ABSTRACT
Rationale: Passive heat therapy improves vascular endothelial function, likely via enhanced nitric oxide (NO) bioavailability, although the mechanistic stimuli driving these changes are unknown. Objective: To determine the isolated effects of circulating (serum) factors on endothelial cell function, particularly angiogenesis, and NO bioavailability. Methods and Results: Cultured human umbilical vein endothelial cells (HUVECs) were exposed to serum collected from 20 healthy young (22 ± 1 years) adults before (0 wk), after one session of water immersion (Acute HT), and after 8 wk of either heat therapy (N = 10; 36 sessions of hot water immersion; session 1 peak rectal temperature: 39.0 ± 0.03°C) or sham (N = 10; 36 sessions of thermoneutral water immersion). Serum collected following acute heat exposure and heat therapy improved endothelial cell angiogenesis (Matrigel bioassay total tubule length per frame, 0 wk: 69.3 ± 1.9 mm vs. Acute HT: 72.8 ± 1.4 mm, p = 0.04; vs. 8 wk: 73.0 ± 1.4 mm, p = 0.03), with no effects of sham serum. Enhanced angiogenesis was NO-mediated, as addition of the NO synthase (NOS) inhibitor L-NNA to the culture media abolished differences in tubule formation across conditions (0 wk: 71.3 ± 1.8 mm, Acute HT: 71.6 ± 1.9 mm, 8 wk: 70.5 ± 1.6 mm, p = 0.69). In separate experiments, we found that abundance of endothelial NOS (eNOS) was unaffected by Acute HT serum (p = 0.71), but increased by 8 wk heat therapy serum (1.4 ± 0.1-fold from 0 wk, p < 0.01). Furthermore, increases in eNOS were related to improvements in endothelial tubule formation (r2 = 0.61, p < 0.01). Conclusions: Passive heat therapy beneficially alters circulating factors that promote NO-mediated angiogenesis in endothelial cells and increase eNOS abundance. These changes may contribute to improvements in vascular function with heat therapy observed in vivo.
Abbreviations: Ang-1: angiopoietin-1; ANOVA: analysis of variance; bFGF: basic fibroblast growth factor; CV: cardiovascular; CVD: cardiovascular diseases; eNOS: endothelial nitric oxide synthase; HSPs: heat shock proteins; HT: heat therapy; HUVECs: human umbilical endothelial cells; L-NNA: Nω-nitro-L-arginine; MnSOD: manganese superoxide dismutase; NO: nitric oxide; NOS: nitric oxide synthase; PBMCs: peripheral blood mononuclear cells; RM: repeated measures; sFlt-1: soluble VEGF receptor; SOD: superoxide dismutase; TGF-β: transforming growth factor- β; VEGF: vascular endothelial growth factor
KEYWORDS: Hot water immersion, vascular function, oxidative stress, endothelial function
Introduction
Heat therapy, in the form of chronic use of hot tubs and saunas, has been gaining attention recently for its utility as a health-promoting lifestyle intervention. Several studies have shown that infrared sauna therapy improves clinical symptoms and outcomes of cardiovascular diseases (CVD) [1–3] and, in epidemiological studies, higher frequency and duration per session of lifelong sauna use has been associated with greatly reduced risk of CVD-related and all-cause mortality [4]. It is likely that these lifelong CV protective effects of heat therapy are related to effects on the vasculature, as the majority of CVDs are characterized and preceded by vascular dysfunction [5–7], including vascular endothelial dysfunction and stiffening of the large elastic arteries. These changes to arteries occur in large part due to superoxide-driven oxidative stress that reduces bioavailability of vasodilatory molecule nitric oxide (NO) [8,9]. We previously demonstrated that 8 wk of heat therapy via hot water immersion induces widespread and robust improvements in vascular function in young, sedentary adults [10], including improved brachial artery flow-mediated dilation (a measure of endothelial function), reduced arterial stiffness, and reduced blood pressure, all of which are independently predictive of CV-related morbidity and mortality [11–14]. Furthermore, using cutaneous microdialysis, we demonstrated that improvements in endothelial function were mediated by enhanced NO-dependent dilation [15].
The mechanistic stimuli that drive these improvements in vascular function with heat therapy are likely multi-fold. First, heat exposure increases cardiac output, blood flow, and therefore shear stress on the vasculature [16,17]. Shear stress is well known to upregulate endothelial NO synthase (eNOS) expression and activity and therefore NO bioavailability through several mechanisms [18–20]. Secondly, elevations in body core temperature induce expression of heat shock proteins (HSPs), which in turn stabilize a variety of proteins important to the CV system. These include eNOS [21,22] and antioxidant enzymes such as superoxide dismutase (SOD) [23], and pharmacological blockade of HSP72 prevents thermoregulatory adaptations to heat acclimation [24,25].
However, a third mechanism, that has received little attention thus far, may play an important role in mediating vascular adaptation – circulating factors. As heat therapy induces widespread systemic effects, factors upregulated elsewhere (e.g. in skeletal muscle or adipocytes) in response to acute and chronic heat therapy may enter the circulation and subsequently come in contact with endothelial cells where they can influence cellular processes, including NO signaling. For example, vascular endothelial growth factor (VEGF) is one circulating factor that is known to increase NO bioavailability and promote angiogenesis [26]. The isolated effects of these circulating factors can be investigated using cell culture models in which cultured endothelial cells are exposed to serum collected from human subjects. Therefore, to investigate the role of heat therapy-induced circulating factors on increasing NO bioavailability, we conducted cell culture experiments in which endothelial cells were incubated with serum collected from young, sedentary human subjects before and after 8 wk of heat therapy. We hypothesized that serum obtained following heat therapy would increase endothelial tubule formation (i.e. angiogenesis), an established in vitro test of NO-mediated endothelial function [27–30] and changes in protein expression associated with improved NO bioavailability.
Methods
Twenty young (22 ± 1 years), healthy (no history of CV-related or other chronic diseases), sedentary (<2 h aerobic exercise per week) male and female subjects who were non-smokers and were not taking any prescription medications except contraceptives participated in the study. All subjects provided oral and written informed consent prior to participation in the study, as set forth by the Declaration of Helsinki. All experimental procedures were approved by the Institutional Review Board at the University of Oregon. The study was registered on ClinicalTrials.gov (identifier: NCT02518399) and other results from this study have been previously published elsewhere [10,15,31].
Subjects participated in 8 wk of either hot (40.5°C; heat therapy) or thermoneutral (36°C; sham group) water immersion (N = 10 per group), as described in detail elsewhere [10,15]. Heat therapy was sufficient to maintain rectal temperature between 38.5°C and 39.0°C for 60 min per session (up to 90 min of hot water immersion per session total); whereas thermoneutral water immersion mimicked the hydrostatic effects of heat therapy while maintaining rectal temperature within 0.2°C of resting. A water temperature of 36°C for the sham condition was selected as it is equivalent to average mean body temperature [32], and was confirmed in pilot testing to be truly thermoneutral based on no changes in rectal temperature, body mass (sweating), or heart rate.
Before (0 wk) and 1 h after (Acute) the first water immersion session and following chronic heat therapy or sham (8 wk; 36–48 h after the last water immersion session to capture the chronic rather than acute effects of heat therapy), venous blood was collected into serum-separating vacutainers (BD, Franklin Lakes, NJ) and Ficoll Hypaque-containing cell preparation tubes with sodium citrate (for isolation of peripheral blood mononuclear cells [PBMCs]; CPT Vacutainer; BD), kept at room temperature for 30 min, then separated by centrifugation, and stored at −80°C until analysis or use in cell culture experiments. Subjects reported to the laboratory fasted from food for at least 2 h and having abstained from exercise for at least 24 h. Sessions were held at the same time of day and subjects were asked to maintain food prior to the 2 h before and caffeine intake similarly between the two sessions. The time frame for acute blood collection was selected based on pilot studies in which we identified 1 h post-hot water immersion as the time point when HSP expression peaked in PBMCs (vs. immediately post, 2 h post, and later time points).
For all cell culture experiments, human umbilical vein endothelial cells (HUVECs; ATCC, Manassas, VA) were cultured in a humidified incubator under standard conditions (37°C, 5% CO2, 20% O2) in vascular cell basal medium supplemented with an endothelial growth kit (ATCC). Cells were used for experiments after 2–3 passages.
As a functional test of NO bioavailability, endothelial tubule formation was quantified using an established Matrigel angiogenesis bioassay [28–30]. HUVECs were plated in 96-well cell culture plates onto solidified phenol-red free growth factor reduced Matrigel (BD Bioscience, San Jose, CA) at a concentration of 1 × 105 cells/ml in basal medium. Cells were treated with 10% serum from human subjects collected at each of the 3 time points and from each of the two groups (in triplicate per sample), and incubated for 10 h. Wells were imaged with a phase-contrasted microscope at 2.5X optical zoom, and total length of tubule formation per frame was assessed with ImageJ analysis software (National Institutes of Health, Bethesda, MD) by two blinded investigators. Results were averaged across investigators and across two separate experiments for verification. In a subset of experiments, cells were additionally treated with 1 mM Nω-nitro-L-arginine (L-NNA; Sigma Aldrich, St. Louis, MO) to inhibit NOS and to determine whether the pro-angiogenic effects of heat therapy were mediated via NOS upregulation. A concentration of 1 mM was selected based on pilot studies in order to reduce NOS activity without completely abolishing angiogenesis.
For quantification of changes in protein abundance with serum exposure, HUVECs were plated in 6-well culture plates and incubated until ~40% confluence. Following 4 h serum starve, cells were incubated for 24 h in basal media supplemented with 10% human sera collected at each of the 3 time points from all 10 subjects in the heat therapy group (3 wells per serum sample), and collected following the 24 h with TrypLETM (trypsin-like enzyme; ThermoFisher, Waltham, MA).
Western immunoblotting was performed in serum-exposed HUVECs and primary PBMC lysates (both lysed via sonication in radioimmunoprecipitation buffer plus protease inhibitor). Protein (20–50 μg) was separated by electrophoresis on 4–20% SDS polyacrylamide separating gels (Life Technologies, Grand Island, NY) and then transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were Ponceau-stained to assess transfer across each gel. Membranes were incubated for 1 h in Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) and then incubated overnight at 4°C in blocking buffer containing primary antibodies. Membranes were then washed and incubated with the appropriate secondary antibodies (LI-COR Biosciences) for 1 h at room temperature. The fluorescent bands were digitized using a LI-COR Odyssey infrared imaging system (LI-COR Biosciences). Digitized images were quantified using LI-COR Image StudioTM software. Antibodies were stripped using NewBlotTM Nitro Stripping Buffer (LI-COR Biosciences) in between probing for primary antibodies. Primary antibodies for HUVECs were: 1) anti-endothelial NO synthase (eNOS) (1:1,000; Cell Signaling Technology, Danvers, MA), 2) anti-Hsp70 [BRM-22] (1:5,000; Abcam), 3) anti-Hsp90 [S88] (1:200; Abcam, Cambridge, MA) and 4) anti-vinculin (loading control; 1:1,000; Cell Signaling Technology). Primary antibodies for PBMCs were: 1) anti-Hsp70 [BRM-22] (1:5,000; Abcam) 2) anti-Hsp90 [S88] (1:200; Abcam), or 3) anti-β-actin (loading control; 1:1,000; Cell Signaling Technology).
To attempt to identify factors in the serum affected by heat therapy that could be responsible for inducing changes in endothelial cells, we measured serum concentrations of free VEGF and soluble VEGF Receptor (sFlt-1) using commercially-available enzyme-linked immunosorbent assay kits (Quantikine, R&D Systems, Minneapolis, MD) according to manufacturer’s instructions. The ratio of sFlt-1 to VEGF was calculated as a marker of bioavailable VEGF.
Statistical analyses were conducted using Prism 7.0e (GraphPad Software, Inc., San Diego, CA). Changes in endothelial tubule formation were compared using two-way mixed design ANOVA with a repeated factor of time/serum condition (0 wk, Acute, or 8 wk) and a between subjects factor of group (heat therapy, heat therapy + L-NNA, or sham). Changes in protein abundance in serum-exposed HUVECs (eNOS, Hsp70, and Hsp90), and serum concentrations of VEGF, sFlt-1 and sFlt-1/VEGF were compared using one-way repeated measures (RM) ANOVA. When significant main effects were observed, pairwise comparisons were made using Tukey’s post hoc test. Comparisons in endothelial tubule formation across groups were made using Student’s paired (heat therapy with vs. without L-NNA) and unpaired (heat therapy vs. sham) t-tests. We were underpowered for comparing changes in Hsp protein in PBMCs using ANOVA (power on two-way RM ANOVA for Hsp90 was 0.21) and so differences were instead compared using Student’s paired t-test for 0 wk vs. Acute and 0 wk vs. 8 wk. Linear regression analysis was used to relate changes in endothelial tubule formation to eNOS abundance, and previously reported changes in endothelial cell protein abundance of manganese SOD (MnSOD; mitochondrial isoform) and superoxide production [31].
Results
Subjects were well-matched across both groups for age, height, weight, body mass index, and resting blood pressure, as presented in Table 1 and reported elsewhere [10,15]. Heat therapy induced physiological adaptations associated with heat acclimation, including a reduction in resting rectal temperature (p = 0.003) and an increase in whole body mean sweat rate during hot water immersion sessions (p < 0.001), whereas no changes were observed in sham subjects (reported elsewhere [15]). We observed a significant elevation in intracellular Hsp70 content in PBMCs following both acute hot water immersion (2.0 ± 0.4-fold change from 0 wk, p = 0.04) and chronic heat therapy (1.6 ± 0.2-fold change from 0 wk, p = 0.04) [31]. Intracellular PBMC Hsp90 content was increased following acute hot water immersion (p = 0.048), but only remained elevated chronically in half of the subjects following 8 wk of heat therapy (p = 0.33 vs. 0 wk) (Figure 1).
Table 1.
Subject demographics and adaptations to heat acclimation.
| Heat therapy group (N = 10) |
Sham group (N = 10) |
|||
|---|---|---|---|---|
| 0 wk | 8 wk | 0 wk | 8 wk | |
| Male/female | 4/6 | - | 4/6 | - |
| Age (years) | 22 ± 1 | - | 22 ± 1 | - |
| Height (cm) | 173 ± 4 | - | 172 ± 3 | - |
| Weight (kg) | 67 ± 3 | - | 66 ± 3 | - |
| Body mass index (kg.m−2) | 22.4 ± 0.6 | - | 22.5 ± 0.6 | - |
| Resting rectal temperature (°C) | 37.3 ± 0.1 | 36.9 ± 0.1*† | 37.2 ± 0.1 | 37.2 ± 0.1 |
| Peak rectal temperature (°C) | 39.0 ± 0.1† | 38.8 ± 0.1*† | 37.5 ± 0.1 | 37.4 ± 0.1 |
| Mean whole body sweat rate (L.h−1) | 0.54 ± 0.20† | 1.29 ± 0.40*† | 0.03 ± 0.04 | 0.02 ± 0.04 |
Data are mean ± SE Mean whole body sweat rate was calculated as body weight loss across the 90-min sessions, after correcting for fluid intake, and normalized for time. * P< 0.05 vs. 0 wk within group. † P < 0.05 vs. sham at the same time point.
Figure 1.
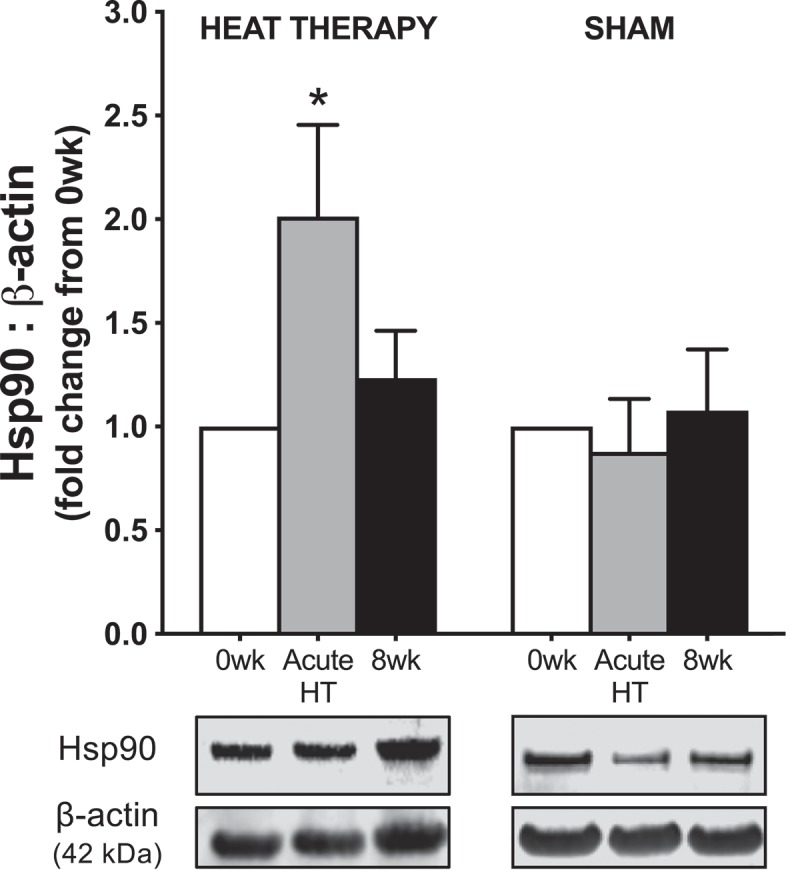
Protein abundance of heat shock protein (Hsp)90 in peripheral blood mononuclear cells collected from subjects before (0 wk) and 1 h after (acute) the first water immersion session and following 8 wk of heat therapy or sham. Representative Western blot images are provided below. Data are mean±SE, *p < 0.05.
Results of the endothelial tubule formation experiments are summarized in Figure 2. Tubule formation was significantly improved in cells exposed to sera from heat therapy subjects following one bout of hot water immersion (Acute HT; p = 0.04) and following heat therapy (8 wk; p = 0.03), relative to cells exposed to sera from the same subjects prior to heat therapy (0 wk). There were no effects of sera from sham subjects on endothelial tubule formation (0 wk vs. Acute HT: p = 0.18; vs. 8 wk: p = 0.99). To investigate whether improvements were NO-mediated, experiments were repeated with the addition of 1 mM L-NNA to the culture media using sera from subjects in the heat therapy group. As intended, 1 mM L-NNA had no effect on endothelial tubule formation in cells exposed to 0 wk sera (heat therapy group, with vs. without L-NNA: p = 0.41), but L-NNA prevented the improvement in tubule formation associated with both acute and chronic heat therapy (main effect: p = 0.69).
Figure 2.
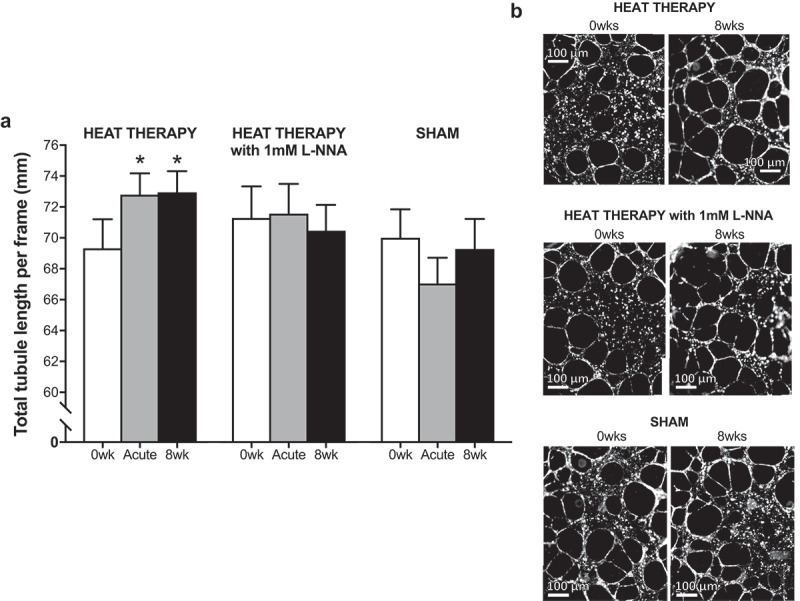
(a) Changes in endothelial cell tubule formation following incubation with sera from heat therapy (with and without 1mM Nω-nitro-L-arginine [L-NNA]) and sham subjects collected before (0 wk) and 1 h after (Acute HT) the first hot water immersion session and following heat therapy (8 wk). Endothelial tubule formation was significantly improved following exposure to sera from subjects in the heat therapy group following acute and chronic hot water immersion. No other significant changes were observed. Data are mean±SE, *p < 0.05. (b) Representative phase contrasted images at 2.5X magnification of endothelial cells exposed to sera collected from the same subject in the heat therapy group before and after 8 wk of heat therapy, with and without L-NNA, and from a subject in the sham group (all pre-water immersion).
Using Western blotting, we found that eNOS protein abundance was unaffected by exposure to acute HT serum (p = 0.71 vs. 0 wk), but significantly increased in cells exposed to 8 wk serum (p < 0.01 vs. 0 wk) (Figure 3(a)). Furthermore, using linear regression analysis, we found that increases in endothelial tubule formation (i.e. NO bioavailability) with chronic heat therapy (8 wk vs. 0 wk serum) were related to increases in eNOS abundance (r2 = 0.61, p < 0.01; Figure 3(b)). We have previously reported that superoxide production, assessed using a real-time fluorescent cell permeable superoxide stain, is lower in cells exposed to both acute HT (p < 0.01) and 8 wk (p = 0.03) heat therapy serum, which was accompanied by increases in protein abundance of the antioxidant enzyme MnSOD (Acute HT: 1.3 ± 0.2-fold change from 0 wk, p = 0.15 vs. 0 wk; 8 wk: 1.7 ± 0.2-fold change from 0 wk, p = 0.02 vs. 0 wk) [31]. Increases in MnSOD with chronic heat therapy (0 wk vs. 8 wk) tended to be related to increases in endothelial tubule formation, but this relation did not reach significance (r2 = 0.25, p = 0.14). We found no relation between changes in endothelial tubule formation following chronic heat therapy and superoxide production (8 wk: r2 = 0.10, p = 0.37) nor with any of these protein-related variables following Acute HT (eNOS: r2 = 0.002, p = 0.90; MnSOD: r2 = 0.07, p = 0.47; superoxide production: r2 = 0.04, p = 0.60).
Figure 3.
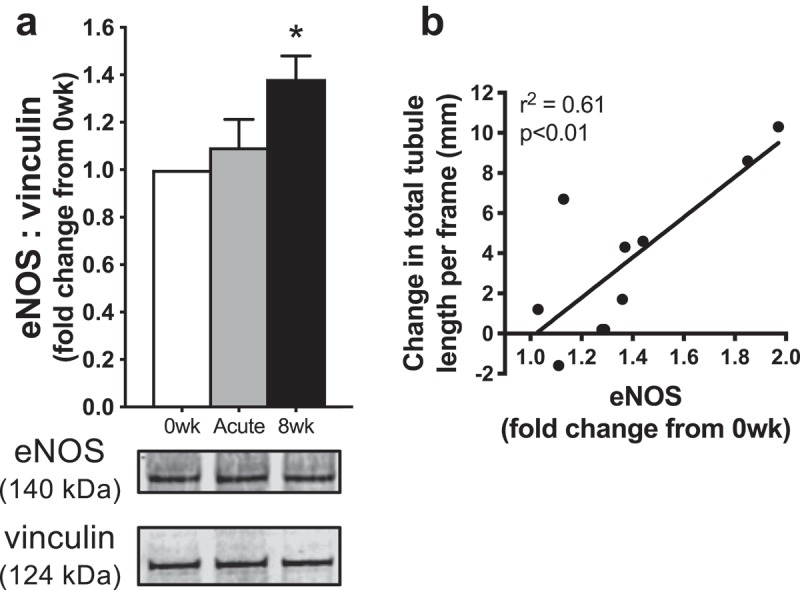
(a) Protein abundance of endothelial nitric oxide synthase (eNOS) in endothelial cells exposed to sera from human subjects collected before (0 wk) and 1 h after (Acute) the first hot water immersion session and following heat therapy (8 wk). Data were normalized to a vinculin loading control and presented as mean±SE fold changes from 0 wk. Representative Western blot images are provided below. (b) Increases in endothelial tubule formation (with exposure to 8 wk vs. 0 wk serum) were significantly related to increases in eNOS.
To investigate potential mechanisms for improvements in NO bioavailability, we first measured abundance of intracellular HSPs, but found no effects of heat therapy serum on either Hsp70 (p = 0.50; Figure 4(a)) or Hsp90 (p = 0.45; Figure 4(b)). We have also previously reported that serum levels of extracellular Hsp70 are unaffected by heat therapy [31]. In addition, we observed no effects of heat therapy on serum levels of pro-angiogenic VEGF (p = 0.16; Figure 4(d)), its soluble receptor sFlt-1 (p = 0.75; Figure 4(e)), or the ratio of sFlt-1/VEGF (i.e. bioavailable VEGF; p = 0.49; Figure 4(f)).
Figure 4.
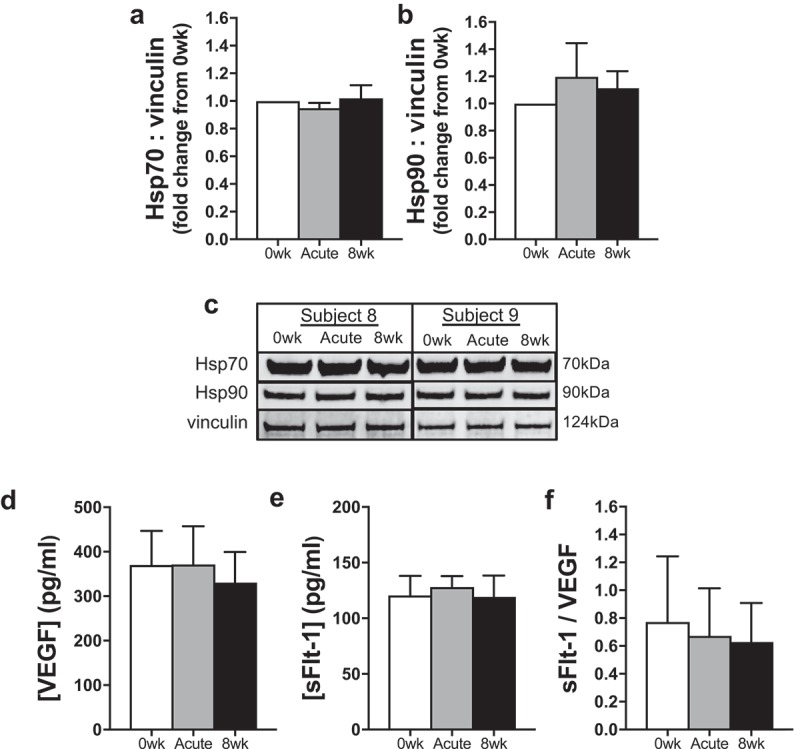
(a–b) Protein abundance of heat shock protein (Hsp) 70 (a) and Hsp90 (b) in endothelial cells exposed to sera from human subjects collected before (0 wk) and 1 h after (Acute) the first hot water immersion session and following 8 wk of heat therapy. Data were normalized to a vinculin loading control and presented as mean±SE fold changes from 0 wk. (c) Representative Western blot images are provided below. (d–f) Serum concentrations of vascular endothelial growth factor (VEGF) (d) and soluble VEGF receptor (sFlt)-1 (e), and the ratio of sFlt-1/VEGF, indicative of bioavailable VEGF, across time points into heat therapy (f). Data are mean±SE No significant differences were observed.
Discussion
We have previously shown that passive heat therapy induces robust and widespread improvements in both macro- and microvascular endothelial function. These improvements, along with reductions in arterial stiffness and blood pressure, likely underlie reductions in CV risk associated with lifelong heat therapy. In the present study, we extended these previous findings by elucidating some of the molecular mechanisms that may mediate improvements in endothelial function. Specifically, we used a cell culture model to isolate the effects of circulating factors upregulated by heat therapy on endothelial function and observed that exposing cultured endothelial cells to serum collected from human subjects who had undergone both acute hot water immersion and chronic heat therapy increased endothelial tubule formation, an established ex vivo test of NO-mediated endothelial function and angiogenesis [27,28,30]. We confirmed that increases in tubule formation with acute and chronic heat therapy serum were in fact mediated by enhanced NO bioavailability by showing that addition of the NOS inhibitor L-NNA eliminated improvements. Furthermore, improvements with chronic heat therapy were likely mediated by increases in eNOS abundance and therefore capacity to produce NO, and may have also been associated with increased antioxidant defenses and/or reduced superoxide production, and therefore reduced scavenging of NO. Interestingly, we did not observe changes in eNOS abundance with serum collected following acute hot water immersion, suggesting improvements in NO bioavailability and angiogenesis may have been primarily mediated by reduced oxidative stress-associated scavenging of NO. It is also possible that acute hot water immersion increased eNOS activation, despite no change in overall enzyme capacity; however, we, unfortunately, were not able to reliably measure abundance of phosphorylated eNOS in our samples. Lastly, all improvements were observed in the absence of any changes in intracellular HSP abundance.
The endothelial tubule formation assay we used involves organized proliferation and migration of endothelial cells to form capillary-like tubules. Thus, it is also considered a test of angiogenesis. When paired with serum exposure, others have used this test as a measure of “serum angiogenic potential” [33,34], concluding that greater serum-induced endothelial cell tubule formation indicates greater angiogenesis in vivo. As such, our results suggest heat therapy may also improve angiogenesis in vivo, which if true, may underscore reductions in blood pressure (secondary to reduced systemic vascular resistance) that we have observed in human subjects. In support of this notion, heat therapy has been reported to improve hindlimb NO-mediated angiogenesis in rats following femoral artery ligation [35] and to upregulate expression of multiple pro-angiogenic factors in human skeletal muscle [36].
Our data also add to the growing evidence that serum from human subjects who have undergone healthy lifestyle interventions can confer cellular protection [37–42]. In general, beneficial changes in cellular phenotypes cannot be attributed to changes in anyone (or several) circulating factors. Rather, it is the culmination of changes in the overall serum milieu that drives phenotypic changes. That said, we attempted to identify factors that may be changing in the serum by measuring circulating levels of HSPs (no changes) and the well-established pro-angiogenic factor VEGF, which induces angiogenesis by increasing eNOS protein abundance and activation [26]. However, we observed no changes in VEGF, nor its soluble receptor sFlt-1, which is consistent with studies investigating the effects of repeated sauna therapy in rats [35] and humans [43]. In particular, Akasaki et al. [35]. observed no change in serum VEGF in rats following 3 wk of sauna therapy, despite NOS-dependent improvements in hindlimb angiogenesis.
There are other known pro-angiogenic factors that can induce NOS-dependent angiogenesis independent of VEGF, including basic fibroblast growth factor (bFGF) [44] and transforming growth factor (TGF)-β[45]; however, there is conflicting evidence on whether these may be upregulated following heat stress [46–49]. Furthermore, enhanced TGF-β signaling is implicated in the development of arterial stiffening [50], which we have observed is reduced in vivo following heat therapy [10]. Another potential candidate is angiopoietin-1 (Ang-1), a ligand for Tie-2 receptors expressed on the surface of endothelial cells that induces angiogenesis by enhancing eNOS activity via the PI 3-kinase, Akt and MAP-kinase pathways [51–53]. Heat stress in cultured cells has been shown to increase Ang-1 mRNA [54], but it is presently unknown whether whole-body heat acclimation would do the same. It is also possible that levels of circulating cells and/or cell fragments may provide more answers. For example, repeated sauna therapy has been shown to increase the number of circulating CD34+ cells in heart failure [55] and peripheral artery disease patients [43]. CD34+ cells are endothelial progenitor cells which are thought to promote angiogenesis through the secretion of angiogenic growth factors and/or their incorporation into developing blood vessels [56]. Although CD34+ cells would presumably (due to centrifugation) not be present in the human sera with which we treated endothelial cells, it is possible mobilized CD34+ cells released signaling molecules that could have been present in the sera.
In conclusion, our data establish a key role of circulating factors in mediating the effects of passive heat therapy on vascular function (and possibly blood pressure). Although a comprehensive investigation into what factors may be changing in the serum was outside of the scope of the present study, we encourage other investigators to perform such studies in the future, as this could help identify targets for the development of CV protective pharmacotherapies. In addition to circulating factors, endothelial cells in vivo are exposed to elevations in blood temperature and increases in arterial wall shear stress during each hot water immersion session. Thus, we believe it is likely that these three mechanisms (i.e. circulating factors, increases in temperature/HSPs, and shear stress) act in combination in vivo to mediate the robust improvements in vascular function that we have observed in human subjects with passive heat therapy.
Funding Statement
This study was supported by American Heart Association (AHA) Grant [14PRE20380300], the Eugene and Clarissa Evonuk Memorial Foundation, and the Kenneth and Kenda Singer Endowment. V.E.B. is currently supported by [F32 HL140875]. C.T.M. is currently supported by the Kenneth and Kenda Singer Endowment, AHA Grant [160016], and [NIH HL144128].
Acknowledgments
The authors would like to sincerely thank the subjects who participated in this study, as well as Dr. Brett R. Ely, Matthew J. Howard and Andrew J. Jeckell for assistance with data collection, and Dr. Hans C. Dreyer and Dr. Jonathan Muyskens for providing laboratory space and support. V.E.B.’s current affiliation is the Department of Integrative Physiology, University of Colorado Boulder.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Kihara T, Miyata M, Fukudome T, et al. Waon therapy improves the prognosis of patients with chronic heart failure. J Cardiol. 2009;53:214–218. [DOI] [PubMed] [Google Scholar]
- [2].Miyata M, Kihara T, Kubozono T, et al. Beneficial effects of Waon therapy on patients with chronic heart failure: results of a prospective multicenter study. J Cardiol. 2008;52:79–85. [DOI] [PubMed] [Google Scholar]
- [3].Sobajima M, Nozawa T, Ihori H, et al. Repeated sauna therapy improves myocardial perfusion in patients with occluded coronary artery-related ischemia. Int J Cardiol. 2013;167:237–243. [DOI] [PubMed] [Google Scholar]
- [4].Laukkanen T, Khan H, Zaccardi F, et al. Association between Sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med. 2015;175:542–548. [DOI] [PubMed] [Google Scholar]
- [5].Benjamin EJ, Muntner P, Alonso A, et al. American Heart Association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;47:CIR0000000000000659. [Google Scholar]
- [6].Lakatta EG, Levy D.. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. [DOI] [PubMed] [Google Scholar]
- [7].Hinderliter AL, Caughey M. Assessing endothelial function as a risk factor for cardiovascular disease. Curr Atheroscler Rep. 2003;5:506–513. [DOI] [PubMed] [Google Scholar]
- [8].Incalza MA, D’Oria R, Natalicchio A, et al. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1–19. [DOI] [PubMed] [Google Scholar]
- [9].Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. [DOI] [PubMed] [Google Scholar]
- [10].Brunt VE, Howard MJ, Francisco MA, et al. Passive heat therapy improves endothelial function, arterial stiffness, and blood pressure in sedentary humans. J Physiol. 2016;594:5329–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shechter M, Issachar A, Marai I, et al. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol. 2009;134:52–58. [DOI] [PubMed] [Google Scholar]
- [12].Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. [DOI] [PubMed] [Google Scholar]
- [14].Mitchell GF, Hwang S-J, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brunt VE, Eymann TM, Francisco MA, et al. Passive heat therapy improves cutaneous microvascular function in sedentary humans via improved nitric oxide-dependent dilation. J Appl Physiol. 2016;121:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Crandall CG, Wilson TE. Human cardiovascular responses to passive heat stress. Compr Physiol. 2015;5:17–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Thomas KN, van Rij AM, Lucas SJE, et al. Substantive hemodynamic and thermal strain upon completing lower-limb hot-water immersion; comparisons with treadmill running. Temperature. 2016;3:286–297. doi: 10.1080/23328940.2016.1156215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Uematsu M, Ohara Y, Navas JP, et al. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol. 1995;269:C1371–8. [DOI] [PubMed] [Google Scholar]
- [19].Ranjan V, Xiao Z, Diamond SL. Constitutive NOS expression in cultured endothelial cells is elevated by fluid shear stress. Am J Physiol. 1995;269:H550–5. [DOI] [PubMed] [Google Scholar]
- [20].Woodman CR, Price EM, Laughlin MH. Shear stress induces eNOS mRNA expression and improves endothelium-dependent dilation in senescent soleus muscle feed arteries. J Appl Physiol. 2005;98:940–946. [DOI] [PubMed] [Google Scholar]
- [21].Pritchard KA, Ackerman AW, Gross ER, et al. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J Biol Chem. 2001;276:17621–17624. [DOI] [PubMed] [Google Scholar]
- [22].Harris MB, Mitchell BM, Sood SG, et al. Increased nitric oxide synthase activity and Hsp90 association in skeletal muscle following chronic exercise. Eur J Appl Physiol. 2008;104:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Choi S, Park KA, Lee HJ, et al. Expression of Cu/Zn SOD protein is suppressed in hsp 70.1 knockout mice. J Biochem Mol Biol. 2005;38:111–114. [DOI] [PubMed] [Google Scholar]
- [24].Amorim FT, Fonseca IT, Machado-Moreira CA. Magalhães F de C. Insights into the role of heat shock protein 72 to whole-body heat acclimation in humans. Temperature. 2015;2:499–505. doi: 10.1080/23328940.2015.1110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kuennen M, Gillum T, Dokladny K, et al. Thermotolerance and heat acclimation may share a common mechanism in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Papapetropoulos A, Garcia-Cardena G, Madri JA, et al. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Goodwin AM. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvas Res. 2007;74:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Murohara T, Asahara T, Silver M, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kubota Y, Kleinman HK, Martin GR, et al. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sun J, Liao JK. Induction of angiogenesis by heat shock protein 90 mediated by protein Kinase Akt and endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2004;24:2238–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brunt VE, Wiedenfeld-Needham K, Comrada LN, et al. Passive heat therapy protects against endothelial cell hypoxia-reoxygenation via effects of elevations in temperature and circulating factors. J Physiol. 2018;596:4831–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Burton AC. Human calorimetry: the average temperature of the tissues of the body. J Nutr. 1935;9:261–280. [Google Scholar]
- [33].Banek CT, Bauer AJ, Needham KM, et al. AICAR administration ameliorates hypertension and angiogenic imbalance in a model of preeclampsia in the rat. Am J Physiol Heart Circ Physiol [Internet] 2013;304:H1159–H1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ben-Shoshan J, Steinvil A, Arbel Y, et al. Sustained elevation of vascular endothelial growth factor and angiopoietin-2 levels after transcatheter aortic valve replacement. Can J Cardiol. 2016;32:1454–1461. [DOI] [PubMed] [Google Scholar]
- [35].Akasaki Y, Miyata M, Eto H, et al. Repeated thermal therapy up-regulates endothelial nitric oxide synthase and augments angiogenesis in a mouse model of hindlimb ischemia. Circ J. 2006;70:463–470. [DOI] [PubMed] [Google Scholar]
- [36].Kuhlenhoelter AM, Kim K, Neff D, et al. Heat therapy promotes the expression of angiogenic regulators in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2016;311:R377–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Weber NC, Riedemann I, Smit KF, et al. Plasma from human volunteers subjected to remote ischemic preconditioning protects human endothelial cells from hypoxia–induced cell damage. Basic Res Cardiol. 2015;110:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tymchuk CN, Barnard RJ, Heber D, et al. Evidence of an inhibitory effect of diet and exercise on prostate cancer cell growth. J Urol. 2001;166:1185–1189. [PubMed] [Google Scholar]
- [39].Soliman S, Aronson WJ, Barnard RJ. Analyzing serum-stimulated prostate cancer cell lines after low-fat, high-fiber diet and exercise intervention. Evid Based Complement Alternat Med. 2011;2011:529053–529057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tymchuk CN, Barnard RJ, Ngo TH, et al. Role of testosterone, estradiol, and insulin in diet- and exercise-induced reductions in serum-stimulated prostate cancer cell growth in vitro. Nutr Cancer. 2002;42:112–116. [DOI] [PubMed] [Google Scholar]
- [41].Zitta K, Meybohm P, Bein B, et al. Serum from patients undergoing remote ischemic preconditioning protects cultured human intestinal cells from hypoxia-induced damage: involvement of matrixmetalloproteinase-2 and −9. Mol Med. 2012;18:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Omodei D, Licastro D, Salvatore F, et al. Serum from humans on long-term calorie restriction enhances stress resistance in cell culture. Aging (Albany NY). 2013;5:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shinsato T, Miyata M, Kubozono T, et al. Waon therapy mobilizes CD34+ cells and improves peripheral arterial disease. J Cardiol. 2010;56:361–366. [DOI] [PubMed] [Google Scholar]
- [44].Babaei S, Teichert-Kuliszewska K, Monge JC, et al. Role of nitric oxide in the angiogenic response in vitro to basic fibroblast growth factor. Circ Res. 1998;82:1007–1015. [DOI] [PubMed] [Google Scholar]
- [45].Inoue N, Venema RC, Sayegh HS, et al. Molecular regulation of the bovine endothelial cell nitric oxide synthase by transforming growth factor-beta 1. Arterioscler Thromb Vasc Biol. 1995;15:1255–1261. [DOI] [PubMed] [Google Scholar]
- [46].Erdös G, Lee YJ, Cho JM, et al. Heat-induced bFGF gene expression in the absence of heat shock element correlates with enhanced AP-1 binding activity. J Cell Physiol. 1995;164:404–413. [DOI] [PubMed] [Google Scholar]
- [47].Sun L, Lamont SJ, Cooksey AM, et al. Transcriptome response to heat stress in a chicken hepatocellular carcinoma cell line. Cell Stress Chaperones. 2015;20:939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yang J, Zhu T, Liu X, et al. Heat shock protein 70 protects rat peritoneal mesothelial cells from advanced glycation end-products-induced epithelial-to-mesenchymal transition through mitogen‑activated protein kinases/extracellular signal-regulated kinases and transforming growth factor-β/Smad pathways. Mol Med Rep. 2015;11:4473–4481. [DOI] [PubMed] [Google Scholar]
- [49].Zhou Y, Cao S, Li H, et al. Heat shock protein 72 antagonizes STAT3 signaling to inhibit fibroblast accumulation in renal fibrogenesis. Am J Pathol. 2016;186:816–828. [DOI] [PubMed] [Google Scholar]
- [50].Fleenor BS, Marshall KD, Durrant JR, et al. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol. 2010;588:3971–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jones N, Master Z, Jones J, et al. Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J Biol Chem. 1999;274:30896–30905. [DOI] [PubMed] [Google Scholar]
- [52].Hwang B, Lee S-H, Kim J-S, et al. Stimulation of angiogenesis and survival of endothelial cells by human monoclonal Tie2 receptor antibody. Biomaterials. 2015;51:119–128. [DOI] [PubMed] [Google Scholar]
- [53].Mofarrahi M, McClung JM, Kontos CD, et al. Angiopoietin-1 enhances skeletal muscle regeneration in mice. Am J Physiol Regul Integr Comp Physiol. 2015;308:R576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Li M, Fuchs S, Böse T, et al. Mild heat stress enhances angiogenesis in a co-culture system consisting of primary human osteoblasts and outgrowth endothelial cells. Tissue Eng Part C Methods. 2014;20:328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ohori T, Nozawa T, Ihori H, et al. Effect of repeated sauna treatment on exercise tolerance and endothelial function in patients with chronic heart failure. Am J Cardiol. 2012;109:100–104. [DOI] [PubMed] [Google Scholar]
- [56].Mackie AR, Losordo DW. CD34-positive stem cells: in the treatment of heart and vascular disease in human beings. Tex Heart Inst J. 2011;38:474–485. [PMC free article] [PubMed] [Google Scholar]


