Abstract
PURPOSE
Tamoxifen administered for 5 years at 20 mg/d is effective in breast cancer treatment and prevention, but toxicity has limited its broad use. Biomarker trials showed that 5 mg/d is not inferior to 20 mg/d in decreasing breast cancer proliferation. We hypothesized that a lower dose given for a shorter period could be as effective in preventing recurrence from breast intraepithelial neoplasia but have a lower toxicity than the standard dose.
PATIENTS AND METHODS
We conducted a multicenter randomized trial of tamoxifen, 5 mg/d or placebo administered for 3 years after surgery in women with hormone-sensitive or unknown breast intraepithelial neoplasia, including atypical ductal hyperplasia and lobular or ductal carcinoma in situ. The primary end point was the incidence of invasive breast cancer or ductal carcinoma in situ.
RESULTS
Five hundred women 75 years of age or younger were included. After a median follow-up of 5.1 years (interquartile range, 3.9-6.3 years), there were 14 neoplastic events with tamoxifen and 28 with placebo (11.6 v 23.9 per 1,000 person-years; hazard ratio, 0.48; 95% CI, 0.26 to 0.92; P = .02), which resulted in a 5-year number needed to treat of 22 (95% CI, 20 to 27). Tamoxifen decreased contralateral breast events by 75% (three v 12 events; hazard ratio, 0.25; 95% CI, 0.07 to 0.88; P = .02). Patient-reported outcomes were not different between arms except for a slight increase in frequency of daily hot flashes with tamoxifen (P = .02). There were 12 serious adverse events with tamoxifen and 16 with placebo, including one deep vein thrombosis and one stage I endometrial cancer with tamoxifen and one pulmonary embolism with placebo.
CONCLUSION
Tamoxifen at 5 mg/d for 3 years can halve the recurrence of breast intraepithelial neoplasia with a limited toxicity, which provides a new treatment option in these disorders.
INTRODUCTION
Breast intraepithelial neoplasia, including atypical ductal hyperplasia (ADH), lobular carcinoma in situ (LCIS), and ductal carcinoma in situ (DCIS), has a five to 10 times higher risk of developing into invasive breast cancer compared with the general population and accounts for 15% to 25% of breast neoplasms on the basis of screening mammography.1 The natural history of breast intraepithelial neoplasia is heterogeneous. Some forms remain indolent, whereas other forms can progress to life-threatening invasive disease, which has led to controversial issues with regard to its optimal approach.1,2
Tamoxifen, a selective estrogen receptor (ER) modulator and a precursor of molecular targeted medicines, is effective in the adjuvant treatment of hormone receptor–positive breast cancer3 and for the prevention of breast cancer in at-risk women on the basis of the Gail model, including those with ADH and LCIS whose risk of subsequent invasive cancer is 10 and 13 per 1,000 per year, respectively.4 However, the toxicity of tamoxifen, including venous thromboembolic events and endometrial cancer, is a significant problem and may explain the low uptake of tamoxifen as preventive therapy.5 The increase of menopausal symptoms during tamoxifen therapy, including vasomotor symptoms and sexual and gynecologic disturbances, is also a cause of decreased quality of life and treatment withdrawal.6,7 The lack of a demonstrated breast cancer mortality benefit for tamoxifen given for primary prevention or after DCIS combined with adverse effects suggests a need for an alternative strategy, such as dose de-escalation. We conducted studies to determine the minimum effective dose of tamoxifen, which was not initially assessed.8,9 Biomarker trials revealed that 5 mg/d was noninferior to 20 mg/d in inhibiting proliferation of breast cancer10 and normal endometrial tissue.11 A prospective cohort study also showed that 10 mg on alternate days halves recurrence of DCIS in postmenopausal women.12
In the present phase III trial, we assessed whether tamoxifen administered for 3 years at 5 mg/d is effective at reducing DCIS, invasive recurrence, or contralateral breast cancer from breast intraepithelial neoplasia without significant toxicity in terms of serious adverse events and patient-reported outcomes.
PATIENTS AND METHODS
Participants and Procedure
We conducted a multicenter phase III trial of tamoxifen 5 mg/d versus placebo administered for 3 years in women 75 years of age or younger with an Eastern Cooperative Oncology Group performance status of 1 or less and operated hormone-sensitive (ER or progesterone receptor ≥ 1%) or unknown breast intraepithelial neoplasia (ADH, DCIS, or LCIS). Women with high-grade or comedo/necrotic DCIS received adjuvant radiotherapy of 50 Gy in 25 courses. Women were visited every 6 months and had a mammography and transvaginal ultrasound annually for 3 years of treatment and 2 years of follow-up. The study (Tam01) was approved by the ethics committee of the sponsor (Galliera Hospital, Genoa, Italy) and participating sites, and all women signed a written informed consent. Main exclusion criteria were any prior cancer, any tamoxifen contraindications, mental disorders, pregnancy, grade 2 or higher biochemical alterations according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4), prior use of anti-estrogens, current use of dicumarols, and CYP2D6 inhibitors such as selective serotonin reuptake inhibitors.
Treatment
Tamoxifen 5 mg or identical placebo tablets were provided by Doppel Farmaceutici (Cortemaggiore, PC, Italy) under good manufacturing practice in bottles containing 210 tablets. Randomization and treatment started after surgery, whereas radiotherapy could be concomitant or before tamoxifen treatment for patients accrued 12 to 60 months after surgery. A review of baseline histologic reports was performed at the coordinating center to check eligibility, but measurements of immunohistochemistry were not centrally repeated. All breast events that occurred during the trial were centrally adjudicated by a clinical committee. Treatment compliance was assessed by pill count. Adherence was defined at each study visit as the use of at least 85% of pills during the 6-month study period.
Study End Points
The primary end point was the incidence of invasive breast cancer or DCIS. Secondary end points were incidence of ADH or LCIS, endometrial cancer, other second primary cancers, deep venous thromboembolic events, coronary heart disease, bone fractures, cataract, and menopausal symptoms. Correlative studies of biomarkers included mammographic density, circulating insulin-like growth factor (IGF) 1 and IGF binding proteins, IGF binding protein 3, sex hormone binding globulin, C-reactive protein, CYP2D6 single nucleotide polymorphisms, tamoxifen, and metabolite blood levels.
Toxicity was assessed by the Common Terminology Criteria for Adverse Events (version 4). Because menopausal symptoms are a major cause of decreased quality of life and treatment dropout from tamoxifen trials,6,7 patient-reported symptoms associated with both menopause and the known adverse effects of tamoxifen were recorded. Hot flashes were evaluated according to Sloan et al,13 where the daily frequency and scores were computed for each patient by combining both severity (mild, moderate, severe, and very severe) and frequency from daily diaries and averaging across the last study week before each semiannual visit. The Breast Cancer Prevention Trial Symptom Scale14 calculates scores on each subscale by averaging a number of items during the last 4 weeks before each semiannual visit, including vasomotor symptoms, sexual and vaginal problems, and musculoskeletal pain/arthralgia.
Randomization
Patients were randomly assigned (1:1) to one of the two treatment arms in a masked fashion by a Web-based system using the minimization algorithm. Random sequences were generated by one of the authors (L.B.) according to the Moses-Oakford algorithm. Only the system administrator had access to the allocation sequences. Stratification criteria were center, time from diagnosis to randomization (within 12 v 12 to 60 months), and histotype (ADH + DCIS v LCIS).
Statistical Analyses
The study was initially powered to detect a 50% reduction in the incidence of invasive breast cancer or DCIS by assuming an annual rate of events of 20 per 1,000 person-years with placebo,4,15,16 5 years of recruitment, 2 years of follow-up, and a 10% dropout rate. With 80% power and a 5% one-sided significance, 1,400 participants were necessary to observe 55 events. Initial statistical assumptions were revised by the independent data monitoring committee according to the lower-than-expected accrual, mainly because of financial constraints, and the higher risk in the placebo arm. On June 28, 2018, the committee recommended the disclosure of results because 80% of the originally expected events were observed, all women had completed the treatment period, and there was a significant benefit and a low toxicity that could change clinical practice.
The Kaplan-Meier cumulative incidence of invasive breast cancer or DCIS (primary end point) and of contralateral breast events was estimated by adopting the reverse Kaplan-Meier technique to calculate median follow-up time.17 Log-rank test was used to test differences between arms. Cox proportional hazards regression modeling was also performed as supportive analysis. We reported P values and hazard ratios (HRs) from nonstratified analyses because stratified log-rank P values and Cox analyses gave superimposable results. Because of the highly overdispersed and zero-inflated distribution of frequency and score of hot flashes, the analysis was performed by adopting a negative binomial regression model; a Poisson model was used for the other vasomotor symptoms (the distribution was less overdispersed), and linear modeling was adopted for the endometrial thickness. In all the regression models, the variance-covariance matrix was estimated to account for repeated measures and provided robust SEs that allowed for intrawoman correlation. Covariates were age and menopausal status. Tests for interaction between time and treatment arm were performed within all the regression models. The Kaplan-Meier method was also used to estimate treatment adherence by pill count at different follow-up times by treatment arm. Effect sizes of clinically relevant outcomes were calculated using the number needed to treat or harm and the likelihood to be of help or harm.18 The primary analysis included all randomly assigned patients according to the intention-to-treat principle. Censoring to last follow-up visit available was applied when patient follow-up data were not complete. The safety analysis included all patients who received at least one dose of study treatment. Prespecified subgroup analyses (≤ 12 v 12 to 60 months from diagnosis, ADH + DCIS v LCIS, ER positive v unknown) and post hoc subgroup analyses, including ADH or LCIS v DCIS per the Gail model,4 were performed only for exploratory purposes and according to the test for interaction approach. Because there was only one noncancer death, competing risk analysis was not performed. No multiplicity adjustment methods were adopted. Although we calculated sample size with a one-sided α-error, results are shown with 95% CIs and two-sided P values. All analyses were performed using Stata 14.2 statistical software (StataCorp, College Station, TX).
RESULTS
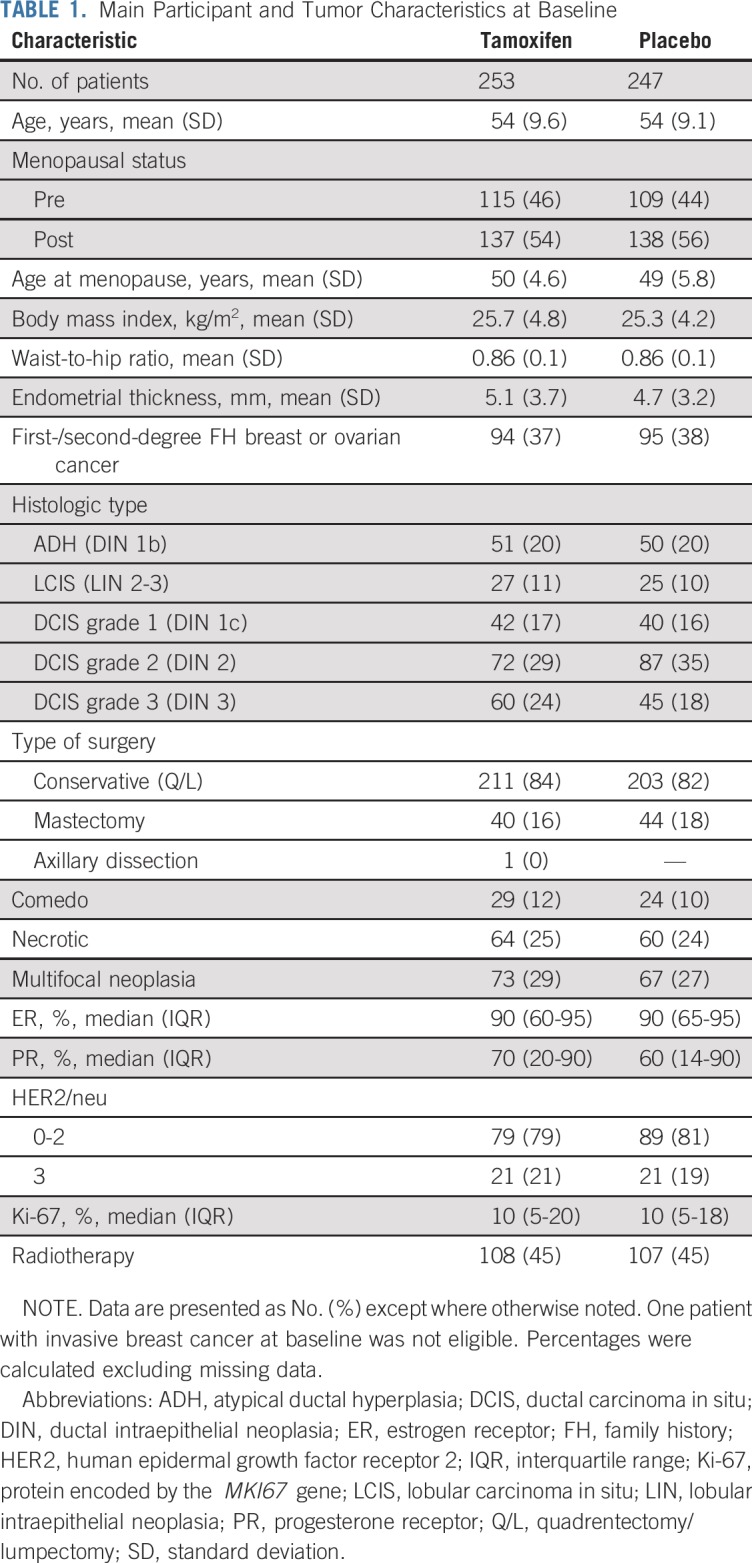
Between November 1, 2008, and March 31, 2015, 1,160 women were screened and 500 75 years of age or younger were included in the study. The participant flow diagram is shown in Figure 1. The main participant and tumor characteristics were evenly distributed between arms (Table 1). The mean age was 54 years (standard deviation, 9 years), and 55% of participants were postmenopausal. Twenty percent had ADH, 11% had LCIS, and the remaining 69% had DCIS. In total, 33% of participants had ER or progesterone receptor unknown neoplasms, of which 63% were among those with ADH or LCIS and 20% among those with DCIS. There were no participants with ER–negative DCIS.
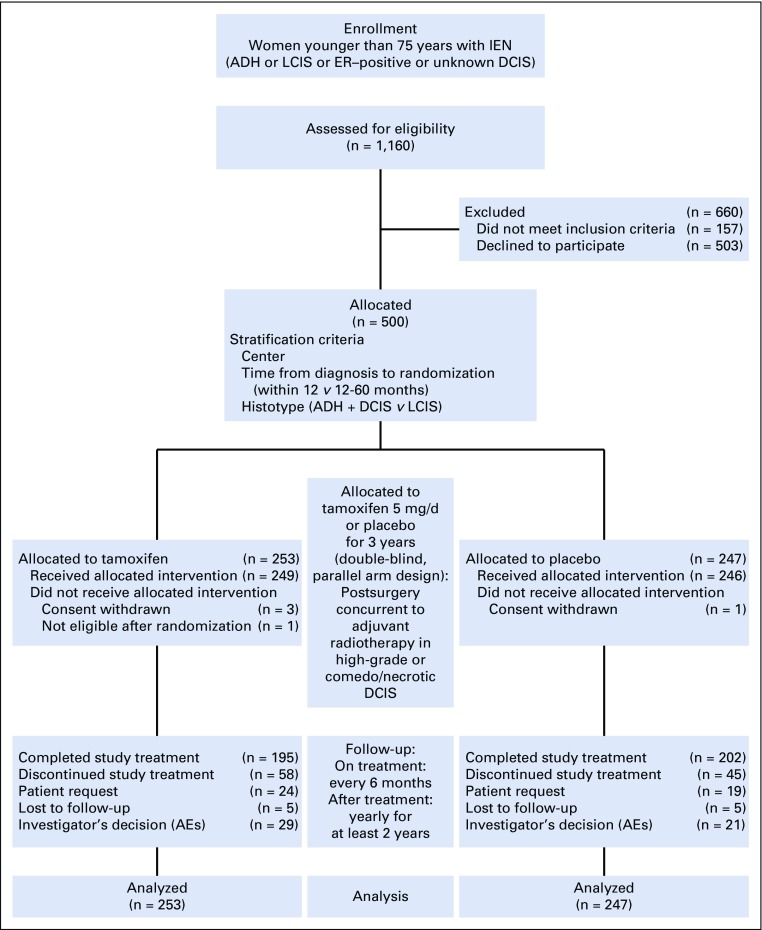
FIG 1.
Participant flow diagram. The intention-to-treat population included all patients who underwent random assignment (n = 253 in the tamoxifen arm; n = 247 in the placebo arm). The safety population included all patients who received at least one dose of trial agent (n = 249 in the tamoxifen arm; n = 246 in the placebo arm). Completion of the study is defined as having completed the 36-month double-blind treatment phase. ADH, atypical ductal hyperplasia; AE, adverse event; DCIS, ductal carcinoma in situ; ER, estrogen receptor; IEN, intraepithelial neoplasia; LCIS, lobular carcinoma in situ.
TABLE 1.
Main Participant and Tumor Characteristics at Baseline
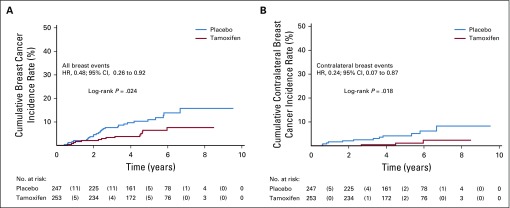
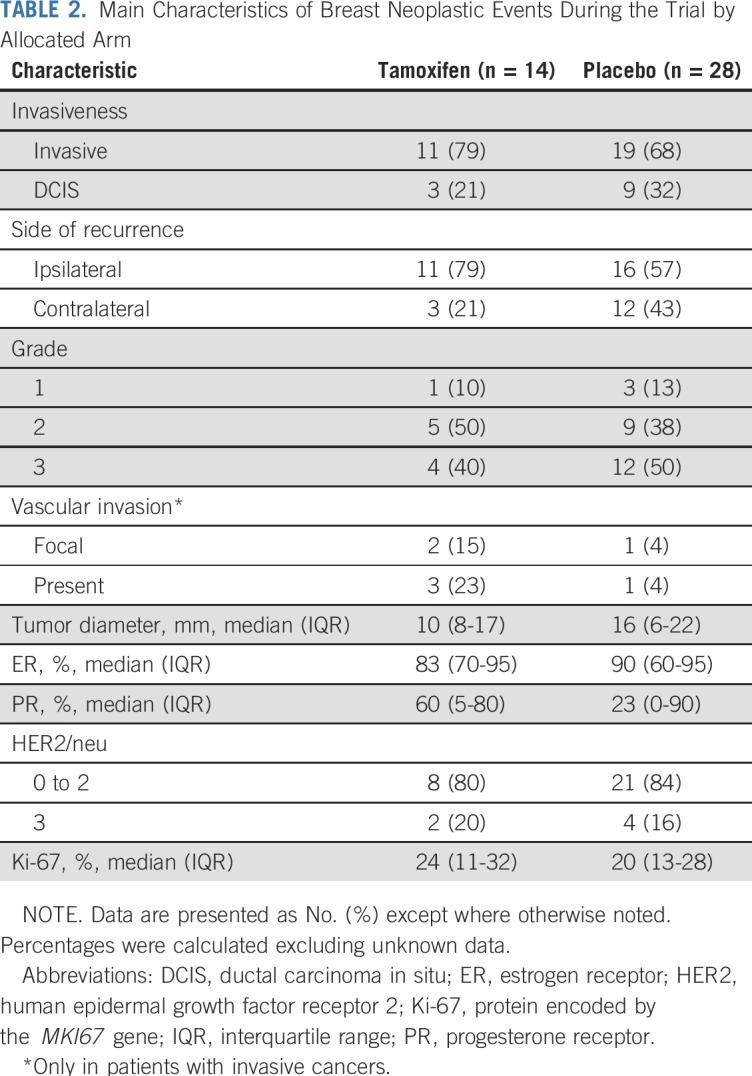
After a median follow-up of 5.1 years (interquartile range, 3.9-6.3 years), there were 14 breast neoplastic events (invasive breast cancer or DCIS) in the tamoxifen arm (rate, 11.6 per 1,000 person-years) and 28 breast events in the placebo arm (rate, 23.9 per 1,000 person-years; HR, 0.48; 95% CI, 0.26 to 0.92; log-rank P = .02). Kaplan-Meier estimates of cumulative incidence rates of breast events (invasive breast cancer or DCIS) in the tamoxifen and placebo arms at 5 years were 6.4% and 11.0%, respectively, resulting in a number needed to treat of 22 (95% CI, 20 to 27). In consideration of contralateral breast cancer incidence, there were three events in the tamoxifen arm and 12 in the placebo arm (HR, 0.25; 95% CI, 0.07 to 0.88; P = .02). The cumulative incidences of the primary end point (invasive breast cancer or DCIS) and contralateral breast events are shown in Figure 2. Table 2 lists the characteristics of the breast neoplastic events. The majority of recurrences were invasive. There was no evidence for a different biology of tumors occurring in the tamoxifen arm compared with the placebo arm. There was one recurrence in the tamoxifen arm versus two in the placebo arm (HR, 0.50; 95% CI, 0.05 to 5.47) in participants with prior ADH, two versus six in those with prior LCIS (HR, 0.31; 95% CI, 0.06 to 1.51), and 11 versus 20 (HR, 0.53; 95% CI, 0.25 to 1.11) in those with DCIS (P for interaction = .8). There was no significant heterogeneity in prespecified subgroups (Appendix Table A1, online only). The risk of invasive or DCIS recurrence in ADH, DCIS, and LCIS strata adjusting for tamoxifen effect is shown in Appendix Figure A1 (online only).
FIG 2.
Kaplan-Meier estimates of (A) cumulative incidence of breast cancer events (invasive breast cancer or ductal carcinoma in situ and of (B) contralateral breast cancer events in the overall intention-to-treat population (ITT) by allocated arm. Numbers in parentheses represent the number of events that occurred within each time interval by treatment arm. The log-rank test was used to draw inferences. The estimate of the hazard ratio (HR) was based on a univariable Cox proportional hazards regression model (ie, with only the treatment arm variable). In the overall ITT population, there were 42 breast cancer events (14 in the tamoxifen arm and 28 in the placebo arm) of which there were 15 contralateral breast cancer events (three in the tamoxifen arm and 12 in the placebo arm). The 3-year cumulative incidence rate of breast cancer events was 2.3% (95% CI, 1.7% to 6.6%) in the tamoxifen arm and 7.6% (95% CI, 4.8% to 11.8%) in the placebo arm, and the 5-year cumulative incidence rate was 6.4% (95% CI, 3.7% to 10.9%) and 11.0% (95% CI, 7.4% to 16.0%), respectively. The 3-year cumulative incidence rate of contralateral breast cancer was 0.4% (95% CI, 0.1% to 3.1%) in the tamoxifen arm and 2.5% (95% CI, 5.5% to 1.1%) in the placebo arm, and the 5-year cumulative incidence rate was 1.1% (95% CI, 0.3% to 4.5%) and 4.2% (95% CI, 2.2% to 8.0%), respectively.
TABLE 2.
Main Characteristics of Breast Neoplastic Events During the Trial by Allocated Arm
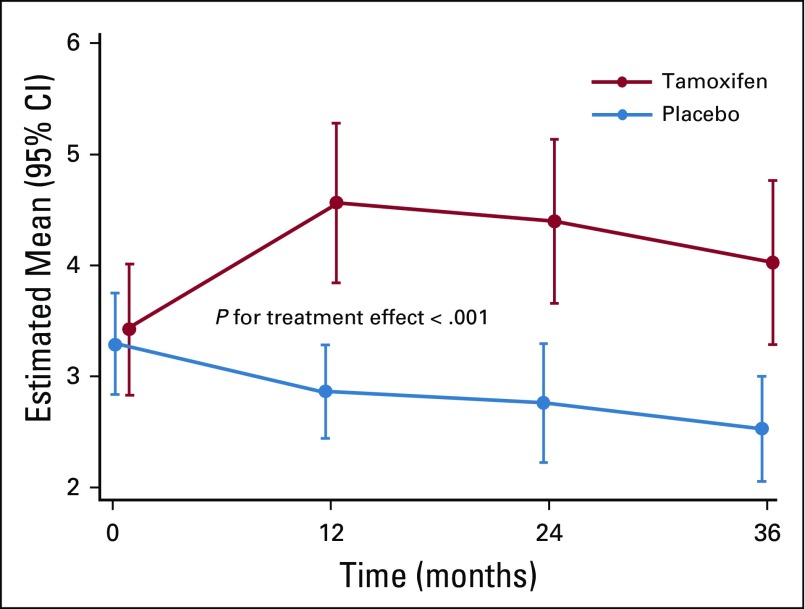
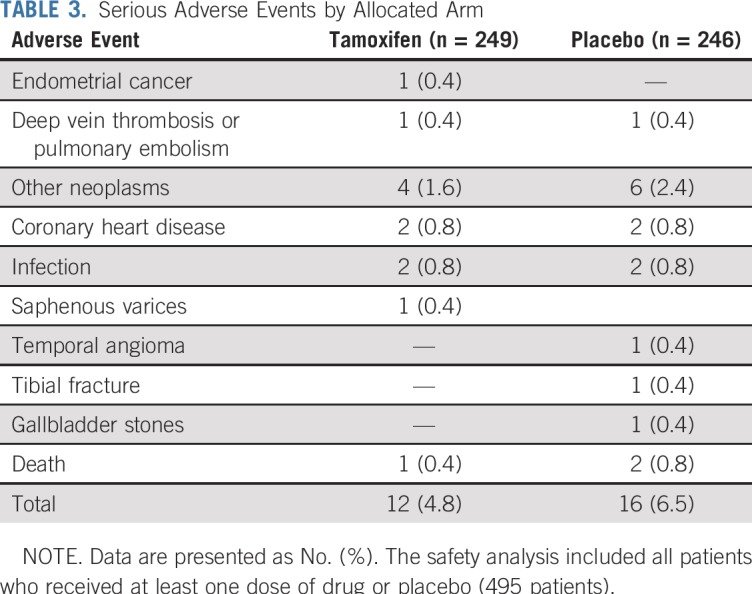
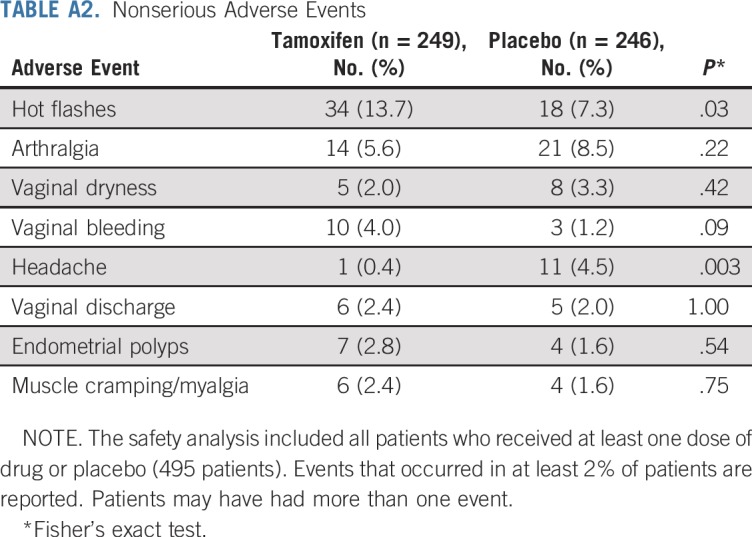
There were 12 serious adverse events in the tamoxifen arm and 16 in the placebo arm, including one deep vein thrombosis, one stage I endometrial cancer in the tamoxifen arm versus one pulmonary embolism in the placebo arm (rate, 0.85 per 1,000 person-years). The 5-year number needed to harm resulting from a cumulative incidence rate of 0.87% with tamoxifen and 0.41% with placebo was 218 (95% CI, 193 to 265), and the likelihood of being helpful was 10 times higher (218 v 22) than being harmful. Second primary cancers were four in the tamoxifen arm and six in the placebo arm. Table 3 lists the serious adverse events. There were two deaths in the placebo arm, one as a result of breast cancer and one as a result of myocardial infarction, and one death was a result of breast cancer in the tamoxifen arm. Appendix Table A2 (online only) lists nonserious investigator-reported adverse events that occurred in more than 2% of participants. More hot flashes and vaginal bleeding and less headache were recorded in the tamoxifen arm, whereas there was no difference in endometrial polyps between tamoxifen and placebo (2.8% v 1.6%). The mean endometrial thickness in postmenopausal women increased significantly by approximately 1 mm while on tamoxifen (Appendix Fig A2, online only).
TABLE 3.
Serious Adverse Events by Allocated Arm
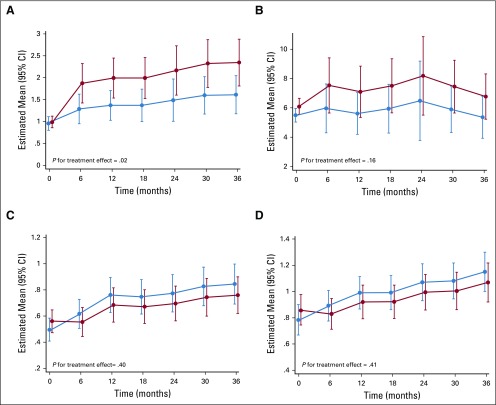
Overall, there was a slight increase in daily frequency of participant-reported hot flashes in the tamoxifen arm over the 3-year treatment period (P = .02; Fig 3A). On average, the incidence rate ratio of daily number of hot flashes in the tamoxifen versus placebo arm was 1.46 (95% CI, 1.05 to 2.00). In the 3-year treatment period, a woman had a mean daily hot flash frequency of 2.1 (95% CI, 1.7 to 2.5) v 1.5 (95% CI, 1.1 to 1.8) in the tamoxifen versus placebo arms, respectively. However, the hot flashes score was not different between arms (P = .16; Fig 3B). Likewise, participant-reported outcomes according to the Breast Cancer Prevention Trial Symptom Scale were not different between the tamoxifen and placebo arms. Specifically, there was no difference in vaginal dryness and pain during sexual intercourse (Fig 3C) or in musculoskeletal symptoms and arthralgia (Fig 3D).
FIG 3.
Marginal predicted means of daily (A) hot flash frequency and (B) score (frequency × intensity), (C) vaginal dryness or pain with intercourse, and (D) musculoskeletal pain or arthralgia during the double-blind treatment phase. Values at baseline (month 0) are observed mean. Daily frequency but not daily score of hot flashes in the tamoxifen arm increased significantly relative to the placebo arm (P for treatment effect = .02 and .16, respectively). There was no difference between arms on vaginal dryness or pain with intercourse and musculoskeletal pain or arthralgia (P for treatment effect = .40 and .41, respectively).
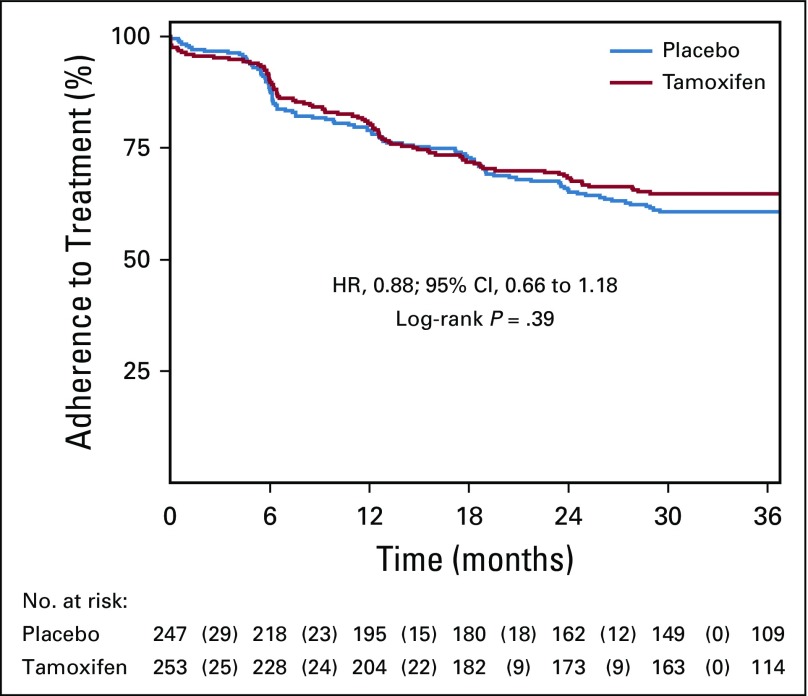
Appendix Figure A3 (online only) shows the time to first nonadherent study visit. Compliance with study therapy was defined as adherence (≥ 85% of pills) for the first 2.5 years of the study; 62.8% of patients overall met criteria for study compliance (65% in the tamoxifen arm and 61% in the placebo arm).
DISCUSSION
Treatment de-escalation is a leading concept of modern medical oncology, particularly in conditions such as noninvasive neoplasia where mortality reduction has not been demonstrated.2,19,20 We assessed the efficacy and safety of a lower dose of tamoxifen given for a shorter period in patients with preinvasive disorders of the breast who have a five to 10 times higher risk of invasive breast cancer.1,2 Our findings indicate that tamoxifen at 5 mg/d for 3 years can halve the recurrence of hormone-sensitive breast intraepithelial neoplasia without noticeable toxicity. Moreover, low-dose tamoxifen decreased by 75% the onset of a contralateral breast cancer, which indicates a potential primary preventive effect, and decreased invasive cancer by more than 40%, which prevents more-intensive adjuvant treatments. After a median of 5 years, serious adverse events were not significantly different from placebo. The relevance of the disease, the trial pragmatic approach that reflects standard clinical procedures, and the feasibility and inexpensiveness of the treatment make our findings generalizable and easily applicable in real-world clinical practice, including large fractions of underserved populations.
Our results are consistent with the effect of 20 mg/d tamoxifen in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-24 subgroup analysis of hormone-sensitive DCIS, where the HR was 0.58 (95% CI, 0.42 to 0.81).21 The similar antiproliferative activity of low-dose tamoxifen compared with the standard dose10 predicted the results of the present trial, confirming the reliability of the proliferation antigen protein encoded by the MKI67 gene as a surrogate biomarker of efficacy. Indeed, the change of protein encoded by the MKI67 gene after a few weeks of hormonal treatment predicted subsequent survival in window-of-opportunity, presurgical trials of breast cancer.22-24 Mortality was not an end point in our population at low risk for early death, where the cumulative risk of recurrence at 5 years in the placebo arm was only 11%. Nevertheless, our results indicate a 5-year number needed to treat of 22 and a likelihood of being helpful 10 times higher than being harmful, a favorable trade-off compared with the full dose.4,25,26
Drug-related serious adverse events were not increased during low-dose tamoxifen therapy. Specifically, there was no increase in deep vein thrombosis and pulmonary embolism. The risk of pulmonary emboli with the full dose of tamoxifen was threefold in the NSABP P-1 trial,5 and the odds ratio of venous thromboembolism in four prevention trials of full-dose tamoxifen was 1.60 (95% CI, 1.21 to 2.12).27,28 On the basis of these findings, we would expect 2.4 cases of deep vein thrombosis and pulmonary embolism with 20 mg/d in our study. In addition, we observed one stage I endometrial cancer in the tamoxifen arm, with a mean rate of 0.85 per 1,000 person-years. In the NSABP B-24 trial of DCIS,9 the risk of endometrial cancer was tripled compared with placebo (1.53 v 0.45 per 1,000 person-years). Likewise, in the NSABP P-1 trial,5 the rate of endometrial cancer was 2.24 per 1,000 person-years in the tamoxifen arm v 0.88 per 1,000 person-years in the placebo arm (rate ratio, 3.28), with a greater difference in women 50 years of age and older. The odds ratio of endometrial cancer with tamoxifen in the prevention trials was 2.18 (95% CI, 1.39 to 3.42).27 We would therefore expect 2.7 cases of endometrial cancer with 20 mg/d in our study, so our data indirectly suggest that the risk of deep vein thrombosis and pulmonary embolism or endometrial cancer is approximately 2.5 times lower with 5 mg/d than with 20 mg/d. Of note, we found no increase in endometrial polyps and a small change of endometrial thickness, two markers of increased endometrial proliferation that occur with 20 mg/d.29
Because menopausal symptoms, including vasomotor symptoms, sexual discomfort, and gynecologic problems have a profound effect on quality of life and adherence to tamoxifen, particularly in the prevention setting,6,7 we paid special attention to these participant-reported outcomes with the use of validated self-administered questionnaires at each visit. Our findings show that the frequency of hot flashes was 1.5-fold higher with tamoxifen compared with placebo. In addition, there was a higher proportion of physician-reported hot flashes with tamoxifen (14%) versus placebo (7%) as an adverse event. However, the hot flash score was not different between arms. The low toxicity of low-dose tamoxifen is confirmed by the same adherence in both arms compared with the full dose where adherence was 10% lower in the tamoxifen arm mostly because of menopausal symptoms.7 In our previous tamoxifen prevention trial of 20 mg/d, the rate of newly diagnosed hot flashes was 67 per 1,000 in the placebo arm and 119 per 1,000 in the tamoxifen arm (rate ratio, 1.78; 95% CI, 1.57 to 2.00).30 Of note, hot flashes have been associated with increased cardiovascular morbidity and mortality31 possibly because of decreased arterial dilation, so our findings with low-dose tamoxifen are encouraging. Other domains of the Breast Cancer Prevention Trial Symptom Scale were not affected by tamoxifen at a low dose, including pain with intercourse, vaginal dryness, and musculoskeletal pain and arthralgia, with the latter being the most frequent adverse effect of aromatase inhibitors. In contrast, the full dose is associated with at least a doubling of vasomotor symptoms, sexual problems, and other participant-reported outcomes compared with placebo.6,7,30,32 Of note, tamoxifen markedly reduced the incidence of headache similarly to the full dose.30
Study limitations include the lower-than-planned number of events, mostly as a result of the financial constraints of this academic, publicly funded trial and the early publication of results because of its practice-changing potential. However, the extended time of recruitment and follow-up and the higher-than-expected risk in the placebo arm made our study sufficiently powered to detect reliable treatment effect estimates without subgroup heterogeneity. The relatively low adherence is also a study weakness, which may have diluted treatment benefit.
The current formulation of tamoxifen on the market is 10- or 20-mg tablets, whereas the 5-mg tablet is not available. Until a new formulation is available, cutting the tablet into two or using 10 mg on alternate days may be reasonable given our prior data and the long half-life of tamoxifen.12,33
In conclusion, a lower dose of tamoxifen (5 mg/d) and a shorter duration of treatment (3 years) can halve the incidence of new breast neoplastic events in women with hormone-sensitive or unknown breast intraepithelial neoplasia. Drug-related toxicity, including venous thromboembolism, endometrial cancer, and menopausal symptoms, was only marginally different from placebo, which provides a new treatment option for women with these disorders.
Appendix
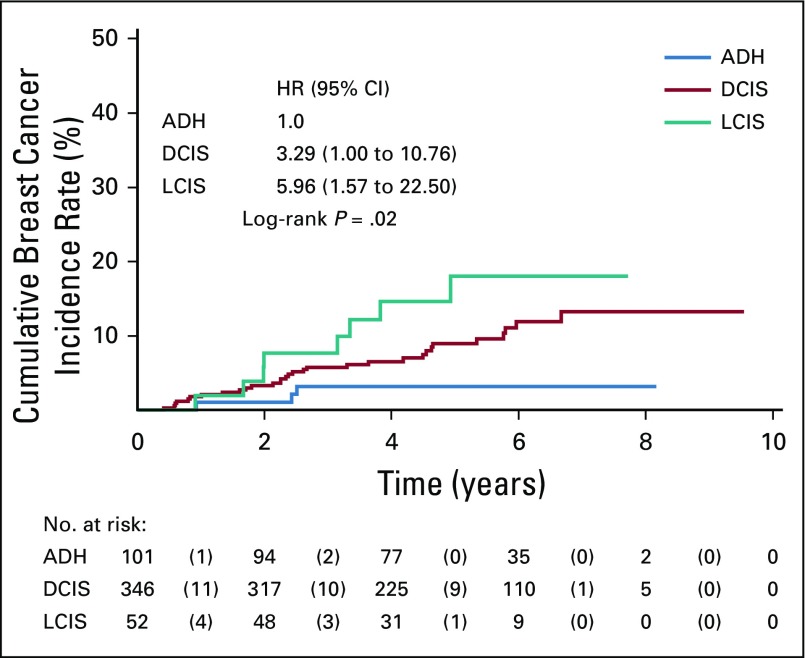
FIG A1.
Kaplan-Meier estimates of cumulative breast cancer incidence by histologic type (atypical ductal hyperplasia [ADH], lobular carcinoma in situ [LCIS], ductal carcinoma in situ [DCIS]) regardless of treatment with tamoxifen. Numbers in parentheses represent the number of events that occurred within each time interval by treatment arm. The log-rank test was used to draw inferences. The hazard ratios (HRs) for LCIS versus ADH and DCIS versus ADH were estimated from a Cox proportional hazards regression model adjusted for treatment arm; the HR of the treatment effect (data not shown), adjusted for the histologic type, was 0.48 (95% CI, 0.25 to 0.91). In the overall intention-to-treat population, there were 42 breast cancer events (three in the ADH group, 31 in the DCIS group, and eight in the LCIS group). The 5-year cumulative incidence rate of breast cancer was 3.2% (95% CI, 1.0% to 9.5%) in the ADH group, 9.0% (95% CI, 6.1% to 13.0%) in the DCIS group, and 18.0% (95% CI, 9.2% to 33.4%) in the LCIS group. The HRs compared with ADH are adjusted for treatment arm.
FIG A2.
Estimated marginal means of endometrial thickness (in millimeters) change in postmenopausal women during the double-blind treatment phase by allocated arm. Endometrial thickness in the tamoxifen arm was significantly increased compared with the placebo arm.
FIG A3.
Kaplan-Meier curve for time to first nonadherent study visit according to treatment arm. Numbers in parentheses represent the number of events that occurred within each time interval by treatment arm. The log-rank test was used to draw inferences. Overall, 62.8% of women were adherent for at least 2.5 years. The mean time on treatment was 2.3 years (standard deviation, 1.1). Kaplan-Meier estimate for adherence was not significantly different in women who took tamoxifen (64.8%) compared with those who took placebo (60.7%). Overall, dropout rates were highest within the first 6 months since randomization (10.3% in the tamoxifen arm; 12.2% in the placebo arm) and decreased thereafter. HR, hazard ratio.
TABLE A1.
Prespecified Subgroup Analyses
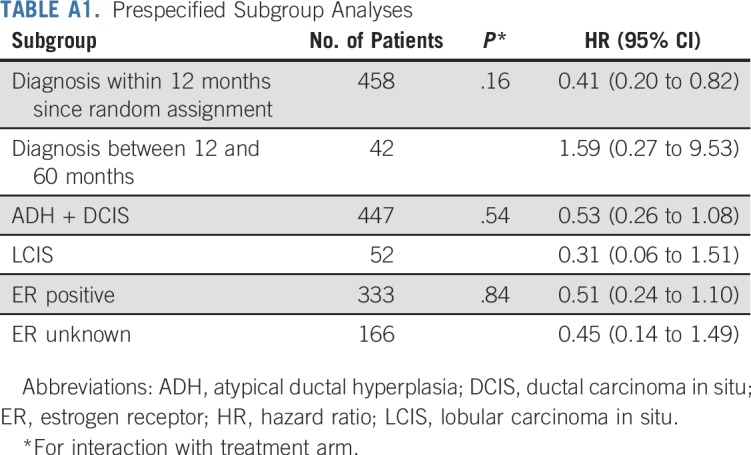
TABLE A2.
Nonserious Adverse Events
Footnotes
Presented at the San Antonio Breast Cancer Symposium 2018, San Antonio, TX, December 4-8, 2018.
Supported by the Italian Ministry of Health (RFPS-2006-1-339898), the Italian Association for Cancer Research (IG 2008 Grant No. 5611), and the Italian League against Cancer (LILT 7-08). These funding sources had no role in the design, execution, analyses, interpretation of the data, or decision to submit results.
Clinical trial information: EudraCT number 2007-007740-10 and NCT01357772.
See accompanying Editorial on page 1595
AUTHOR CONTRIBUTIONS
Conception and design: Andrea DeCensi, Matteo Puntoni, Aliana Guerrieri-Gonzaga, Luca Boni, Bernardo Bonanni
Financial support: Andrea DeCensi
Administrative support: Andrea DeCensi, Silvia Caviglia, Daniela Branchi, Sara Campora, Marilena Petrera, Tania Buttiron Webber
Provision of study material or patients: Andrea DeCensi, Franca Avino, Laura Cortesi, Cristiana Taverniti, Maria Grazia Pacquola, Fabio Falcini, Marcella Gulisano, Maria Digennaro, Anna Cariello, Katia Cagossi, Graziella Pinotti, Matteo Lazzeroni, Davide Serrano
Collection and assembly of data: Andrea DeCensi, Silvia Caviglia, Daniela Branchi, Sara Campora, Marilena Petrera, Tania Buttiron Webber
Data analysis and interpretation: Andrea DeCensi, Matteo Puntoni, Aliana Guerrieri-Gonzaga, Luca Boni, Bernardo Bonanni
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Local and Contralateral Recurrence in Breast Intraepithelial Neoplasia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Andrea DeCensi
Research Funding: Ente Ospedaliero Ospedali Galliera (Inst)
Laura Cortesi
Honoraria: AstraZeneca, Pfizer
Consulting or Advisory Role: Pfizer, Amgen, Novartis
Marcella Gulisano
Travel, Accommodations, Expenses: Roche, Pfizer, Eli Lilly, Angelini Pharma
Marilena Petrera
Employment: Chiesi Farmaceutici
Luca Boni
Patents, Royalties, Other Intellectual Property: International patent number PCT/EP2012/065661
No other potential conflicts of interest were reported.
REFERENCES
- 1.Lazzeroni M, Dunn BK, Pruneri G, et al. Adjuvant therapy in patients with ductal carcinoma in situ of the breast: The Pandora’s box. Cancer Treat Rev. 2017;55:1–9. doi: 10.1016/j.ctrv.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Merrill AL, Esserman L, Morrow M. Ductal carcinoma in situ. N Engl J Med. 2016;374:390–392. doi: 10.1056/NEJMclde1512213. [DOI] [PubMed] [Google Scholar]
- 3.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 5.Noonan S, Pasa A, Fontana V, et al. A survey among breast cancer specialists on the low uptake of therapeutic prevention with tamoxifen or raloxifene. Cancer Prev Res (Phila) 2018;11:38–43. doi: 10.1158/1940-6207.CAPR-17-0162. [DOI] [PubMed] [Google Scholar]
- 6.Land SR, Walcott FL, Liu Q, et al. Symptoms and QOL as predictors of chemoprevention adherence in NRG Oncology/NSABP Trial P-1. J Natl Cancer Inst. 2015;108:djv365. doi: 10.1093/jnci/djv365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith SG, Sestak I, Howell A, et al. Participant-reported symptoms and their effect on long-term adherence in the International Breast Cancer Intervention Study I (IBIS I) J Clin Oncol. 2017;35:2666–2673. doi: 10.1200/JCO.2016.71.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazzeroni M, DeCensi A. Alternate dosing schedules for cancer chemopreventive agents. Semin Oncol. 2016;43:116–122. doi: 10.1053/j.seminoncol.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Costantino JP, Redmond CK, et al. Endometrial cancer in tamoxifen-treated breast cancer patients: Findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86:527–537. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- 10.Decensi A, Robertson C, Viale G, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95:779–790. doi: 10.1093/jnci/95.11.779. [DOI] [PubMed] [Google Scholar]
- 11.Decensi A, Gandini S, Serrano D, et al. Randomized dose-ranging trial of tamoxifen at low doses in hormone replacement therapy users. J Clin Oncol. 2007;25:4201–4209. doi: 10.1200/JCO.2006.09.4318. [DOI] [PubMed] [Google Scholar]
- 12.Guerrieri-Gonzaga A, Sestak I, Lazzeroni M, et al. Benefit of low-dose tamoxifen in a large observational cohort of high risk ER positive breast DCIS. Int J Cancer. 2016;139:2127–2134. doi: 10.1002/ijc.30254. [DOI] [PubMed] [Google Scholar]
- 13.Sloan JA, Loprinzi CL, Novotny PJ, et al. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 14.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: A measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97:448–456. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 15.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 16.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J Clin Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 17.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 18.Citrome L, Ketter TA. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract. 2013;67:407–411. doi: 10.1111/ijcp.12142. [DOI] [PubMed] [Google Scholar]
- 19.Narod SA, Iqbal J, Giannakeas V, et al. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1:888–896. doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
- 20.Benson JR, Jatoi I, Toi M. Treatment of low-risk ductal carcinoma in situ: Is nothing better than something? Lancet Oncol. 2016;17:e442–e451. doi: 10.1016/S1470-2045(16)30367-9. [DOI] [PubMed] [Google Scholar]
- 21.Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: A study based on NSABP protocol B-24. J Clin Oncol. 2012;30:1268–1273. doi: 10.1200/JCO.2010.34.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeCensi A, Guerrieri-Gonzaga A, Gandini S, et al. Prognostic significance of Ki-67 labeling index after short-term presurgical tamoxifen in women with ER-positive breast cancer. Ann Oncol. 2011;22:582–587. doi: 10.1093/annonc/mdq427. [DOI] [PubMed] [Google Scholar]
- 23.Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99:167–170. doi: 10.1093/jnci/djk020. [DOI] [PubMed] [Google Scholar]
- 24.Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100:1380–1388. doi: 10.1093/jnci/djn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31:2942–2962. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 26.Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327–2333. doi: 10.1200/JCO.2010.33.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuzick J, Sestak I, Bonanni B, et al. Selective oestrogen receptor modulators in prevention of breast cancer: An updated meta-analysis of individual participant data. Lancet. 2013;381:1827–1834. doi: 10.1016/S0140-6736(13)60140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Decensi A, Maisonneuve P, Rotmensz N, et al. Effect of tamoxifen on venous thromboembolic events in a breast cancer prevention trial. Circulation. 2005;111:650–656. doi: 10.1161/01.CIR.0000154545.84124.AC. [DOI] [PubMed] [Google Scholar]
- 29. doi: 10.1016/j.ajog.2004.12.083. Chalas E, Costantino JP, Wickerham DL, et al: Benign gynecologic conditions among participants in the Breast Cancer Prevention Trial. Am J Obstet Gynecol 192:1230-1237, 2005; discussion 1237-1239. [DOI] [PubMed] [Google Scholar]
- 30.Veronesi U, Maisonneuve P, Rotmensz N, et al. Tamoxifen for the prevention of breast cancer: Late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. 2007;99:727–737. doi: 10.1093/jnci/djk154. [DOI] [PubMed] [Google Scholar]
- 31.Thurston RC, Johnson BD, Shufelt CL, et al. Menopausal symptoms and cardiovascular disease mortality in the Women’s Ischemia Syndrome Evaluation (WISE) Menopause. 2017;24:126–132. doi: 10.1097/GME.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day R, Ganz PA, Costantino JP, et al. Health-related quality of life and tamoxifen in breast cancer prevention: A report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17:2659–2669. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 33.Guerrieri-Gonzaga A, Baglietto L, Johansson H, et al. Correlation between tamoxifen elimination and biomarker recovery in a primary prevention trial. Cancer Epidemiol Biomarkers Prev. 2001;10:967–970. [PubMed] [Google Scholar]