Abstract
Aberrant protein aggregation is a defining feature of most neurodegenerative diseases. During pathological aggregation, key proteins transition from their native state to alternative conformations, which are prone to oligomerize into highly ordered fibrillar states. As part of the cellular quality control machinery, molecular chaperones can intervene at many stages of the aggregation process to inhibit or reverse aberrant protein aggregation or counteract the toxicity associated with amyloid species. Although the action of chaperones is considered cytoprotective, essential housekeeping functions can be hijacked for the propagation and spreading of protein aggregates, suggesting the cellular protein quality control system constitutes a double-edged sword in neurodegeneration. Here, we discuss the various mechanisms used by chaperones to influence protein aggregation into amyloid fibrils to understand how the interplay of these activities produces specific cellular outcomes and to define mechanisms that may be targeted by pharmacological agents for the treatment of neurodegenerative conditions.
The accumulation of protein aggregate deposits in the brain is a hallmark of most neurodegenerative conditions including Alzheimer's (AD), Parkinson's (PD), and Huntington's disease (HD) and amyotrophic lateral sclerosis (ALS) (Chiti and Dobson 2017). Although many neurodegenerative conditions arise sporadically, hereditary cases link increased concentrations or aggregation propensity of signature proteins to disease severity and early onset, suggesting a causal link between protein aggregation and disease phenotypes (Soto 2003).
Protein aggregates typically arise from the failure of the cellular protein quality control (PQC) machinery to refold or degrade misfolded species in a timely manner (Tyedmers et al. 2010; Labbadia and Morimoto 2015). During aging, the capacity of the PQC network is decreased and becomes less responsive to protein misfolding stress signals, while damaged protein species accumulate (Blake et al. 1991; Ben-Zvi et al. 2009). The age-related decline of the PQC machinery has therefore been proposed to be a contributing factor to the late onset of protein aggregation in neurodegeneration (Labbadia and Morimoto 2015). The flip side to this hypothesis is that in early life, efficient PQC systems are in place to delay the onset of protein aggregation and, consequently, neurodegeneration. The effect of aging on protein homeostasis is further explored in Hartl (2018). Molecular chaperones play central roles in maintaining protein homeostasis in the cell as modulators of protein conformational states (Bukau et al. 2006; Kampinga and Craig 2010; Saibil 2013; Balchin et al. 2016). Chaperone action is therefore a key regulator in the pathological transition from the native protein conformation into the amyloid fibril (Hipp et al. 2014; Kampinga and Bergink 2016).
In this review, we discuss the various strategies used by chaperones to interfere with protein aggregation into amyloid fibrils and how their action may affect the progression of disease phenotypes. We illustrate molecular mechanisms of amyloid handling by the human chaperone machinery with specific case studies to provide an overview of the various ways in which protein aggregates can be targeted for therapeutic intervention in the context of neurodegenerative diseases.
PROTEIN AGGREGATION IN NEURODEGENERATION
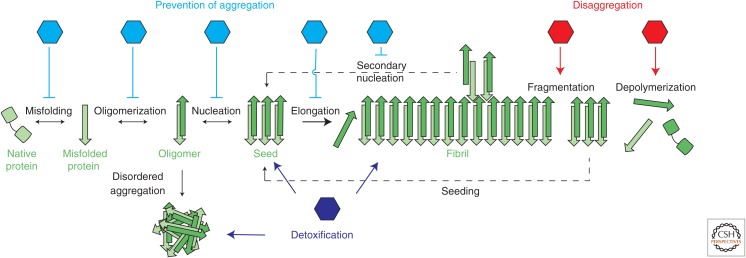
Protein aggregates associated with neurodegeneration are typically characterized by a highly ordered, β-sheet-rich structure, termed amyloid (Chiti and Dobson 2017; Eisenberg and Sawaya 2017) (for additional details on the link between the amyloid state and human disease, see Dobson 2018). In vitro, the molecular mechanism of protein aggregation into amyloid fibrils has been described as a multistep pathway (Morris et al. 2009; Eichner and Radford 2011; Knowles et al. 2014) initiated by the stochastic or stress-induced conversion of a native protein into a mis- or unfolded and aggregation-prone state (Chen et al. 2002). The accumulation, subsequent oligomerization, and conformational rearrangement (Cremades et al. 2012; Neudecker et al. 2012) of such species leads to the formation of aggregation nuclei or “seeds” that act as templates of the amyloid conformation (Serio et al. 2000; Tanaka et al. 2006). The rapid templated incorporation of native monomers dramatically accelerates fibril growth. Fragmentation (Knowles et al. 2009) and secondary nucleation (Cohen et al. 2013), in which the surface of existing fibrils facilitates the formation of additional fibrillar seeds, exacerbate the phenotype rapidly by multiplying the number of ends from which aggregates can grow. Although the amyloid structure is energetically highly favorable (Baldwin et al. 2011), amyloid fibrils are not static entities but exist in equilibrium with monomer and oligomeric species (Fig. 1) (Carulla et al. 2005).
Figure 1.
The amyloid equilibrium presents multiple opportunities for chaperone interference. Molecular chaperones can affect the various conformational rearrangements undergone by an amyloidogenic protein along the aggregation pathway. Broadly speaking, chaperone activities can be divided into three categories: prevention of aggregation (blue), disaggregation (red), or neutralization of toxic species (dark blue). Chaperone disaggregation enhances both depolymerization and fragmentation events, producing monomers and potentially seeding competent oligomers.
In vivo, interaction with cellular factors, molecular crowding, as well as posttranslational modifications and enzymatic processing all influence the aggregation behavior of amyloidogenic proteins and peptides (Oueslati et al. 2010; Stroo et al. 2017). Off-pathway oligomers and disordered aggregates are likely to coexist with the highly ordered amyloid conformation, each constituting their own challenge to the cellular PQC machinery (Lashuel et al. 2013; Kampinga and Bergink 2016).
The self-templating properties of the amyloid conformation are central to the prion hypothesis, which states that prions, the infectious particles causing a number of fatal transmissible neurodegenerative diseases, solely consist of an alternative β-sheet-rich, self-propagating amyloid conformation (PrPSc) of the α-helix-rich cellular prion protein PrPC (Prusiner 1998). The characteristic feature of the amyloid fold provides an explanation of how a single protein conformation can propagate without the transmission of genetic information. On infection, prions replicate not only at the molecular level, but also at the cellular level. This involves intercellular transmission of fibrillar seeds and the subsequent initiation and propagation of the amyloid conformation in naïve cells (Prusiner 1998). Although this phenomenon is a defining feature of infectious prion diseases, there is currently no epidemiological evidence that other prevalent human neurodegenerative diseases are contagious. However, a similar “prion-like” propagation has been described for other amyloid-forming proteins, including AD-associated amyloid β (Aβ) and tau and PD-associated α-synuclein (αsyn), with aggregation initially developing in particular brain regions and subsequently spreading toward more distal interconnected regions in a stereotypic manner as disease progresses (Brundin et al. 2010; Goedert et al. 2010). Hence, given the similarities in aggregate assembly, growth, and spreading, it is not surprising that many amyloidogenic proteins associated with neurodegenerative diseases are now considered to be prion-like.
A complex relationship exists between the various states of the amyloid equilibrium and cellular toxicity, with both on- and off-pathway oligomers as well as fibrillar species assigned to pathogenicity (Cohen and Dillin 2008; Sun et al. 2015; Tipping et al. 2015; Ries and Nussbaum-Krammer 2016). Moreover, as discussed in Eisenberg (2018) and Otzen and Riek (2018), not all amyloid-forming proteins are linked to disease. Diverging observations may be explained by inherent differences between various amyloidogenic proteins (Chiti and Dobson 2017), conformational differences in the resultant structures of their aggregation (Gath et al. 2014; Melki 2018), as well as the properties of flexible regions accessible for interaction with other cellular factors (Olzscha et al. 2011; Kim et al. 2016). For example, the amyloid structures of Aβ peptides integrate the entire amino acid sequence into their β-sheet folds (Luhrs et al. 2005; Paravastu et al. 2008; Gremer et al. 2017). Larger amyloidogenic proteins such as tau and αsyn only partially bury their polypeptide sequence in the final amyloid core, exposing “fuzzy” coats of disordered overhang sequences (Vilar et al. 2008; Tuttle et al. 2016; Fitzpatrick et al. 2017; Guerrero-Ferreira et al. 2018). Smaller fibrillar and less-ordered oligomeric species, formed along the aggregation pathway, display proportionally larger hydrophobic surfaces that may make aberrant interactions with other cellular factors (Olzscha et al. 2011; Kim et al. 2016), sequester PQC machinery (Park et al. 2013; Yu et al. 2014; Guo et al. 2018), or disrupt cellular membranes (Quist et al. 2005; Campioni et al. 2010). The different properties of the oligomeric states that accumulate during protein aggregation provide multiple opportunities for the interaction with PQC components. Such oligomers are thought to be the dominant cytotoxic species, whereas larger amyloid aggregates are mainly regarded as inert and relatively cytoprotective because their size and highly ordered packing minimizes interactions with the cellular environment.
MOLECULAR CHAPERONES AND PROTEIN QUALITY CONTROL
In response to the threat of protein conformational instability, cells have evolved elaborate networks of chaperone proteins responsible for the folding, subcellular targeting, sequestration, disaggregation, and degradation of aggregation-prone, unfolded, and misfolded protein species (Bukau et al. 2006; Kampinga and Craig 2010; Saibil 2013; Balchin et al. 2016). The expression of chaperones is up-regulated in a cell compartment–specific manner in response to protein misfolding stresses such as heat shock, oxidative stress, nutrient starvation, or genomic instability, to compensate for compromised protein homeostasis. The mechanisms of stress-induced up-regulation of chaperone expression and the complex coordination of this response across tissues in multicellular organisms are discussed in more detail in Joutsen and Sistonen (2018) and Morimoto (2018). Failure to elicit the cytosolic/nuclear heat shock response results in large-scale protein aggregation and possibly cell death. It is therefore noteworthy that some aggregation events associated with neurodegeneration, such as that of the polyglutamine (polyQ) expanded Huntingtin protein (Htt) in HD, suppress this cellular response (Chafekar and Duennwald 2012), leading to further exacerbation of the already impaired proteostasis. Nevertheless, genetic or chemical activation of the heat shock response reduces aggregation in cell and animal models of PD and AD (Paris et al. 2010; Pierce et al. 2013). Moreover, the neuronal toxicity of aggregating αsyn, tau, and Htt was reduced in animal model of neurodegeneration on Hsf1 overexpression or local administration (Hay et al. 2004; Fujikake et al. 2008; Liangliang et al. 2010; Jiang et al. 2013).
The central cytosolic members of the chaperone network are the ATP-independent small heat shock proteins (sHSPs) and several ATP-dependent chaperone families: Hsp70s with their cochaperones, in particular J-domain proteins (Hsp40s) and nucleotide exchange factors (NEFs) such as Hsp110, Hsp90 and its cochaperones, and the chaperonin TriC/CCT (Bukau et al. 2006; Kampinga and Craig 2010; Hartl and Bracher 2011; Li et al. 2012). These chaperone systems differ fundamentally in their modes of substrate binding, the conformational states of the substrates they recognize, and the changes they impose on their substrates. Figure 2 provides an overview of the key members of this chaperone network, their domain organization, and their substrate interaction modes. For further details, Biebl and Buchner (2018) and Janowska et al. (2018) elaborate on the complex functional regulation of Hsp90 and sHsps, respectively.
Figure 2.
The cytosolic chaperone network. Chaperones in the human cytosol collaborate to identify, sequester, and refold misfolded proteins back to their native state. Molecular chaperones recognize similar hydrophobic motifs in substrates that are typically buried but become accessible on misfolding. Despite their overlapping substrate pools, their domain organization and substrate-binding modes differ significantly. sHsps rely on a complex oligomerization equilibrium to regulate their chaperoning activity in response to misfolding stressors and to sequester misfolded proteins. The Hsp70 family chaperoning conformational cycle is regulated by J-domain proteins, which target substrates to Hsp70, and NEFs that promote substrate release. Although J-domain proteins can act as chaperones independently from Hsp70, chaperoning activity by NEFs is limited to the Hsp110-type only. Additional cellular factors serve to bridge the Hsp70 machine to downstream chaperones: Hsp90 and/or the class II chaperonin TriC/CCT. The nature of collaboration between the ATP-independent sHSPs and the Hsp70 machinery remains unknown. Nucleotide states throughout the chaperone functional cycles are indicated with “D” or “T” for ADP- or ATP-bound, respectively. CTD, carboxy-terminal domain; DD, dimerization domain; inter, intermediate domain; JD, J domain; MD, middle domain; NBD, nucleotide-binding domain; NTD, amino-terminal domain; NEF, nucleotide exchange factor; SBD, substrate-binding domain; ZFD, zinc finger domain.
Amyloid-type aggregates present unique challenges for the cellular PQC machinery. In particular, the constituent proteins of amyloids do not display the typical characteristic of a chaperone substrate, that is, high exposed hydrophobicity, but instead present highly repetitive sequence and structural motifs resulting from the ordered stacking of monomers in the amyloid fibril. The cellular chaperone machinery, therefore, has to rely on unconventional and sometimes specialized molecular mechanisms to counteract protein aggregation in the context of neurodegeneration.
Chaperones appear to differ strongly in their effect on the various amyloidogenic proteins, highlighting both the strong similarities but also differences between the aggregation events underlying associated neurodegenerative conditions (Kakkar et al. 2014). αsyn and tau intracellular aggregation, signatures of PD and AD, respectively, are both reduced on induction of the heat shock response in cell culture models (Kakkar et al. 2014). Although generalized ATP-dependent chaperones can act at all stages of polyQ aggregation, noncanonical Hsp70 cochaperones, as well as sHSPs are more effective at preventing polyQ protein aggregation or counteracting disease phenotypes (Kakkar et al. 2014). Aβ aggregation was shown to be strongly affected by a range of sHSPs in vitro; however, the physiological relevance of these observations is unknown because of the extracellular localization of Aβ aggregates. Known in vitro chaperone interactions with amyloidogenic proteins are summarized in Table 1.
Table 1.
Chaperones described to modulate amyloid aggregation in vitro
| Chaperone family | Member | Substrate protein | Activity described | Interacting species | References |
|---|---|---|---|---|---|
| Small heat shock proteins (sHSPs) | HSPB1 (hsp27) | αsyn Aβ40/42 tau Htt |
Prevention of aggregation Neutralization |
Oligomer Fibrils |
Wilhelmus et al. 2006a; Ojha et al. 2011; Baughman et al. 2018; Cox et al. 2018 |
| HSPB2/3 | αsyn insulin |
Prevention of aggregation | Bruinsma et al. 2011; Prabhu et al. 2012 | ||
| HSPB5 (αB-crystallin) |
αsyn Aβ40/42 tau β2-M |
Prevention of aggregation Neutralization Disaggregation |
Monomer Oligomer Fibril |
Bruinsma et al. 2011; Duennwald et al. 2012; Mannini et al. 2012; Cox et al. 2016 | |
| HSPB6 | Aβ40/42 αsyn |
Prevention of aggregation | Monomer | Lee et al. 2005; Wilhelmus et al. 2006a; Bruinsma et al. 2011 | |
| HSPB8 | Aβ40 polyQ αsyn |
Prevention of aggregation | Monomer | Wilhelmus et al. 2006b; Bruinsma et al. 2011 | |
| J-domain proteins (Hsp40) | DNAJA1 | tau | Prevention of aggregation | Monomer | Mok et al. 2018 |
| DNAJA2 | tau | Prevention of aggregation | Monomer | Mok et al. 2018 | |
| DNAJB1 | αsyn polyQ IAPP |
Prevention of aggregation Disaggregation |
Fibril | Pemberton et al. 2011; Duennwald et al. 2012; Gao et al. 2015 | |
| DNAJB2 | polyQ | Prevention of aggregation | Oligomer | Labbadia et al. 2012 | |
| DNAJB6 | Aβ αsyn polyQ |
Prevention of aggregation | Oligomer | Månsson et al. 2014a,b; Aprile et al. 2017 | |
| DNAJB8 | αsyn polyQ |
Prevention of aggregation | Oligomer | Månsson et al. 2014b; Aprile et al. 2017 | |
| Hsp70 | HSPA1A (Hsp70) |
Aβ αsyn IAPP IgG tau |
Prevention of aggregation | Monomer Oligomer |
Wacker et al. 2004; Dedmon et al. 2005; Evans et al. 2006; Chien et al. 2010; Voss et al. 2012; Rosas et al. 2016 |
| HSPA5 (Bip) |
IAPP IgG |
Prevention of aggregation | Davis et al. 2000; Chien et al. 2010 | ||
| HSPA8 (Hsc70) |
HttEx1 αsyn tau |
Prevention of aggregation Disaggregation | Monomer Oligomer Fibril |
Muchowski et al. 2000; Pemberton et al. 2011; Duennwald et al. 2012; Gao et al. 2015; Monsellier et al. 2015; Mok et al. 2018; Scior et al. 2018 | |
| NEFs | HSPH2 (Apg2) |
αsyn HttEx1 |
Disaggregation | Fibril | Duennwald et al. 2012; Gao et al. 2015 |
| Hsp90 | HSPC1 | Aβ αsyn tau |
Prevention of aggregation | Oligomers | Evans et al. 2006; Falsone et al. 2009; Daturpalli et al. 2013; Schirmer et al. 2016 |
| Chaperonin | TriC/CCT | αsyn polyQ |
Prevention of aggregation Neutralization |
Monomer Oligomer Fibril |
Behrends et al. 2006; Tam et al. 2009; Shahmoradian et al. 2013; Sot et al. 2017 |
| Hsp60 | tau | Prevention of aggregation | Monomer Oligomer | Mok et al. 2018 | |
| Ribosome-associated chaperones | NAC | αsyn | Prevention of aggregation | Monomer | Martin et al. 2018 |
| Brichos-domain proteins | proSP-C | SP-C Aβ40/42 | Prevention of aggregation | Fibril | Willander et al. 2012 |
| gastrokine1 | Aβ40 | Prevention of aggregation | Monomer | Altieri et al. 2014 | |
| Bri2 | Aβ40/42 IAPP |
Prevention of aggregation | Fibril | Willander et al. 2012; Oskarsson et al. 2018 | |
| Extracellular chaperones | α2-macro-globulin | Aβ42 Calcitonin HypF-N Lysozyme |
Prevention of aggregation Neutralization |
Oligomer | Hughes et al. 1998; Yerbury et al. 2009; Mannini et al. 2012 |
| Clusterin | Aβ40/42 ApoCII αsyn Calcitonin Lysozyme tau TDP43 TTR |
Prevention of aggregation Neutralization | Monomer Oligomer |
Kumita et al. 2007; Narayan et al. 2012; Greene et al. 2015; Mok et al. 2018 | |
| Haptoglobin | Aβ42 Calcitonin Lysozyme |
Prevention of aggregation | Oligomer | Yerbury et al. 2009 | |
| Other | Prefoldin | Aβ42 Htt |
Prevention of aggregation | Oligomer | Sakono et al. 2008; Tashiro et al. 2013 |
| 7B2 | αsyn Aβ40/42 IAPP |
Prevention of aggregation | Helwig et al. 2013; Peinado et al. 2013 | ||
| ApoE | Aβ40 | Prevention of aggregation | Evans et al. 1995 |
A pattern begins to emerge where peptide amyloids including Aβ and unflanked polyQ stretches do not interact with the canonical Hsp70 chaperone machinery (Kakkar et al. 2014). sHSPs and specialized J domain chaperones interact predominantly with oligomeric states of these species to prevent further aggregation and/or suppress cytotoxicity. In contrast, relatively larger amyloidogenic proteins such as αsyn, tau, and as notable exception Htt Exon1 that contain flexible flanking sequences in addition to the amyloid forming polyQ region, do interact with canonical Hsp70 cochaperones, including in their monomeric state, suggesting that it is not their amyloid-forming sequences but rather neighboring regions that allow binding of these chaperones (Monsellier et al. 2015).
PREVENTION OF AGGREGATION BY MOLECULAR CHAPERONES
As first line of defense against the formation of amyloid fibrils, the cellular PQC machinery employs various molecular strategies to prevent the (further) aggregation of the native protein pool. Classical chaperone folding and refolding activities rescue destabilized or misfolded protein species back to their native state. Transient interactions with misfolded proteins or small oligomers prevent their coalescence into seeding competent nuclei, while reducing accessibility to fibril ends prevents the further conversion of the native protein into amyloid fibrils. Finally, the decoration by chaperones along the fibrillar axis may interfere with the process of secondary nucleation, which can be a dominant driving force in accelerating protein aggregation under conditions where fibril fragmentation is limited (Fig. 1) (Cohen et al. 2013).
Chaperone “profolding” activities concern only the ∼35% subset of amyloidogenic proteins that attain a stable tertiary structure when folded to their native state (Chiti and Dobson 2017). Examples are the secreted immunoglobin light chain (Ig LC) and transthyretin (TTR) proteins that require the endoplasmic reticulum (ER) resident Hsp70 chaperone, HSPA5 (BIP), for folding into the IgG quaternary-structure and native tetramer, respectively (Blancas-Mejía and Ramirez-Alvarado 2013). Although unassembled Ig LC and TTR monomers are typically retained in the ER, reduced proteostasis capacity leads to their aberrant secretion and subsequent extracellular aggregation into amyloid fibrils (Lee et al. 1999; Sorgjerd et al. 2006). Destabilization of their native folds by mutation or the absence of partner subunits promotes sustained HSPA5 (BIP) interaction (Melnick et al. 1994; Lee et al. 1999; Quintas et al. 2001; Sorgjerd et al. 2006). This more stringent quality control reduces the secretion of destabilized TTR and Ig LC and thus suppresses extracellular protein aggregation (Melnick et al. 1994; Davis et al. 2000). Conversely, chemical disruption of ER proteostasis, reducing chaperone availability, results in an accumulation of extracellular aggregates (Chen et al. 2016), demonstrating that indeed the preservation of native protein folds, in conjunction with the timely ER retention and degradation of nonnative folds by ER-associated degradation (ERAD) is an efficient mechanism to suppress amyloid formation (Fig. 1, “misfolding”).
Chaperones can prevent aggregation of misfolded proteins that escape the PQC machinery, or of the predominant intrinsically disordered, low-complexity proteins associated with neurodegeneration, through interference with primary nucleation. Transient interactions with aggregation-prone regions in monomeric proteins or sequences in close proximity, can prevent their initial oligomerization into seeding competent aggregates. This strategy corresponds to the classical “holding” activity (Mattoo and Goloubinoff 2014) of sHSPs and canonical J-domain proteins as well as the activity of other HSPs, including Hsp70 and Hsp110, where the binding to a substrate sequence sterically interferes with the aggregation process (Fig. 1, “oligomerization”). The native tau interactions with the Hsp70 and Hsp90 chaperone machinery illustrates this concept well. As an intrinsically disordered protein, tau does not rely on these chaperones for folding; however, its interaction with chaperones is essential to regulate tau microtubule association, phosphorylation status, and degradation (Jinwal et al. 2010; Thompson et al. 2012; Karagoz et al. 2014). Perturbations in these interactions result in the accumulation and aggregation of hyperphosphorylated tau (Jinwal et al. 2013; Wang et al. 2013; Young et al. 2018). Chaperone “holding” activities may also compete with the further elongation of preexisting fibrils, as discussed in further detail below.
Alternatively, primary nucleation can be suppressed through the stabilization of intermediate, non-seeding-competent oligomeric states before their conversion into aggregation “seeds.” This particular mechanism appears to be used by the noncanonical J-domain proteins, DNAJB6, and DNAJB8. DNAJB6 effectively suppresses the aggregation of various members of the polyQ expanded protein family (Månsson et al. 2014b) as well as Aβ42 (Månsson et al. 2014a) in vitro and in cell culture models on overexpression (Gillis et al. 2013), and improves cognitive function in a mouse model of HD (Kakkar et al. 2016). Kinetic analysis of the in vitro aggregation behavior of polyQ proteins and Aβ in the presence of DNAJB6 revealed that the chaperone predominantly inhibits primary nucleation through interaction with small oligomers, rather than monomeric species (Månsson et al. 2014a; Arosio et al. 2016; Kakkar et al. 2016). DNAJB6/8 contain, in addition to classical structural features of the DNAJB class (Fig. 2; Kampinga and Craig 2010), a signature region rich in serine and threonine residues that is hypothesized to specifically interact with the β-hairpin hydrogen bonding network of polyQ-containing protein and possibly other amyloidogenic protein aggregates (Kakkar et al. 2016; Månsson et al. 2018; Soderberg et al. 2018). DNAJB6 forms higher oligomeric complexes and therefore presents multiple substrate interfaces (Soderberg et al. 2018), suggesting that its oligomeric status conveys the ability to specifically recognize amyloidogenic oligomers (Fig. 1, “nucleation”).
Once nucleation occurs, aggregation “seeds” can rapidly elongate through the templated incorporation of monomers. Monomer sequestration by chaperones can compete with this process through the depletion of the available monomer pool (Wacker et al. 2004; Luheshi et al. 2010). Conversely, chaperones may act on the fibrillar state to sterically hinder further monomer incorporation. The sHSPs HSPB1 and HSPB5 have been described to act in this way, preventing the further elongation of Aβ40/42 and αsyn amyloid fibrils at substoichiometric chaperone concentrations (Waudby et al. 2010; Shammas et al. 2011). The β-sandwich folds of the α-crystallin domains of HSPB1/5 (Fig. 2) are crucial for the interaction with amyloid fibrils. It is likely that the β-sheet of the sHSP dimerization interface in the α-crystallin domain matches the hydrogen bonding register of preformed fibrils, effectively capping the fibrils (Mainz et al. 2015). A side effect of this interaction is that preformed fibrils are stabilized, as preintegrated monomers can no longer dissociate (Fig. 1, “elongation”) (Raman et al. 2005).
Finally, chaperones can suppress aggregation through the inhibition of secondary nucleation. Secondary nucleation events are aggregation events catalyzed by the surface of preformed fibrils, which rapidly creates new fibrillar seeds and thus increases protein aggregation rates. Chaperones that interact with the surface of amyloid fibrils can interfere with this phenomenon (Table 1). The best characterized example of a chaperone family that uses this mechanism is the BRICHOS domain-containing proteins. BRICHOS domains appear to have evolved to shield β-sheet-prone regions of their respective proproteins from aggregation (Mao et al. 2016), but additionally interfere in the oligomerization of other amyloidogenic proteins, in particular Aβ42 (Willander et al. 2012). In vitro, Aβ42 aggregation is reduced predominantly through the inhibition of secondary nucleation (Cohen et al. 2015); BRICHOS domains thus do not prevent the initial formation of amyloid fibrils but suppress the amplification effects of new aggregation seeds. The underlying molecular interactions have thus far not been described. Overexpression of the Bri2 BRICHOS domain strongly inhibits Aβ aggregation in a Drosophila model of AD (Fig. 1: “secondary nucleation”) (Poska et al. 2016).
The development of detailed kinetic analyses of in vitro aggregation data has greatly contributed to our understanding of the chaperoning mechanisms that act to prevent the aggregation of amyloidogenic proteins and the oligomeric state that they act on (Arosio et al. 2016). The inherent promiscuity of chaperone interactions with their substrates means that chaperones often act on multiple conformational species that accumulate during aggregation and thus interfere with various microscopic processes simultaneously (Table 1). A case in point are the sHSPs that not only act to prevent elongation through interaction at fibril ends but also reduce secondary nucleation through similar interactions along the fibril axis. Moreover, their presence makes preformed fibrillar species more susceptible to disaggregation by the Hsp70 machinery (see section on Chaperone-Driven Disaggregation of Amyloid Species). Their interaction with oligomeric species stabilizes non-seeding-competent conformations, and a subset of sHSP may act to neutralize such oligomeric species through the regulated aggregation and sequestration into quality control compartments (see next section).
NEUTRALIZATION/DETOXIFICATION OF PROTEIN AGGREGATES
An additional consequence of the interaction of chaperones with oligomers and fibrils of amyloidogenic proteins is the detoxification of such species in the cell. A first approach to counteracting the toxicity of, in particular, smaller oligomeric states is reducing the total exposed hydrophobicity by increasing the size of aggregates. Indeed, formation of large intracellular inclusion bodies correlates with increased survival of neurons expressing mutant Htt (Arrasate et al. 2004).
Chaperones, including sHSP HSPB1 and extracellular chaperones clusterin, α2 macroglobulin, and haptaglobin (Ojha et al. 2011; Mannini et al. 2012), interact with protein oligomers in vitro to form higher-order complexes of amyloid-forming proteins, including Aβ42, IAPP, and HypF-N. Such sequestration does not dramatically alter the structure of the aggregates (as judged by thioflavin T fluorescence and circular dichroism analysis) but strongly reduces their cellular toxicity (Ojha et al. 2011). Detoxification relies not only on the increased size of the aggregate, but also on the shielding action of chaperone binding, which prevents unspecific interactions with other cellular proteins (Beeg et al. 2016). Notably, the chaperonin TRiC/CCT, which prevents the aggregation of Htt Exon1 through transient interactions with oligomeric and fibrillar species (Tam et al. 2006; Shahmoradian et al. 2013; Pavel et al. 2016), can additionally act to detoxify polyQ proteins by promoting the assembly of soluble, nontoxic oligomeric species that are larger in size (∼500 kDa) than their toxic counterparts (∼200 kDa) (Behrends et al. 2006).
A “sequestrase” activity of sHSPs, which mediates the formation of organized protein foci (Specht et al. 2011; Escusa-Toret et al. 2013; Miller et al. 2015), was shown to be essential for survival of yeast cells subjected to severe and repetitive protein misfolding stresses (Escusa-Toret et al. 2013; Ungelenk et al. 2016; Mogk et al. 2018). Although an analogous role for their sHSP repertoire has not yet been shown, mammalian cells respond similarly to the proteotoxic stress associated with proteasomal impairment, by sequestering misfolded proteins into large deposits termed aggresomes located near the centrosome (Johnston et al. 1998). Polyubiquitinated substrates are targeted to this site by Hsp70 and its NEF cochaperone BAG3 as well as the adaptor proteins 14-3-3 or HDAC6, which couple the aggregate cargo to dynein for trafficking (Gamerdinger et al. 2011; Xu et al. 2013). The aggresome is considered a storage site for aggregates facilitating clearance by autophagy (Kopito 2000). It should be noted that aggresome structures have mainly been described in cell culture models and do not necessarily correspond to inclusion bodies associated with neurodegenerative diseases. However, they clearly represent a cytoprotective stress response and their studies shed light on how cells manage acute protein misfolding stress. Finally, in Caenorhabditis elegans, long-lived daf-2 mutants show increased accumulation of large insoluble aggregates as compared with wild type (WT), suggesting that intracellular sequestration of aggregation-prone proteins is beneficial to organismal longevity (Walther et al. 2015). Nevertheless, the biological relevance of (sHSP-mediated) sequestration of aggregates formed by amyloidogenic proteins in mammalian cells remains to be established.
Detoxification of amyloids by chaperones can also follow an indirect mechanism, in which rather than acting on the aggregate itself, chaperones such as the Hsp70 system extract coaggregated protein species from deposits. This may counteract cellular toxicity induced by the sequestration of essential proteins (e.g., transcription factors) (Olzscha et al. 2011). Conversely, such extraction activity may sequester essential PQC components toward amyloid-induced deposits and away from essential housekeeping functions (Hinault et al. 2010). That chaperone sequestration by aggregates can perturb housekeeping functions was shown for the role of the constitutive Hsp70, HSPA8 (Hsc70), in endocytosis on protein aggregation (Yu et al. 2014). In yeast, the aggregation of overexpressed polyQ proteins results in the sequestration of J-domain protein DNAJB1 homolog Sis1, blocking a PQC pathway that shuttles target proteins for proteasomal degradation in the nucleus (Park et al. 2013). Moreover, oligomeric species of amyloidogenic proteins can block proteasomal activity through direct ubiquitin-independent interaction with the 20S subunit, reducing overall cellular degradative capacity (Thibaudeau et al. 2018). Beyond its burden on the PQC network as a substrate, oligomer and amyloid species formed by amyloidogenic proteins can thus interfere with standard housekeeping functions of the PQC in unexpected ways.
CHAPERONE-DRIVEN DISAGGREGATION OF AMYLOID SPECIES
Once amyloid fibrils are formed, molecular chaperones may act to reverse protein aggregation. A priori, amyloid disaggregation by molecular chaperones can occur through either passive or active processes. In the passive process, monomers that spontaneously dissociate from the fibrils are captured by chaperones, thus shifting the amyloid equilibrium to a soluble state. In the active process, chaperones act on the fibrillar state to accelerate depolymerization of monomers or destabilize the fibril core to such extent that fibrils fragment.
To date, the best characterized and apparent most versatile disaggregase activity in the human cell is provided by the Hsp70 machinery (Fig. 3). The Hsp70 chaperone family acts through the regulated conformational cycling of Hsp70 between an open ATP-bound state and a closed, high-substrate affinity ADP-bound state. Substrate binding and trapping through ATP hydrolysis is assisted by J-domain proteins, while ADP release, and timed substrate release on ATP rebinding are driven by NEFs (Fig. 2). Hsp70s recognize a degenerate motif of about five residues enriched in hydrophobic amino acids, flanked by positively charged amino acids (statistically occurring every 30–40 residues in protein sequences) (Rudiger et al. 1997). Surface exposure of this peptide motif in protein substrates allows for Hsp70 binding to a wide range of conformers from unfolded nascent peptides and protein aggregates to near native conformations. Importantly, the J-domain proteins belonging to the class A and class B families have substrate-binding activities themselves (Rudiger et al. 2001). The engagement of substrates by J-domain proteins, at binding sites in proper distance to Hsp70-binding sites, precedes or coincides with the association of Hsp70 partner chaperones. J-domain proteins are therefore substrate-targeting factors for Hsp70s.
Figure 3.
Amyloid disaggregation by the human Hsp70 machinery. DNAJB1 identifies substrates as Hsp70 machinery targets based on sequence and structural features (1). DNAJB1 substrate engagement allows the recruitment of HSPA8–ATP to the fibril surface (2). Catalyzed ATP hydrolysis converts the HSPA8/fibril complex to a long-lived, high-affinity state (3). Recruitment of the Hsp110-type nucleotide exchange factor HSPH2 (Apg2) displaces ADP and allows ATP rebinding to HSPA8, resulting in substrate release (4). Reinitiated recruitment of HSPA8 by DNAJB1 to remaining fibrillar fragments allows for complete solubilization of amyloid fibrils. The pulling energies required for amyloid disaggregation are putatively generated during phase 2 and 3 as indicated by the “>” symbols.
Because of their broad substrate specificity, J-domain proteins and Hsp70s act at multiple levels of the amyloid aggregation equilibrium in vitro, including preventing the aggregation of αsyn, Aβ, tau, as well as polyQ-containing aggregates through transient interaction with monomers and oligomers, removal of coaggregated protein species, and solubilization of preformed amyloid fibrils (Table 1).
Efficient disaggregation of αsyn (Duennwald et al. 2012; Gao et al. 2015) and Htt Exon1 (Scior et al. 2018) amyloid fibrils by human HSPA8 (Hsc70), relies on the canonical class B J-domain protein DNAJB1 and Hsp110-type NEFs (Fig. 2). Other J-domain proteins such as DNAJA1 and DNAJA2, implicated in the dissolution of disordered protein aggregates (Nillegoda et al. 2015), cannot substitute in vitro, suggesting a specific function for DNAJB1 in the recognition of amyloid substrates. In C. elegans, however, J-domain proteins of both class A and class B appear to act synergistically to reduce polyQ aggregate load (Kirstein et al. 2017). To what extent this observation can be attributed to differential roles of the J-domain protein classes in prevention of aggregation versus disaggregation remains to be characterized in further detail. A recent study on chaperone modulators of tau aggregation (Mok et al. 2018) corroborates the idea that J-domain proteins of class A may preferentially act to prevent amyloid formation, acting predominantly on small aggregate species or monomers, while members of class B target fibrils and large oligomers to disaggregation (Nillegoda et al. 2015).
The disaggregation activity of Hsp70 further relies on the NEF activity of Hsp110-type cochaperones. It is worth noting that other NEFs (Fig. 2) cannot complement well in disaggregation activity, suggesting that a particular feature of the Hsp110 family is essential to tuning the Hsp70 machinery for disaggregation. One such defining feature may be the unique ability of Hsp110s to bind to substrates themselves (Xu et al. 2012). Hsp110-substrate interactions are typically short-lived but can provide additional “holding” activity to the existing J-domain protein and Hsp70 substrate interactions. Alternatively, the HSPH2-mediated release of HSPA8 from αsyn fibrils was strongly accelerated compared with that mediated by BAG1, even after compensating for differences in NEF activity (Gao et al. 2015). Kinetic factors relating to the rate of Hsp70 recycling may therefore provide an alternative explanation for the unique ability of Hsp110s to support disaggregation.
In bacteria, yeast, and plant cells, the Hsp70 machinery is additionally complemented by the Hsp100 AAA+ ATPase that provides considerable threading power to extract polypeptides from protein aggregates (Mogk et al. 2018; Shorter and Southworth 2018). The molecular mechanism of the stand-alone HSPA8 disaggregation activity of amyloid fibrils in metazoan is, however, poorly understood. The high stability of amyloid fibrils, and the observation that the Hsp70 machinery can act along the axis of fibrils suggests that the conformational cycle of Hsp70 generates considerable pulling forces. It has been proposed that cochaperone-assisted Hsp70 binding may exert pulling forces on flexible sequences within the amyloid fibrils owing to the strong reduction in available conformational space at the fibril surface. In an attempt to reduce the entropic penalty associated with such binding events, entropic pulling forces up to 10–20 pN, which corresponds approximately to the stability of a small protein domain, may be generated to extract the bound fragment from the fibrillar core (De Los Rios et al. 2006; Goloubinoff and De Los Rios 2007; Mogk et al. 2018; Nillegoda et al. 2018). Alternative mechanisms rely on the coordinated action of Hsp70 and Hsp110 to either generate a sudden power stroke on coordinated substrate release (Gao et al. 2015), or alternate binding and release cycles that act as a ratchet for random fluctuation in the amyloid structure (Mattoo et al. 2013).
Hsp70-mediated disaggregation of αsyn fibrils produces both monomeric and oligomeric species that, overall, have reduced cytotoxicity compared with the untreated fibrils on extracellular application (Gao et al. 2015). The oligomeric species produced may, however, act as a conformational template for further protein aggregation within the cell. Indeed, such a mechanism seems to be essential for the maintenance of prions in yeast, in which the disaggregating activity of Hsp104 produces smaller seeds that are more efficiently transmitted to daughter cells and recruit soluble proteins into the growing aggregates (Chernoff et al. 1995). Consequently, inhibiting Hsp104 function cures yeast cells of multiple yeast prions. The effect of Hsp70 disaggregation on the propagation of amyloids within metazoan host cells and the resulting cytotoxicity has thus far not been probed.
ROLE OF MOLECULAR CHAPERONES IN THE DEGRADATION OF AMYLOID FORMING PROTEINS
Alternative PQC strategies to counteract the cytotoxicity associated with protein aggregation into amyloid fibrils relies on the timely degradation of aggregation-prone soluble or aggregated species. Here, chaperones again play an essential role in substrate selection and facilitation of the degradation process.
Terminally misfolded proteins are targeted for degradation via the ubiquitin–proteasome system (UPS) or the autophagy–lysosomal pathways (ALPs). The cochaperones CHIP and BAG1 convey functional specificity to Hsp70 in targeting substrates toward proteasomal degradation (Kästle and Grune 2012). Their binding to Hsp70 slows down the protein-refolding process, which is necessary for the switch from refolding to degradation (Kästle and Grune 2012). CHIP is an E3 ubiquitin ligase that ubiquitinates both Hsp90/Hsp70/BAG1-bound substrate complexes as well as the chaperones themselves, and mediates the targeting of the complexes to the proteasome (Kästle and Grune 2012). The NEF activity of BAG1 is responsible for the release of substrates to the proteasome by decreasing the affinity of Hsp70 to the substrate (Lüders et al. 2000; Kästle and Grune 2012).
Although the UPS is only able to process monomeric protein species, it may still act on aggregates with the help of the Hsp70/Hsp110 disaggregase machinery (Hjerpe et al. 2016). Polyubiquitinated proteins that have been extracted from an aggregate by the action of Hsp70 and Hsp110 are recognized by ubiquilin 2 (UBQLN2) and shuttled to the 26S proteasome (Hjerpe et al. 2016). Mutations in UBQLN2 have been linked to familial cases of ALS, highlighting the significance of this pathway for maintaining protein homeostasis in motor neurons (Deng et al. 2011).
The lysosome constitutes the cellular organelle for a second pathway of protein degradation, which not only handles monomeric substrates but also multimeric assemblies as a whole, such as oligomers and larger aggregates. Cytosolic cargo is delivered to the lysosome by a process termed autophagy, which comprises three major pathways: macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA). In both microautophagy and CMA, the constitutively expressed Hsp70, HSPA8, recognizes cargo proteins by directly binding to specific motifs such as the five amino acids “KFERQ” in RNase A (Chiang and Dice 1988). In CMA, HSPA8 delivers the substrate to the lysosomal membrane protein LAMP2A (Tekirdag and Cuervo 2018). Subsequent translocation requires multimerization of LAMP2A and a pool of HSPA8, which resides in the lysosomal lumen. The exact mechanisms by which HSPA8 is transported to the lysosome and how it is able to facilitate translocation within this acidic environment are unknown (Tekirdag and Cuervo 2018). Other cochaperones may be implicated in this process as well, but their exact role remains elusive (Agarraberes and Dice 2001).
In microautophagy, HSPA8 (Hsc70) targets its substrates to the endosomal membrane by electrostatic interactions with a cationic region in the substrate-binding domain (Sahu et al. 2011). Although microautophagy was first observed in yeast and describes a process in which the vacuole directly engulfs cytosolic cargo, in mammalian cells it refers to a similar process that occurs not in lysosomes, but instead in late endosomes (LEs)/multivesicular bodies (MVBs) in an ESCRT-dependent manner (Sahu et al. 2011). Therefore, it is also referred to as endosomal microautophagy in mammalian cells (eMI). In contrast to CMA, the KFERQ-like targeting motif is not sufficient for directing protein cargo to mammalian endosomes. Moreover, an unfolding of substrates is not required, allowing the recruitment of higher molecular weight complexes (Tekirdag and Cuervo 2018). Although the exact structural requirements and additional cofactors and cochaperones still await discovery, it is tempting to speculate that eMI might share components with the “misfolding-associated protein secretion” (MAPS) pathway, in which cytosolic cargo proteins likewise get targeted to the endosome, albeit for secretion (see below). This might (partially) explain the considerable cross talk between (autophagic) degradation and intercellular spreading of disease proteins (Lopes da Fonseca et al. 2015).
In addition to a sequence-mediated selection process, chaperones also play a role in targeting specific proteins to macroautophagy. This pathway involves the formation of a limiting membrane, and the sequestration of substrates within a double-membrane-bound autophagosome, which eventually fuses with the lysosome for the degradation of its contents. Macroautophagy was long considered to be an unspecific bulk degradation process of cytoplasmic material, but there is increasing evidence that cargo can be removed selectively. This is accomplished by specific adaptor proteins that recruit the substrate protein to lipidated LC3 in the autophagosomal membrane. For the selective clearance of aggregated proteins, including those of amyloidogenic proteins, Hsp70 functions in concert with the sHsp HSPB8 and the NEF BAG3, as well as the ubiquitin adaptor p62/SQSTM1 to deliver ubiquitinated aggregates to the autophagosome (Gamerdinger et al. 2009).
Of note, the same set of chaperones is also involved in a pathway termed “chaperone-assisted selective autophagy” (CASA) (Arndt et al. 2010), which mediates the disposal of damaged components of the Z disc, a large protein complex essential for muscle function, by the actinin-associated LIM protein (ALP). These results imply that this chaperone network acts preferentially on large protein assemblies regardless of whether they consist of amorphous aggregates or highly ordered structures such as the Z disc or amyloid fibers.
In closing, it is worth mentioning that proteasomal and lysosomal degradation pathways are not mutually exclusive but rather compensate for each other (Ji and Kwon 2017). It is therefore not surprising that amyloidogenic proteins have been shown to be subjected to both proteasomal or lysosomal degradation, depending on the physiological state of the cell. Nevertheless, it is clear that the function of both pathways declines as a result of a chronic challenge by misfolded proteins or during aging. Boosting these systems might help to delay the onset of age-related diseases. The further roles of protein degradative pathways in human disease are examined in Finley (2018) and Finkbeiner (2018).
SPREADING OF AMYLOID FIBRILS IS FACILITATED BY CHAPERONE MACHINERY
In stark contrast to the various strategies by which molecular chaperones modulate the accumulation of oligomers and fibrils of amyloidogenic species in the cytoplasm stands the putative role that chaperones play in the cell-to-cell transmission of protein aggregates (Fig. 4).
Figure 4.
Role of chaperones in cellular trafficking and propagation of amyloid fibrils. Amyloidogenic species can spread from cell to cell, hijacking preexisting cellular trafficking pathways (light blue). Additional, misfolded substrate-specific pathways exist to release protein aggregates into the extracellular space. Extracellular chaperones can sequester these species into larger deposits that are less readily taken up by recipient cells (red). The successful transmission of aggregation-prone species to a naïve cell can trigger amyloid formation in this cell through templated incorporation of native monomers (orange).
Molecular chaperones, as part of their functional versatility, play important roles in processes related to cellular trafficking. Although the precise mechanism of aggregate spreading to neighboring cells is still subject of current research, different intercellular transport pathways have been identified that involve chaperones. These include a transfer of lysosome-resident aggregates through tunneling nanotubes (TNTs), which are membrane-enclosed protrusions connecting the cytosol of two cells (Fig. 4; Victoria and Zurzolo 2017). This pathway requires the accumulation of misfolded proteins within lysosomes, which at least in part requires HSPA8 (as outlined above).
An alternative route appears to be the aforementioned MAPS pathway, which mediates the secretion of cytosolic misfolded proteins by an unconventional, ER/Golgi-independent pathway (Lee et al. 2016). This pathway involves the ER-associated deubiquitinase USP19 and the pair of J-domain proteins DNAJC5 (also known as cysteine string protein α [CSPα]) and HSPA8 (Fontaine et al. 2016; Lee et al. 2016; Xu et al. 2018). MAPS substrates are first recognized by USP19 and recruited to the ER surface, and subsequently transferred to DNAJC5 (and presumably HSPA8), which localizes to LEs and mediates the uptake of cargo into the vesicle lumen. Endosomes then fuse with the plasma membrane resulting in the release of the DNAJC5/client complex into the extracellular space (Xu et al. 2018). Although these results showed the export of free proteins, another recent study found that the coexpression of DNAJC5 leads to the secretion of misfolded proteins via extracellular vesicles (Deng et al. 2017), suggesting that some cargo proteins might get sorted into intraluminal vesicles of MVBs for their release (Fig. 4).
Of note, long before being implicated in MAPS, DNAJC5 has been intensively investigated for its function in synaptic vesicle exocytosis. Genetic studies suggest that it is essential for the assembly of the soluble NSF attachment protein receptor (SNARE) complex (Sharma et al. 2012; Burgoyne and Morgan 2015). This body of work suggests that DNAJC5 could also play a more indirect role in MAPS by facilitating secretory vesicle fusion through mediating correct SNARE complex assembly.
Outside the cell, disease proteins encounter extracellular chaperones that modulate their toxicity, infectivity, and oligomeric status. ER-resident chaperone DNAJB11 (Erdj3) can even be cosecreted with destabilized proteins under conditions of ER misfolding stress in an apparent attempt to reduce extracellular proteotoxicity (Genereux et al. 2015). A similar function can be attributed to the extracellular chaperone clusterin. Both chaperones function in an ATP-independent manner to suppress the extracellular aggregation of Aβ and TTR and reduce toxicity (Yerbury et al. 2007; Narayan et al. 2012; Greene et al. 2015). Clusterin additionally acts to sequester small oligomers into large extracellular aggregates (Narayan et al. 2012) that are no longer readily taken up by recipient cells (Whiten et al. 2018). Although elevated clusterin levels in the brain of AD patients and the association of clusterin variants with AD in multiple genome-wide association studies (GWAS) highlight the importance of clusterin in AD, its exact role in disease progression needs further investigation (Yu and Tan 2012).
According to current knowledge, multiple pathways for the uptake of disease-associated proteins into recipient cells exist (Brettschneider et al. 2015). The ones involving chaperones include clathrin-mediated endocytosis (CME), in which DNAJC6 (auxilin) and HSPA8 (Hsc70) function by uncoating clathrin-coated vesicles during endocytosis (Sousa and Lafer 2015). CME is one of the pathways that has been linked to the internalization of αsyn both, as free protein and as exosome-associated cargo (Oh et al. 2016; Ngolab et al. 2017). Moreover, mutations in DNAJC6 have been recently identified in GWAS for juvenile parkinsonism (PARK19) (Lin and Farrer 2014). DNAJC13 (receptor-mediated endocytosis-8, RME-8) is another J-domain-protein-type cochaperone of HSPA8 that has been recently linked to familial forms of PD (PARK21) (Lin and Farrer 2014). It was first discovered in a C. elegans screen for genes involved in receptor-mediated endocytosis (Zhang et al. 2001). Subsequent studies point toward a more indirect effect on endocytosis by regulating downstream endosomal tubulation and sorting of cargo proteins (Shi et al. 2009; Freeman et al. 2014). However, how alterations in endosomal trafficking and homeostasis caused by dysfunctional DNAJC13 might aggravate αsyn-induced neurotoxicity, and whether it is implicated in αsyn spreading needs further investigation. Moreover, although these reports underscore the significance of the endocytic/endosomal vesicle trafficking pathway in synucleinopathies and reveal a role of particular Hsp70 cochaperones specific to these diseases, their implication in the toxicity and spreading of amyloidogenic proteins associated with other neurodegenerative diseases remains to be proven.
CONCLUSION AND PERSPECTIVES
Amyloid formation is a complex process that presents a multitude of opportunities for intervention. What becomes apparent is both the diversity in molecular mechanisms and the functional versatility of chaperones that interfere in this process. With this flexibility comes the caveat that the cellular outcome of chaperone action is a complex summation of various, often competing activities. The therapeutic potential of chaperones to suppress protein aggregation in the context of neurodegeneration will therefore hinge on our ability to grasp the complex interplay of the various chaperoning mechanisms and the identification of factors that may provide functional specificity such as the cochaperones. A promising example of such an approach is the development of a small molecule inhibitor of the Hsp90 cochaperone Aha1, which reduces tau aggregation in vitro, cell culture, and mouse model of AD by enhancing monomeric tau degradation (Shelton et al. 2017).
An additional layer of complexity stems from our current inability to unequivocal attribute cytotoxicity to one or more aggregate states produced along the amyloid formation pathway. The emerging theory that the cytotoxicity observed in neurodegeneration is predominantly caused by oligomers or prefibrillar species rather than amyloid fibrils would suggest that while preventing initial aggregation is the holy grail to combat aggregation associated diseases, promoting the more rapid conversion to the amyloid state by suppressing chaperoning activities that prevent fibril elongation may be an alternative strategy to minimize cytotoxicity in later stages of disease progression.
With our current level of mechanistic insight, we can begin to envisage specific therapeutic strategies designed to replicate chaperone activities by small molecules. This includes chemicals and peptides designed to inhibit aggregation (Briggs et al. 2016; Valera and Masliah 2016) or small protein domains engineered to sequester and neutralize protein aggregates or assist in the intra- and extracellular clearance (Messer and Joshi 2013; Manoutcharian et al. 2017). All these developments will be much aided by a better understanding of the effects of the global cellular environment on the aggregation process, as the highly ordered amyloid fibrils discussed in this review are likely to represent only a fraction of the protein aggregate populations present in neurodegeneration.
ACKNOWLEDGMENTS
This work was supported by grants of the Deutsche Forschungsgemeinschaft (DFG) Collaborative Research Center SFB1036 TP8 and TP20 to B.B. and C.N.-K., respectively, the Baden-Württemberg Stiftung (BWST-ISFIII-029), and the Alzheimer Forschung Initiative e.V. (AFI, 17054). We thank Axel Mogk for critical reading of the manuscript.
Footnotes
Editors: Richard I. Morimoto, F. Ulrich Hartl, and Jeffery W. Kelly
Additional Perspectives on Protein Homeostasis available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Agarraberes FA, Dice JF. 2001. A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J Cell Sci 114: 2491–2499. [DOI] [PubMed] [Google Scholar]
- Altieri F, Di Stadio CS, Severino V, Sandomenico A, Minopoli G, Miselli G, Di Maro A, Ruvo M, Chambery A, Quagliariello V, et al. 2014. Anti-amyloidogenic property of human gastrokine 1. Biochimie 106: 91–100. 10.1016/j.biochi.2014.08.004 [DOI] [PubMed] [Google Scholar]
- Aprile FA, Källstig E, Limorenko G, Vendruscolo M, Ron D, Hansen C. 2017. The molecular chaperones DNAJB6 and Hsp70 cooperate to suppress α-synuclein aggregation. Sci Rep 7: 9039 10.1038/s41598-017-08324-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M, Furst DO, Saftig P, Saint R, Fleischmann BK, et al. 2010. Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr Biol 20: 143–148. 10.1016/j.cub.2009.11.022 [DOI] [PubMed] [Google Scholar]
- Arosio P, Michaels TC, Linse S, Månsson C, Emanuelsson C, Presto J, Johansson J, Vendruscolo M, Dobson CM, Knowles TP. 2016. Kinetic analysis reveals the diversity of microscopic mechanisms through which molecular chaperones suppress amyloid formation. Nat Commun 7: 10948 10.1038/ncomms10948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. 2004. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431: 805–810. 10.1038/nature02998 [DOI] [PubMed] [Google Scholar]
- Balchin D, Hayer-Hartl M, Hartl FU. 2016. In vivo aspects of protein folding and quality control. Science 353: aac4354 10.1126/science.aac4354 [DOI] [PubMed] [Google Scholar]
- Baldwin AJ, Knowles TP, Tartaglia GG, Fitzpatrick AW, Devlin GL, Shammas SL, Waudby CA, Mossuto MF, Meehan S, Gras SL, et al. 2011. Metastability of native proteins and the phenomenon of amyloid formation. J Am Chem Soc 133: 14160–14163. 10.1021/ja2017703 [DOI] [PubMed] [Google Scholar]
- Baughman HER, Clouser AF, Klevit RE, Nath A. 2018. HspB1 and Hsc70 chaperones engage distinct tau species and have different inhibitory effects on amyloid formation. J Biol Chem 293: 2687–2700. 10.1074/jbc.M117.803411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeg M, Stravalaci M, Romeo M, Carra AD, Cagnotto A, Rossi A, Diomede L, Salmona M, Gobbi M. 2016. Clusterin binds to Aβ1-42 oligomers with high affinity and interferes with peptide aggregation by inhibiting primary and secondary nucleation. J Biol Chem 291: 6958–6966. 10.1074/jbc.M115.689539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends C, Langer CA, Boteva R, Bottcher UM, Stemp MJ, Schaffar G, Rao BV, Giese A, Kretzschmar H, Siegers K, et al. 2006. Chaperonin TRiC promotes the assembly of polyQ expansion proteins into nontoxic oligomers. Mol Cell 23: 887–897. 10.1016/j.molcel.2006.08.017 [DOI] [PubMed] [Google Scholar]
- Ben-Zvi A, Miller EA, Morimoto RI. 2009. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci 106: 14914–14919. 10.1073/pnas.0902882106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Biebl MM, Buchner J. 2018. Structure, function, and regulation of the Hsp90 machinery. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a034017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake MJ, Udelsman R, Feulner GJ, Norton DD, Holbrook NJ. 1991. Stress-induced heat shock protein 70 expression in adrenal cortex: An adrenocorticotropic hormone-sensitive, age-dependent response. Proc Natl Acad Sci 88: 9873–9877. 10.1073/pnas.88.21.9873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancas-Mejía LM, Ramirez-Alvarado M. 2013. Systemic amyloidoses. Annu Rev Biochem 82: 745–774. 10.1146/annurev-biochem-072611-130030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J, Del Tredici K, Lee VM, Trojanowski JQ. 2015. Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nat Rev Neurosci 16: 109–120. 10.1038/nrn3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R, Kennelly SP, O'Neill D. 2016. Drug treatments in Alzheimer's disease. Clin Med (Lond) 16: 247–253. 10.7861/clinmedicine.16-3-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinsma IB, Bruggink KA, Kinast K, Versleijen AA, Segers-Nolten IM, Subramaniam V, Kuiperij HB, Boelens W, de Waal RM, Verbeek MM. 2011. Inhibition of α-synuclein aggregation by small heat shock proteins. Proteins 79: 2956–2967. 10.1002/prot.23152 [DOI] [PubMed] [Google Scholar]
- Brundin P, Melki R, Kopito R. 2010. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol 11: 301–307. 10.1038/nrm2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B, Weissman J, Horwich A. 2006. Molecular chaperones and protein quality control. Cell 125: 443–451. 10.1016/j.cell.2006.04.014 [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. 2015. Cysteine string protein (CSP) and its role in preventing neurodegeneration. Semin Cell Dev Biol 40: 153–159. 10.1016/j.semcdb.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioni S, Mannini B, Zampagni M, Pensalfini A, Parrini C, Evangelisti E, Relini A, Stefani M, Dobson CM, Cecchi C, et al. 2010. A causative link between the structure of aberrant protein oligomers and their toxicity. Nat Chem Biol 6: 140–147. 10.1038/nchembio.283 [DOI] [PubMed] [Google Scholar]
- Carulla N, Caddy GL, Hall DR, Zurdo J, Gairí M, Feliz M, Giralt E, Robinson CV, Dobson CM. 2005. Molecular recycling within amyloid fibrils. Nature 436: 554–558. 10.1038/nature03986 [DOI] [PubMed] [Google Scholar]
- Chafekar SM, Duennwald ML. 2012. Impaired heat shock response in cells expressing full-length polyglutamine-expanded huntingtin. PLoS ONE 7: e37929 10.1371/journal.pone.0037929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Ferrone FA, Wetzel R. 2002. Huntington's disease age-of-onset linked to polyglutamine aggregation nucleation. Proc Natl Acad Sci 99: 11884–11889. 10.1073/pnas.182276099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Genereux JC, Suh EH, Vartabedian VF, Rius B, Qu S, Dendle MTA, Kelly JW, Wiseman RL. 2016. Endoplasmic reticulum proteostasis influences the oligomeric state of an amyloidogenic protein secreted from mammalian cells. Cell Chem Biol 23: 1282–1293. 10.1016/j.chembiol.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff YO, Lindquist SL, Ono B, Inge-Vechtomov SG, Liebman SW. 1995. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. Science 268: 880–884. 10.1126/science.7754373 [DOI] [PubMed] [Google Scholar]
- Chiang HL, Dice JF. 1988. Peptide sequences that target proteins for enhanced degradation during serum withdrawal. J Biol Chem 263: 6797–6805. [PubMed] [Google Scholar]
- Chien V, Aitken JF, Zhang S, Buchanan CM, Hickey A, Brittain T, Cooper GJ, Loomes KM. 2010. The chaperone proteins HSP70, HSP40/DnaJ and GRP78/BiP suppress misfolding and formation of β-sheet-containing aggregates by human amylin: A potential role for defective chaperone biology in type 2 diabetes. Biochem J 432: 113–121. 10.1042/BJ20100434 [DOI] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. 2017. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu Rev Biochem 86: 27–68. 10.1146/annurev-biochem-061516-045115 [DOI] [PubMed] [Google Scholar]
- Cohen E, Dillin A. 2008. The insulin paradox: Aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci 9: 759–767. 10.1038/nrn2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SI, Linse S, Luheshi LM, Hellstrand E, White DA, Rajah L, Otzen DE, Vendruscolo M, Dobson CM, Knowles TP. 2013. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc Natl Acad Sci 110: 9758–9763. 10.1073/pnas.1218402110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SIA, Arosio P, Presto J, Kurudenkandy FR, Biverstal H, Dolfe L, Dunning C, Yang X, Frohm B, Vendruscolo M, et al. 2015. A molecular chaperone breaks the catalytic cycle that generates toxic Aβ oligomers. Nat Struct Mol Biol 22: 207–213. 10.1038/nsmb.2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Selig E, Griffin MD, Carver JA, Ecroyd H. 2016. Small heat-shock proteins prevent α-synuclein aggregation via transient interactions and their efficacy is affected by the rate of aggregation. J Biol Chem 291: 22618–22629. 10.1074/jbc.M116.739250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Whiten DR, Brown JWP, Horrocks MH, San Gil R, Dobson CM, Klenerman D, van Oijen AM, Ecroyd H. 2018. The small heat shock protein Hsp27 binds α-synuclein fibrils, preventing elongation and cytotoxicity. J Biol Chem 293: 4486–4497. 10.1074/jbc.M117.813865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremades N, Cohen SI, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne P, Aprile FA, et al. 2012. Direct observation of the interconversion of normal and toxic forms of α-synuclein. Cell 149: 1048–1059. 10.1016/j.cell.2012.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daturpalli S, Waudby CA, Meehan S, Jackson SE. 2013. Hsp90 inhibits α-synuclein aggregation by interacting with soluble oligomers. J Mol Biol 425: 4614–4628. 10.1016/j.jmb.2013.08.006 [DOI] [PubMed] [Google Scholar]
- Davis PD, Raffen R, Dul LJ, Vogen MS, Williamson KE, Stevens JF, Argon Y. 2000. Inhibition of amyloid fiber assembly by both BiP and its target peptide. Immunity 13: 433–442. 10.1016/S1074-7613(00)00043-1 [DOI] [PubMed] [Google Scholar]
- Dedmon MM, Christodoulou J, Wilson MR, Dobson CM. 2005. Heat shock protein 70 inhibits α-synuclein fibril formation via preferential binding to prefibrillar species. J Biol Chem 280: 14733–14740. 10.1074/jbc.M413024200 [DOI] [PubMed] [Google Scholar]
- De Los Rios P, Ben-Zvi A, Slutsky O, Azem A, Goloubinoff P. 2006. Hsp70 chaperones accelerate protein translocation and the unfolding of stable protein aggregates by entropic pulling. Proc Natl Acad Sci 103: 6166–6171. 10.1073/pnas.0510496103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng HX, Chen W, Hong ST, Boycott KM, Gorrie GH, Siddique N, Yang Y, Fecto F, Shi Y, Zhai H, et al. 2011. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 477: 211–215. 10.1038/nature10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Koutras C, Donnelier J, Alshehri M, Fotouhi M, Girard M, Casha S, McPherson PS, Robbins SM, Braun JEA. 2017. Neurons export extracellular vesicles enriched in cysteine string protein and misfolded protein cargo. Sci Rep 7: 956 10.1038/s41598-017-01115-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Dobson C. 2018. Amyloid phenomenon and significance for human disease. Cold Spring Harb Perspect Biol 10.1101/cshpersect.a033878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duennwald ML, Echeverria A, Shorter J. 2012. Small heat shock proteins potentiate amyloid dissolution by protein disaggregases from yeast and humans. PLoS Biol 10: e1001346 10.1371/journal.pbio.1001346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner T, Radford SE. 2011. A diversity of assembly mechanisms of a generic amyloid fold. Mol Cell 43: 8–18. 10.1016/j.molcel.2011.05.012 [DOI] [PubMed] [Google Scholar]
- *.Eisenberg D. 2018. Structural insights in amyloids and prion diseases. Cold Spring Harb Perspect Biol 7 10.1101/cshpersect.a033852 [DOI] [Google Scholar]
- Eisenberg DS, Sawaya MR. 2017. Structural studies of amyloid proteins at the molecular level. Annu Rev Biochem 86: 69–95. 10.1146/annurev-biochem-061516-045104 [DOI] [PubMed] [Google Scholar]
- Escusa-Toret S, Vonk WI, Frydman J. 2013. Spatial sequestration of misfolded proteins by a dynamic chaperone pathway enhances cellular fitness during stress. Nat Cell Biol 15: 1231–1243. 10.1038/ncb2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KC, Berger EP, Cho CG, Weisgraber KH, Lansbury PT. 1995. Apolipoprotein E is a kinetic but not a thermodynamic inhibitor of amyloid formation: Implications for the pathogenesis and treatment of Alzheimer disease. Proc Natl Acad Sci 92: 763–767. 10.1073/pnas.92.3.763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CG, Wisén S, Gestwicki JE. 2006. Heat shock proteins 70 and 90 inhibit early stages of amyloid β-(1-42) aggregation in vitro. J Biol Chem 281: 33182–33191. 10.1074/jbc.M606192200 [DOI] [PubMed] [Google Scholar]
- Falsone SF, Kungl AJ, Rek A, Cappai R, Zangger K. 2009. The molecular chaperone Hsp90 modulates intermediate steps of amyloid assembly of the Parkinson-related protein α-synuclein. J Biol Chem 284: 31190–31199. 10.1074/jbc.M109.057240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Finkbeiner S. 2018. Autophagy lysosomal pathways and neurodegeneration. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a033993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Finley D. 2018. Ubiquitin proteasome and the remodeling of life. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a033985 [DOI] [Google Scholar]
- Fitzpatrick AWP, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ, Crowther RA, Ghetti B, Goedert M, Scheres SHW. 2017. Cryo-EM structures of tau filaments from Alzheimer's disease. Nature 547: 185–190. 10.1038/nature23002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine SN, Zheng D, Sabbagh JJ, Martin MD, Chaput D, Darling A, Trotter JH, Stothert AR, Nordhues BA, Lussier A, et al. 2016. DnaJ/Hsc70 chaperone complexes control the extracellular release of neurodegenerative-associated proteins. EMBO J 35: 1537–1549. 10.15252/embj.201593489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman CL, Hesketh G, Seaman MN. 2014. RME-8 coordinates the activity of the WASH complex with the function of the retromer SNX dimer to control endosomal tubulation. J Cell Sci 127: 2053–2070. 10.1242/jcs.144659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikake N, Nagai Y, Popiel HA, Okamoto Y, Yamaguchi M, Toda T. 2008. Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. J Biol Chem 283: 26188–26197. 10.1074/jbc.M710521200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C. 2009. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J 28: 889–901. 10.1038/emboj.2009.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamerdinger M, Kaya AM, Wolfrum U, Clement AM, Behl C. 2011. BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO Rep 12: 149–156. 10.1038/embor.2010.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Carroni M, Nussbaum-Krammer C, Mogk A, Nillegoda NB, Szlachcic A, Guilbride DL, Saibil HR, Mayer MP, Bukau B. 2015. Human Hsp70 disaggregase reverses Parkinson's-linked α-synuclein amyloid fibrils. Mol Cell 59: 781–793. 10.1016/j.molcel.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gath J, Bousset L, Habenstein B, Melki R, Bockmann A, Meier BH. 2014. Unlike twins: An NMR comparison of two α-synuclein polymorphs featuring different toxicity. PLoS ONE 9: e90659 10.1371/journal.pone.0090659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genereux JC, Qu S, Zhou M, Ryno LM, Wang S, Shoulders MD, Kaufman RJ, Lasmezas CI, Kelly JW, Wiseman RL. 2015. Unfolded protein response-induced ERdj3 secretion links ER stress to extracellular proteostasis. EMBO J 34: 4–19. 10.15252/embj.201488896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis J, Schipper-Krom S, Juenemann K, Gruber A, Coolen S, van den Nieuwendijk R, van Veen H, Overkleeft H, Goedhart J, Kampinga HH, et al. 2013. The DNAJB6 and DNAJB8 protein chaperones prevent intracellular aggregation of polyglutamine peptides. J Biol Chem 288: 17225–17237. 10.1074/jbc.M112.421685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Clavaguera F, Tolnay M. 2010. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci 33: 317–325. 10.1016/j.tins.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Goloubinoff P, De Los Rios P. 2007. The mechanism of Hsp70 chaperones: (Entropic) pulling the models together. Trends Biochem Sci 32: 372–380. 10.1016/j.tibs.2007.06.008 [DOI] [PubMed] [Google Scholar]
- Greene MJ, Klimtchuk ES, Seldin DC, Berk JL, Connors LH. 2015. Cooperative stabilization of transthyretin by clusterin and diflunisal. Biochemistry 54: 268–278. 10.1021/bi5011249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremer L, Scholzel D, Schenk C, Reinartz E, Labahn J, Ravelli RBG, Tusche M, Lopez-Iglesias C, Hoyer W, Heise H, et al. 2017. Fibril structure of amyloid-β(1-42) by cryo-electron microscopy. Science 358: 116–119. 10.1126/science.aao2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Ferreira R, Taylor NMI, Mona D, Ringler P, Lauer ME, Riek R, Britschgi M, Stahlberg H. 2018. Cryo-EM structure of α-synuclein fibrils. eLife 7: e36402 10.7554/eLife.36402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Lehmer C, Martinez-Sanchez A, Rudack T, Beck F, Hartmann H, Perez-Berlanga M, Frottin F, Hipp MS, Hartl FU, et al. 2018. In situ structure of neuronal C9orf72 poly-GA aggregates reveals proteasome recruitment. Cell 172: 696–705.e12. 10.1016/j.cell.2017.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hartl U. 2018. Protein folding and misfolding in the cytoplasm. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a033951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F, Bracher A. 2011. Molecular chaperones in protein folding and proteostasis. Nature 475: 324–332. 10.1038/nature10317 [DOI] [PubMed] [Google Scholar]
- Hay DG, Sathasivam K, Tobaben S, Stahl B, Marber M, Mestril R, Mahal A, Smith DL, Woodman B, Bates GP. 2004. Progressive decrease in chaperone protein levels in a mouse model of Huntington's disease and induction of stress proteins as a therapeutic approach. Hum Mol Genet 13: 1389–1405. 10.1093/hmg/ddh144 [DOI] [PubMed] [Google Scholar]
- Helwig M, Hoshino A, Berridge C, Lee SN, Lorenzen N, Otzen DE, Eriksen JL, Lindberg I. 2013. The neuroendocrine protein 7B2 suppresses the aggregation of neurodegenerative disease-related proteins. J Biol Chem 288: 1114–1124. 10.1074/jbc.M112.417071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinault MP, Cuendet AF, Mattoo RU, Mensi M, Dietler G, Lashuel HA, Goloubinoff P. 2010. Stable α-synuclein oligomers strongly inhibit chaperone activity of the Hsp70 system by weak interactions with J-domain co-chaperones. J Biol Chem 285: 38173–38182. 10.1074/jbc.M110.127753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp MS, Park SH, Hartl FU. 2014. Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol 24: 506–514. 10.1016/j.tcb.2014.05.003 [DOI] [PubMed] [Google Scholar]
- Hjerpe R, Bett JS, Keuss MJ, Solovyova A, McWilliams TG, Johnson C, Sahu I, Varghese J, Wood N, Wightman M, et al. 2016. UBQLN2 mediates autophagy-independent protein aggregate clearance by the proteasome. Cell 166: 935–949. 10.1016/j.cell.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SR, Khorkova O, Goyal S, Knaeblein J, Heroux J, Riedel NG, Sahasrabudhe S. 1998. α2-macroglobulin associates with β-amyloid peptide and prevents fibril formation. Proc Natl Acad Sci 95: 3275–3280. 10.1073/pnas.95.6.3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Janowska MK, Baughman HER, Woods CN, Klevit RE. 2018. Mechanisms of small heat shock proteins. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a034025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji CH, Kwon YT. 2017. Crosstalk and interplay between the ubiquitin–proteasome system and autophagy. Mol Cells 40: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YQ, Wang XL, Cao XH, Ye ZY, Li L, Cai WQ. 2013. Increased heat shock transcription factor 1 in the cerebellum reverses the deficiency of Purkinje cells in Alzheimer's disease. Brain Res 1519: 105–111. 10.1016/j.brainres.2013.04.059 [DOI] [PubMed] [Google Scholar]
- Jinwal UK, Koren J III, Borysov SI, Schmid AB, Abisambra JF, Blair LJ, Johnson AG, Jones JR, Shults CL, O'Leary JC III, et al. 2010. The Hsp90 cochaperone, FKBP51, increases Tau stability and polymerizes microtubules. J Neurosci 30: 591–599. 10.1523/jneuroscI.4815-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinwal UK, Akoury E, Abisambra JF, O'Leary JC III, Thompson AD, Blair LJ, Jin Y, Bacon J, Nordhues BA, Cockman M, et al. 2013. Imbalance of Hsp70 family variants fosters tau accumulation. FASEB J 27: 1450–1459. 10.1096/fj.12-220889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JA, Ward CL, Kopito RR. 1998. Aggresomes: A cellular response to misfolded proteins. J Cell Biol 143: 1883–1898. 10.1083/jcb.143.7.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Joutsen J, Sistonen L. 2018. Tailoring of proteostasis networks with heat shock factors. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a034066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar V, Meister-Broekema M, Minoia M, Carra S, Kampinga HH. 2014. Barcoding heat shock proteins to human diseases: looking beyond the heat shock response. Dis Model Mech 7: 421–434. 10.1242/dmm.014563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar V, Mansson C, de Mattos EP, Bergink S, van der Zwaag M, van Waarde MA, Kloosterhuis NJ, Melki R, van Cruchten RT, Al-Karadaghi S, et al. 2016. The S/T-rich motif in the DNAJB6 chaperone delays polyglutamine aggregation and the onset of disease in a mouse model. Mol Cell 62: 272–283. 10.1016/j.molcel.2016.03.017 [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Bergink S. 2016. Heat shock proteins as potential targets for protective strategies in neurodegeneration. Lancet Neurol 15: 748–759. 10.1016/S1474-4422(16)00099-5 [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA. 2010. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11: 579–592. 10.1038/nrm2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagoz GE, Duarte AMS, Akoury E, Ippel H, Biernat J, Luengo TM, Radli M, Didenko T, Nordhues BA, Veprintsev DB, et al. 2014. Hsp90–tau complex reveals molecular basis for specificity in chaperone action. Cell 156: 963–974. 10.1016/j.cell.2014.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kästle M, Grune T. 2012. Interactions of the proteasomal system with chaperones: Protein triage and protein quality control. Prog Mol Biol Transl Sci 109: 113–160. 10.1016/B978-0-12-397863-9.00004-3 [DOI] [PubMed] [Google Scholar]
- Kim YE, Hosp F, Frottin F, Ge H, Mann M, Hayer-Hartl M, Hartl FU. 2016. Soluble oligomers of polyQ-expanded huntingtin target a multiplicity of key cellular factors. Mol Cell 63: 951–964. 10.1016/j.molcel.2016.07.022 [DOI] [PubMed] [Google Scholar]
- Kirstein J, Arnsburg K, Scior A, Szlachcic A, Guilbride DL, Morimoto RI, Bukau B, Nillegoda NB. 2017. In vivo properties of the disaggregase function of J-proteins and Hsc70 in Caenorhabditis elegans stress and aging. Aging Cell 16: 1414–1424. 10.1111/acel.12686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles TP, Waudby CA, Devlin GL, Cohen SI, Aguzzi A, Vendruscolo M, Terentjev EM, Welland ME, Dobson CM. 2009. An analytical solution to the kinetics of breakable filament assembly. Science 326: 1533–1537. 10.1126/science.1178250 [DOI] [PubMed] [Google Scholar]
- Knowles TP, Vendruscolo M, Dobson CM. 2014. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol 15: 384–396. 10.1038/nrm3810 [DOI] [PubMed] [Google Scholar]
- Kopito RR. 2000. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol 10: 524–530. 10.1016/S0962-8924(00)01852-3 [DOI] [PubMed] [Google Scholar]
- Kumita JR, Poon S, Caddy GL, Hagan CL, Dumoulin M, Yerbury JJ, Stewart EM, Robinson CV, Wilson MR, Dobson CM. 2007. The extracellular chaperone clusterin potently inhibits human lysozyme amyloid formation by interacting with prefibrillar species. J Mol Biol 369: 157–167. 10.1016/j.jmb.2007.02.095 [DOI] [PubMed] [Google Scholar]
- Labbadia J, Morimoto RI. 2015. The biology of proteostasis in aging and disease. Annu Rev Biochem 84: 435–464. 10.1146/annurev-biochem-060614-033955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbadia J, Novoselov SS, Bett JS, Weiss A, Paganetti P, Bates GP, Cheetham ME. 2012. Suppression of protein aggregation by chaperone modification of high molecular weight complexes. Brain 135: 1180–1196. 10.1093/brain/aws022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashuel HA, Overk CR, Oueslati A, Masliah E. 2013. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat Rev Neurosci 14: 38–48. 10.1038/nrn3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Brewer JW, Hellman R, Hendershot LM. 1999. BiP and immunoglobulin light chain cooperate to control the folding of heavy chain and ensure the fidelity of immunoglobulin assembly. Mol Biol Cell 10: 2209–2219. 10.1091/mbc.10.7.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Carson K, Rice-Ficht A, Good T. 2005. Hsp20, a novel α-crystallin, prevents Aβ fibril formation and toxicity. Protein Sci 14: 593–601. 10.1110/ps.041020705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JG, Takahama S, Zhang G, Tomarev SI, Ye Y. 2016. Unconventional secretion of misfolded proteins promotes adaptation to proteasome dysfunction in mammalian cells. Nat Cell Biol 18: 765–776. 10.1038/ncb3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Soroka J, Buchner J. 2012. The Hsp90 chaperone machinery: Conformational dynamics and regulation by co-chaperones. Biochim Biophys Acta 1823: 624–635. 10.1016/j.bbamcr.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Liangliang X, Yonghui H, Shunmei E, Shoufang G, Wei Z, Jiangying Z. 2010. Dominant-positive HSF1 decreases α-synuclein level and α-synuclein-induced toxicity. Mol Biol Rep 37: 1875–1881. 10.1007/s11033-009-9623-2 [DOI] [PubMed] [Google Scholar]
- Lin MK, Farrer MJ. 2014. Genetics and genomics of Parkinson's disease. Genome Med 6: 48 10.1186/gm566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Fonseca T, Villar-Piqué A, Outeiro TF. 2015. The Interplay between α-synuclein clearance and spreading. Biomolecules 5: 435–471. 10.3390/biom5020435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders J, Demand J, Hohfeld J. 2000. The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/Hsp70 and the proteasome. J Biol Chem 275: 4613–4617. 10.1074/jbc.275.7.4613 [DOI] [PubMed] [Google Scholar]
- Luheshi LM, Hoyer W, de Barros TP, van Dijk Hard I, Brorsson AC, Macao B, Persson C, Crowther DC, Lomas DA, Stahl S, et al. 2010. Sequestration of the Aβ peptide prevents toxicity and promotes degradation in vivo. PLoS Biol 8: e1000334 10.1371/journal.pbio.1000334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhrs T, Ritter C, Adrian M, Riek-Loher D, Bohrmann B, Dobeli H, Schubert D, Riek R. 2005. 3D structure of Alzheimer's amyloid-β(1-42) fibrils. Proc Natl Acad Sci 102: 17342–17347. 10.1073/pnas.0506723102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainz A, Peschek J, Stavropoulou M, Back KC, Bardiaux B, Asami S, Prade E, Peters C, Weinkauf S, Buchner J, et al. 2015. The chaperone αB-crystallin uses different interfaces to capture an amorphous and an amyloid client. Nat Struct Mol Biol 22: 898–905. 10.1038/nsmb.3108 [DOI] [PubMed] [Google Scholar]
- Mannini B, Cascella R, Zampagni M, van Waarde-Verhagen M, Meehan S, Roodveldt C, Campioni S, Boninsegna M, Penco A, Relini A, et al. 2012. Molecular mechanisms used by chaperones to reduce the toxicity of aberrant protein oligomers. Proc Natl Acad Sci 109: 12479–12484. 10.1073/pnas.1117799109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoutcharian K, Perez-Garmendia R, Gevorkian G. 2017. Recombinant antibody fragments for neurodegenerative diseases. Curr Neuropharmacol 15: 779–788. 10.2174/1570159X01666160930121647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Månsson C, Arosio P, Hussein R, Kampinga HH, Hashem RM, Boelens WC, Dobson CM, Knowles TP, Linse S, Emanuelsson C. 2014a. Interaction of the molecular chaperone DNAJB6 with growing amyloid-β 42 (Aβ42) aggregates leads to sub-stoichiometric inhibition of amyloid formation. J Biol Chem 289: 31066–31076. 10.1074/jbc.M114.595124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Månsson C, Kakkar V, Monsellier E, Sourigues Y, Harmark J, Kampinga HH, Melki R, Emanuelsson C. 2014b. DNAJB6 is a peptide-binding chaperone which can suppress amyloid fibrillation of polyglutamine peptides at substoichiometric molar ratios. Cell Stress Chaperones 19: 227–239. 10.1007/s12192-013-0448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Månsson C, van Cruchten RTP, Weininger U, Yang X, Cukalevski R, Arosio P, Dobson CM, Knowles T, Akke M, Linse S, et al. 2018. Conserved S/T residues of the human chaperone DNAJB6 are required for effective inhibition of Aβ42 amyloid fibril formation. Biochemistry 57: 4891–4902. 10.1021/acs.biochem.8b00353 [DOI] [PubMed] [Google Scholar]
- Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin JH, et al. 2016. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353: aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Jackson MP, Gamerdinger M, Gense K, Karamonos TK, Humes JR, Deuerling E, Ashcroft AE, Radford SE. 2018. Conformational flexibility within the nascent polypeptide-associated complex enables its interactions with structurally diverse client proteins. J Biol Chem 293: 8554–8568. 10.1074/jbc.RA117.001568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo RU, Goloubinoff P. 2014. Molecular chaperones are nanomachines that catalytically unfold misfolded and alternatively folded proteins. Cell Mol Life Sci 71: 3311–3325. 10.1007/s00018-014-1627-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo RU, Sharma SK, Priya S, Finka A, Goloubinoff P. 2013. Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with Hsp70 to solubilize protein aggregates. J Biol Chem 288: 21399–21411. 10.1074/jbc.M113.479253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki R. 2018. How the shapes of seeds can influence pathology. Neurobiol Dis 109: 201–208. 10.1016/j.nbd.2017.03.011 [DOI] [PubMed] [Google Scholar]
- Melnick J, Dul JL, Argon Y. 1994. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature 370: 373–375. 10.1038/370373a0 [DOI] [PubMed] [Google Scholar]
- Messer A, Joshi SN. 2013. Intrabodies as neuroprotective therapeutics. Neurotherapeutics 10: 447–458. 10.1007/s13311-013-0193-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SB, Ho CT, Winkler J, Khokhrina M, Neuner A, Mohamed MY, Guilbride DL, Richter K, Lisby M, Schiebel E, et al. 2015. Compartment-specific aggregases direct distinct nuclear and cytoplasmic aggregate deposition. EMBO J 34: 778–797. 10.15252/embj.201489524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk A, Bukau B, Kampinga HH. 2018. Cellular handling of protein aggregates by disaggregation machines. Mol Cell 69: 214–226. 10.1016/j.molcel.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Mok SA, Condello C, Freilich R, Gillies A, Arhar T, Oroz J, Kadavath H, Julien O, Assimon VA, Rauch JN, et al. 2018. Mapping interactions with the chaperone network reveals factors that protect against tau aggregation. Nat Struct Mol Biol 25: 384–393. 10.1038/s41594-018-0057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsellier E, Redeker V, Ruiz-Arlandis G, Bousset L, Melki R. 2015. Molecular Interaction between the chaperone Hsc70 and the N-terminal flank of Huntingtin exon 1 modulates aggregation. J Biol Chem 290: 2560–2576. 10.1074/jbc.M114.603332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Morimoto RI. 2018. Cell non-autonomous regulation of proteostasis in aging and disease. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a034074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AM, Watzky MA, Finke RG. 2009. Protein aggregation kinetics, mechanism, and curve-fitting: A review of the literature. Biochim Biophys Acta 1794: 375–397. 10.1016/j.bbapap.2008.10.016 [DOI] [PubMed] [Google Scholar]
- Muchowski PJ, Schaffar G, Sittler A, Wanker EE, Hayer-Hartl MK, Hartl FU. 2000. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc Natl Acad Sci 97: 7841–7846. 10.1073/pnas.140202897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P, Orte A, Clarke RW, Bolognesi B, Hook S, Ganzinger KA, Meehan S, Wilson MR, Dobson CM, Klenerman D. 2012. The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-β(1-40) peptide. Nat Struct Mol Biol 19: 79–83. 10.1038/nsmb.2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudecker P, Robustelli P, Cavalli A, Walsh P, Lundstrom P, Zarrine-Afsar A, Sharpe S, Vendruscolo M, Kay LE. 2012. Structure of an intermediate state in protein folding and aggregation. Science 336: 362–366. 10.1126/science.1214203 [DOI] [PubMed] [Google Scholar]
- Ngolab J, Trinh I, Rockenstein E, Mante M, Florio J, Trejo M, Masliah D, Adame A, Masliah E, Rissman RA. 2017. Brain-derived exosomes from dementia with Lewy bodies propagate α-synuclein pathology. Acta Neuropathol Commun 5: 46 10.1186/s40478-017-0445-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillegoda NB, Kirstein J, Szlachcic A, Berynskyy M, Stank A, Stengel F, Arnsburg K, Gao X, Scior A, Aebersold R, et al. 2015. Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature 524: 247–251. 10.1038/nature14884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillegoda NB, Wentink AS, Bukau B. 2018. Protein disaggregation in multicellular organisms. Trends Biochem Sci 43: 285–300. 10.1016/j.tibs.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Oh SH, Kim HN, Park HJ, Shin JY, Bae EJ, Sunwoo MK, Lee SJ, Lee PH. 2016. Mesenchymal stem cells inhibit transmission of α-synuclein by modulating clathrin-mediated endocytosis in a parkinsonian model. Cell Rep 14: 835–849. 10.1016/j.celrep.2015.12.075 [DOI] [PubMed] [Google Scholar]
- Ojha J, Masilamoni G, Dunlap D, Udoff RA, Cashikar AG. 2011. Sequestration of toxic oligomers by HspB1 as a cytoprotective mechanism. Mol Cell Biol 31: 3146–3157. 10.1128/MCB.01187-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olzscha H, Schermann SM, Woerner AC, Pinkert S, Hecht MH, Tartaglia GG, Vendruscolo M, Hayer-Hartl M, Hartl FU, Vabulas RM. 2011. Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell 144: 67–78. 10.1016/j.cell.2010.11.050 [DOI] [PubMed] [Google Scholar]
- Oskarsson ME, Hermansson E, Wang Y, Welsh N, Presto J, Johansson J, Westermark GT. 2018. BRICHOS domain of Bri2 inhibits islet amyloid polypeptide (IAPP) fibril formation and toxicity in human β cells. Proc Natl Acad Sci 115: E2752–E2761. 10.1073/pnas.1715951115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Otzen D, Riek R. 2018. Functional amyloids. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a033860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oueslati A, Fournier M, Lashuel HA. 2010. Role of post-translational modifications in modulating the structure, function and toxicity of α-synuclein: implications for Parkinson's disease pathogenesis and therapies. Prog Brain Res 183: 115–145. 10.1016/S0079-6123(10)83007-9 [DOI] [PubMed] [Google Scholar]
- Paravastu AK, Leapman RD, Yau WM, Tycko R. 2008. Molecular structural basis for polymorphism in Alzheimer's β-amyloid fibrils. Proc Natl Acad Sci 105: 18349–18354. 10.1073/pnas.0806270105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D, Ganey NJ, Laporte V, Patel NS, Beaulieu-Abdelahad D, Bachmeier C, March A, Ait-Ghezala G, Mullan MJ. 2010. Reduction of β-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer's disease. J Neuroinflammation 7: 17 10.1186/1742-2094-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Kukushkin Y, Gupta R, Chen T, Konagai A, Hipp MS, Hayer-Hartl M, Hartl FU. 2013. PolyQ proteins interfere with nuclear degradation of cytosolic proteins by sequestering the Sis1p chaperone. Cell 154: 134–145. 10.1016/j.cell.2013.06.003 [DOI] [PubMed] [Google Scholar]
- Pavel M, Imarisio S, Menzies FM, Jimenez-Sanchez M, Siddiqi FH, Wu X, Renna M, O'Kane CJ, Crowther DC, Rubinsztein DC. 2016. CCT complex restricts neuropathogenic protein aggregation via autophagy. Nat Commun 7: 13821 10.1038/ncomms13821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado JR, Sami F, Rajpurohit N, Lindberg I. 2013. Blockade of islet amyloid polypeptide fibrillation and cytotoxicity by the secretory chaperones 7B2 and proSAAS. FEBS Lett 587: 3406–3411. 10.1016/j.febslet.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton S, Madiona K, Pieri L, Kabani M, Bousset L, Melki R. 2011. Hsc70 protein interaction with soluble and fibrillar α-synuclein. J Biol Chem 286: 34690–34699. 10.1074/jbc.M111.261321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A, Podlutskaya N, Halloran JJ, Hussong SA, Lin PY, Burbank R, Hart MJ, Galvan V. 2013. Over-expression of heat shock factor 1 phenocopies the effect of chronic inhibition of TOR by rapamycin and is sufficient to ameliorate Alzheimer's-like deficits in mice modeling the disease. J Neurochem 124: 880–893. 10.1111/jnc.12080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poska H, Haslbeck M, Kurudenkandy FR, Hermansson E, Chen G, Kostallas G, Abelein A, Biverstal H, Crux S, Fisahn A, et al. 2016. Dementia-related Bri2 BRICHOS is a versatile molecular chaperone that efficiently inhibits Aβ42 toxicity in Drosophila. Biochem J 473: 3683–3704. 10.1042/BCJ20160277 [DOI] [PubMed] [Google Scholar]
- Prabhu S, Raman B, Ramakrishna T, Rao CM. 2012. HspB2/myotonic dystrophy protein kinase binding protein (MKBP) as a novel molecular chaperone: structural and functional aspects. PLoS ONE 7: e29810 10.1371/journal.pone.0029810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. 1998. Prions. Proc Natl Acad Sci 95: 13363–13383. 10.1073/pnas.95.23.13363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintas A, Vaz DC, Cardoso I, Saraiva MJ, Brito RM. 2001. Tetramer dissociation and monomer partial unfolding precedes protofibril formation in amyloidogenic transthyretin variants. J Biol Chem 276: 27207–27213. 10.1074/jbc.M101024200 [DOI] [PubMed] [Google Scholar]
- Quist A, Doudevski I, Lin H, Azimova R, Ng D, Frangione B, Kagan B, Ghiso J, Lal R. 2005. Amyloid ion channels: A common structural link for protein-misfolding disease. Proc Natl Acad Sci 102: 10427–10432. 10.1073/pnas.0502066102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman B, Ban T, Sakai M, Pasta SY, Ramakrishna T, Naiki H, Goto Y, Rao Ch M. 2005. αB-crystallin, a small heat-shock protein, prevents the amyloid fibril growth of an amyloid β-peptide and β2-microglobulin. Biochem J 392: 573–581. 10.1042/BJ20050339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries HM, Nussbaum-Krammer C. 2016. Shape matters: The complex relationship between aggregation and toxicity in protein-misfolding diseases. Essays Biochem 60: 181–190. 10.1042/EBC20160008 [DOI] [PubMed] [Google Scholar]
- Rosas PC, Nagaraja GM, Kaur P, Panossian A, Wickman G, Garcia LR, Al-Khamis FA, Asea AA. 2016. hsp72 (hspa1a) prevents human islet amyloid polypeptide aggregation and toxicity: A new approach for type 2 diabetes treatment. PLoS ONE 11: e0149409 10.1371/journal.pone.0149409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudiger S, Germeroth L, Schneider-Mergener J, Bukau B. 1997. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J 16: 1501–1507. 10.1093/emboj/16.7.1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudiger S, Schneider-Mergener J, Bukau B. 2001. Its substrate specificity characterizes the DnaJ co-chaperone as a scanning factor for the DnaK chaperone. EMBO J 20: 1042–1050. 10.1093/emboj/20.5.1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, Potolicchio I, Nieves E, Cuervo AM, Santambrogio L. 2011. Microautophagy of cytosolic proteins by late endosomes. Dev Cell 20: 131–139. 10.1016/j.devcel.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibil H. 2013. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol 14: 630–642. 10.1038/nrm3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakono M, Zako T, Ueda H, Yohda M, Maeda M. 2008. Formation of highly toxic soluble amyloid β oligomers by the molecular chaperone prefoldin. FEBS J 275: 5982–5993. 10.1111/j.1742-4658.2008.06727.x [DOI] [PubMed] [Google Scholar]
- Schirmer C, Lepvrier E, Duchesne L, Decaux O, Thomas D, Delamarche C, Garnier C. 2016. Hsp90 directly interacts, in vitro, with amyloid structures and modulates their assembly and disassembly. Biochim Biophys Acta 1860: 2598–2609. 10.1016/j.bbagen.2016.07.033 [DOI] [PubMed] [Google Scholar]
- Scior A, Buntru A, Arnsburg K, Ast A, Iburg M, Juenemann K, Pigazzini ML, Mlody B, Puchkov D, Priller J, et al. 2018. Complete suppression of Htt fibrilization and disaggregation of Htt fibrils by a trimeric chaperone complex. EMBO J 37: 282–299. 10.15252/embj.201797212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serio TR, Cashikar AG, Kowal AS, Sawicki GJ, Moslehi JJ, Serpell L, Arnsdorf MF, Lindquist SL. 2000. Nucleated conformational conversion and the replication of conformational information by a prion determinant. Science 289: 1317–1321. 10.1126/science.289.5483.1317 [DOI] [PubMed] [Google Scholar]
- Shahmoradian SH, Galaz-Montoya JG, Schmid MF, Cong Y, Ma B, Spiess C, Frydman J, Ludtke SJ, Chiu W. 2013. TRiC's tricks inhibit huntingtin aggregation. eLife 2: e00710 10.7554/eLife.00710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammas SL, Waudby CA, Wang S, Buell AK, Knowles TP, Ecroyd H, Welland ME, Carver JA, Dobson CM, Meehan S. 2011. Binding of the molecular chaperone αB-crystallin to Aβ amyloid fibrils inhibits fibril elongation. Biophys J 101: 1681–1689. 10.1016/j.bpj.2011.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Burre J, Bronk P, Zhang Y, Xu W, Sudhof TC. 2012. CSPα knockout causes neurodegeneration by impairing SNAP-25 function. EMBO J 31: 829–841. 10.1038/emboj.2011.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton LB, Baker JD, Zheng D, Sullivan LE, Solanki PK, Webster JM, Sun Z, Sabbagh JJ, Nordhues BA, Koren J III, et al. 2017. Hsp90 activator Aha1 drives production of pathological tau aggregates. Proc Natl Acad Sci 114: 9707–9712. 10.1073/pnas.1707039114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi A, Sun L, Banerjee R, Tobin M, Zhang Y, Grant BD. 2009. Regulation of endosomal clathrin and retromer-mediated endosome to Golgi retrograde transport by the J-domain protein RME-8. EMBO J 28: 3290–3302. 10.1038/emboj.2009.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Shorter J, Southworth DR. 2018. Spiraling in control: Structures and mechanisms of the Hsp104 disaggregase. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a034033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg CAG, Mansson C, Bernfur K, Rutsdottir G, Harmark J, Rajan S, Al-Karadaghi S, Rasmussen M, Hojrup P, Hebert H, et al. 2018. Structural modelling of the DNAJB6 oligomeric chaperone shows a peptide-binding cleft lined with conserved S/T-residues at the dimer interface. Sci Rep 8: 5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgjerd K, Ghafouri B, Jonsson BH, Kelly JW, Blond SY, Hammarstrom P. 2006. Retention of misfolded mutant transthyretin by the chaperone BiP/GRP78 mitigates amyloidogenesis. J Mol Biol 356: 469–482. 10.1016/j.jmb.2005.11.051 [DOI] [PubMed] [Google Scholar]
- Sot B, Rubio-Muñoz A, Leal-Quintero A, Martínez-Sabando J, Marcilla M, Roodveldt C, Valpuesta JM. 2017. The chaperonin CCT inhibits assembly of α-synuclein amyloid fibrils by a specific, conformation-dependent interaction. Sci Rep 7: 40859 10.1038/srep40859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto C. 2003. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci 4: 49–60. 10.1038/nrn1007 [DOI] [PubMed] [Google Scholar]
- Sousa R, Lafer EM. 2015. The role of molecular chaperones in clathrin mediated vesicular trafficking. Front Mol Biosci 2: 26 10.3389/fmolb.2015.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht S, Miller SBM, Mogk A, Bukau B. 2011. Hsp42 is required for sequestration of protein aggregates into deposition sites in Saccharomyces cerevisiae. J Cell Biol 195: 617–629. 10.1083/jcb.201106037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroo E, Koopman M, Nollen EA, Mata-Cabana A. 2017. Cellular regulation of amyloid formation in aging and disease. Front Neurosci 11: 64 10.3389/fnins.2017.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CS, Lee CC, Li YN, Yao-Chen Yang S, Lin CH, Chang YC, Liu PF, He RY, Wang CH, Chen W, et al. 2015. Conformational switch of polyglutamine-expanded huntingtin into benign aggregates leads to neuroprotective effect. Sci Rep 5: 14992 10.1038/srep14992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam S, Geller R, Spiess C, Frydman J. 2006. The chaperonin TRiC controls polyglutamine aggregation and toxicity through subunit-specific interactions. Nat Cell Biol 8: 1155–1162. 10.1038/ncb1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam S, Spiess C, Auyeung W, Joachimiak L, Chen B, Poirier MA, Frydman J. 2009. The chaperonin TRiC blocks a huntingtin sequence element that promotes the conformational switch to aggregation. Nat Struct Mol Biol 16: 1279–1285. 10.1038/nsmb.1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Collins SR, Toyama BH, Weissman JS. 2006. The physical basis of how prion conformations determine strain phenotypes. Nature 442: 585–589. 10.1038/nature04922 [DOI] [PubMed] [Google Scholar]
- Tashiro E, Zako T, Muto H, Itoo Y, Sorgjerd K, Terada N, Abe A, Miyazawa M, Kitamura A, Kitaura H, et al. 2013. Prefoldin protects neuronal cells from polyglutamine toxicity by preventing aggregation formation. J Biol Chem 288: 19958–19972. 10.1074/jbc.M113.477984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekirdag K, Cuervo AM. 2018. Chaperone-mediated autophagy and endosomal microautophagy: Jointed by a chaperone. J Biol Chem 293: 5414–5424. 10.1074/jbc.R117.818237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaudeau TA, Anderson RT, Smith DM. 2018. A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nat Commun 9: 1097 10.1038/s41467-018-03509-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AD, Scaglione KM, Prensner J, Gillies AT, Chinnaiyan A, Paulson HL, Jinwal UK, Dickey CA, Gestwicki JE. 2012. Analysis of the tau-associated proteome reveals that exchange of Hsp70 for Hsp90 is involved in tau degradation. ACS Chem Biol 7: 1677–1686. 10.1021/cb3002599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping KW, Karamanos TK, Jakhria T, Iadanza MG, Goodchild SC, Tuma R, Ranson NA, Hewitt EW, Radford SE. 2015. pH-induced molecular shedding drives the formation of amyloid fibril-derived oligomers. Proc Natl Acad Sci 112: 5691–5696. 10.1073/pnas.1423174112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle MD, Comellas G, Nieuwkoop AJ, Covell DJ, Berthold DA, Kloepper KD, Courtney JM, Kim JK, Barclay AM, Kendall A, et al. 2016. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat Struct Mol Biol 23: 409–415. 10.1038/nsmb.3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyedmers J, Mogk A, Bukau B. 2010. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol 11: 777–788. 10.1038/nrm2993 [DOI] [PubMed] [Google Scholar]
- Ungelenk S, Moayed F, Ho CT, Grousl T, Scharf A, Mashaghi A, Tans S, Mayer MP, Mogk A, Bukau B. 2016. Small heat shock proteins sequester misfolding proteins in near-native conformation for cellular protection and efficient refolding. Nat Commun 7: 13673 10.1038/ncomms13673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E, Masliah E. 2016. Therapeutic approaches in Parkinson's disease and related disorders. J Neurochem 139 (Suppl 1): 346–352. 10.1111/jnc.13529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria GS, Zurzolo C. 2017. The spread of prion-like proteins by lysosomes and tunneling nanotubes: Implications for neurodegenerative diseases. J Cell Biol 216: 2633–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar M, Chou HT, Luhrs T, Maji SK, Riek-Loher D, Verel R, Manning G, Stahlberg H, Riek R. 2008. The fold of α-synuclein fibrils. Proc Natl Acad Sci 105: 8637–8642. 10.1073/pnas.0712179105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss K, Combs B, Patterson KR, Binder LI, Gamblin TC. 2012. Hsp70 alters tau function and aggregation in an isoform specific manner. Biochemistry 51: 888–898. 10.1021/bi2018078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker JL, Zareie MH, Fong H, Sarikaya M, Muchowski PJ. 2004. Hsp70 and Hsp40 attenuate formation of spherical and annular polyglutamine oligomers by partitioning monomer. Nat Struct Mol Biol 11: 1215–1222. 10.1038/nsmb860 [DOI] [PubMed] [Google Scholar]
- Walther DM, Kasturi P, Zheng M, Pinkert S, Vecchi G, Ciryam P, Morimoto RI, Dobson CM, Vendruscolo M, Mann M, et al. 2015. Widespread proteome remodeling and aggregation in aging C. elegans. Cell 161: 919–932. 10.1016/j.cell.2015.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JZ, Xia YY, Grundke-Iqbal I, Iqbal K. 2013. Abnormal hyperphosphorylation of tau: Sites, regulation, and molecular mechanism of neurofibrillary degeneration. J Alzheimers Dis 33 (Suppl 1): S123–139. [DOI] [PubMed] [Google Scholar]
- Waudby CA, Knowles TP, Devlin GL, Skepper JN, Ecroyd H, Carver JA, Welland ME, Christodoulou J, Dobson CM, Meehan S. 2010. The interaction of αB-crystallin with mature α-synuclein amyloid fibrils inhibits their elongation. Biophys J 98: 843–851. 10.1016/j.bpj.2009.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiten DR, Cox D, Horrocks MH, Taylor CG, De S, Flagmeier P, Tosatto L, Kumita JR, Ecroyd H, Dobson CM, et al. 2018. Single-molecule characterization of the interactions between extracellular chaperones and toxic α-synuclein oligomers. Cell Rep 23: 3492–3500. 10.1016/j.celrep.2018.05.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmus MM, Boelens WC, Otte-Höller I, Kamps B, de Waal RM, Verbeek MM. 2006a. Small heat shock proteins inhibit amyloid-β protein aggregation and cerebrovascular amyloid-β protein toxicity. Brain Res 1089: 67–78. 10.1016/j.brainres.2006.03.058 [DOI] [PubMed] [Google Scholar]
- Wilhelmus MM, Boelens WC, Otte-Höller I, Kamps B, Kusters B, Maat-Schieman ML, de Waal RM, Verbeek MM. 2006b. Small heat shock protein HspB8: its distribution in Alzheimer's disease brains and its inhibition of amyloid-β protein aggregation and cerebrovascular amyloid-β toxicity. Acta Neuropathol 111: 139–149. 10.1007/s00401-005-0030-z [DOI] [PubMed] [Google Scholar]
- Willander H, Presto J, Askarieh G, Biverstål H, Frohm B, Knight SD, Johansson J, Linse S. 2012. BRICHOS domains efficiently delay fibrillation of amyloid β-peptide. J Biol Chem 287: 31608–31617. 10.1074/jbc.M112.393157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Sarbeng EB, Vorvis C, Kumar DP, Zhou L, Liu Q. 2012. Unique peptide substrate binding properties of 110-kDa heat-shock protein (Hsp110) determine its distinct chaperone activity. J Biol Chem 287: 5661–5672. 10.1074/jbc.M111.275057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Graham K, Foote M, Liang F, Rizkallah R, Hurt M, Wang Y, Wu Y, Zhou Y. 2013. 14-3-3 protein targets misfolded chaperone-associated proteins to aggresomes. J Cell Sci 126: 4173–4186. 10.1242/jcs.126102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Cui L, Dibello A, Wang L, Lee J, Saidi L, Lee JG, Ye Y. 2018. DNAJC5 facilitates USP19-dependent unconventional secretion of misfolded cytosolic proteins. Cell Discov 4: 11 10.1038/s41421-018-0012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerbury JJ, Poon S, Meehan S, Thompson B, Kumita JR, Dobson CM, Wilson MR. 2007. The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J 21: 2312–2322. 10.1096/fj.06-7986com [DOI] [PubMed] [Google Scholar]
- Yerbury JJ, Kumita JR, Meehan S, Dobson CM, Wilson MR. 2009. α2-Macroglobulin and haptoglobin suppress amyloid formation by interacting with prefibrillar protein species. J Biol Chem 284: 4246–4254. 10.1074/jbc.M807242200 [DOI] [PubMed] [Google Scholar]
- Young ZT, Mok SA, Gestwicki JE. 2018. Therapeutic strategies for restoring tau homeostasis. Cold Spring Harb Perspect Med 8: a024612 10.1101/cshperspect.a024612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JT, Tan L. 2012. The role of clusterin in Alzheimer's disease: pathways, pathogenesis, and therapy. Mol Neurobiol 45: 314–326. 10.1007/s12035-012-8237-1 [DOI] [PubMed] [Google Scholar]
- Yu A, Shibata Y, Shah B, Calamini B, Lo DC, Morimoto RI. 2014. Protein aggregation can inhibit clathrin-mediated endocytosis by chaperone competition. Proc Natl Acad Sci 111: E1481–E1490. 10.1073/pnas.1321811111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Grant B, Hirsh D. 2001. RME-8, a conserved J-domain protein, is required for endocytosis in Caenorhabditis elegans. Mol Biol Cell 12: 2011–2021. 10.1091/mbc.12.7.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]