessment criteria, applicability to tasks involving high metabolic workloads, and the lack of follow-up analyses to determine its accuracy for determining recovery in order to diminish the likelihood of a second EHS occurrence.
KEYWORDS: Exertional heat stroke, heat tolerance test, heat illness, return to play, return to duty
Introduction
Heat illness is a multi-faceted syndrome whose diagnosis and recovery remain controversial. Heat illnesses remain a significant topic, as exertional heat stroke (EHS), the most severe form of heat illness, is the third most prevalent cause of death in athletes [1]. The athletic community is not the only population affected; others such as military troops, and agricultural and factory workers [2–5] are also at risk of experiencing EHS. Multiple factors increase the risk of EHS such as high intensity physical activity, long duration of environmental heat exposure, low fitness level, recent or concurrent illness, and poor hydration, with increasing risk as factors are combined [6].
EHS results in hospitalization and can even cause death under the most serious circumstances [7]. Between 2012–2013, the Occupational Safety and Health Administration reported 13 deaths which occurred as a result of moderate to heavy work performed outdoors or in hot indoor environments (e.g. factories) [4]. In 2017, there were a total of 464 EHS cases in active duty military members [5]. Although the EHS death rate in the military has decreased over the past 20 years due to enhanced prevention and treatment [8], the absolute number of EHS cases increased by ~16% from 2016, demonstrating that despite knowledge and guidance put forth to the troops, EHS remains a significant problem [5,9].
In addition to the negative and potentially long term health effects, EHS can also result in loss of time from competition, recreational activities or work days as well as incur significant medical costs. Loss of duty time can occur over a span of days, months, or even result in discharge from military service [7]. Carter [10] reported that in a group of 994 U.S. Army patients with EHS, 37% were hospitalized for 2–4 days and 26% were hospitalized for 5–6 days. In addition to the significant physical burden on the patient, the financial burden for treatment from EHS can also be quite substantial, costing as much as $5,000 for hospital admittance [7].
Unfortunately, when an individual has experienced an EHS, there is poor guidance regarding the criteria that are needed to determine return to activity (RTA) without adverse consequences, such as causing further organ damage or a subsequent event. Here, RTA can refer to military duty, occupational work, and professional or recreational athletic competition and exercise training. Current RTA guidance is based on subjective physician assessments, measurement of clinical blood biomarkers, and/or performance of a heat tolerance test (HTT). In this review we will discuss the physiological responses to exercise in the heat, the etiology of EHS and current RTA guidelines through in-depth examination of blood biomarkers and biophysics. Additionally, this review will assess the most commonly utilized HTT, identify gaps in its assessment value and make recommendations for future research in this area. As the HTT is a tool commonly used by many as part of a RTA assessment, it is important to more fully understand criticisms of the test and its limitations.
Basics of heat storage and physiological responses to exercise in the heat
Thermoregulation in hot environments, in its simplest form, is the ability of the body to dissipate heat gained due to physical activity, environmental exposure, and their combination. Storage of body heat, and the consequential increases in body temperature, occur when heat gain exceeds heat loss. The majority of energy used for metabolism is converted to heat and delivered from the body core to the skin. Heat exchange with the environment is influenced by air temperature, air water vapor pressures (absolute humidity), air flow, radiation (solar, sky, and ground) and clothing. Body heat exchanges occur by convection, radiation, conduction, and evaporation. The biophysics of heat balance can be written in simplified form as: S = M-Work ± (R + C)-E, where M is heat production, Work is external work performed, (R + C) is the sum of radiation and convection (loss or gain), and E is evaporative heat loss. During vigorous exercise/work, heat production can be increased by 15–20 times compared to at rest, and can increase body core temperature by 1°C every 5 minutes [6]. High air temperatures, solar loads, and absolute humidity combined with low air flows and heavy clothing can make R + C positive and drastically reduce E, thus increasing S and body core temperature. Body heat storage will plateau when there is a balance between heat gain and heat loss. Physiological responses permitting heat loss include sudomotor (sweating) and vasomotor (vasodilation) reflexes stimulated via the hypothalamus. Increased skin blood flow carries heat from the body core to the skin, where the temperature gradient between air and skin will determine dry heat loss or gain. Evaporation of sweat from the skin carries away heat and lowers skin temperature. However, during exercise, cutaneous vasodilation must be balanced with the need for increased blood flow at the skeletal muscle. Increases in heart rate (HR), and subsequently cardiac output, along with vasoconstriction at the level of the splanchnic organs are necessary to accomplish these hemodynamic adaptations.
Healthy individuals performing steady-state exercise who are unable to reach a thermal steady-state are subject to a greater amount of physiological strain and are at a potentially greater risk for EHS. These individuals can be considered thermally intolerant [11,12]. The most common means of assessing heat intolerance is via measurement of HR and body core temperature. If an individual is heat intolerant, they will have a higher HR and body core temperature and will only be able to complete shorter work durations compared to those who are tolerant [13,14]. Heat intolerance may be due to low cardiorespiratory fitness levels (VO2max), older age, or lack of heat acclimatization [15–17].
Heat illness & exertional heat stroke
The combination of increased metabolic heat production and exposure to hot environmental conditions increases susceptibility to exertional heat illness. There can be confusion regarding the relationship among the categories of heat illnesses (exhaustion, injury, and stroke). It is important to understand that one illness does not “progress” into the next (Figure 1). However, within each category of illness, there is a spectrum of severity which can contribute to difficulty in diagnosis because signs and symptoms of each illness can overlap. True forms of heat illness include heat exhaustion, heat injury, and heat stroke. Less severe conditions, such as miliaria rubra (heat rash) and heat syncope are often inappropriately grouped with other heat related illnesses because of their tendency to occur in warm environments. Heat exhaustion is generally thought of as a moderate form of heat illness in which elevated body temperature and reduced organ perfusion result in fatigue. Organ damage and central nervous system dysfunction with heat exhaustion are absent or extremely mild and recovery occurs rapidly with the cessation of heat stress. Exertional heat injury is a more severe form of heat illness that presents with reversible organ damage. The most severe, and potentially lethal, form of heat injury is heat stroke, which is characterized by profound central nervous system dysfunction in combination with severe hyperthermia and often with end organ damage.
Figure 1.
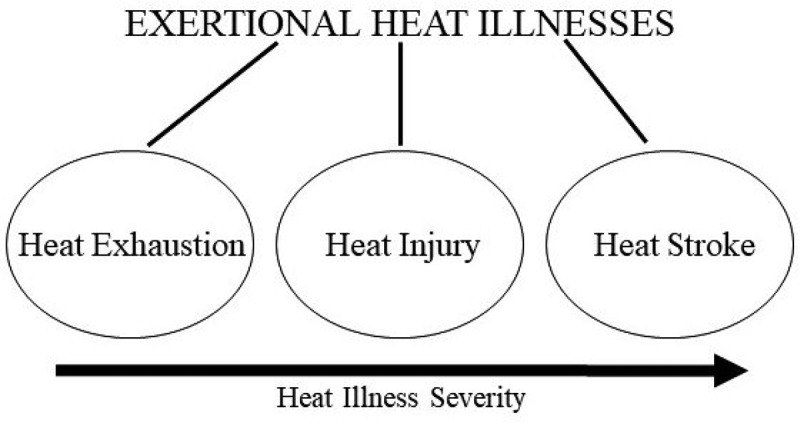
Categories of exertional heat illnesses.
Heat stroke is commonly divided into two types: classic (also referred to as passive) which is not associated with physical activity, or exertional which occurs during exercise or physical activity. Classic heat stroke generally affects elderly individuals with underlying comorbidities during unseasonably warm environmental temperatures, such as those witnessed during heat waves. Classic heat stroke can also affect the very young that do not have fully developed behavioral or physiological adaptations or mechanisms to adequately adapt to the heat. Classic heat stroke will not be discussed in the present review. On the other hand, EHS typically occurs in younger, healthy individuals engaging in rigorous physical exertion [18]. Although body core temperature with classic heat stroke or EHS is often greater than 40°C, reliance on a single temperature value is not recommended for diagnosis. Rather, central nervous system dysfunction is the clinical diagnostic hallmark of heat stroke with a variety of manifestations that may include confusion, delirium, combativeness, ataxia, seizures, or coma.
Pathophysiology of heat illness
Although the exact mechanism for EHS is unknown, several possible mechanisms, or a combination of mechanisms, are hypothesized to be responsible for the uncontrolled increase in body temperature and subsequent organ damage that occurs (Figure 2). Direct thermal injury to the cell is one potential pathway that can lead to the adverse sequelae and multi-organ dysfunction seen in EHS. It is clear that prolonged excessive elevations in body temperature result in direct thermal injury to tissues, as cells begin to degrade and proteins unfold around 42°C [19,20]. However, it has been established that a core temperature of up to 41.9°C can be well tolerated in exercising individuals with little or no adverse sequela [21]. It should also be noted that while the most direct estimate of tissue temperature in human EHS victims is currently body core temperature, this may not be an exact measure of the specific tissue temperature. A second pathway proposes that ischemia reperfusion is concurrently responsible for cell damage and the subsequent inflammatory response. The prevailing theory surmises that prolonged intestinal ischemia due to the redirection of blood flow to the skin and exercising skeletal muscle causes a breakdown of the gut membrane that increases permeability and allows endotoxin and other bacterial products to leak into the circulation to induce a systemic inflammatory response syndrome [22,23]. Coagulopathies represent a complication of the systemic inflammatory response syndrome that can progress to disseminated intravascular coagulation. Tissue damage from the above mechanisms can result in organ dysfunction or failure of the liver, kidneys, intestines, lungs, heart, vascular tissue, and brain [24]. Frequent complications of EHS include acute hepatic and renal dysfunction or failure, disseminated intravascular coagulation, metabolic acidosis, and electrolyte imbalances [24]. Rhabdomyolysis has anecdotally been regarded as a frequent comorbidity of EHS, particularly among Service Members and athletes undergoing vigorous training, and could further compromise renal function. Although, by definition, EHS always presents with central nervous system dysfunction at the time of collapse, structural damage to the brain is rare and reserved to the most severe cases [24]. Among EHS fatalities, structural brain damage was most evident within the cerebellum but less evident or nonexistent in other regions [25,26]. Because the preoptic anterior hypothalamus is responsible for temperature regulation where thermosensory neurons reside, it has been hypothesized that damage to this structure is responsible for loss of thermoregulation resulting in EHS, although this has never been demonstrated [25–28]. As such, it is unlikely that temperature fluctuations witnessed during EHS or in recovery are due to a dysfunctional preoptic anterior hypothalamus [24].
Figure 2.
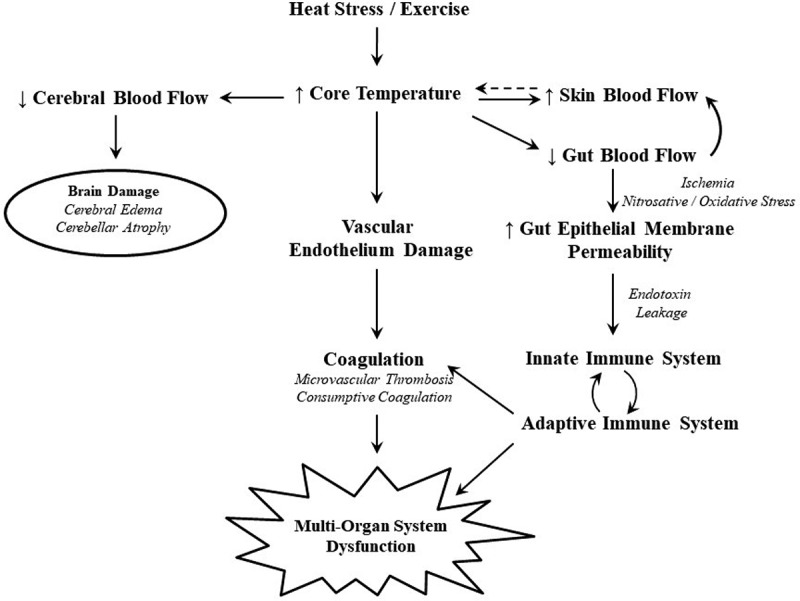
Pathophysiology of heat stress. Reprinted with permission [47].
Prolonged hyperthermia with EHS has been shown to increase severity of organ damage and mortality rates. Therefore, immediate rapid cooling is crucial for minimizing injury and promoting survival [29–31]. Subsequent treatment should focus on acquiring clinical labs, rehydration, restoring electrolytes, and monitoring and supporting organs at risk for damage. Apparent recovery from EHS often takes weeks or months to occur. An in-depth focus on the treatment of EHS is beyond the scope of this review, but additional information may be found in the following references [18,24,29].
Return to activity
After an EHS episode, there is minimal guidance on when an individual can RTA without the risk of additional organ damage or a second EHS occurrence. Due to significant inter-individual variability of EHS risk and severity, current research suggests that each RTA case should be reviewed on an individualized basis [32–36]. Symptoms of EHS range drastically between individuals, with the most common injuries occurring to the hepatic, renal, musculoskeletal, and thermoregulatory systems. However, although more rare, case reports have found lasting damage to the cardiovascular and neurological systems as well [33]. Current guidance from the National Athletic Trainer’s Association proposes that individuals who experience EHS should complete a 7–21 day rest period, be asymptomatic, have normal blood-work values, and obtain a physician’s clearance prior to beginning a gradual RTA [36]. Presently, the U.S. Military RTA guidelines follow Army Regulation 40–501, which utilizes physician judgement and acclimatization protocols as described in TB MED 507 [37] prior to returning to duty [12,37–39].
As previously discussed, EHS can result in damage and dysfunction to multiple organs and tissues, and as such, various biomarkers in the blood are utilized in an attempt to predict the severity of injury and monitor clinical recovery. Aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, blood urea nitrogen, and creatine kinase have all been found to be elevated in the serum in response to heat stroke [40–43]. However, the diagnostic value of these blood biomarkers is somewhat limited by their response to a variety of causes and release by multiple tissues/organs. For example, mild skeletal muscle injury from exercise or severe skeletal muscle injury from rhabdomyolysis can result in increases in creatine kinase, lactate dehydrogenase, aspartate aminotransferase, and alanine aminotransferase [44,45]. Additionally, elevated blood urea nitrogen can be indicative of renal or hepatic failure, but can also result from dehydration, increased amino acid catabolism, or gastrointestinal bleeding [46]. While clinical blood biomarkers are currently the best determinant of organ function recovery, symptoms may persist and organ damage may still be present even after blood biomarkers have returned to baseline values [24,47–49]. Increased rates of mortality years after EHS also suggest that residual organ damage is present [50]. Hence, determination of full and complete recovery after EHS remains difficult.
As a result of the limitations in using blood biomarkers to predict the severity of EHS, biomarkers with greater specificity are currently under investigation (e.g. cardiac troponin I) [24]. Unfortunately, blood biomarkers may be unable to give a complete picture of the extent of injury. It is possible that a fraction of biomarkers remain cell-associated and thus never appear in the circulation for detection [51]. This could explain why, after blood biomarkers have returned to baseline levels and clinical symptoms are no longer present, residual organ damage may still be present and the risk of early death is increased [47,48,50]. Blood biomarkers can offer select information regarding injury status but clearly have a limited ability to delineate full recovery from EHS.
Given these limitations, once an individual’s biomarkers have returned to normal, gradual RTA and heat acclimatization are recommended [35,36]. As heat intolerance can be present for up to 5 years post EHS episode, it is important to determine the associated sequelae and cause of EHS [33,36]. Premature RTA guidance can put an individual at extreme risk for short or long term organ damage [35]. The HTT has been proposed as a method of determining RTA, but practical application (i.e. does not replicate activity-specific metabolic demand) and feasibility (i.e. lack of facilities) have been challenged for sport and military settings alike [32]. Consequentially, some physicians are seeking additional approaches, such as a HTT for those who have the appropriate testing facilities, to combine with biomarker analyses to determine RTA guidance.
Heat tolerance test
What is the HTT?
There are many modalities and protocols of HTTs (see Table 1); however, because the RTA literature most commonly utilizes the HTT created by the Israeli Defense Force (IDF), this review will solely focus on this protocol. The HTT was created by the IDF as an objective screening tool to determine heat tolerance after an episode of EHS and to prevent reoccurrence in military forces [52,53]. The modality and tolerance/intolerance criteria of the IDF HTT has evolved numerous times over the previous 30 years (see Table 2). The original HTT test was a bench step test (60 min @ 40 Watts (W) followed by 20 min @ 80 W) performed in a temperate environment (23°C, 60% relative humidity (RH)) and then completed four weeks later in a hot environment (40°C, 50% RH; 180 min @ 40 W). The test was stopped when rectal temperature (Trec) exceeded 39.6°C with no designated maximal HR endpoint [14]. The current HTT test was designed for those between the ages of 17–30 years who are 6–8 weeks post-hyperthermic incident. This test consists of 120 minutes treadmill walking at 5 km/hr at 2% grade in 40°C, 40% RH. Wind speed is not explicitly stated in the current test, however, in order to maintain chamber conditions, a wind speed of 0.4–1.0 m/s can be assumed. Testing is discontinued if Trec reaches 39°C or HR reaches 180 bpm, or if the volunteer experiences dizziness or fainting.
Table 1.
Various modalities of the heat tolerance test.
| Citation | Activity | Intensity | Ambient Temperature, RH | Details |
|---|---|---|---|---|
| Dreosti 1935 [56] | Shoveling rocks | ~9000 ft*lbs/hr for 1hr | 35°C, 100% | Classified subjects as heat tolerant or intolerant based on final oral temperature |
| Piwonka et al 1965 [57] | Walking | 5.6 km/hr for 85 min | 40°C, 25% | Compared Trec, HR, and sweating responses of trained athletes and untrained controls |
| Armstrong et al 1991 [58] | Walking in insulating gear | 4 km/hr for 50 min, rest for 10 min; repeated over 6 hr | 33°C, 20% | Low exercise heat tolerance if unable to complete test (HR ≥ 180bpm, Trec ≥ 39.5ºC, fatigue, or discomfort) |
| Johnson et al 2013 [37] | Stationary bike | 70% VO2 max for 90min | 36ºC, 50% | Subject (prior heat stroke) reached safety endpoint (Gastrointestinal temperature ≥39.5ºC); 10 day heat acclimation protocol was able to lengthen time before reaching safety endpoint |
| Mee et al 2015 [65] | Running | 9 km/hr for 30 min | 40ºC, 40% | Demonstrated repeatability of a running HTT |
| Roberts et al 2016 [63] | Running | 10.5–12.9 km/hr | 25ºC, 60% | Subject (prior HS) reached safety endpoint (Trec = 39.5ºC) after 70min |
| Sagui et al 2017 [59] | Running in full combat gear | 15 min jog then 8 km race | 11–24°C, 47–90% range | Divided subjects by whether gastrointestinal temperature plateaued over last 10min |
Table 2.
IDF heat tolerance tests.
| Citation | Activity | Intensity | Ambient Temperature, RH | Details |
|---|---|---|---|---|
| Shvartz et al 1977 [16] | Bench Stepping | 80 W for 15 min | 23ºC, 48% | HR and Trec combined into score ranging from 10–100; heat intolerant if score <35 |
| 40 W for 180 min | 39ºC, 54% | HR and Trec combined into score ranging from 10–100; heat intolerant if score <35 | ||
| Shapiro et al 1979 [14] | Bench Stepping | 40 W for 60 min, 15 min rest, 80 W for 20 min | 23ºC, 60% | Determination of intolerance criteria not provided |
| 40 W for 180 min | 40ºC, 50% | Determination of intolerance criteria not provided | ||
| Moran et al 2004 [11] | Walking | 5 km/hr for 120 min | 20ºC, 50% | Comfort tolerance test; not predictive of heat intolerance |
| 40ºC, 40% | Heat intolerant if Trec ≥ 38.6°C, HR ≥ 160 bpm, or unable to complete test | |||
| Moran et al 2007 [52] | Walking | 5 km/hr for 12 0min | 40ºC, 40% | Heat intolerant if Trec > 38.5°C, HR > 145 bpm, or unable to complete test |
| Druyan et al 2013 [17] | Walking | 5 km/hr for 120 min | 40ºC, 40% | Heat intolerant if Trec ≥ 38.5°C or HR ≥ 150 bpm or Tre fails to plateau (increase >0.45°C during hour 2 of HTT) |
| Ketko et al 2014 [62] | Walking | 5 km/hr for 120 min | 40ºC, 40% | Heat intolerant if TCR ≤ 0.320ºC/bpm after 60min or TCR ≤ 0.279ºC/bpm after 120 min (TCR = Trec/HR) |
| Schermann et al 2018 [53] | Walking | 5 km/hr for 120 min | 40ºC, 40% | PHT- algorithm developed to estimate the probability of heat tolerance |
In the Israeli military, after an individual has experienced an EHS, they will complete a HTT after 6–8 weeks of convalescence in which they have not engaged in any physical activity. If classified as heat intolerant (criteria to be discussed), they are placed on a temporary medical profile and are retested after three months of rest. If found to be heat intolerant again at the three month mark, they are given a permanent profile, which reduces work in hot environments, or assigns the individual to a new occupation within the military [49]. If classified as heat tolerant after three months, then they are allowed to RTA after completing progressive exercise and heat acclimation [12]. Only 10% of individuals who perform the IDF’s HTT fail on the first attempt, and less than 2% of individuals fail after the retest three months later [54]. Additionally, once cleared to return to duty, it is reported that only two heat tolerant and four heat intolerant individuals (out of 145 individuals tested) have experienced a recurring exertional heat illness [55].
IDF’s methods of determining heat intolerance
The IDF’s method of determining heat tolerance has changed over the years (see Table 2). The primary method of classifying heat intolerance is to assess Trec and HR values at the end of exercise (120 minute mark). Heat intolerance is classified when an individual has a rectal temperature greater than 38.5°C or HR above 145 bpm [52].
Another method of evaluating heat tolerance is via a composite score which ranges from 10–100 [16] in both temperate and hot environments. A value of 10 demonstrates high HR and Trec and a score of 100 demonstrates low HR and Trec values. The composite scores are then summed and divided by 2 ([HR score + Trec score]/2). A score below 35 represents those who are likely to experience heat intolerance while those who score above a 75 are considered heat acclimated [16]. Studies performed by Armstrong [60,61], which replicated Shvartz’s [16] work, demonstrated high variability in composite score outcomes. Additionally, due to the high cardiorespiratory fitness level of Armstrong’s volunteers, individuals were able to achieve low HR scores on the first day which therefore increased the composite score supporting the concept that composite score ratings can be misleading as a result of the population tested. Armstrong [61] concluded that because the HTT was performed in a relatively thermoneutral environment, it cannot accurately represent HR or Trec compared to standardized HTTs. Therefore, in thermoneutral environments, composite scores may not be considered indicative of true heat tolerance status.
Occasionally there is ambiguity in the determination of classifying heat tolerance due to the plateauing of Trec. Therefore, another way of determining heat intolerance is to examine Trec during the second hour of testing. Heat intolerance can be classified if body core temperature increases by 0.45°C or greater [17,53], nevertheless, this does not consider cardiovascular strain. Consequentially, Ketko proposed that the determination of heat intolerance should be based upon the thermal-circulatory ratio (TCR = Trec/HR) which is calculated as the mean of the last 5 minutes of the test. Heat intolerance is then determined as having a ratio higher than 0.279°C/bpm. However, the TCR has only been tested in a young, fit male population with the precise environmental conditions previously published [62].
In 2018, the IDF published guidelines for determining HTT intolerance criteria [53]; while guidelines 1 and 2 are similar to previous IDF HTT protocols, guidelines 3 and 4 are new additions. The HTT should be analyzed in the following order: 1). Evaluate Trec and HR at the cessation of exercise (120 minute mark). Individuals are classified as heat intolerant if Trec is greater than 38.5°C or HR is greater than 150 bpm. 2). Evaluate the change in Trec (ΔTrec = Trec @ 120 minutes – Trec @ 60 minutes). Individuals are classified as heat intolerant if ΔTrec > 0.45. 3). Individuals should be potentially classified as heat intolerant if ΔTrec is between 0.25 and 0.45, have a HR between 120–150 bpm and if Trec is greater than 38.2°C. 4). An individual should be considered heat tolerant if ΔTrec is less than 0.25, if HR is less than 120 bpm, and if Trec is less than 38.2°C [53]. A value of 38.2°C was determined as a threshold value based on previous work completed by the IDF demonstrating that heat tolerant individuals tend to reach a Trec plateau at 37.78–38.38°C [52] (R. Yanovich, personal communication, 25 July 2018). However, it can be difficult to determine heat tolerance classification for those who reach 38.2°C. Therefore, the Probability of Heat Tolerance (PHT) model was created to eliminate subjective determinations on borderline cases. The PHT uses an algorithm to output one single value to provide more specific HTT determination guidelines. Additionally, Schermann proposed that rather than being wrongly categorized as tolerant/intolerant, the heat tolerance status of some individuals may in fact be classified as uncertain or modifiable [53]. Clearly, definitive pass/fail criteria for the HTT remains unresolved.
Considerations of the HTT
While physicians seek a practical method for aiding in RTA decisions, the HTT has limitations that go beyond the absence of firmly established test criteria. Due to the inability of the HTT to detect acute fluctuations in HR and core temperature, the sensitivity of the current HTT has been challenged [60]. Others also argue that the HTT cannot predict reoccurring EHS cases and is therefore an ineffective tool for RTA [15]. In general, it is unrealistic to propose that a test can predict every future EHS episode. An individual may be considered heat tolerant after performing a HTT, yet at a later date have an EHS due to having risk factors (e.g. dehydration, bacterial/viral illness, poor sleep, etc.) which were not present at the time of testing [10]. Furthermore, it is theorized that a prior EHS may be a risk factor for a reoccurring episode due to a potential increase in C-reactive proteins, which consequentially increases the hyperthermic response to exercise, or that a previous EHS episode may weaken a cells ability to counteract the effects of high temperatures [10]. However, there is no experimentally validated literature that supports that a prior EHS will lead to a reoccurring event.
Additionally, the HTT does not consider seasonal weather variations, fitness, or acclimatization status [12,63]. Druyan [17] concludes that the season of testing (summer vs. winter) has yielded no difference in results, and therefore acclimatization status does not alter HTT responses. This is additionally supported by Kazman [64] who found that there were no differences in body core temperature and HR between seasons; however, 49% of the tests were completed in the summer and only 7% were completed in winter. Both of these conclusions are strictly at odds with decades of heat acclimatization research findings, but the degree to which “true” acclimatization occurs in the summer when most living is done indoors with air conditioning may help reconcile the differences.
The HTT also does not account for organ damage after an EHS, as it does not capture heat adaptations vs. acute responses to heat [37]. However, Epstein [54] recently notes that a Soldier is only allowed to RTA once normal laboratory values have been established; additionally, the prolonged organ damage has yet to be proven in humans but rather a non-EHS rat model [48]. Although prolonged organ damage has not been experimentally validated in human studies, and due to ethics it likely never will, case studies and correlational epidemiological studies do note that lasting damage may be observed [28,50].
The current IDF HTT cannot be effectively utilized for all populations. For example, the HTT is inappropriate for endurance athletes or those completing higher workloads due to its moderate intensity (~30–40% VO2max) and relatively short duration [53,63,65,66]. Fit individuals can pass the HTT only to find themselves once again at risk of a heat illness when performing the more difficult tasks that produced their heat illness in the first place [63]. Consequentially, more fit individuals will exceed the HTT’s capability and will need the workload to be normalized for VO2max [67]. The HTT test may produce false positive results in female Soldiers as the test does not consider several physiological (lower aerobic fitness) and anthropometric (higher body surface area to mass ratio) differences between women and men. Druyan [68] found a significantly higher rate of heat intolerance in females compared to males yet no significant differences between average HR, Trec or sweating rate. Of those previously diagnosed with EHS, or suspected of EHS, 67% of women and only 26% of men were classified as heat intolerant via the HTT [64]. Using the current HTT, women are 3.68 times more likely to be categorized as heat intolerant compared to men due to a HR greater than 150 bpm, but not due to differences in Trec, therefore signifying that thermal strain is less of a factor in females [64]. Therefore, the HTT fails to be applicable to the general military population which reported 86% of hospitalizations between 1980 and 2002 were males, with 18% of the hospitalizations being attributed to EHS [69]. Consequentially, the HTT may only be applicable for males who are in a controlled environment (e.g. environmental chamber) while wearing minimal clothing compared to combat situations.
Biophysics and heat acclimatization
In order to fully appreciate the interaction of the environment and thermoregulation, the basics of the biophysics of heat exchange should be understood. Heat stress refers to environmental and host conditions that tend to increase body temperature (i.e. body heat storage). Heat strain refers to physiological (e.g. HR) and or psychological consequences of heat stress (e.g. perceived effort). During the HTT, a relatively small amount of energy is used to perform work (e.g. 2% treadmill grade) while the vast majority of energy generated by treadmill walking (e.g. 5 km/hr) takes the form of metabolic heat (Hprod) that is generated and released from active skeletal muscles and transferred from the body core to skin (skin blood flow). It is important to consider that heat exchange during the HTT is limited exclusively to sweat evaporation because the test is performed at an air temperature in excess of skin temperature (40°C) but leaves room for sweat evaporation (40% RH or ambient air water vapor pressure, Pa = 22 mmHg) with minimal room airflow (usually ~1 m/s). The requirement for heat loss is therefore synonymous with the requirement for evaporative cooling (Ereq), which can be written as the expression:
where (R + C) is the sum of heat gain by both radiation and convection (temperature of air and surrounding walls). The degree to which evaporation can occur depends on the maximal evaporative capacity of the environment (Emax), which can be written as the expression:
where he is the evaporative heat transfer coefficient and Psk,s is saturated water vapor pressure at skin temperature. The ratio Ereq/Emax defines the skin wettedness (ω) that is required to achieve the Ereq rate as related to Emax. When Ereq/Emax reaches unity, it can also describe heat tolerance whereby a ratio >1.0 generally indicates that heat balance cannot be achieved while a ratio <1.0 generally indicates that heat balance can be achieved [70]. The closer Ereq/Emax gets to 1.0, the greater the requirement for wet skin. As the ratio exceeds ~0.50, evaporative efficiency declines and sweat may begin to drip. The formula 1.0-ω2/2 describes evaporative efficiency (η) and the required evaporative cooling power to achieve thermal balance is: Ereq/η [71], which can be converted to a required sweating rate for thermal balance (SRreq).
Table 3 applies rational modeling [71,72] of heat balance to illustrate that the HTT requires ω values of 0.59–0.72, depending on body size. It is therefore a compensable heat stress test so long as adequate sweat secretion and evaporation can take place. Based upon evaporative inefficiency, the SRreq needed to achieve heat balance in the HTT ranges from 0.870 to 1.387 L/h, depending on body size (Table 3). In untrained (or de-conditioned) persons who are non-heat acclimatized, ωmax ≈ 0.70; in trained persons ≈ 0.85; in heat acclimatized persons ≈ 0.95, whereby ω ≈ 1.0 is a theoretical maximum [73]. Higher ωmax values are related to greater density of activated sweat glands [73]. Therefore, trained and heat acclimatized persons should be better able to wet a larger percentage of their body surface area, increase evaporative heat loss, and complete the HTT.
Table 3.
Biophysical explanation of heat balance requirements for the HTT.
| Ereq (W) | Emax (W) | ω | η | Ereq/η (W) | SRreq (L/h) | |
|---|---|---|---|---|---|---|
| 60 kg | 485 | 823 | 0.59 | 83% | 587 | 0.870 |
| 90 kg | 695 | 968 | 0.72 | 74% | 936 | 1.387 |
Symbols as described in text.
The HTT pass/fail criteria, a passed test indicating heat tolerance and a failed test indicating intolerance, are predicated on body core temperature (Tc) and HR responses to a standardized set of exercise-heat stress conditions. It is very well established that both measures decrease in response to repeated exercise-heat exposures. Figure 3 applies an empirical heat strain model to illustrate the differences in body core temperature response to HTT before and after heat acclimatization [72]. Details are provided in the figure legend. Numerous adaptations occur to reduce heat strain [74]. One of the most important, mentioned in the basics of heat storage section, is an increase in evaporative heat loss potential [73], which reduces Ereq – Emax (i.e. heat storage) and facilitates greater skin cooling. Both have been demonstrated many times in combination to decrease body Tc (~0.5°C) and HR (~30 bpm) responses to a standard exercise-heat stress [74]. Thus, heat acclimatization state should fundamentally influence both the HTT outcomes, pass/fail rates, and consequently test interpretations. If heat acclimatization or fitness are not the explanation, it is possible that a genetic propensity for having fewer sweat glands or any sudomotor signal interference, such as pharmaceutical-induced, could create a similar failed HTT result. Anything more than minimal clothing (shorts and t-shirt), such as a combat uniform, will also increase the ratio Ereq/Emax by reducing the ratio denominator (greater thermal and evaporative resistances), thus increasing the required ω beyond the range reported here for nearly nude virtual persons (Table 3). Despite these or even other possibilities, it is intuitive that heat acclimatization (and fitness) status hold significant potential to influence HTT outcomes and interpretations. The HTT is arguably more a test of heat acclimatization status and physical fitness than heat tolerance, per se.
Figure 3.
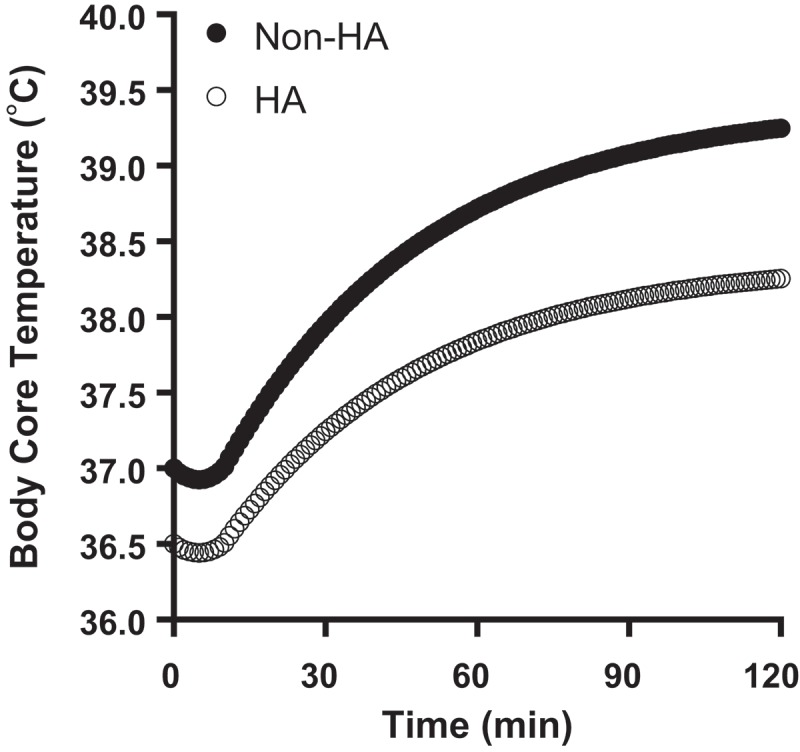
Empirical prediction of the body core temperature response to HTT before (Non-HA) and after (HA) heat acclimatization. Data modeled using the Heat Strain Decision Aid (HSDA). HTT conditions were as described by Moran et al. [52]. The lower final body core temperatures in HA (−0.98°C) is due to a lower starting temperature (51%) and slower rate of rise (49%). Data are for an 80 kg person wearing an Army physical fitness uniform (shorts and t-shirt) with typical thermal and evaporative resistances. For HSDA algorithm details, see Potter et al., 2017 [72].
Gaps in knowledge
Sensitivity/specificity of the test & follow up data
The standard of care for return to duty in the United States Army is a graduated RTA over the course of several weeks to months, dependent on severity of injury, as detailed in Army Regulation 40–501 [38]. The HTT in the U.S. Military Services is currently and principally used as an investigational tool for advancing research. However, the HTT is also being utilized by some foreign Services and in the U.S. Military Services to assist in decision making for RTA. Unfortunately, there is currently no evidence that the HTT provides any diagnostic utility for assessing recovery from exertional heat illness or risk of incurring subsequent exertional heat illness. “Tolerance” demonstrated for Ereq/Emax ≤ 0.70 will never be synonymous with tolerance to more extreme (yet common) circumstances that pose exertional heat illness risk, nor will it ever reveal specific recovery from organ damage or general readiness for duty in the heat. For the HTT to be used as described [52] for RTA or retention in the armed services, these issues must first be resolved.
Proposed recommendations
Table 3 illustrates that the standardized HTT produces different biophysical requirements for sweating based on differences in body size alone. Since the test is really designed to determine the ability to produce and evaporate sweat of sufficient quantities to produce heat balance (plateaus in body Tc and HR), a better test might involve a non-body weight dependent mode of exercise (e.g. cycle ergometer) whereby Ereq could be fixed [75] or Ereq/Emax fixed to a value that anyone should be able to achieve (e.g. <0.50) unless sudomotor function is truly dysfunctional. A different approach might be to use a more stressful test (higher Ereq or lower Emax) requiring greater ω – particularly if the patient is being assessed for RTA in stressful physical and environmental circumstances. Indeed, prior heat illness victims of considerable fitness have been able to pass the HTT only to demonstrate trends towards overheating when exposed to more demanding, but realistic, combinations of exercise and environmental stressors [63]. It would also be beneficial to determine if an individual is able to acclimatize to exercising in the heat in general [37]. The idea of accompanying the HTT with a blood biomarker measurement following exercise is intriguing as it could potentially increase the ability to diagnose individuals who are not ready to RTA. While recovery of blood biomarkers following EHS is required prior to undergoing the HTT (discussed previously), it is possible that residual organ damage could still exist. It is possible that exercise/heat stress associated with the HTT could result in the elevation of these biomarkers if the stress was robust enough to induce organ ischemia. However, it should be noted that the most commonly used HTT may not have the degree of exercise/heat stress to elicit this response. Because many of the most commonly measured clinical blood biomarkers lack the clinical sensitivity and specificity required to determine RTA status, it is questionable whether measurement of these indices prior or during the HTT will provide the diagnostic value that is needed to protect against further organ damage.
Conclusions
Proper assessment for RTA decision following an exertional heat illness is important as the determination has numerous personal, professional and legal ramifications. The RTA decision is made more difficult due to the lack of specific biomarkers (physiological and/or blood) and established criteria. Conceptually, the idea of a RTA test that assesses specific physiological and thermoregulatory responses during exercise heat-stress makes sense. However, current versions of the HTT have severe limitations as discussed. Although the HTT does inform the tester of whether or not an individual is able to perform the unique HTT at the time the test is administered, it would seem that this information provides only cautious sensitivity and low (or absent) specificity for true RTA status/readiness. One large knowledge gap in the HTT literature is the lack of follow-up data on individuals who have passed the HTT and returned to duty/activity and diagnostic sensitivity and specificity of the test itself. Lastly, it is important to recognize that no test can be predictive of future injury particularly in the case of EHS where numerous individual, environmental and situational factors contribute to injury risk.
Funding Statement
This work was supported by the U.S. Army Medical Research and Materiel Command.
Abbreviations
- Bpm
beats/minute
- °C
celsius
- C
convection
- E
evaporative heat loss
- EHS
exertional heat stroke
- Emax
maximal evaporative capacity of the environment
- Ereq
amount of evaporation required to achieve heat balance
- He
evaporative heat transfer coefficient
- Hprod
metabolic heat
- HR
heart rate
- HTT
Heat Tolerance Test
- IDF
Israeli Defense Force
- km/hr
kilometer/hour
- M
heat production
- m/s
meters/second
- Pa
ambient air water vapor pressure
- PHT
probability of heat tolerance
- Psks
saturated water vapor pressure at skin temperature
- R
radiation
- %RH
relative humidity
- RTA
return to activity
- S
heat balance
- SRreq
required sweating rate for thermal balance
- Tc
core temperature
- TCR
thermal circulatory ratio
- Trec
rectal temperature
- VO2max
maximal oxygen consumption
- W
watts
- Work
external work performed
- η
evaporative efficiency
- ω
skin wettedness
- ωmax
maximal skin wettedness
Declarations of Interest & Disclaimer
The authors report no conflicts of interest.
The opinions or assertions contained herein are the private views of the author(s) and are not to be construed as official or as reflecting the views of the U.S. Army or the Department of Defense.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Howe AS, Boden BP.. Heat-related illness in athletes. Am J Sports Med. 2007;35(8):1384–1395. PubMed PMID: 17609528. [DOI] [PubMed] [Google Scholar]
- [2].CDC. Heat-related deaths among crop workers–United States, 1992–2006. MMWR. 2008;57(24):649–653. PubMed PMID: 18566563. [PubMed] [Google Scholar]
- [3].(NIOSH) D Preventing heat related illness or death of outdoor workers. Workplace Solutions. 2013. [cited].
- [4].Arbury S, Jacklitsch B, Farquah O, et al. Heat illness and death among workers - United States, 2012–2013. MMWR Morb Mortal Wkly Rep. 2014;63(31):661–665. PubMed PMID: 25102413; PubMed Central PMCID: PMCPMC4584656. [PMC free article] [PubMed] [Google Scholar]
- [5].Armed Forces Health Surveillance Center:Update: heat illness, active component, U.S. Armed Forces, 2017. Msmr 2018;25(4):6–12. PubMed PMID: 29696983. [Google Scholar]
- [6].Armstrong LE, Casa DJ, Millard-Stafford M, et al. American college of sports medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556–572. PubMed PMID: 17473783. [DOI] [PubMed] [Google Scholar]
- [7].DeGroot DW, Kenefick RW, Sawka MN. Impact of arm immersion cooling during ranger training on exertional heat illness and treatment costs. Mil Med. 2015;180(11):1178–1183. PubMed PMID: 26540710. [DOI] [PubMed] [Google Scholar]
- [8].Abriat A, Brosset C, Bregigeon M, et al. Report of 182 cases of exertional heatstroke in the French Armed Forces. Mil Med. 2014;179(3):309–314. PubMed PMID: 24594466. [DOI] [PubMed] [Google Scholar]
- [9].Armed Forces Health Surveillance Center: Update: heat illness, active component, U.S. Armed Forces, 2016. Msmr 2017;24(3):9–13. PubMed PMID: 28358520. [PubMed] [Google Scholar]
- [10].Carter R 3rd, Cheuvront S, Sawka M. A case report of idiosyncratic hyperthermia and review of U.S. Army heat stroke hospitalizations. J Sport Rehabil. 2007;16:237–243. [DOI] [PubMed] [Google Scholar]
- [11].Moran DS, Heled Y, Still L, et al. Assessment of heat tolerance for post exertional heat stroke individuals. Med Sci Monit. 2004;10(6):Cr252–Cr257. PubMed PMID: 15173669. [PubMed] [Google Scholar]
- [12].Kazman JB, Heled Y, Lisman PJ, et al. Exertional heat illness: the role of heat tolerance testing. Curr Sports Med Rep. 2013;12(2):101–105. PubMed PMID: 23478560. [DOI] [PubMed] [Google Scholar]
- [13].Hori S. Index for the assessment of heat tolerance. J Human Ergol. 1978;7(2):135–144. PubMed PMID: 756447. [PubMed] [Google Scholar]
- [14].Shapiro Y, Magazanik A, Udassin R, et al. Heat intolerance in former heatstroke patients. Ann Intern Med. 1979;90(6):913–916. PubMed PMID: 443686. [DOI] [PubMed] [Google Scholar]
- [15].Lisman P, Kazman JB, O’Connor FG, et al. Heat tolerance testing: association between heat intolerance and anthropometric and fitness measurements. Mil Med. 2014;179(11):1339–1346. PubMed PMID: 25373064. [DOI] [PubMed] [Google Scholar]
- [16].Shvartz E, Shibolet S, Meroz A, et al. Prediction of heat tolerance from heart rate and rectal temperature in a temperate environment. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(4):684–688. PubMed PMID: 908684. [DOI] [PubMed] [Google Scholar]
- [17].Druyan A, Ketko I, Yanovich E, et al. Redefining the distinction between heat tolerant and intolerant individuals during a heat tolerance test. J Therm Biol. 2013;38(8):539–542. [Google Scholar]
- [18].Sawka MN, O’Connor FG. Disorders due to heat and cold. Goldman-Cecil Medicine. 25th ed., Vol. 1, Philadelphia: Elsevier Saunders; 2016. p. 691–695. [Google Scholar]
- [19].Bynum GD, Pandolf KB, Schuette WH, et al. Induced hyperthermia in sedated humans and the concept of critical thermal maximum. A J Physiol. 1978;235(5):R228–R236. PubMed PMID: 727284. [DOI] [PubMed] [Google Scholar]
- [20].Burger FJ, Fuhrman FA. Evidence of injury by heat in mammalian tissues. A J Physiol. 1964;206:1057–1061. PubMed PMID: 14208940. [DOI] [PubMed] [Google Scholar]
- [21].Maron MB, Wagner JA, Horvath SM. Thermoregulatory responses during competitive marathon running. J Appl Physiol Respir Environ Exerc Physiol. 1977;42(6):909–914. PubMed PMID: 881391. [DOI] [PubMed] [Google Scholar]
- [22].Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54(1):75–159. PubMed PMID: 4587247. [DOI] [PubMed] [Google Scholar]
- [23].Kregel KC, Wall PT, Gisolfi CV. Peripheral vascular responses to hyperthermia in the rat. J Appl Physiol. 1988;64(6):2582–2588. PubMed PMID: 3403442 [DOI] [PubMed] [Google Scholar]
- [24].Leon LR, Bouchama A. Heat stroke. Compr Physiol. 2015;5(2):611–647. PubMed PMID: 25880507. [DOI] [PubMed] [Google Scholar]
- [25].Malamud N, Haymaker W, Custer RP. Heat stroke; a clinico-pathologic study of 125 fatal cases. Mil Surgeon. 1946;99(5):397–449. PubMed PMID: 20276794. [PubMed] [Google Scholar]
- [26].Shibolet S, Coll R, Gilat T, et al. Heatstroke: its clinical picture and mechanism in 36 cases. Q J Med. 1967;36(144):525–548. PubMed PMID: 6077230. [PubMed] [Google Scholar]
- [27].Szold O, Reider G,II, Ben Abraham R, et al. Gray-white matter discrimination–a possible marker for brain damage in heat stroke? Eur J Radiol. 2002;43(1):1–5. PubMed PMID: 12065113. [DOI] [PubMed] [Google Scholar]
- [28].Albukrek D, Bakon M, Moran DS, et al. Heat-stroke-induced cerebellar atrophy: clinical course, CT and MRI findings. Neuroradiology. 1997;39(3):195–197. PubMed PMID: 9106293. [DOI] [PubMed] [Google Scholar]
- [29].Casa DJ, McDermott BP, Lee EC, et al. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35(3):141–149. PubMed PMID: 17620933. [DOI] [PubMed] [Google Scholar]
- [30].Vicario SJ, Okabajue R, Haltom T. Rapid cooling in classic heatstroke: effect on mortality rates. Am J Emerg Med. 1986;4(5):394–398. PubMed PMID: 3741557. [DOI] [PubMed] [Google Scholar]
- [31].Yaqub BA, Al-Harthi SS, Al-Orainey IO, et al. Heat stroke at the Mekkah pilgrimage: clinical characteristics and course of 30 patients. Q J Med. 1986;59(229):523–530. PubMed PMID: 3763815. [PubMed] [Google Scholar]
- [32].Lopez RM, Tanner P, Irani S, et al. A functional return-to-play progression after exertional heat stroke in a high school football player. J Athl Train. 2018;53(3):230–239. PubMed PMID: 29373058; PubMed Central PMCID: PMCPMC5894373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McDermott BP, Casa DJ, Yeargin SW, et al. Recovery and return to activity following exertional heat stroke: considerations for the sports medicine staff. J Sport Rehabil. 2007;16(3):163–181. PubMed PMID: 17923722. [DOI] [PubMed] [Google Scholar]
- [34].O’Connor FG, Casa DJ, Bergeron MF, et al. American college of sports medicine roundtable on exertional heat stroke–return to duty/return to play: conference proceedings. Curr Sports Med Rep. 2010;9(5):314–321. PubMed PMID: 20827100. [DOI] [PubMed] [Google Scholar]
- [35].Asplund CA, O’Connor FG. Challenging return to play decisions: heat stroke, exertional rhabdomyolysis, and exertional collapse associated with sickle cell trait. Sports Health. 2016;8(2):117–125. PubMed PMID: 26896216; PubMed Central PMCID: PMCPMC4789928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Casa DJ, DeMartini JK, Bergeron MF, et al. National athletic trainers’ association position statement: exertional heat illnesses. J Athl Train. 2015;50(9):986–1000. PubMed PMID: 26381473; PubMed Central PMCID: PMCPMC4639891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Headquarters, Department of the Army and Air Force: Heat stress control and casualty management Departments of the Army and Air Force Technical Bulletin Medical 507 (TBMED 507). Air Force Pam, 2003;48-152.
- [38].Johnson EC, Kolkhorst FW, Richburg A, et al. Specific exercise heat stress protocol for a triathlete’s return from exertional heat stroke. Curr Sports Med Rep. 2013;12(2):106–109. PubMed PMID: 23478561. [DOI] [PubMed] [Google Scholar]
- [39].Army US Standards of Medical Fitness. 2017.
- [40].O’Connor FG, Williams AD, Blivin S, et al. Guidelines for return to duty (play) after heat illness: a military perspective. J Sport Rehabil. 2007;16(3):227–237. PubMed PMID: 17923729. [DOI] [PubMed] [Google Scholar]
- [41].Kew M, Bersohn I, Seftel H. The diagnostic and prognostic significance of the serum enzyme changes in heatstroke. Trans R Soc Trop Med Hyg. 1971;65(3): 325–330. PubMed PMID: 5559748. [DOI] [PubMed] [Google Scholar]
- [42].King MA, Leon LR, Mustico DL, et al. Biomarkers of multiorgan injury in a preclinical model of exertional heat stroke. J Appl Physiol. 2015;118(10):1207–1220. PubMed PMID: 25814640. [DOI] [PubMed] [Google Scholar]
- [43].Mellor PJ, Mellanby RJ, Baines EA, et al. High serum troponin I concentration as a marker of severe myocardial damage in a case of suspected exertional heatstroke in a dog. J Vet Cardiol. 2006;8(1):55–62. PubMed PMID: 19083337. [DOI] [PubMed] [Google Scholar]
- [44].Quinn CM, Duran RM, Audet GN, et al. Cardiovascular and thermoregulatory biomarkers of heat stroke severity in a conscious rat model. J Appl Physiol. 2014;117(9):971–978. PubMed PMID: 25123200. [DOI] [PubMed] [Google Scholar]
- [45].Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med. 2010;48(6):757–767. PubMed PMID: 20518645. [DOI] [PubMed] [Google Scholar]
- [46].Romagnoli M, Alis R, Aloe R, et al. Influence of training and a maximal exercise test in analytical variability of muscular, hepatic, and cardiovascular biochemical variables. Scand J Clin Lab Invest. 2014;74(3):192–198. PubMed PMID: 24484196. [DOI] [PubMed] [Google Scholar]
- [47].Mitch WE. Chronic Kidney disease In: Goldman L, Schafer AI, editors. Goldman-Cecil medicine. 2nd ed. Piladelphia: Elsevier Saunders; 2016. p.833–841. [Google Scholar]
- [48].Leon LR, Helwig BG. Heat stroke: role of the systemic inflammatory response. J Appl Physiol. 2010;109(6):1980–1988. PubMed PMID: 20522730. [DOI] [PubMed] [Google Scholar]
- [49].Bianchi L, Ohnacker H, Beck K, et al. Liver damage in heatstroke and its regression: a biopsy study. Hum Pathol. 1972;3(2):237–248. PubMed PMID: 5028621. [DOI] [PubMed] [Google Scholar]
- [50].Schermann H, Sherman M, Rutenberg R. Case report of a new headache developed by a combat soldier after an episode of exertional heat illness. Front Neurol. 2017;8:383 PubMed PMID: 28824539; PubMed Central PMCID: PMCPMC5545587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wallace RF, Kriebel D, Punnett L, et al. Prior heat illness hospitalization and risk of early death. Environ Res. 2007;104(2):290–295. PubMed PMID: 17306249. [DOI] [PubMed] [Google Scholar]
- [52].Auron PE, Warner SJ, Webb AC, et al. Studies on the molecular nature of human interleukin 1. J Immunol. 1987;138(5):1447–1456. PubMed PMID: 3492551. [PubMed] [Google Scholar]
- [53].Moran DS, Erlich T, Epstein Y. The heat tolerance test: an efficient screening tool for evaluating susceptibility to heat. J Sport Rehabil. 2007;16(3):215–221. PubMed PMID: 17923727. [DOI] [PubMed] [Google Scholar]
- [54].Schermann H, Craig E, Yanovich E, et al. Probability of heat intolerance: standardized interpretation of heat-tolerance testing results versus specialist judgment. J Athl Train. 2018;53(4):423–430. PubMed PMID: 29775421; PubMed Central PMCID: PMCPMC5967286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Epstein Y, Heled Y. Back to play of athletes after exertional heat stroke. Curr Sports Med Rep. 2013;(5):346 PubMed PMID: 24030310 Doi: 10.1249/JSR.0b013e3182a630ac [DOI] [PubMed] [Google Scholar]
- [56].Schermann H, Heled Y, Fleischmann C, et al. The validity of the heat tolerance test in prediction of recurrent exertional heat illness events. J Sci Med Sport. 2018;21(6):549–552. PubMed PMID: 29066054. [DOI] [PubMed] [Google Scholar]
- [57].Dreosti AO. The results of some investigations into the medical aspect of deep mining on the witwatersrand. J Chem Metall Mining Soc South Afr. 1935;36(5):102–109. [Google Scholar]
- [58].Piwonka RW, Robinson S, Gay VL, et al. Preacclimatization of men to heat by training. J Appl Physiol. 1965;20(3):379–383. PubMed PMID: 5837555. [DOI] [PubMed] [Google Scholar]
- [59].Armstrong LE, Szlyk PC, Sils IV, et al. Prediction of the exercise-heat tolerance of soldiers wearing protective overgarments. Aviat Space Environ Med. 1991;62(7):673–677. PubMed PMID: 1898304. [PubMed] [Google Scholar]
- [60].Sagui E, Beighau S, Jouvion A, et al. Thermoregulatory response to exercise after exertional heat stroke. Mil Med. 2017;182(7):e1842–e1850. PubMed PMID: 28810981. [DOI] [PubMed] [Google Scholar]
- [61].Armstrong LE, Hubbard RW, DeLuca JP, et al. Evaluation of a temperate environment test to predict heat tolerance. Eur J Appl Physiol Occup Physiol. 1987;56(4):384–389. PubMed PMID: 3622480. [DOI] [PubMed] [Google Scholar]
- [62].Armstrong LE, Hubbard RW, Christensen EL, et al. Evaluation of a temperate-environment test of heat tolerance in prior heatstroke patients and controls. Eur J Appl Physiol Occup Physiol. 1990;60(3):202–208. PubMed PMID: 2347323. [DOI] [PubMed] [Google Scholar]
- [63].Ketko I, Eliyahu U, Epstein Y, et al. The thermal-circulatory ratio (TCR): an index to evaluate the tolerance to heat. Temperature. 2014;1(2):101–106. PubMed PMID: 27583291; PubMed Central PMCID: PMCPMC4977162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Roberts WO, Dorman JC, Bergeron MF. recurrent heat stroke in a runner: race simulation testing for return to activity. Med Sci Sports Exerc. 2016;48(5):785–789. . PubMed PMID: 26694842. [DOI] [PubMed] [Google Scholar]
- [65].Kazman JB, Purvis DL, Heled Y, et al. Women and exertional heat illness: identification of gender specific risk factors. US Army Med Dep j. 2015:58–66.PubMed PMID: 26101907. [PubMed] [Google Scholar]
- [66].Mee JA, Doust J, Maxwell NS. Repeatability of a running heat tolerance test. J Therm Biol. 2015;91–97. PubMed PMID: 25774031 DOI: 10.1016/j.jtherbio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- [67].O’Connor FG, Heled Y, Deuster PA. Exertional heat stroke, the return to play decision, and the role of Heat Tolerance Testing: a clinician’s dilemma. Curr Sports Med Rep. 2018;17(7):244–248. PubMed PMID: 29994825. [DOI] [PubMed] [Google Scholar]
- [68].Epstein Y, Yanovich R, Heled Y. Heat Tolerance Test or race simulation test for return to activity after heat stroke. Med Sci Sports Exerc. 2016;48(7):1428 PubMed PMID: 27308730. [DOI] [PubMed] [Google Scholar]
- [69].Druyan A, Makranz C, Moran D, et al. Heat tolerance in women–reconsidering the criteria. Aviat Space Environ Med. 2012;83(1):58–60. PubMed PMID: 22272518. [DOI] [PubMed] [Google Scholar]
- [70].Carter R 3rd, Cheuvront SN, Williams JO, et al. Epidemiology of hospitalizations and deaths from heat illness in soldiers. Med Sci Sports Exerc. 2005;37(8):1338–1344. PubMed PMID: 16118581. [DOI] [PubMed] [Google Scholar]
- [71].Havenith G, Fiala D. Thermal indices and thermophysiological modeling for heat stress. Compr Physiol. 2015;6(1):255–302. PubMed PMID: 26756633. [DOI] [PubMed] [Google Scholar]
- [72].Malchaire J, Piette A, Kampmann B, et al. Development and validation of the predicted heat strain model. Ann Work Expo Health. 2001;45(2):123–135. PubMed PMID: 11182426. [PubMed] [Google Scholar]
- [73].Potter AW, Blanchard LA, Friedl KE, et al. Mathematical prediction of core body temperature from environment, activity, and clothing: the heat strain decision aid (HSDA). J Therm Biol. 2017;64:78–85. PubMed PMID: 28166950. [DOI] [PubMed] [Google Scholar]
- [74].Ravanelli N, Coombs GB, Imbeault P, et al. Maximum skin wettedness after aerobic training with and without heat acclimation. Med Sci Sports Exerc. 2018;50(2):299–307. PubMed PMID: 28991042. [DOI] [PubMed] [Google Scholar]
- [75].Taylor NA. Human heat adaptation. Compr Physiol. 2014;4(1):325–365. PubMed PMID: 24692142. [DOI] [PubMed] [Google Scholar]
- [76].Gagnon D, Jay O, Kenny GP. The evaporative requirement for heat balance determines whole-body sweat rate during exercise under conditions permitting full evaporation. J Physiol. 2013;591(11):2925–2935. PubMed PMID: 23459754; PubMed Central PMCID: PMCPMC3690695. [DOI] [PMC free article] [PubMed] [Google Scholar]


