ABSTRACT
The superfamily of Transient Receptor Potential (TRP) channels is composed by a group of calcium-permeable ionic channels with a generally shared topology. The thermoTRP channels are a subgroup of 11 members, found in the TRPA, TRPV, TRPC, and TRPM subfamilies. Historically, members of this subgroup have been classified as cold, warm or hot-specific temperature sensors. Recently, new experimental results have shown that the role that has been given to the thermoTRPs in thermosensation is not necessarily strict. In addition, it has been shown that these channels activate over temperature ranges, which can have variations depending on the species and the interaction with a specific biological context. Investigation of these interactions could help to elucidate the mechanisms of activation by temperature, which remains uncertain.
Abbreviations: Cryo-EM: Cryogenic electron microscopy; DRG: Dorsal root ganglia; H: Human; ROS: Reactive Oxygen Species; TG: Trigeminal ganglia; TRP: Transient Receptor Potential; TRPA: TRP ankyrin; TRPV: TRP vanilloid; TRPC: TRP canonical; TRPM: TRP melastatin
KEYWORDS: ThermoTRP, TRP channels, TRPV1, TRPA1, TRPM8, temperature sensing, enthalpy
Introduction
Thermosensation is the ability of organisms to detect and codify both environmental and internal temperature, and it is indispensable for survival. Thermosensation in different higher animal species depends mostly on the activation of ion channels, some of which are members of the superfamily of Transient Receptor Potential (TRP) channels known as thermoTRPs. These membrane proteins serve as molecular thermal sensors and are also polymodal channels that are activated by different physical and chemical stimuli. Most of them are cation permeable, non-selective channels with varying preference for calcium. All TRP channels have a shared general topology, assembling as tetramers, with each subunit consisting of six transmembrane segments, where the channel pore is formed by the transmembrane segments five and six and the loop between them [1–4]. The regions with the largest structural divergences are the intracellular domains. While TRPV channels are almost structurally identical [3,5–11] and share homology with TRPA1 [12], the TRPM and TRPC channels have amino and carboxy-termini with very different topology [13–19] (Figure 1). The thermoTRP channels are a subgroup of 11 members of the TRPA, TRPV, TRPC, and TRPM subfamilies. However, despite major recent progress in understanding their structural biology and the molecular determinants of some of their functions, the molecular mechanisms by which their temperature sensing function is accomplished remain mostly unknown [20].
Figure 1.
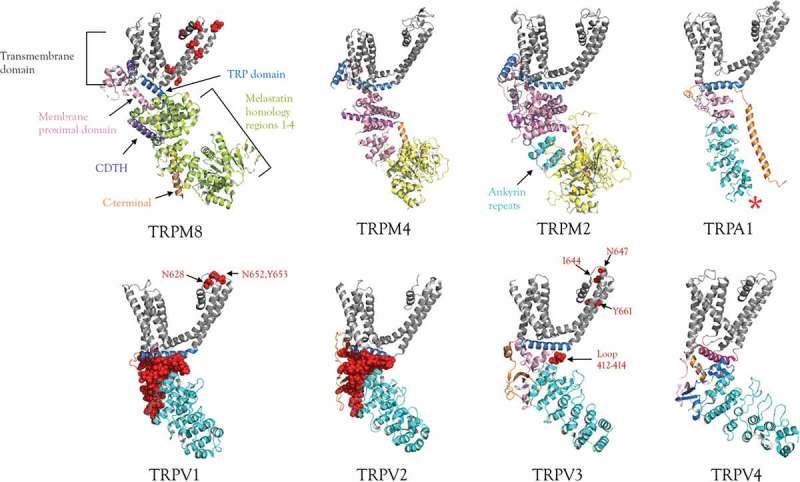
Structures of some thermoTRPs determined by single-particle cryo-EM.
The details of a single subunit are shown, the different colors match specific domains. The amino acids shown as red spheres represent the sites proposed as essential for the thermosensation. The asterisk in the structure of TRPV1 symbolizes some amino acids related to thermosensation (D261, M258, S250) that are not resolved in the available structures. Structural files from the Protein Data Bank and appropriate reference for each channel are as follows. TRPV1 (PDB 3J5P) [3]; TRPV2 (PDB 5AN8) [6]; TRPV3 (PDB 6MH0) [11]; TRPV4 (PDB 6BBJ) [7]; TRPA1 (PDB 3J9P) [12]; TRPM2 (PDB 6CO7) [17]; TRPM4 (PDB 6BCL) [18]; TRPM8 (PDB 6BPQ) [16]; TRPC5 structure (PDB 6AEI, is not available yet).
ThermoTRP channels have been identified in diverse animal species over the past several years, underscoring the diversification of these channels in the course of evolution [21]. However, their physiological function has only been analyzed in detail in a few model organisms such as mice and the fruit fly Drosophila. Thus, functional aspects of thermosensation in vivo have not been well understood [21]. In mammals, the expression of these channels occurs mainly in the membrane of sensory neurons from the trigeminal ganglia (TG) and dorsal root ganglia (DRG), but are also distributed in various other tissues [22,23]. A complicating factor is the fact that for almost all thermoTRPs, it has been shown that the temperature that elicits 50% of the response is variable and can fall in a range that varied from 10°C to 20°C. Further, orthologous channels can also have variations in the range of activation, which are dependent on the species and cellular context [21,24,25].
Recently, two important papers have proposed that only some thermoTRP channels are responsible for thermosensation in mammals; among the channels that have been put forward are TRPV1, TRPM3, and TRPA1 for sensing mild and noxious heat [26]. Another previous paper found that only TRPM8, TRPA1, and TRPV1 channels are necessary for all thermosensation (cold, warm and noxious heat) also in mice [27]. In this review, we take a look at the known function and assigned roles of different thermoTRP channels in thermosensation, in relationship with the newly reported findings.
Thermotrp channels
The thermoTRP channels belong to different TRP subfamilies: TRPC (TRPC5) [28]; TRPV (TRPV1-TRPV4) [29–32]; TRPM (TRPM2 – TRPM5 and TRPM8) [26,33–37]; and TRPA (TRPA1) [38]. They are collectively called thermoTRPs because these membrane proteins are unique in responding by opening when the temperature is changed. This is not just modulation of gating by temperature, as can be observed in all ion channels, but actual transitioning between states induced by temperature. Although the thermodynamics of activation by temperature can be complicated, a few generalizations can be made. The heat involved in channel opening is reflected in the steepness with which current increases and can be quantified by an apparent enthalpy (ΔH) with higher values of ΔH corresponding to increased steepness. Hot activated channels have positive values of ΔH while cold activated channels are associated with negative ΔH. The heat of activation of thermoTRP channels can be very high. The apparent enthalpy associated with opening can be as large as 100 kcal/mol [39].
ThermoTRPs can be classified considering the temperature that evokes 50% of the maximal current. TRPV1 and TRPV2 are activated by high temperatures, while other thermoTRPs (TRPV3, TRPV4, TRPM2, TRPM4, and TRPM5) are activated by warm temperatures [21,32]. It is known that TRPM8 is activated by cold. However, it is also known that different thermoTRP channels possess distinctive ranges of activation temperatures, which may vary depending on the species [21] (Figure 2). TRPA1 was earlier proposed to be a cold sensor, but it is now understood that, in mammals, it activates by increasing temperature from intermediate temperatures (17 ≥ °C).
Figure 2.
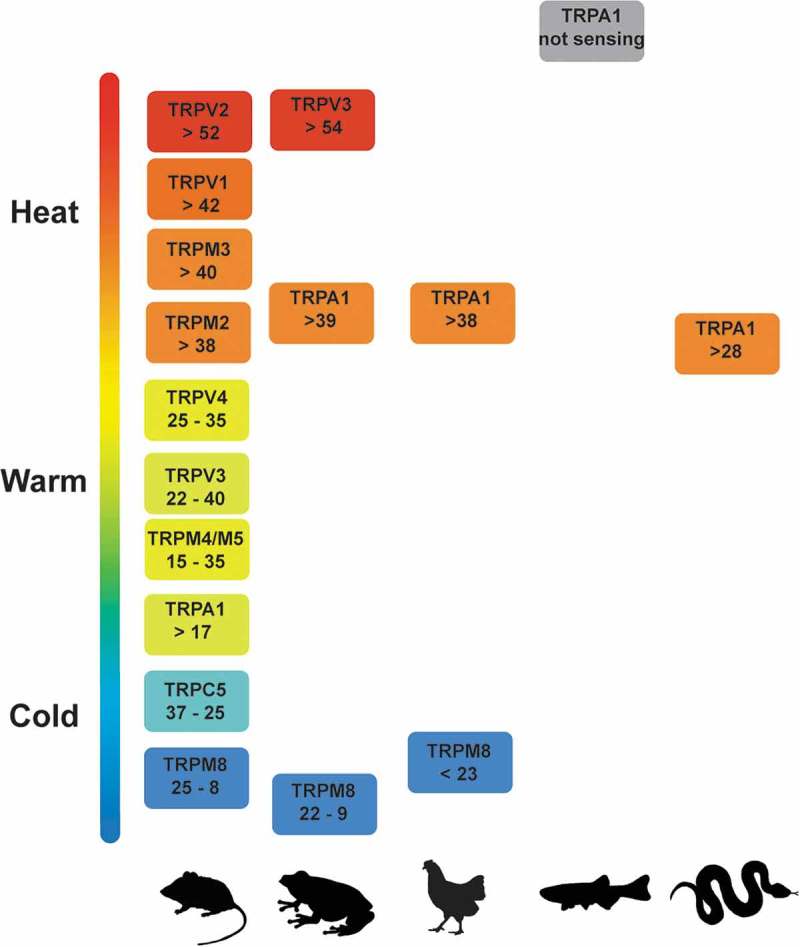
Range of temperature of activation of thermoTRP channels and its variation in different species.
The range of activation temperature for TRP channels in mammalians, amphibians, birds, fish, and reptiles is shown in °C. The most complete picture of the functions of thermoTRPs is only available in mammals. In most other species knowledge is incomplete and some thermoTRP channels show significant differences in their range of activation by temperature.
Thermotrp channels in humans
The distribution and function of thermoTRP channels in humans have not been well characterized. However, it is known that these channels are present in the brain, peripheral nervous system, pulmonary system, cardiovascular system, renal system, liver, digestive system, reproductive system, and skin (see Rosasco and Gordon, 2017) [40] and have been proposed as therapeutic targets in different diseases.
The channels TRPV1, TRPV2, TRPV4, TRPM3, and TRPM8 channels, have been implicated in the perception of pain in humans, because these channels are expressed in sensory nerve endings [41]. There is also the possibility that thermoTRP channels are involved in diseases like respiratory disorders (TRPV1, TRPV4, TRPA1, and TRPM8), because the human respiratory tract is innervated by sensory fibers which are activated by irritant stimuli [41]. Other thermoTRPs are also related to diseases like diabetes, for example, TRPM2, TRPM3, TRPM4/M5, and TRPA1, which are expressed in beta-cells and are directly involved in insulin secretion [42]. It has been hypothesized that some thermoTRP channels (TRPV1, TRPV2, TRPV4, TRPM2, TRPM4, TRPM8 and TRPM8) could be involved in various kinds of cancers [43,44]. Recently TRPV2 and TPV4 were implicated in mediating human melanoma cell death [45], but the relation between cancer and TRP-channels is still controversial. One thermoTRP channel in the brain, TRPC5 which is highly expressed in hippocampus and amygdala, has been proposed as a therapeutic target for anxiety [46].
All thermoTRPs channels in humans seem to be related to some disorder or disease [41], which increases the importance of research into the physiology of these channels and their possible use as pharmacologic targets.
Thermotrp channels variation in range of temperature activation
An important note of caution should be discussed first. The range of temperatures for activation of thermoTRP channels is not measured in the same way by all authors. Since thermoTRP channels are polymodal, voltage is an important modulator of the temperature response [47,48] and not all channel activation temperatures are measured at the same voltage and sometimes the voltages used are not reported. For example, some channels, such as TRPM5 [34,48], can activate below 20°C at very positive voltages but do not produce currents at the same temperature at intermediate or physiological voltages. For this reason, the definition of cold, warm, and noxious heat sensors can be complicated and not unique.
In mammals such as rodents, TRPV1/V2, TRPM3 and TRPM2 channels are activated by hot temperatures (upwards of 38°C), while TRPM4/TRPM5 [34], TRPV3 [31,49], and TRPV4 channels are considered activated by warm temperatures, activating by [48] temperatures higher than 15°C, 22°C and 23°C, respectively, and TRPC5, TRPM8, and TRPA1 have been classified as cold receptors. However, these ranges of temperature activation are not the same in all species. For example, when the TRPA1 channel was characterized for the first time, it was reported as a cold receptor, being activated at temperatures below 17°C [38]. Since then, TRPA1 has been considered as a cold sensing channel, but this is controversial because the temperature sensitivity of TRPA1 is an example of how a function like temperature sensitivity has changed along the phylogenetic scale [21]. This channel is expressed in animals like caterpillars [50], mosquito, fruit fly, snake [51], chicken [25], frog and lizard [52], where it is clearly activated by heat, while in zebrafish, human, and rhesus monkey, it is apparently insensitive to temperature [53].
Recently, TRPA1 has been postulated, using calcium imaging of neurons from double and triple knockout mice, to have an important role in noxious heat sensing in mice [26]. In behavioral experiments, TRPA1 and TRPV1 contribute essentially to noxious heat sensitivity in mice, but there is no additive effect of deleting both genes [54]. In addition, human TRPA1 channels seem to be a bidirectional thermosensor when reconstituted in an artificial membrane [55]. These authors postulate that TRPA1 is intrinsically thermo-sensitive and this characteristic has been conserved in evolution, such that we can see TRPA1 being activated by hot temperatures in different species. It has been proposed too that its intrinsic capacity for sensing cold and hot temperature is regulated by specific cellular proprieties of the organism that is expressing the channel. TRPA1 is strongly modulated by the redox state and by diverse ligands [55]. In addition, these authors have reported that different TRPA1 channel conformations are involved in its cold and heat sensitivity [55]. Also, single-point mutations in the ankyrin domain can change its threshold for temperature activation [56]. For these reasons, we can suppose that minimal posttranslational modifications and/or the cellular environment, could be major determinants for the observed temperature response of TRPA1 in different organisms, while previously several papers have only reported cold sensitivity [38,53,57].
The fact that in TRPV1 knockout mice only a small effect on noxious heat responses is observed [26,27,58,59], indicates the presence of compensatory mechanisms. This coincides with the observation that in TRPV1 knockout mice, there is an increased locomotor activity [60,61], producing no changes in body temperature in response to increased or decreased ambient temperature.
Other channels in the TRPV family are activated by temperature. For example, TRPV2 is expressed in numerous organ system and is activated by noxious heat >52°C [30] and its structure was determined by cryo-EM in 2016 [5]. It has been shown that TRPV2 channel has two distinct roles, depending on developmental stage; during embryonic development, it regulates axon outgrowth as a mechanosensor and in adults, it participates in thermosensation [62]. However, it was found that mice deficient in TRPV2 failed to display an altered heat-sensing phenotype [63], maybe because compensatory mechanisms, i.e. other heat sensitive ion channels.
TRPV3 has been well characterized as a calcium-permeable, temperature sensitive channel, responding in the temperature range from 22°C to 40°C in mammalian cells transfected with hTRPV3 [31,64]. Its presence has been reported in other species like fish, mouse (where apparently it does not have an important contribution to thermosensation [49]) and frogs; in this last one, it was initially characterized as a cold sensor with a temperature threshold 16°C [24]. Recently, other researchers showed that when frog TRPV3 channel is expressed in mammalian HEK293 cells, significant activity is observed at temperatures >54°C. They argue that the previous study failed to detect heat sensitivity in Xenopus oocytes injected with frog TRPV3 because the temperatures tested were lower than 40°C in their study [65]. The apparent sensitivity to cold temperatures in this channel remains controversial. Also, the mechanism of heat activation in TRPV2 and TRPV3 seems to be different to TRPV1, showing a response to temperature in the warm range that needs to be sensitized by a high-temperature stimulus, and thus making their response dependent on the stimulus history [65,66].
Another TRPV channel, TRPV4 is activated by warm stimuli >25°C to >34°C [32,67], although not much is known about this channel`s temperature sensitivity. However, this may soon change, as the recent structures of TRPV4 and TRPV2 and TRPV3 [5–7,11] solved by cyo-EM, might give new impetus to this area of research. Up until now, it is not known if TRPV3 and 4 have a physiological function as thermosensors in sensory neurons, but it is known that TRPV4 is expressed in the nerve fibers of human dental pulp [68], where it might contribute to temperature sensitivity. Recently, TRPV4 was shown to be the temperature receptor in human sperm and it is involved in regulating sperm thermotaxis [69]. On the other hand, the expression of TRPV3 and 4 in keratinocytes [22] rises the exciting possibility that skin epithelial cells can mediate the perception of warm temperatures directly and also mediate cold sensing via an isoform of TRPM8 [70].
Other channels like TRPM4 and TRPM5 are two temperature-sensitive channels that are activated by heating in the mild temperature range between 15°C and 35°C [34].
TRPM2 channels mediate responses to heat stress at temperatures above 38°C [36,37]. In addition, it is known that there is significant co-expression of TRPM2 with TRPV1 and TRPM3 in mouse DRG neurons [71], indicating that these channels lower the heat threshold of neurons and may act as a break to prevent overheating and tissue damage [36]. In mice, the genetic deletion of TRPM2 has an impact of thermal preference, suggesting this channel is important for thermoregulation in this rodent [71].
A first physiological function of TRPM3 was described in the pancreas, where the channel is expressed in insulin-secreting β-cells and was suggested to modulate insulin release under specific conditions [72]. It was established as a heat-sensitive ion channel with a temperature threshold >40°C, in sensory neurons where it is coexpressed with TRPV1 [35,73].
The TRPM8 channel was cloned in 2001 and characterized as a cold- and menthol-sensitive receptor from DRG and trigeminal neurons in mice and retains these characteristics when expressed in CHO and HEK 293 cells [33,74], but it was not until 2007 that the channel was shown to be necessary for cold temperature sensing [75]. The TRPM8 channel has been identified in different species such as rat, mouse, chicken, frog, and all orthologues of TRPM8 channels are cold sensitive [46]. It has been shown that chicken TRPM8 has smaller cold responses compared to mouse TRPM8, suggesting that the cold sensing in chicken is not mainly given by TRPM8 channel, while in frog, TRPM8 responds to colder temperatures [46]. These differences are being used to try to resolve questions such as what are the molecular determinants that explain the gaiting by temperature in this channel [76]. These studies could be compared and complemented by the new information provided by the recent TRPM8 channel structure obtained for Cryo-EM [16].
Among the TRPC channels, TRPC5 has been shown to be highly sensitive to mild cooling in the range from 37°C to 25°C in DRG neurons in mice, also it is known that it is present in human tissues [28]. The structure of TRPC5 was obtained by cryo-EM at 2.9 Å resolution, however the PDB is not available yet [19]. A hypothesis regarding why TRPC5 does not seem to be an important ion channel for thermosensation is that deleting TRPC5, in the mouse, results in compensatory replacement by functionally overlapping cold transducers. For example, TRPM8 channels seem to increase their functional availability. This may explain the presence of cold sensing in TRPC5 knockout mice [28].
As can be seen, although thermoTRPs seem to have relatively well-defined ranges of temperature response, there can be considerable overlap. Moreover, there is no consensus on the heat or cold activation of some thermoTRPs, such as TRPV3 and TRPA1, as originally postulated and it might turn out that the heat sensitivity of all or many thermoTRPs could be dynamically regulated.
Thermotrp channels and sensitization in chronic pain
Because thermoTRP channels show a response to heat of varying degrees, several of them are implicated in sensitization processes in pain-related disorders. TRPV channels are generally activated by temperatures above 40°C at positive voltages, but upon modulation by phosphorylation or binding of ligands such as PIP2 [77], proalgesic lipids [78] or modulation by cysteine-oxidizing reagents and ROS [79,80] the midpoint of temperature activation can be shifted to lower temperatures by as much as 20°C, producing activation of the channels and pain pathways at normal body temperatures, a pathological condition known as thermal hyperalgesia [30]. TRPV2 and TRPV3 channels are sensitized in a use-dependent manner [66] in such a way that the current carried by these channels increases with repetitive heat stimulation. This sensitization can contribute to thermal hyperalgesia after sufficient stimulation and in the presence of proinflammatory mediators. In a similar manner, sensitization of TRPV4 channels by the anandamide-arachidonic acid pathway can contribute to hyperalgesia and has been implicated in pain produced by chemotherapy drugs such as taxol [81].
Sensitization to cold stimuli or cold allodynia occurs as a result of chronic nerve injury. This form of sometimes-intractable chronic pain has been reported to be mediated by increased expression and sensitized responses to cold by the TRPM8 channel [82]. Overall, thermoTRP channels are some of the principal mediators of in several chronic pain conditions and are continually sought after as possible therapeutic targets.
Role of thermotrps in cold, warm, and noxious heat sensing
A recent study has postulated that TRPV1 channels, which were initially proposed as noxious temperature sensors, are important for warm temperature sensitivity, more than noxious heat stimulus sensing in vivo [27]. This finding was reported in trigeminal ganglion neurons, where TRPV1 is present, but is not highly expressed. Other groups have reported that the expression profile of TRP channels is different in the trigeminal or dorsal root ganglion. They noted that TRPV1 expression was significantly greater in DRG segments compared to TG or other regions of the spinal cord [23,83]. This lower expression makes it possible that the contribution of TRPV1 to the thermosensitive response in TG neurons could be lower than in cells where its expression is more significant, i.e. the DRG neuron. These differences, speak to a possible cellular specificity of the contribution of TRP channels to the responses to even the same stimulus.
The study of Yarmolinsky [27] suggests that TRPV1 channels are subjected to modulation of the range of temperatures where they respond, perhaps through posttranslational modification or other modulatory mechanisms, making TRPV1 responsive even in the range of temperatures considered mild (below 43°C). Another possible mechanism of TRPV1 modulation was recently suggested. Sanchez-Moreno and coworkers have shown that the range of temperatures for the TRPV1 response is dynamic, shifting to lower temperatures in a use-dependent manner [84]. The range of temperature activation of TRPV1 seems to be modulated by the local structure of the ankyrin repeat domains, raising the possibility that subtle rearrangements in this region, in response to modulatory stimuli, can alter the temperature range of activation of this channel [85].
More recently, and in contrast with the Yarmolinsky et al. study, the Voets group [26] reported that only three thermo TRP channels, TRPV1, TRPM3, and TRPA1 are needed for noxious temperature sensitivity in DRG neurons of mice. These authors report that the response to mild stimuli is mostly intact and depends on a small subset of neurons and might originate from thermal responses in keratinocytes, presumably mediated by other thermoTRP channels or even other heat-sensitive membrane proteins. These two papers present evidence for two different interpretations of the heat response in sensory neurons. On the one hand, the data of Vandewauw et al. seem to support the strict separation of temperature sensitivity among different TRP channels and thus, an exclusive line communication of temperature information. The work by Yarmolinsky and collaborators would suggest that TRPV1 is capable of supporting both noxious and mild temperature sensation. It seems that the mild and high-temperature-sensitive TRPV1 is expressed in different subsets of neurons. In this case, the type of sensory nerve cell and not a particular compliment of ion channels would determine the communication line of sensory information.
Conclusions
The role of thermoTRP channels in temperature sensing is well established; however, a number of controversies still stand. In particular, the jury seems to still be out regarding the heat or cold sensitivity of TRPA1 channels. It seems that the heat response of TRPA1 is strongly dependent on the redox environment of the cell or the presence of modulatory intracellular cysteine-reactive species. Both the Yarmolinsky and Vandewauw studies coincide in that TRPA1 is not a cold sensor in mice and instead participates in heat sensing.
What are all the other thermoTRP channels doing if they do not participate in peripheral thermosensation in mice, as these studies seem to suggest? One possibility is that they play a role in thermosensation in other organs or that their sensitivity to temperature is residual. TRPV2 has a very high-temperature activation threshold and residual vanilloid sensitivity [86,87], which makes it improbable that it participates in mild or even noxious thermosensation. TRPV3 also has a high activation threshold and its more likely involved in pruritus in the skin.
It is also possible that these channels have roles in thermosensitivity in animals other than mammals, and this function is still to be revealed. It seems likely that the evolution of thermoTRP channels among a wide variety of animal species has produced a lot of variability in the processes involved as molecular determinants in thermosensation. An important lesson from the recent studies seems to be that we cannot generalize the functions of thermoTRP channels among different organisms.
Funding Statement
This work was supported by the DGAPA-PAPIIT [IN209515]; CONACyT [252644].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Clapham DE, Runnels LW, Strübing C.. The TRP ion channel family. Nat Rev Neurosci. 2001;2(6):387–396. [DOI] [PubMed] [Google Scholar]
- [2].Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liao M, Cao E, Julius D, et al. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504(7478):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cao E, Liao M, Cheng Y, et al. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504(7478):113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huynh KW, Cohen MR, Jiang J, et al. Structure of the full-length TRPV2 channel by cryo-EM. Nat Commun. 2016;7: 11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zubcevic L, Herzik MA, Chung BC, et al. Cryo-electron microscopy structure of the TRPV2 ion channel. Nat Struct Mol Biol. 2016;23(2):180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Deng Z, Paknejad N, Maksaev G, et al. Cryo-EM and X-ray structures of TRPV4 reveal insight into ion permeation and gating mechanisms. Nat Struct Mol Biol. 2018;25(3):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hughes TET, Lodowski DT, Huynh KW, et al. Structural basis of TRPV5 channel inhibition by econazole revealed by cryo-EM. Nat Struct Mol Biol. 2018;25(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McGoldrick LL, Singh AK, Saotome K, et al. Opening of the human epithelial calcium channel TRPV6. Nature. 2018;553(7687):233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Madej MG, Ziegler CM. Dawning of a new era in TRP channel structural biology by cryo-electron microscopy. Pflugers Arch. 2018;470(2):213–225. [DOI] [PubMed] [Google Scholar]
- [11].Singh AK, McGoldrick LL, Sobolevsky AI. Structure and gating mechanism of the transient receptor potential channel TRPV3. Nat Struct Mol Biol. 2018;25(9):805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Paulsen CE, Armache J-P, Gao Y, et al. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520(7548):511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Feng S. TRPC channel structure and properties. Adv Exp Med Biol. 2017;976:9–23. [DOI] [PubMed] [Google Scholar]
- [14].Vinayagam D, Mager T, Apelbaum A, et al. Electron cryo-microscopy structure of the canonical TRPC4 ion channel. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Duan J, Li J, Zeng B, et al. Structure of the mouse TRPC4 ion channel. Nat Commun. 2018;9(1):3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yin Y, Wu M, Zubcevic L, et al. Structure of the cold- and menthol-sensing ion channel TRPM8. Science. 2018;359(6372):237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang Z, Tóth B, Szollosi A, et al. Structure of a TRPM2 channel in complex with Ca2+ explains unique gating regulation. eLife. 2018;DOI: 10.7554/eLife.36409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guo J, She J, Zeng W, et al. Structures of the calcium-activated, non-selective cation channel TRPM4. Nature. 2017;552(7684):205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Duan J, Li J, Chen G-L, et al. Cryo-EM structure of the receptor-activated TRPC5 ion channel at 2.9 angstrom resolution. bioRxiv. 2018;467969. [Google Scholar]
- [20].Castillo K, Diaz-Franulic I, Canan J, et al. Thermally activated TRP channels: molecular sensors for temperature detection. Phys Biol. 2018;15(2):021001. [DOI] [PubMed] [Google Scholar]
- [21].Saito S, Tominaga M. Functional diversity and evolutionary dynamics of thermoTRP channels. Cell Calcium. 2015;57(3):214–221. [DOI] [PubMed] [Google Scholar]
- [22].Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. [DOI] [PubMed] [Google Scholar]
- [23].Jang Y, Lee Y, Kim SM, et al. Quantitative analysis of TRP channel genes in mouse organs. Arch Pharm Res. 2012;35(10):1823–1830. [DOI] [PubMed] [Google Scholar]
- [24].Saito S, Fukuta N, Shingai R, et al. Evolution of vertebrate transient receptor potential Vanilloid 3 channels: opposite temperature sensitivity between mammals and western clawed frogs. PLoS Genet. 2011;7(4):e1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Saito S, Banzawa N, Fukuta N, et al. Heat and noxious chemical sensor, chicken TRPA1, as a target of bird repellents and identification of its structural determinants by multispecies functional comparison. Mar Mol Biol Evol. 2014;313:708–722. [DOI] [PubMed] [Google Scholar]
- [26].Vandewauw I, De Clercq K, Mulier M, et al. Publisher correction: a TRP channel trio mediates acute noxious heat sensing. Nature. 2018. doi: 10.1038/s41586-018-0100-8. [DOI] [PubMed] [Google Scholar]
- [27].Yarmolinsky DA, Peng Y, Pogorzala LA, et al. Coding and plasticity in the mammalian thermosensory system. Neuron. 2016;92(5):1079–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zimmermann K, Lennerz JK, Hein A, et al. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc Natl Acad Sci USA. 2011. November 01;108(44):18114–18119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. [DOI] [PubMed] [Google Scholar]
- [30].Caterina MJ, Rosen TA, Tominaga M, et al. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398(6726):436–441. [DOI] [PubMed] [Google Scholar]
- [31].Xu H, Ramsey IS, Kotecha SA, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418(6894):181–186. [DOI] [PubMed] [Google Scholar]
- [32].Prober DA, Zimmerman S, Myers BR, et al. Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J Neurosci. 2008;28(40):10102–10110. . en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. [DOI] [PubMed] [Google Scholar]
- [34].Talavera K, Yasumatsu K, Voets T, et al. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438(7070):1022–1025. .en [DOI] [PubMed] [Google Scholar]
- [35].Vriens J, Owsianik G, Hofmann T, et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron. 2011;70(3):482–494. [DOI] [PubMed] [Google Scholar]
- [36].Song K, Wang H, Kamm GB, et al. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science. 2016;353(6306):1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kamm GB, Siemens J. The TRPM2 channel in temperature detection and thermoregulation. Temperature. 2017;4(1):21–23. doi: 10.1080/23328940.2016.1258445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Story GM, Peier AM, Reeve AJ, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112(6):819–829. [DOI] [PubMed] [Google Scholar]
- [39].Islas LD. Molecular mechanisms of temperature gating in TRP channels In: Emir TLR, editor. Neurobiology of TRP channels. Frontiers in neuroscience. 2nd ed. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. p. 11–25. [PubMed] [Google Scholar]
- [40].Rosasco MG, Gordon SE. TRP channels. In: Emir TLR, editor. Neurobiology of TRP channels. Frontiers in neuroscience. 2nd ed. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. p.1–9. [Google Scholar]
- [41].Kaneko Y, Szallasi A. Transient receptor potential (TRP) channels: a clinical perspective. Br J Pharmacol. 2014;171(10):2474–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Uchida K, Dezaki K, Yoneshiro T, et al. Involvement of thermosensitive TRP channels in energy metabolism. J Physiol Sci. 2017;67(5):549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Santoni G, Farfariello V. TRP channels and cancer: new targets for diagnosis and chemotherapy. Endocr Metab Immune Disord Drug Targets. 2011;11(1):54–67. [DOI] [PubMed] [Google Scholar]
- [44].Hantute-Ghesquier A, Haustrate A, Prevarskaya N, et al. TRPM Family Channels in Cancer. Pharmaceuticals (Basel). 2018;11(2):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zheng J, Liu F, Du S, et al. Mechanism for regulation of melanoma cell death via activation of thermo-TRPV4 and TRPV2. J Oncol. 2019;2019:7362875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Myers BR, Sigal YM, Julius D. Evolution of thermal response properties in a cold-activated TRP channel. PLoS ONE. 2009;4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jara-Oseguera A, Islas LD. The role of allosteric coupling on thermal activation of thermo-TRP channels. Biophys J. 2013;104(10):2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Latorre R, Brauchi S, Orta G, et al. ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium. 2007;42(4):427–438. [DOI] [PubMed] [Google Scholar]
- [49].Huang SM, Li X, Yu Y, et al. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain. 2011;7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wei JJ, Fu T, Yang T, et al. A TRPA1 channel that senses thermal stimulus and irritating chemicals in Helicoverpa armigera. Insect Mol Biol. 2015;24(4):412–421. [DOI] [PubMed] [Google Scholar]
- [51].Gracheva EO, Ingolia NT, Kelly YM, et al. Molecular basis of infrared detection by snakes. Nature. 2010;464(7291):1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Saito S, Nakatsuka K, Takahashi K, et al. Analysis of transient receptor potential ankyrin 1 (TRPA1) in Frogs and Lizards Illuminates both nociceptive heat and chemical sensitivities and coexpression with TRP Vanilloid 1 (TRPV1) in ancestral vertebrates. J Biol Chem. 2012;287(36):30743–30754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chen J, Kang D, Xu J, et al. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun. 2013. ;4:2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hoffmann T, Kistner K, Miermeister F, et al. TRPA1 and TRPV1 are differentially involved in heat nociception of mice. Eur J Pain. 2013;17(10):1472–1482. [DOI] [PubMed] [Google Scholar]
- [55].Moparthi L, Kichko TI, Eberhardt M, et al. Human TRPA1 is a heat sensor displaying intrinsic U-shaped thermosensitivity. Sci Rep. 2016;6: 28763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Jabba S, Goyal R, Sosa-Pagán JO, et al. Directionality of temperature activation in mouse TRPA1 ion channel can be inverted by single-point mutations in ankyrin repeat six. Neuron. 2014;82(5):1017–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Karashima Y, Talavera K, Everaerts W, et al. TRPA1 acts as a cold sensor in vitro and in vivo. Pnas. 2009;106(4):1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. [DOI] [PubMed] [Google Scholar]
- [59].Davis JB, Gray J, Gunthorpe MJ, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–187. [DOI] [PubMed] [Google Scholar]
- [60].Garami A, Pakai E, Oliveira DL, et al. Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci. 2011;31(5):1721–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wanner SP, Garami A, Romanovsky AA. Hyperactive when young, hypoactive and overweight when aged: connecting the dots in the story about locomotor activity, body mass, and aging in Trpv1 knockout mice. Aging (Albany NY). 2011;3(4):450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Shibasaki K. Physiological significance of TRPV2 as a mechanosensor, thermosensor and lipid sensor. J Physiol Sci. 2016;66(5):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Park U, Vastani N, Guan Y, et al. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J Neurosci. 2011;31(32):11425–11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Smith GD, Gunthorpe MJ, Kelsell RE, et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418(6894):186–190. [DOI] [PubMed] [Google Scholar]
- [65].Liu B, Qin F. sThe Xenopus tropicalis orthologue of TRPV3 is heat sensitive. J Gen Physiol. 2015;146(5):411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Liu B, Qin F. Use dependence of heat sensitivity of vanilloid receptor TRPV2. Biophys J. 2016;110(7):1523–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Shibasaki K, Tominaga M, Ishizaki Y. Hippocampal neuronal maturation triggers post-synaptic clustering of brain temperature-sensor TRPV4. Biochem Biophys Res Commun. 2015;458(1):168–173. [DOI] [PubMed] [Google Scholar]
- [68].Bakri MM, Yahya F, Munawar KMM, et al. Transient receptor potential vanilloid 4 (TRPV4) expression on the nerve fibers of human dental pulp is upregulated under inflammatory condition. Arch Oral Biol. 2018;89:94–98. [DOI] [PubMed] [Google Scholar]
- [69].Mundt N, Spehr M, Lishko PV. TRPV4 is the temperature-sensitive ion channel of human sperm. eLife. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bidaux G, Borowiec A-S, Gordienko D, et al. Epidermal TRPM8 channel isoform controls the balance between keratinocyte proliferation and differentiation in a cold-dependent manner. Proc Natl Acad Sci U S A. 2015;112(26):E3345–E3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tan C-H, McNaughton PA. The TRPM2 ion channel is required for sensitivity to warmth. Nature. 2016;536(7617):460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Held K, Voets T, Vriens J. TRPM3 in temperature sensing and beyond. Temperature. 2015;2(2):201–213. doi: 10.4161/23328940.2014.988524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Vriens J, Voets T. Sensing the heat with TRPM3. Pflugers Arch. 2018;470(5):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. [DOI] [PubMed] [Google Scholar]
- [75].Dhaka A, Murray AN, Mathur J, et al. TRPM8 is required for cold sensation in mice. Neuron. 2007;54(3):371–378. [DOI] [PubMed] [Google Scholar]
- [76].Pertusa M, Rivera B, González A, et al. Critical role of the pore domain in the cold response of TRPM8 channels identified by ortholog functional comparison. J Biol Chem. 2018;293(32):12454–12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lishko PV, Procko E, Jin X, et al. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54(6):905–918. [DOI] [PubMed] [Google Scholar]
- [78].Morales-Lázaro SL, Lemus L, Rosenbaum T. Regulation of thermoTRPs by lipids. Temperature. 2017;4(1):24–40. dio: 10.1080/23328940.2016.1254136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Salazar H, Llorente I, Jara-Oseguera A, et al. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat Neurosci. 2008;11(3):255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Trevisani M, Siemens J, Materazzi S, et al. 2007; 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007; 10433:13519–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Todaka H, Taniguchi J, Satoh J-I, et al. Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J Biol Chem. 2004;279(34):35133–35138. [DOI] [PubMed] [Google Scholar]
- [82].Xing H, Chen M, Ling J, et al. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007;27(50):13680–13690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Vandewauw I, Owsianik G, Voets T. Systematic and quantitative mRNA expression analysis of TRP channel genes at the single trigeminal and dorsal root ganglion level in mouse. BMC Neurosci. 2013;14(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sánchez-Moreno A, Guevara-Hernández E, Contreras-Cervera R, et al. Irreversible temperature gating in trpv1 sheds light on channel activation. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Laursen WJ, Schneider ER, Merriman DK, et al. Low-cost functional plasticity of TRPV1 supports heat tolerance in squirrels and camels. Proc Natl Acad Sci U S A. 2016;113(40):11342–11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Yang F, Vu S, Yarov-Yarovoy V, et al. Rational design and validation of a vanilloid-sensitive TRPV2 ion channel. Proc Natl Acad Sci U S A. 2016;113(26):E3657–E3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zhang F, Hanson SM, Jara-Oseguera A, et al. Engineering vanilloid-sensitivity into the rat TRPV2 channel. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]


