Abstract
Outer hair cells (OHCs) of the mammalian cochlea behave like actuators: they feed energy into the cochlear partition and determine the overall mechanics of hearing. They do this by generating voltage-dependent axial forces. The resulting change in the cell length, observed by microscopy, has been termed “electromotility.” The mechanism of force generation OHCs can be traced to a specific protein, prestin, a member of a superfamily SLC26 of transporters. This short review will identify some of the more recent findings on prestin. Although the tertiary structure of prestin has yet to be determined, results from the presence of its homologs in nonmammalian species suggest a possible conformation in mammalian OHCs, how it can act like a transport protein, and how it may have evolved.
The outer hair cells (OHCs) of the mammalian cochlea are an identifiable group of cells of the inner ear, which are responsible for many of the distinct features of our hearing (Fig. 1). These features include absolute sensitivity to low sound pressure levels, selectivity to frequencies over many octaves, and the dependence of cochlear performance on physiological status. These properties have collectively defined a process known as “cochlear amplification.” Although other orders of terrestrial animals also have cells homologous to the mammalian OHCs, it seems that the evolution of hearing organs has taken slightly different routes in different species. This review will focus specifically on mammalian OHCs, their loss or functional failure being a major cause of hearing deficiency, including that caused by age. Thus, although hair cells in all vertebrate species share common molecular features (see Köppl and Manley 2018), there have been particular specializations in mammalian hearing to enable and favor high-frequency hearing. Although several extensive reviews have been published (Ashmore 2008; He et al. 2014; Corey et al. 2017; Santos-Sacchi et al. 2017), the emphasis here will be on a number of outstanding issues.
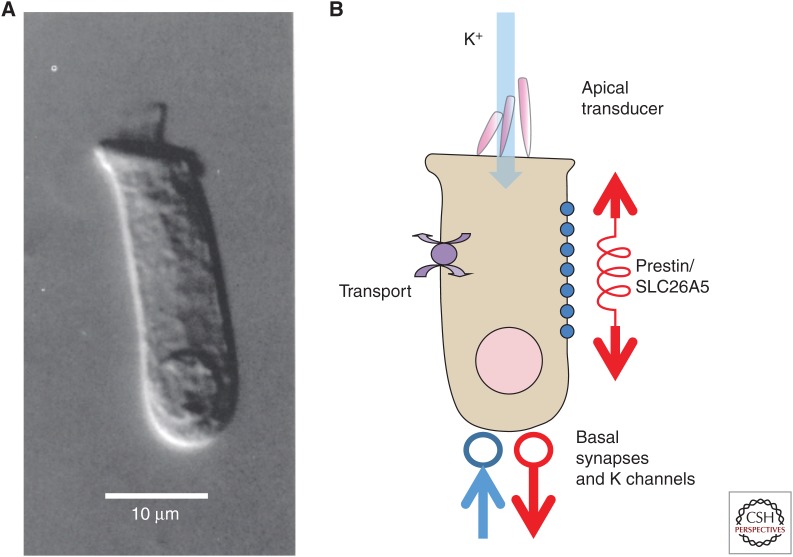
Figure 1.
The elements of the mammalian outer hair cell (OHC). (A) An isolated OHC from turn 2 (∼5–10 kHz region) of the guinea pig cochlea. The apical stereocilia are apparent at the apical surface. (B) Schematic OHC showing the location of prestin/SLC26A5 down the basolateral surface, generating longitudinal forces. Transport to regulate pHi is presumed to be collocated as a parallel property of prestin. K+ ions from scala media enter through the mechanoelectric transducer channels at the apex and exit through the basally located K+ channels. Both afferent (red) and efferent (blue) terminals are located at the base of the cell.
ELECTROMOTILITY
The OHCs of the organ of Corti, the neurosensory epithelium of the mammalian cochlea, are organized in three (and sometimes four) rows along the full length of the cochlear partition. Viewing the cochlear partition in cross section suggests that OHCs are placed in a position to have the maximal mechanical influence on the flexure of the basilar membrane.
The original observation that OHCs were cells that were “motile” was made by Brownell and his coworkers in Geneva (Brownell et al. 1985). They showed, using intracellular recording electrodes, that the cells could be driven to change length when the membrane potential was changed. The term “electromotility” was used as shorthand to describe this behavior and has stuck, even though it might imply that “motile” means that the cells are migrating somewhere. It required the widest electrical bandwidth possible with patch clamp recording to show that such length changes were rapid and certainly fast enough to claim that OHCs could be force-generating elements and are involved in shaping cochlear mechanics at acoustic frequencies (Ashmore 1987). Since then, a variety of different biophysical techniques have shown that the electromotile mechanism can be driven over the full range of frequencies known to be encoded by most mammalian hearing organs, and certainly up to 80 kHz (Frank et al. 1999).
It is strictly more accurate to describe the OHC as an “actuator” as the source of the energy because the force generation derives from the potential across the mechanoelectrical transducer channels: it is not produced seemingly from energy sources within the cell. A back-of-the envelope calculation using the stiffness of the cell shows that the maintenance of the cell membrane potential at −50 mV allows sufficient extraction of energy from the electric field to explain the work done by the OHC against the mechanical constraints of the tissue in which they are embedded (Dallos 1991).
Mammalian OHCs possess a very characteristic V-shaped bundle of stereocilia, serving to inject current into the cells when deflected. As sensors of the movement of the basilar membrane, OHCs thus form part of a local mechanical-electrical-mechanical feedback loop to control basilar membrane tuning. The intimate role of OHCs in such feedback has meant that their function in the in vivo cochlea often has to be interpreted by appeal to cochlear models. When studied in isolation a property such as electromotility gives an exaggerated impression of the length change that an OHC may exhibit in a fully functioning cochlea. In vivo, when cells are constrained by their surrounding cells, they operate more like force-generating elements.
A number of other features of OHCs should also be considered when discussing electromotility. First, although they are neuroepithelial cells specialized so that the apical surface facing scala media is mechanosensitive and the basal surface contains synaptic machinery, OHCs are relatively sparsely innervated by afferent fibers in comparison to inner hair cells. It is clear that OHCs can generate activity in the type II auditory nerve fibers (Weisz et al. 2009), but this information may be used in a very different way from the major sensory pathways from the sharply tuned type I fibers forming the majority of the auditory nerve. Second, OHCs are the target of a descending pathway, the fibers of the medial olivocochlear bundle. These fibers, releasing acetylcholine (ACh), activate heteromeric α9/10 AChRs on OHCs and serve to control the membrane conductance of the cell (to K+). The net effect is to both hyperpolarize the cells and to reduce the amplitude of the receptor potentials in the cell and thus to reduce the mechanical OHC loop gain and overall cochlear sensitivity. The role of the efferent system is contentious but its effect on mechanical tuning (Murugasu and Russell 1996) and distortion product emissions (e.g., Maison et al. 2007) is clear. There is also the suggestion, from studies in a mouse where the efferent cholinergic receptor had been deleted, that the efferent system activity might even slow auditory aging (Liberman et al. 2014).
IDENTIFICATION OF THE MECHANISM
A number of hypotheses could explain the phenomenon of OHC electromotility. There are several constraining observations: (1) the lateral membrane of the OHC is packed with a particle about 8 nm in diameter; (2) a change in the OHC membrane potential is accompanied by a gating charge movement, or equivalently the cell membrane capacitance is voltage dependent; (3) the charge movement is blocked by the amphiphilic anion salicylate (aspirin, with an additional methyl group has the same effect); and (4) OHCs only acquire motile properties progressively during a short period of development.
The identification of prestin (Zheng et al. 2000) by using a subtracted complementary DNA (cDNA) library prepared from the messenger RNA (mRNA) of isolated hair cells in principle solves most of these problems. Mammalian prestin is a 744 amino-acid protein with a predicted molecular mass of 81 kDa, and when it is expressed in heterologous cells they exhibit voltage-dependent movements and a nonlinear capacitance (NLC), which are indicative of protein rearrangements when under the influence of the membrane potential. The surprise is that prestin is member of a superfamily of membrane transporters SLC26A5, a family whose other members are chloride-bicarbonate exchangers (Fig. 2) (Lohi et al. 2000).
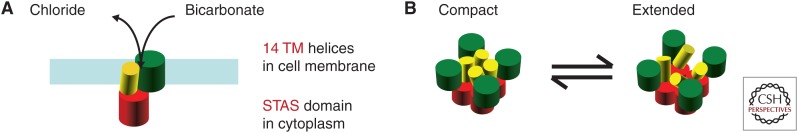
Figure 2.
Models for prestin. (A) Organization of prestin/SLC26A5 in the membrane as a monomer. Two mobile components of the protein are placed in the membrane (Gorbunov et al. 2014; Geertsma et al. 2015), whereas the carboxy-terminal region, containing the sulfate transporter and anti-sigma factor antagonist (STAS) domain is cytoplasmic (Mio et al. 2008). (B) Hypothetical model for coassembly into a tetramer. The carboxy-terminal region (red) forms a base against which the oligomer can deform, rapidly shifting between a compact (cell depolarized) and an extended configuration (cell hyperpolarized).
There are some differences in the behavior of the NLC when it is studied in heterologous cells (e.g., HEK293, TSA201, or CHO cells) and in isolated OHCs ex vivo. The difference seems to depend on the molecular packing density: in cell systems where the prestin is expressed at a relatively low level (e.g., giving rise to a maximum NLC of 0.4 pF) corresponding to a copy number of about 2.5 × 105 prestin molecules/cell (equivalent to ∼100 prestins/μm2), the peak capacitance is close to −75 mV (Oliver et al. 2001); in OHCs, the voltage at the peak progressively increases during maturation of the cell to reach a steady state at −40 mV in the mouse at P12, where the density in the OHC membrane is estimated to be about 4000 prestins /µm2 (Oliver and Fakler 1999).
AN INCOMPLETE TRANSPORTER
How a transport protein could give rise to a motor was solved soon after the discovery of prestin with the proposal that prestin/SLC26A5 was an incomplete transporter (Oliver et al. 2001). The idea is that the protein acts like a mechanoezyme as part of its transport cycle, but that the cycle is incomplete. As a result of binding of an intracellular anion (choride) the conformational change in the protein is sufficient to produce a length change in the plane of the membrane and hence in the length of the OHC. The maximum electromotile change in an OHC is 4%, so the change in the particle diameter would be 0.04 × 8 nm = 0.3 nm, not impossibly small but close to the limit currently observable by structural techniques such as x-ray crystallography or cryoelectron microscopy.
ANTIPORTER ACTIVITY
Prestin homologs have been detected in every vertebrate hair cell wherever they have been looked for (He et al. 2014). None of the nonmammalian cells, however, show the same degree of electromotility as OHCs, although chick hair cells exhibit a limited degree of motility (Beurg et al. 2013). In chick and in zebrafish, the prestin homologs clearly act like transporters for anions (Schaechinger and Oliver 2007). In both these species, although in different orders, transport is electrogenic, with two bicarbonates being exchanged for one chloride and currents are readily observable. The transport can be detected as an electrogenic a SO42−: Cl− antiporter (the doubly charged sulfate ion being transported in place of two bicarbonates) (Schaechinger and Oliver 2007). Even though mammalian prestin/SLC26A5 resembles an antiporter for chloride and bicarbonate, it has proved more difficult to show that any transport by the protein does occur.
If other anions are used instead of chloride, then transport can be measured either by electrical methods or by using the classical methods of radioactive ion uptake. Thus, cells transfected with mammalian prestin take up 14C-formate as an ion, but at a rate that is only weakly inhibited by 10 mm salicylate, the inhibitor of NLC (Bai et al. 2009).
Electrophysiological studies have shown that mammalian prestin/SLC26A5 is electrogenic. Using similar methods as those used by Schaechinger and Oliver, exposure of prestin expressing CHO cells to a low chloride–high bicarbonate gradient produces a change in the membrane potential compatible with a 2:1 HCO3−: Cl− stoichiometry (Mistrik et al. 2012). It can also be shown, using a form of prestin tagged with the fluorescent pH sensor pHluorin to monitor the influx near the membrane, that the presence of prestin in the cells allows for a faster recovery from an acid load by allowing an influx of bicarbonate.
Although comparable experiments have not been completed fully to monitor choride, a movement of chloride movement can be detected using yellow fluorescent protein (YFP), which is chloride sensitive, when the cells are exposed to low (6 mm) chloride and high (23 mm) bicarbonate on the outside favoring exchange. An anion transport current can be detected, however, with the much more permeant anion thiocyanate, SCN− (Schanzler and Fahlke 2012). Both SCN− and Cl− give rise to comparable NLC in transfected HEK293 cells, although replacing external Cl− with SCN− shifts the peak of the NLC curve more negatively from −69 mV (typical of a cell expression system) to −102 mV. The authors conclude, however, that there is a transport pathway for anions and, on the basis of parallel studies with zSLC26A5 (from zebrafish) and hSLC26A7 (from human), a pathway that may be conserved between many members of the SLC26 family.
Although SCN− is used as permeant anion in many transport studies, its transport pathway in prestin may differ from that of chloride (Bai et al. 2017). In a tet-inducible, prestin-expressing human embryonic kidney (HEK) cell line, which shows a large NLC, a current carried by chloride is also present whenever the NLC can be measured. Such observations only become apparent with high expression levels, and so possibly have been missed in early experiments (where the peak NLC was 10%–15% of what can now be achieved). The evidence presented supports the idea that chloride is able to pass through a separate pathway intimately linked to the NLC, and most probably linked to a stretch-sensitive chloride channel reported for prestin (Rybalchenko and Santos-Sacchi 2003). Other members of the SLC26 family (SLC26A3 and SLC26A6), as well as some ion channels (manifesting as a so-called omega or gating-pore currents [Moreau et al. 2015]), also show evidence for a similar sensitive leakage current. Whether this influences the gain and function of OHCs in cochlea mechanics is undecided, but it does suggest that there may be several osmoregulating mechanisms present in the cell.
PHARMACOLOGY
The pharmacological manipulations of prestin have suffered from a shortage of reagents. The best known is salicylate, which is effective at mm levels from the extracellular surface, although the binding site is thought to be on the cytoplasmic surface. Ingestion of aspirin is known to (reversibly) elevate auditory thresholds (and lead to tinnitus as a consequence). As a competitive antagonist to chloride, the dissociation constant of salicylate is estimated from the NLC to be KD = 21 µm. (Oliver et al. 2001). Nonsteroidal anti-inflammatories have little or no effect.
GENETICS: MOUSE HEARING
In mouse, there is ample evidence that prestin determines cochlear sensitivity. The first knockouts of prestin clearly showed that OHCs isolated from these mice are nonmotile and that there is a 40–60 dB loss of cochlear sensitivity in vivo (Liberman et al. 2002). A cochlear microphonic could still be measured so the OHC transduction was not affected, although there appeared to be a progressive apoptosis of the cells. A more direct measurement of basilar membrane motion in the hook region (60–70 kHz) of the cochlea also showed that the sharp mechanical tuning characteristic of the wild types was absent in the prestin knockout mouse but, curiously, did not show a significant threshold loss (Mellado Lagarde et al. 2008). The possible explanation is that prestin contributes to the stiffness of the OHCs, and that removal of prestin alters the coupling of sound to basilar membrane mechanics.
The issue of possible changes in the mechanical properties of the OHCs can be circumvented by using a prestin mutant mouse where two residues are substituted near the presumed last transmembrane helix (V499G and Y501H), yielding OHCs that are structurally and biophysically near normal but have an NLC shifted very positively and outside the physiological operating range (Dallos et al. 2008). In this mutant indeed, the basilar membrane is no longer sharply tuned and there is a reduction in both frequency selectivity and cochlear sensitivity, which are the major consequences with no assumed alterations in cochlear impedance matching (Weddell et al. 2011).
GENETICS: HUMAN HEARING
There has been some dispute about the prevalence of prestin/SLC26A5 mutations in humans. An early report suggested that a single nucleotide splice acceptor site mutation in the second intron of SLAC26A5 was responsible for a hearing deficit at the recessive DFNB61 locus (Liu et al. 2003). However, it was subsequently argued that this variation occurs no more frequently in the hearing impaired than in controls and therefore precludes a causative role (Tang et al. 2005).
To date, therefore, human prestin mutations appear quite rare. A single case report identifies two profoundly deaf sisters with compound heterozygote mutations, one (p.W70X) in the amino-terminal region, which is expected to inactivate the protein, and the other (p.R130S), which is within the sulfate transport motif of the SLC26 genes (Matsunaga and Morimoto 2016). In all other respects, the subjects were found to be normal. In a transgenic mouse model for the latter mutation membrane, targeting is impaired, kinetics are slowed (producing a reduced NLC at the measurement frequencies), and transport for thiocyanate is enhanced (Takahashi et al. 2016). Apart from one other candidate mutation reported (p.R150Q) (Toth et al. 2007), the p.W70X and the p.R130S are the only deafness-causing mutations identified so far.
EVOLUTIONARY ASPECTS OF PRESTIN
Is mammalian prestin really a modified transporter? SLC26A5 homologs have been found in a remarkably wide range of sensory hair cells both in chordates (He et al. 2014) as well as in invertebrates, for example, in the hearing organs of Drosophila melanogaster (Kavlie et al. 2015). The conservation of SLAC26A5 suggests that it has a particular role that has been conserved through evolutionary changes and one suggestion is that it might play a role in controlling cellular pH or perhaps in the osmotic regulation of these cells.
There have been relatively few reports that SLC26A5 gives rise to any form of hair cell motility except in the mammals. Where they have been studied biophysically, these prestin homologs in other species either seem to exhibit significantly reduced NLC at acoustic frequencies, as for zebrafish prestin (Schaechinger and Oliver 2007), and/or are inserted into the membrane at much lower levels than could generate motility, as in chick hair cells (Beurg et al. 2013). What many of these homologs do exhibit, however, is ion transport.
As sequence data for the SLC26 super-family became more common, and for SLC26A5 in particular, bioinformatics revealed that there have been some significant evolutionary changes. The main differences between mammalian and nonmammalian prestin are found in the cytoplasmic portion of the resulting protein, rather than in those regions assumed to form the transmembrane loops (Franchini and Elgoyhen 2006). Predictions of adaptive changes in such genes are based on whether there is a sufficiently large synonymous versus nonsynonymous ratio; by the same measure, it also appears that the ACh α9 receptor, found in OHCs, has also undergone adaptive evolution in mammals, whereas pendrin (SLC26A4), also implicated in cochlear function, has not. It is not immediately clear functionally what such changes in mammalian prestin indicate, although it is tempting to speculate that it is the modification to the carboxy-terminal “sulfate transporter and anti-omega factor antagonist” (STAS) domain that confers a special role.
Within mammals there are examples of adaptive convergence in the auditory system. Some species of bat and all the toothed whales use echolocation to find prey. All of these species use ultrasonic sounds with a matched requirement to be able to detect such frequencies. Remarkably both dolphin and Doppler-sensitive CF bats exhibit what appears to be an example of convergent molecular evolution of prestin, even though these animals have been separated phylogenetically for many tens of millions of years (Liu et al. 2010). Four of these specific convergent amino acid sites (G167S, I348T, A565S, and E700D) are of particular interest as the latter two are in the STAS domain, suggesting this domain, which may play a special role in the function of prestin.
STRUCTURE
The structure of prestin remains unresolved. The original sequence data suggested that the molecule had 12 transmembrane regions (Zheng et al. 2000; Oliver et al. 2001). Other proposals, including a model with 10 transmembrane regions have been made (Bai et al. 2009). The current best predictions for the structure, based on homology modeling with the uracil transport family, suggest that there are instead 14 transmembrane domains with a duplication of a 7 transmembrane motif (Gorbunov et al. 2014).
Because the size of the particle in the OHC lateral membrane is 8 nm in diameter, it is thus highly unlikely that prestin functions as a monomer, but is oligomeric, most probably a tetramer. Some compelling evidence for this conclusion comes from single-molecule photobleaching experiments. Successive bleaching of green fluorescent protein (GFP) tagged prestin expressed in HEK reveals a series of equal amplitude steps that is most consistent with a tetrameric structure (Hallworth and Nichols 2012). These results are compatible with earlier results that suggest that prestin, at least when expressed in mammalian cell lines, acts as a high-order oligomer based on a stable dimer formed by disulfide bonds between single molecules (Zheng et al. 2006).
The amino acid sequence of prestin also predicts a cytoplasmic carboxy-terminal region, accounting for nearly 30% of the peptide, which contains a distinguishing STAS domain in common with other members of the SLC26 anion transporters. The role of this domain is unclear. The crystal structure of the cytoplasmic domain is known both in mammals and in chick and there are subtle differences. The mammalian domain contains an unanticipated anion binding site that may act as a reservoir for anions involved in the rapid conformational changes. In distinction, the chick homolog contains no such site.
Although there is not a full structure, with transmembrane and STAS units together, for mammalian prestin, there is for a bacterial member of the SLC26 family. The structure at 3.2 Å resolution of SLC26Dg, the facilitator (via bicarbonate) of the proton-coupled fumarate symporter from the bacterium Deinococcus gethermalis reveals that this molecule forms an obligate dimer (Geertsma et al. 2015). The dimeric feature is shared by all members of the SLC26 family and the NLC evidence based on independent manipulation of the two components suggest that the two units do not function independently (Detro-Dassen et al. 2008). Thus, structural studies need to identify the nature of interface between the component proteins.
In support of this proposal, the most complete image of the mammalian prestin is obtained by negative staining of prestin particles and reconstruction with a fourfold symmetry at a resolution of 2 nm (Mio et al. 2008). The size of the resulting particle is compatible with the images obtained by electron microscopy of the OHC lateral membrane, but surprisingly about 60% of the particle mass appears within the cell on the cytoplasmic surface of the membrane, suggesting this is where the STAS domains are assembled. It is therefore tempting to suggest that prestin is a dimer of dimers, with the cytoplasmic STAS domain region perhaps forming a plate against which the membrane-bound units distort.
ORGANIZATION OF PRESTIN IN THE LATERAL MEMBRANE
The high density of prestin in the OHC lateral membrane is quite surprising. The packing is tight so that the lateral membrane particulate density covers about 60% of the surface. This high packing ensures that such molecular crowding amplifies up any small changes in surface area of the protein. Even so, to act as a force-generating element, it is necessary to ensure that the membrane does not buckle, thereby allowing all force to be generated within the plane of the membrane. OHCs have evolved a submembranous cortical network to ensure that the membrane retains a degree of rigidity (Kalinec et al. 1992).
The question of how membrane-associated prestin links to the underlying cytoskeleton remains unresolved. The spacing between the plasma membrane and the cytoskeleton is about 50 nm and, although structures linking the two are often termed “pillars” and apparent in electron micrographs (Holley et al. 1992), the nature of the linkage is unknown. The puzzle is that the prestin-related particles outnumber the pillars by an order of magnitude. A particular form of spectrin, βV, forms cytoskeletal meshwork with actin, and further builds up as prestin levels rapidly increase during early postnatal development (Legendre et al. 2008). Although spectrin βV interacts directly with F-actin and band 4.1, it does not interact with prestin. Interestingly, an unidentified component in lysates derived from mature auditory organs does promote a prestin–spectrin interaction, whereas lysates from other tissues do not. It has been suggested that the spectrin βV isoform, like that of prestin itself, has undergone adaptive evolution from a form involved in a molecular trafficking network, to one that allows it to provide the molecular support for the OHC mechanisms (Cortese et al. 2017).
CONCLUDING REMARKS
There is sometimes a perception that we now have a molecule that can be assigned the role of a molecular motor that drives electromotility in OHCs, and that all is solved. Although it is true that many of the low hanging fruits have been picked, there remain a number of outstanding questions.
The first is the so-called RC time constant problem that arises because the membrane of an OHC has an electrical filtering effect on any changes in the membrane potential driving the prestin. Although many ingenious solutions have been proposed (see Corey et al. 2017), it has proved remarkably difficult technically to study OHCs from the basal high-frequency end of the cochlea. Not only are electrical recording bandwidths limited (for patch clamp systems to below 10 kHz), but basal hair cells have proved difficult to handle. A partial answer, however, may be that basal hair cells have a high ionic channel density, mainly of the Kv7.4 channel, and therefore a small electrical time constant (Johnson et al. 2011). It also seems likely that OHCs are not driven exclusively by intracellular potentials, but by transmembrane potential (Mistrik et al. 2009) and so the space around the OHCs in the organ of Corti becomes an essential aspect of their function.
The molecular basis for the actuator behavior of prestin/SLC26A5 remains unclear, although inasmuch as all transporters can be considered as mechanoenzymes, a conformational change of the molecule with a component acting in the plane of the membrane seems to be the correct starting point. The reason for the current uncertainty is that the molecular structure of prestin remains unresolved. It clearly seems to form a tetrameric molecule in the membrane, but how it is trafficked and how the interfaces between the monomers are formed has so far not been addressed. A number of studies have endeavored to identify the “motor” motif by swapping motifs in prestin. A span of 11 amino acids (158–168) near the sulfate transport motif swapped into nonmammalian orthologs is sufficient to confer NLC and motor properties in cells transfected with such modified proteins (Tan et al. 2012). Further chimeric prestins have been constructed, containing sequences from both mammalian and nonmammalian prestins, which show both characteristics of NLC and of transport (Schaechinger et al. 2011).
A number of fundamental biophysical challenges in cochlear neurobiology arise from hearing performance at the upper end of the auditory range. What sets the upper limit to mammalian hearing? Is it the maximum operating frequency of the hair cell transduction channels? Or is it a structural problem of how to evolve a sufficiently high-frequency cochlea? Or is it that the feedback loop we have discussed above has an inherent bandwidth, traceable in part to the properties of prestin/SLC26A5? Some of the answers depend on developing tools to explore ultrafast structural processes. For that, the prestin/SLC26A5 system provides a useful laboratory.
ACKNOWLEDGMENTS
The work is supported by grants from the Medical Research Council (MRC) (MR/K015826), Biotechnology and Biological Sciences Research Council (BBSRC) (BB/M00659), and the Royal Society.
Footnotes
Editors: Guy P. Richardson and Christine Petit
Additional Perspectives on Function and Dysfunction of the Cochlea available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Ashmore JF. 1987. A fast motile response in guinea-pig outer hair cells: The cellular basis of the cochlear amplifier. J Physiol 388: 323–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. 2008. Cochlear outer hair cell motility. Physiol Rev 88: 173–210. [DOI] [PubMed] [Google Scholar]
- Bai JP, Surguchev A, Montoya S, Aronson PS, Santos-Sacchi J, Navaratnam D. 2009. Prestin’s anion transport and voltage-sensing capabilities are independent. Biophys J 96: 3179–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai JP, Moeini-Naghani I, Zhong S, Li FY, Bian S, Sigworth FJ, Santos-Sacchi J, Navaratnam D. 2017. Current carried by the Slc26 family member prestin does not flow through the transporter pathway. Sci Rep 7: 46619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Tan X, Fettiplace R. 2013. A prestin motor in chicken auditory hair cells: Active force generation in a nonmammalian species. Neuron 79: 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. 1985. Evoked mechanical responses of isolated cochlear outer hair cells. Science 227: 194–196. [DOI] [PubMed] [Google Scholar]
- Corey DP, O Maoleidigh D, Ashmore JF. 2017. Mechanical transduction processes in the hair cell. In Understanding the cochlea (ed. Manley GA, et al. ), pp. 75–111. Springer, New York. [Google Scholar]
- Cortese M, Papal S, Pisciottano F, Elgoyhen AB, Hardelin JP, Petit C, Franchini LF, El-Amraoui A. 2017. Spectrin βV adaptive mutations and changes in subcellular location correlate with emergence of hair cell electromotility in mammalians. Proc Natl Acad Sci 114: 2054–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. 1991. Neurobiology of cochlear hair cells. In Auditory physiology and perception (ed. Cazals Y, Demany L, Horner K), pp. 3–17. Pergamon, Oxford. [Google Scholar]
- Dallos P, Wu X, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, et al. 2008. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron 58: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detro-Dassen S, Schanzler M, Lauks H, Martin I, zu Berstenhorst SM, Nothmann D, Torres-Salazar D, Hidalgo P, Schmalzing G, Fahlke C. 2008. Conserved dimeric subunit stoichiometry of SLC26 multifunctional anion exchangers. J Biol Chem 283: 4177–4188. [DOI] [PubMed] [Google Scholar]
- Franchini LF, Elgoyhen AB. 2006. Adaptive evolution in mammalian proteins involved in cochlear outer hair cell electromotility. Mol Phylogenet Evol 41: 622–635. [DOI] [PubMed] [Google Scholar]
- Frank G, Hemmert W, Gummer AW. 1999. Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proc Natl Acad Sci 96: 4420–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geertsma ER, Chang YN, Shaik FR, Neldner Y, Pardon E, Steyaert J, Dutzler R. 2015. Structure of a prokaryotic fumarate transporter reveals the architecture of the SLC26 family. Nat Struct Mol Biol 22: 803–808. [DOI] [PubMed] [Google Scholar]
- Gorbunov D, Sturlese M, Nies F, Kluge M, Bellanda M, Battistutta R, Oliver D. 2014. Molecular architecture and the structural basis for anion interaction in prestin and SLC26 transporters. Nat Commun 5: 3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallworth R, Nichols MG. 2012. Prestin in HEK cells is an obligate tetramer. J Neurophysiol 107: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DZ, Lovas S, Ai Y, Li Y, Beisel KW. 2014. Prestin at year 14: Progress and prospect. Hear Res 311: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley MC, Kalinec F, Kachar B. 1992. Structure of the cortical cytoskeleton in mammalian outer hair cells. J Cell Sci 102: 569–580. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Beurg M, Marcotti W, Fettiplace R. 2011. Prestin-driven cochlear amplification is not limited by the outer hair cell membrane time constant. Neuron 70: 1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec F, Holley MC, Iwasa KH, Lim DJ, Kachar B. 1992. A membrane-based force generation mechanism in auditory sensory cells. Proc Natl Acad Sci 89: 8671–8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlie RG, Fritz JL, Nies F, Gopfert MC, Oliver D, Albert JT, Eberl DF. 2015. Prestin is an anion transporter dispensable for mechanical feedback amplification in Drosophila hearing. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 201: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Köppl C, Manley GA. 2018. A functional perspective on the evolution of the cochlea. Cold Spring Harb Perspect Med 10.1101/cshperspect.a033241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre K, Safieddine S, Kussel-Andermann P, Petit C, El-Amraoui A. 2008. αII-βV spectrin bridges the plasma membrane and cortical lattice in the lateral wall of the auditory outer hair cells. J Cell Sci 121: 3347–3356. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. 2002. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 419: 300–304. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Liberman LD, Maison SF. 2014. Efferent feedback slows cochlear aging. J Neurosci 34: 4599–4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Ouyang XM, Xia XJ, Zheng J, Pandya A, Li F, Du LL, Welch KO, Petit C, Smith RJ, et al. 2003. Prestin, a cochlear motor protein, is defective in non-syndromic hearing loss. Hum Mol Genet 12: 1155–1162. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cotton JA, Shen B, Han X, Rossiter SJ, Zhang S. 2010. Convergent sequence evolution between echolocating bats and dolphins. Curr Biol 20: R53–R54. [DOI] [PubMed] [Google Scholar]
- Lohi H, Kujala M, Kerkela E, Saarialho-Kere U, Kestila M, Kere J. 2000. Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics 70: 102–112. [DOI] [PubMed] [Google Scholar]
- Maison SF, Parker LL, Young L, Adelman JP, Zuo J, Liberman MC. 2007. Overexpression of SK2 channels enhances efferent suppression of cochlear responses without enhancing noise resistance. J Neurophysiol 97: 2930–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga T, Morimoto N. 2016. The auditory phenotype of children harboring mutations in the prestin gene. Acta Otolaryngol 136: 397–401. [DOI] [PubMed] [Google Scholar]
- Mellado Lagarde MM, Drexl M, Lukashkin AN, Zuo J, Russell IJ. 2008. Prestin’s role in cochlear frequency tuning and transmission of mechanical responses to neural excitation. Curr Biol 18: 200–202. [DOI] [PubMed] [Google Scholar]
- Mio K, Kubo Y, Ogura T, Yamamoto T, Arisaka F, Sato C. 2008. The motor protein prestin is a bullet-shaped molecule with inner cavities. J Biol Chem 283: 1137–1145. [DOI] [PubMed] [Google Scholar]
- Mistrik P, Mullaley C, Mammano F, Ashmore J. 2009. Three-dimensional current flow in a large-scale model of the cochlea and the mechanism of amplification of sound. J R Soc Interface 6: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistrik P, Daudet N, Morandell K, Ashmore JF. 2012. Mammalian prestin is a weak Cl–/HCO3− electrogenic antiporter. J Physiol 590: 5597–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau A, Gosselin-Badaroudine P, Delemotte L, Klein ML, Chahine M. 2015. Gating pore currents are defects in common with two Nav1.5 mutations in patients with mixed arrhythmias and dilated cardiomyopathy. J Gen Physiol 145: 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugasu E, Russell IJ. 1996. The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J Neurosci 16: 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, Fakler B. 1999. Expression density and functional characteristics of the outer hair cell motor protein are regulated during postnatal development in rat. J Physiol 519: 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, He DZ, Klocker N, Ludwig J, Schulte U, Waldegger S, Ruppersberg JP, Dallos P, Fakler B. 2001. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science 292: 2340–2343. [DOI] [PubMed] [Google Scholar]
- Rybalchenko V, Santos-Sacchi J. 2003. Cl– flux through a non-selective, stretch-sensitive conductance influences the outer hair cell motor of the guinea-pig. J Physiol 547: 873–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Navratanam D, Raphael R, Oliver D. 2017. Prestin: Molecular mechanisms underlying outer hair cells electromotility. In Understanding the cochlea (ed. Manley G, Gummer A, Popper A, Fay R), pp. 113–145. Springer, New York. [Google Scholar]
- Schaechinger TJ, Oliver D. 2007. Nonmammalian orthologs of prestin (SLC26A5) are electrogenic divalent/chloride anion exchangers. Proc Natl Acad Sci 104: 7693–7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechinger TJ, Gorbunov D, Halaszovich CR, Moser T, Kugler S, Fakler B, Oliver D. 2011. A synthetic prestin reveals protein domains and molecular operation of outer hair cell piezoelectricity. EMBO J 30: 2793–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanzler M, Fahlke C. 2012. Anion transport by the cochlear motor protein prestin. J Physiol 590: 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Cheatham MA, Zheng J, Homma K. 2016. The R130S mutation significantly affects the function of prestin, the outer hair cell motor protein. J Mol Med (Berl) 94: 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Pecka JL, Tang J, Lovas S, Beisel KW, He DZ. 2012. A motif of eleven amino acids is a structural adaptation that facilitates motor capability of eutherian prestin. J Cell Sci 125: 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HY, Xia A, Oghalai JS, Pereira FA, Alford RL. 2005. High frequency of the IVS2-2A>G DNA sequence variation in SLC26A5, encoding the cochlear motor protein prestin, precludes its involvement in hereditary hearing loss. BMC Med Genet 6: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth T, Deak L, Fazakas F, Zheng J, Muszbek L, Sziklai I. 2007. A new mutation in the human pres gene and its effect on prestin function. Int J Mol Med 20: 545–550. [PubMed] [Google Scholar]
- Weddell TD, Mellado-Lagarde M, Lukashkina VA, Lukashkin AN, Zuo J, Russell IJ. 2011. Prestin links extrinsic tuning to neural excitation in the mammalian cochlea. Curr Biol 21: R682–R683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz C, Glowatzki E, Fuchs P. 2009. The postsynaptic function of type II cochlear afferents. Nature 461: 1126–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. 2000. Prestin is the motor protein of cochlear outer hair cells. Nature 405: 149–155. [DOI] [PubMed] [Google Scholar]
- Zheng J, Du GG, Anderson CT, Keller JP, Orem A, Dallos P, Cheatham M. 2006. Analysis of the oligomeric structure of the motor protein prestin. J Biol Chem 281: 19916–19924. [DOI] [PubMed] [Google Scholar]