ABSTRACT
A cold–induced vasodilation (CIVD) test was administered to 113 Canadian Armed Forces (CAF) soldiers (age 25.6 ± 6 yrs) during pre-deployment to a Canadian Arctic training exercise. The incidence and rates/types of subsequent peripheral cold injuries, as well as the relationship of CIVD responses against other hypothesized/reported risk factors (smoking, gender, age, ethnicity and prior cold injury), were analyzed. Although there was a wide range of CIVD RIF (resistance index to frostbite) scores (mean = 5.0 ± 1.5), there were no systematic relationships between RIF and injury type/location and rate, and the other risk factors analyzed. The absence of physiological links to cold injury occurrence suggests that in a military cold deployment setting, other factors are in play, which might include clothing, training, leadership and doctrine. These factors should be examined in future work.
KEYWORDS: Arctic, cold weather injuries, frostbite, cold-induced vasodilation, Canadian Armed Forces, military operations, cold
Introduction
Incidences of cold weather injuries (CWI), including frostbite and frostnip (precursor tissue injury to frostbite) during military operations has been a medical issue that dates back decades, and continues to be a challenge for Canadian Armed Forces members participating in Arctic field exercises during the winter months [1–4]. CAF units deployed to the north often face extreme cold weather, frequently experiencing daily lows approaching −35°C and below, with wind chill equivalent temperatures lower than −60°C [5]. These conditions can make even simple tasks, such as erecting shelter, preparing and consuming rations, and operating specialized equipment (especially equipment requiring fine motor dexterity and thus un-gloved hands) extremely challenging.
Since 2016, data has been collected on the health risks and human performance challenges on Arctic exercises and notably, a high incident rate of cold weather injuries [3] was found. Many modifiable risk factors can contribute to CWI, such as inadequate protective clothing (which has not improved markedly over this time frame for CAF members in Arctic deployments), as well as behavioral factors (e.g., successful clothing layering knowledge, energy conservation strategies, and CWI symptom reporting). However, other, non-modifiable inherent physiological risk factors may also contribute, such as inter-individual differences in cold-induced vasodilation (CIVD) response [6].The CIVD reflex is an acute autonomous local neuro-cardiovascular adaptation which involves the cyclic opening and closing of arterio-venous anastomoses located in the fingers and toes, allowing blood flow to periodically enter the peripheral tissues, maintaining blood flow, and thus, minimizing cold- induced tissue freezing [1].
In 2005, Daanen & Van Der Struijs [6] characterized peripheral tissue’s ability to withstand cold exposure by re-introducing the concept of a “resistance index” (RIF) of frostbite based on the CIVD response. The authors observed that low RIF values were linked to high occurrence of local cold injuries during military deployments, and suggested that the CIVD test and resultant RIF score could be employed as a risk tool to predict an individual’s risk of incurring frostbite. They also reported that RIF scores were higher (i.e. better) in Caucasians and smokers and that subjects who experienced higher pain after 10 min (min) of immersion had lower RIF scores and therefore were possibly at risk for cold injuries. Since CIVD is presumed to play a role in providing protection against CWI’s [1,6], it follows that the utilization of the RIF might have application in a military context by predicting the predisposition of individual soldiers for incurring CWIs during military exercises in extreme cold conditions.
Recognizing the differences in their study population, the Arctic clothing used, the operational tasks (intensity and types), and duration of cold exposures, the main purpose of this study was to replicate the work of Daanen & Van Der Struijs [6] in a Canadian operational context, and test the hypothesis that RIF scores predict who would sustain a CWI during an extreme cold exposure deployment.
Methods
This study was granted approval by the Human Research Ethics Committee of Defence Research and Development Canada (DRDC), Toronto Research Centre (TRC).
113 volunteers were recruited from the Canadian Armed Forces (CAF) OPERATION NUNALIVUT 2018 (OP NU 2018) training exercise, and any member aged 18–65 without pre- existing health conditions or previous cold-induced injury involving the hands and fingers was eligible to participate. Volunteers read the study information and were given the opportunity to ask questions before providing written, voluntary consent to participate in the thirty minute CIVD test. Participants were assured that while pain, numbness, and discomfort are normal responses, the chance of permanent finger tissue damage as a result of the CIVD test is very remote [6,7]. Prior to beginning the CIVD test, all participants completed a short survey regarding their previous cold weather experiences.
All CIVD testing was performed at Canadian Forces Base (CFB) Shilo, MB, CA in a temperature-controlled room (22 ± 1°C), set up with tables to accommodate 30 participants at one time. The volunteers reported to the indoor testing site in the morning after consuming a normal breakfast., Upon arrival, oral temperature was measured to rule out fever (>37.8°C).
The CIVD procedure used is well documented [1,6–12]. A thermistor (ACR Systems Incorporated Surrey, British Columbia) was attached to the third finger of the left hand. It was secured to the middle of the palmar surface of the distal phalanx using a single layer of transparent, waterproof tape (3MTMTransporeTM Surgical Tape). The thermistor was connected to a portable data logger capable of up to 8 independent signal inputs (ACR Systems Incorporated SmartReaderPlus 8 Surrey, British Columbia), and one logger was shared for groups of up to six participants. Temperature data obtained from the thermistors was measured and stored at a rate of once every 8 seconds.
Each participant was assigned an individual double-walled polystyrene foam cup filled with small ice cubes (1cm3) and cold water, to create a volume of approximately 400 ml of a 0–0.5°C ice slurry, according to the methods described by Daanen and Struijs [6]. Following five min of baseline temperature measurement, participants submerged their finger past the proximal interphalangeal joint, with their elbow supported such that their wrist and hand were suspended in a relaxed and comfortable position. Every two min, each participant stirred their own ice slurry bath using their free hand and a provided pen, to ensure uniform temperature throughout the ice slurry solution [1]. Every five min, they rated their level of finger pain using a Numeric Pain Distress scale [6], which is a 10-point scale from “0” (no pain) to “10” (unbearable pain). Participants were instructed to maintain submersion for 30 min, but were
free to withdraw their finger if the test became intolerable. After 30 min, participants were instructed to withdraw their finger from the water bath, but temperature and pain scale ratings were measured for another 10 min.
Approximately one month following the baseline CIVD screening test, participants embarked on the OP NU 2018 training course in northern Nunavut, Canada. This exercise involved a six day field survival component, during which participants were outside for 14–18 hours per day, completing various manual tasks, commuting on snowmobile, and nights were spent in either snow shelters or small heated tents. Over the 6-day field exercise, the average daily high was – 30.3°C, the average daily low was −39.8°C, and average windchill equivalent temperature was – 44.6°C [13] During the course, participants were monitored by field medics and any suspected CWI was referred to the course medical officer for diagnosis. In the event of injury, the location, severity and mechanism of injury and other details were recorded by the participant and medical team. All cold weather injuries were recorded, but injuries to the fingers and toes were of particular interest, as the CIVD response only occurs in the extremities (9).
Individual CIVD response curves for each volunteer’s CIVD test were constructed from the raw finger thermistor temperature data, and plotted as a function of time (min) (Figure 1). These data were then used to calculate a Resistance Index of Frostbite (RIF) ratings, as described by Yoshimura and Iida [7] and Daanen and van der Struijs [6], and modified slightly. The rational for modifying the RIF was to ensure that all values of Tmin, Tonset, and Tmean are included in the calculation for the number of points. For example, in the ranges noted in previous papers, values for Tmin between 1.5 and 1.6 would not be included. The modifications were as follows:
Figure 1.
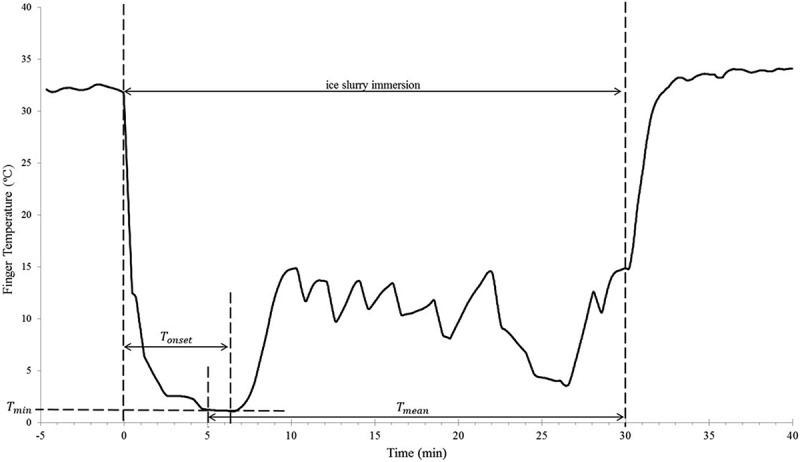
Fingertip temperature response to 30 mins of immersion in ice slurry. Tmin= minimum finger temperature before CIVD reaction; Tonset= time immersed before CIVD reaction begins; Tmean= average temperature from 5−30 mins of immersion. RIF is calculated using Tmin, Tonset, and Tmean.
The three variables utilized to construct the RIF were:
Where:
= the minimum skin temperature prior to CIVD response
≤ 1.55°C = 1 point
1.55°C < <4.05°C = 2 points
≥ 4.05°C = 3 points
= onset time of CIVD (determined by examining graphs and consensus by investigators)
> 11.5 min = 1 point
11.5 min >> 7.5 min = 2 points
< 7.5 min = 3 points
= mean finger temperature from minute 5–30
≤ 4.0°C = 1 point 4.0°C>
<< 7.05°C = 2 points
≥ 7.05°C = 3 points
RIF scores could range from 3–9, with 3 being the weakest response to cold (high risk of frostbite), and 9 being the strongest reaction (low risk of frostbite).
Statistical analyses were performed using GraphPad Prism (version 7.00 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com). An alpha level of 0.05 was used for all statistical analyses. The differences in RIF scores between injured and non-injured between RIF scores and incidence of CWI was determined using a t-test. A one-way analysis of variance (ANOVA) was used to determine the response pattern between CWI location and RIF score. Additional ANOVAs were used to examine the differences between RIF scores and smoking behaviour, ethnicity, and military rank. A Pearson’s product-moment correlation coefficient (PPMCC) test was used to estimate the correlation between RIF and age, as well as RIF and oral temperature. An ANOVA test was used to examine the changes in Borg Pain Rating throughout the 40-minute test. A PPMCC test was used to examine the correlation between pain ratings and RIF.
Two survey questions were used to examine the relation between self-assessed cold tolerance and RIF. Question 1 (How do you generally feel in the cold?) used a 4-point scale between 1 (“Very Uncomfortable”) and 4 (“Very Comfortable”), for 5 different locations (whole body, ears, toes, fingers, and face). The ratings from each location were totaled, and then compared to the RIF score using a PPMC. Question 3 inquired, “Are you exceptionally sensitive to the cold?”. A t-test was used to compare the RIF scores of those who answered “Yes” with those who answered “No”.
Results
109 of 113 participants completed the CIVD test (Mean age = 25.6 ± 5.8 years). Four participants withdrew from the test (two due to discomfort; two due to scheduling conflicts) and are not included in the final analysis. All but one of the participants was male. The mean RIF score of all the participants was 5.0 ± 1.5.
Ratings of pain were highest 5 min after finger immersion and decreased over time, with a small increase initially after withdrawal at 35 min (Figures 1 and 2). Pain rating correlated weakly and negatively with RIF scores, with strongest correlations shown at 35 min (r =−0.445) and 40 min (r =−0.290).
Figure 2.
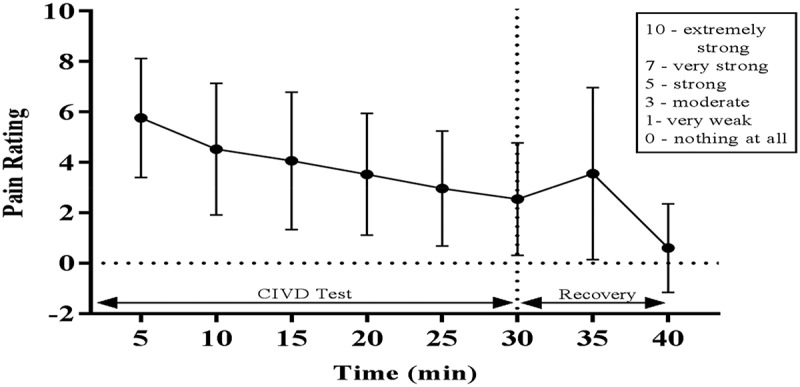
Borg pain rating every 5 min during 30 min of finger immersion (0–3 mins) and 10 mins of recovery (30–40 mins).
34 participants declined to identify their ethnicity. A t-test was performed to compare RIF scores the remaining participants (n =75, Mean RIF = 5.2 ± 1.5) with the total group mean (n =109, Mean RIF = 5.0 ± 1.5), and no significant difference was found. Of those who provided their ethnicity, 60 identified as Caucasian. In the Non-Caucasian group (n =15), 5 responded as Asian, 4 as Aboriginal, 4 as mixed ethnicity, and 2 as African descent. A t-test was then performed to examine differences in RIF between Caucasian and Non-Caucasian participants, which revealed no significant difference. An ANOVA, followed by Tukey’s multiple comparisons test, was done to examine differences between the subgroups within the Non-Caucasian participants, and found no significant differences.
104 participants completed the survey in its entirety. A t-test found no difference in RIF between those who answered “No” to the question #3 regarding sensitivity to cold (n =92, RIF =5.0 ± 1.5) and those who answered “Yes” (n =13, RIF =4.7 ± 1.0). A PPMCC found no significant correlation between RIF and total comfort score in Question 1 (r =−0.08).
Of the 109 participants for whom RIF scores were calculated, 40 experienced a CWI during the Arctic training exercise. Mean RIF scores are shown in Table 1. No significant difference was found between the RIF scores of injured and uninjured participants . Of the 40 injuries, 14 were sustained in the extremities (hands and feet). When RIF scores were compared across injury locations, no significant differences were found.
Table 1.
Mean resistance index to frostbite (RIF) scores.
| N | RIF (Mean ± SD) | |
|---|---|---|
| All Participants | 109 | 5.0 ± 1.5 |
| Injury Status | ||
| Injured | 40 | 5.1 ± 1.5 |
| Uninjured | 69 | 4.9 ± 1.4 |
| Injury Severity | ||
| Frostbite | 34 | 4.9 ± 1.4 |
| Frostnip | 6 | 5.7 ± 2.0 |
| Injury Location | ||
| Non-Extremity | 26 | 5.2 ± 1.7 |
| All Extremities | 14 | 4.9 ± 1.3 |
| Feet | 1 | 4.0 ± 0.0 |
| Hands | 9 | 5.3 ± 1.2 |
| Both Hands and Feet | 4 | 4.3 ± 1.3 |
| Smoking Behaviour | ||
| Smoker | 47 | 5.3 ± 1.6 |
| Non-Smoker | 62 | 4.8 ± 1.3 |
| Rank | ||
| Junior NCM | 94 | 5.0 ± 1.5 |
| Senior NCM | 12 | 5.0 ± 1.3 |
| Junior Officer | 3 | 4.7 ± 0.6 |
| Ethnicity | ||
| Caucasian | 60 | 5.0 ± 1.5 |
| Non-Caucasian | 15 | 5.9 ± 1.3 |
| Asian | 5 | 5.0 ± 1.0 |
| Aboriginal | 4 | 6.5 ± 1.7 |
| Mixed ethnicity | 4 | 6.5 ± 1.3 |
| African | 2 | 6.0 ± 0.0 |
| Declined to Answer | 34 | 4.7 ± 1.2 |
No significant difference was found based on smoking behaviour or military rank. No significant correlation was found between RIF and age (r =0.003), RIF and number of cigarettes smoked per day (r =0.226), or RIF and oral temperature (r =0.114).
Discussion
The goal of the current study was to test whether the RIF could serve as a tool to predict susceptibility to peripheral CWIs during CAF Arctic Operations with the intention of helping to reduce the incidence of CWIs during future Arctic Operations. The institution of the RIF rating would complement other methods of preventing CWIs – perhaps even on an individual basis by implementing either extra cold weather protection or other technical or administrative/training/doctrinal measures. The other key finding however is the overall rate of CWIs in the study – a 33% casualty rate. We suspect that this magnitude of injuries in a temperate temperature deployment would be generally considered unacceptable by most militaries.
CWIs
As we have presented in this study, the RIF-value of the 14 soldiers that were diagnosed with a CWI on the extremities was 4.9 ± 1.3. The RIF- value of the 69 soldiers that were not diagnosed with a CWI was 4.9 ± 1.4. These values were not significantly different. These results conflict with previous findings from Daanen and van der Struijs [6] who reported significantly lower RIF scores in marines who suffered cold injuries vs. those who did not. RIF scores also did not appear to correlate with injury severity, as there was no significant difference between those who experienced frostbite (N =34, RIF =4.9 ± 1.4) and those who experienced frostnip (N =6, RIF =5.7 ± 2.0).
The CIVD response has been shown in temperatures as high as 8°C [14], but in extremely cold and windy conditions, the CIVD mechanism may be unable to prevent freezing and subsequent CWIs [15]. Wilson et al. [15] examined the effects of different air temperatures and wind speeds on the CIVD response in the finger, and found that none of their participants exhibited CIVD at air temperatures below −16°C. In the present study, the participants spent six days in northern Nunavut, Canada, experiencing daily temperatures ranging from −23°C to −43°C, with wind chill equivalent temperatures of less than −50°C [13] In addition, many of their operational tasks exposed them to higher wind speeds (long snowmobile trips), and compromised personal protection (removing gloves to perform fine-motor tasks). It is possible that in this scenario, the cold exposure was too great to be mitigated by the CIVD response, thus negating the predictive potential of the individual RIF scores.
Park and Lee [16] found that self-identified cold intolerance significantly correlated with a weaker CIVD response. Similarly, Daanen and van der Struijs [6] noted that those with a low RIF score tended to be aware of their susceptibility to CWIs. Even though these subjects avoided hazardous situations, they were more at risk. In our study, the risk avoidance may also be a plausible explanation for the absence of a relationship between RIF and CWI incidence. If those with low RIF scores are aware of their poor response and heightened risk, they may behave more cautiously to avoid situations that may elicit a CWI. However, the relevant survey questions in the present study showed no relationship between RIF and self-assessed cold sensitivity.
The CIVD test was performed in fingers, while cold injuries also occurred at other body locations like the toes. It has been shown that the physiological behaviour of fingers and toes is often unrelated [17] and therefore the CIVD test in the fingers may not have been specific enough for other cold injuries [12]. Additionally, a total of 40 soldiers were diagnosed with CWIs (mean RIF =5.1 ±1.5), however, only the extremity CWIs are relatable to the CIVD test, as arteriovenous anastomoses exist only in the fingers and toes [9]. Thus the relation between RIF and the 26 soldiers who were diagnosed with CWIs on the face, head, and/or neck cannot be assessed in this study.
Smoking
The relationship between smoking and RIF was assessed due to the well-documented peripheral vasoconstriction that occurs temporarily after tobacco smoking [18]. Previous studies have reported impaired rewarming in smokers [19,20]. Interestingly, Daanen and van der Struijs [6] reported increased RIF scores in smokers compared to non-smokers. In the present study, there was no significant difference in RIF scores between smokers and non-smokers, and no correlation with number of cigarettes smoked per day. Despite being permitted to smoke ad libitum until arrival for the study, the acute vasoconstriction resulting from tobacco smoke did not appear to influence the CIVD response. Of the 40 participants who suffered a CWI, 22 were non-smokers and 18 were smokers, indicating smoking did not increase incidence of CWI in this study, contrary to previous reports [21–23].
Ethnicity
There was no significant difference in RIF based on self-identified ethnicity. Previous studies have reported significant differences in RIF [6, 24] and CIVD response [25–27] across difference ethnicities, with weaker CIVD responses typically being found in non-Caucasians. In the present study, the population was predominantly Caucasian (N =60), which may have masked potential significant differences with the underrepresented non-Caucasian group (N =15). For instance, previous studies have reported lower CIVD responses in individuals of African descent [27,28], but the present study had only two participants of African descent, and found no significant difference from the Caucasian group.
Pain ratings
Pain is known to vary during typical CIVD tests, peaking during vasoconstriction and decreasing during vasodilation [29]. The pain ratings given during and after the CIVD test showed only a weak to moderate correlation with RIF. The strongest correlations were shown at 35 min (r =- 0.45) and 40 min (r =−0.29). All correlations were negative, meaning higher pain ratings correlated with lower RIF (higher risk). Daanen and van der Struijs [6] also found moderate negative correlations between pain and RIF, noting that pain could be taken as a warning sign before a CWI occurs.
Limitations
This study only included one female participant, thus sex-related differences in RIF were not examined. While this may be a limitation, a previous study [11] found CIVD response was not influenced by sex.
Participants were permitted to smoke and consume caffeine ad libitum until arrival for the CIVD test. As described above, previous studies have demonstrated the acute vasoconstriction and CIVD responses of smoking. While no difference in RIF was found between smokers and non- smokers, the acute effects of smoking were not controlled in this study. Kim et al. [30] also noted a diminished CIVD response following caffeine consumption, which may have impacted the RIF scores seen presently. Future studies should ensure a uniform period of pre-study smoking and caffeine abstinence in all subjects.
Despite a large sample size and a wide distribution of CIVD responses, this study did not find any systematic relationships between cold injury rate/type and CIVD score, nor were there any relationships with several of the factors in the literature which have linked susceptibility to incurring peripheral cold injuries with CIVD scores. These factors included age, prior injury, smoking status, and ethnicity.
These findings suggest that other factors more strongly predict risk of incurring peripheral cold freezing injuries: clothing choice, type, medical and individual self-surveillance of symptoms, cold weather deployment training, leadership and military activities/doctrine (vs. civilian settings). These factors should be examined in more detail in future work in an effort to minimize the debilitating effects of cold peripheral injuries.
Disclosure statement
The authors report no conflict of interest.
Abbreviations
- ANOVA
Analysis of variance
- CA
Canada
- CAF
Canadian Armed Forces
- CFB
Canadian Forces Base
- CIVD
Cold-induced vasodilation
- CWI
Cold weather injuries
- DRDC
Defence Research and Development Canada
- Minutes
min
- OP NU 2018
OPERATION NUNALIVUT 2018
- PPMCC
Pearson’s product-moment correlation coefficient
- RIF
Resistance index to frostbite
- TRC
Toronto Research Centre
References
- [1].Cheung SS. Responses of the hands and feet to cold exposure. Temperature. 2015;2(1):105–120. doi: 10.1080/23328940.2015.1008890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Imray CHE, Oakley EHN. Cold still kills: cold-related illnesses in military practice freezing and non-freezing cold injury. J R Army Med Corps. 2006;152:218–222. [DOI] [PubMed] [Google Scholar]
- [3].Sullivan-Kwantes W, Dhillon P, Goodman L, et al. Medical encounters during a Joint Canadian/US exercise in the high arctic (Exercise Arctic Ram). Mil Med. 2017;182(9/10):e1764. [DOI] [PubMed] [Google Scholar]
- [4].Sullivan-Kwantes W, Goodman L. The new cold war. Temperature. 2017;4(4):341–344. doi: 10.1080/23328940.2017.1381799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Climate Change Canada Canadian climate normals 1981-2010 station data: resolute NU. [Accessed 2018 Apr 2]. http://climate.weather.gc.ca/climate_normals/results_1981_2010_e.html?searchType=stnProv&lstProvince=NU&txtCentralLatMin=0&txtCentralLatSec=0&txtCentralLongMin=0&txtCentralLongSec=0&stnID=1776&dispBack=0
- [6].Daanen H., Van Der Struijs NR.. Resistance index of frostbite as a predictor of cold injury in arctic operations. Aviat Space Environ Med. 2005;76:1119–1122. [PubMed] [Google Scholar]
- [7].Yoshimura H, Iida T. Studies on the reactivity of skin vessels to extreme cold. Part 1 a point test on the resistance against frostbite. Jap J Physiol. 1950;1:147–159. [DOI] [PubMed] [Google Scholar]
- [8].Daanen HA, Koedam J, Cheung SS. Trainability of cold induced injuries in finger in fingers and toes. J Appl Physiol. 2012;112:2595–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Daanen H. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411–426. [DOI] [PubMed] [Google Scholar]
- [10].Kunkle EC. Phasic pains induced by cold. J Appl Physiol. 1949;1(12):811–824. [DOI] [PubMed] [Google Scholar]
- [11].Tyler CJ, Reeve T, Cheung SS. Cold-induced vasodilation during single digit immersion in 0°C and 8°C water in men and women. PLoS One. 2015;10(4):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kingma CF, Hofman II, Daanen HM. Relation between finger cold-induced vasodilation and rewarming speed after cold exposure. Eur J Appl Physiol. 2018. DOI: 10.1007/s00421-018-4012-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Climate Change Canada Daily data report for March 2018. [Accessed 2018 Apr 2]. http://climate.weather.gc.ca/climate_data/daily_data_e.html?StationID=53060&timeframe=2&StartYear=1840&EndYear=2018&Day=20&Year=2018&Month=3
- [14].Mekjavic IB, Dobnikar U, Kounalakis SN. Cold-induced vasodilation response in the fingers at 4 different water temperatures. Appl Physiol Nutr Metab. 2013;38:14–20. [DOI] [PubMed] [Google Scholar]
- [15].Wilson O, Goldman RF. Role of air temperature and wind in the time necessary for a finger to freeze. J Appl Physiol. 1970;29(5):658–664. [DOI] [PubMed] [Google Scholar]
- [16].Park J, Lee JL. Relationships of self-identified cold tolerance and cold-induced vasodilation in the finger. Int J Biometerol. 2016;60:521–529. [DOI] [PubMed] [Google Scholar]
- [17].Cheung SS, Mekjavic IB. Cold-induced vasodilatation is not homogenous or generalizable across the hand and feet. Eur J Appl Physiol. 2007;99:701–705. [DOI] [PubMed] [Google Scholar]
- [18].Midttun J, Serjrsen P, Paaske WP. Smokers have severely disturbed peripheral micro-circulation. Int Angiol. 2006;25(3):293–296. [PubMed] [Google Scholar]
- [19].Cleophas TJM, Fennis JFM, Van’tLaar A. Finger temperature after a finger- cooling test: influence of air temperature and smoking. J Appl Physiol. 1982;52(5):1167–1171. [DOI] [PubMed] [Google Scholar]
- [20].Miland AO, Mercer JM. Effect of a short period of abstinence from smoking on rewarming patterns of the hands following local cooling. Eur J Appl Physiol. 2006;98(2):161–168. [DOI] [PubMed] [Google Scholar]
- [21].Ervasti O, Juopperi K, Kettunen P, et al. The occurrence of frostbite and its risk factors in young men. Int J Circumpolar Health. 2004;63(1):71–80. [DOI] [PubMed] [Google Scholar]
- [22].Murphy JV, Banwell PE, Roberts AHN, et al. Frostbite: pathogenesis and treatment. J Trauma. 2000;48(1):171–178. [DOI] [PubMed] [Google Scholar]
- [23].Hassi J, Makinen TM. Frostbite: occurrence, risk factors, and consequences. Int J Circumpolar Health. 2000;59(2):92–98. [PubMed] [Google Scholar]
- [24].Hirai K, Horvath SM, Weinstein V. Differences in the vascular hunting reaction between Caucasians and Japanese. Angiology. 1970;8:502–510. [DOI] [PubMed] [Google Scholar]
- [25].Khatun A, Ashikaga S, Nagano H, et al. Cold-induced vasodilation comparison between Bangladeshi and Japanese natives. J Physiol Anthropol. 2016;35:13 DOI: 10.1186/2Fs40101-016-0095-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee J-Y, Bakri I, Matsuo A, et al. Cold-induced vasodilation and vasoconstriction in the finger of tropical and temperate indigenes. J Therm Biol. 2013;38:70–78. [Google Scholar]
- [27].Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Pflugers Arch. 2014;114:2369–2379. [DOI] [PubMed] [Google Scholar]
- [28].Maley MJ, House JR, Tipton MJ, et al. Vascular responses of the extremities to transdermal application of vasoactive agents in Caucasian and African descent individuals. Eur J Appl Physiol. 2015;115(8):1801–1811. [DOI] [PubMed] [Google Scholar]
- [29].Kreh A, Anton F, Gilly H, et al. Vascular reactions correlated with pain due to cold. Exp Neurol. 1984;85:533–546. [DOI] [PubMed] [Google Scholar]
- [30].Bj K, Seo Y, Kim JH, et al. Effect of caffeine intake on finger cold- induced vasodilation. Wilderness Environ Med. 2013;24:328–336. [DOI] [PubMed] [Google Scholar]


