Abstract
Purpose
Light input, via the eyes, is essential for regulating circadian rhythms. Eye diseases can cause disruption of vital biological rhythms. Of totally blind people, 87% report sleep problems. There are no UK guidelines for visual disturbance–related circadian rhythm disruption. Our objective was to systematically review the literature to determine the effectiveness of pharmacological agents on the sleep quality of patients with sleep disturbance related to ocular disease.
Methods
We searched CENTRAL, MEDLINE, EMBASE, PsycINFO, and CINAHL alongside protocol registries and citation searches. We assessed the risk of bias using the Cochrane Risk of Bias Assessment Tool and assessed the strength of overall evidence using GRADE criteria.
Results
Four studies (n=116) met the inclusion criteria. Low-quality evidence showed that melatonin can cause entrainment (1 study), increases in total sleep time (all 3 studies), and reduction in sleep latency (1 study). Low-to-moderate quality evidence showed tasimelteon causes a significant improvement in entrainment, midpoint of sleep timing, lower-quartile of night-time sleep, and upper-quartile of daytime sleep.
Conclusions
Results should be treated with caution as the melatonin studies had risks of bias due to inadequate reporting of randomization and masking procedures. The tasimelteon trial had a risk of reporting bias due to changing the outcomes after enrolling participants. Despite the paucity of trials, melatonin and tasimelteon may cause entrainment and improve subjective sleep measures with limited side effects.
Translational Relevance
Given the relative cost melatonin may be a viable choice for treatment of circadian rhythm sleep disorders in the blind and warrants further research.
Keywords: sleep, melatonin, circadian rhythm
Introduction
Of blind people, 87% report at least one sleep problem,1 compared with 30% of sighted people,2 and sleep–wake disruption is frequently reported as a significant side-effect of visual impairment.3 Sleep is essential for maintaining good health. Severe sleep disturbance is associated with cardiovascular problems, type II diabetes, immunosuppression, cancer,4,5 depression, and cognitive impairment6 and therefore has a significant economic impact.7
The suprachiasmatic nucleus (SCN) regulates 24-hour physiological cycles such as sleep–wake cycles. As the circadian period (τ) of the SCN is typically longer than 24 hours (23.8–25.1 hours)8 the SCN requires synchronization (entrainment) to the Earth's 24-hour light–dark cycle via input from circadian time cues (zeitgebers). The most powerful zeitgeber is light exposure, through photosensitive retinal ganglion cells (pRGC), which express the photopigment melanopsin. Depending on timing, light exposure causes phase advances (earlier sleep onset) or phase delays (later sleep onset). Misalignment between the external 24-hour environment and the endogenous circadian timing causes circadian rhythm sleep disorders (CRSD).
Exogenous melatonin has been reported to be able to re-entrain blind patients in nonrandomized trials, and case reports9–12 and is the current treatment of choice for CRSD in the blind.13 Ramelteon (Rozerem; Takeda Pharmaceuticals North America, Inc., Deerfield, IL), the first FDA-approved melatonin agonist,14 has been shown to phase shift circadian rhythms in sighted individuals.15 Tasimelteon (Vanda Pharmaceuticals, Washington, DC), a melatonin agonist, has received FDA16 and European Medicines Agency (EMA)17 approval for treatment of CRSD in blind patients. Caffeine has been shown to phase shift circadian rhythms in humans,18 animal,19 and plant models20 (Table 1).
Table 1.
Current Evidence for Phase-Shifting Therapies
| Intervention |
What We Already Know |
| Exogenous melatonin | Exogenous melatonin was first demonstrated as having phase-shifting effects in 1984 and since then daily administration of exogenous melatonin has been shown to cause re-entrainment in blind patients.9–12 Exogenous melatonin has been described as the current treatment of choice for non–24-hour sleep–wake disorder in the blind.21 Several low-quality investigations compared melatonin with placebo in single masked, nonrandomized trials with small sample sizes (5–10 participants) of blind participants.10,12,22 |
| Sack et al.22 demonstrated melatonin inducing a phase advancement in four of five participants, with an average cumulative phase advancement of 8.41 hours but no participants were entrained. Melatonin treatment was restricted to 21 days so participants were not treated for a full circadian beat. | |
| Lockley et al.10 was the first study to demonstrate entrainment by melatonin in blind patients, with entrainment achieved in four of seven participants. They found that the participants who initiated melatonin in the advance phase of the melatonin phase response curve (PRC) entrained and the participants who initiated melatonin in the delay phase of the PRC did not entrain. | |
| Hack et al.12 reported six of 10 participants entrained with melatonin. Entrainment was more likely when melatonin treatment was initiated in the advance phase of the PRC. They demonstrated that melatonin can entrain individuals when administered in the delay phase of the PRC if treatment is continued for a sufficient length of time. The use of subjective reporting measures increased the likelihood of bias due to inaccurate reporting. | |
| Ramelteon | Ramelteon (Rozerem) is the first FDA-approved melatonin receptor agonist.14 It has been shown to advance the phase of circadian rhythms in healthy individuals15 and several randomized controlled trials have shown it can significantly reduce the latency to sleep time in primary insomnia in sighted individuals.23–26 However, there is a lack of trials investigating ramelteon in CRSD related to ocular disease. |
| Tasimelteon | Tasimelteon (Hetlioz) is a selective agonist for melatonin and has received FDA16 and EMA17 approval for treatment of CRSD in blind patients. However, due to cost its use is limited. Currently, tasimelteon and ramelteon appear to be the only melatonin agonists that have been reported in CRSD related to ocular disease. |
| Caffeine | Although not usually considered as a treatment for CRSD, caffeine has been shown to alter the phase of circadian rhythms in animal19 and plant models.20 Caffeine has been shown to be able to delay melatonin rhythms in a double-blind, placebo-controlled trial of sighted humans.18 The largest trial of caffeine in nonentrained blind participants investigated just three blind participants comparing a placebo run in to a 150-mg dose of caffeine. It found that while the morning administration of caffeine can mitigate some of the negative impact of nonentrained rhythms (i.e., poor daytime alertness and poor mood) it does not appear to address the underlying circadian disorder.27 However, this trial was clearly underpowered. It is unclear what effect different doses of caffeine would have, or the effect of administration at different circadian phases. |
This review summarizes the evidence of phase-shifting pharmacological agents' effects on the sleep quality of patients with CRSD related to ocular disease in order to guide clinicians and future research.
Methods
We conducted this systematic review according to PRISMA guidelines.28 We included published and unpublished randomized controlled trials (RCT) and crossover trials, involving participants with ocular disease or anophthalmia, comparing the use of a phase-shifting pharmaceutical agent against any comparator. The primary outcome was any validated or unvalidated measures of quantity and/or quality of sleep. The secondary outcome was reported adverse events (AE).
We identified studies through electronic searches of the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, PsycINFO, and CINAHL from inception until September 2, 2017. Core key words searched were the following: “blindness,” “circadian rhythm sleep disorder,” “melatonin,” “caffeine,” “tasimelteon,” and “ramelteon.” The full search strategies are presented in Supplementary File S1. Due to resource limitations, we excluded non-English language papers. We identified additional trials through bibliographic searches. We searched www.clinicaltrials.gov, the International Standard Randomized Controlled Trial Number (ISRCTN) registry, and the World Health Organization International Clinical Trials Registry Platform for relevant ongoing studies and http://www.opengrey.eu for gray literature.
We extracted data using a customized form (Supplementary File S2). Key information extracted from each paper included details of study design, setting, dates of study, location, number of centers and number of withdrawals, baseline participant characteristics, details of intervention and comparison agents (e.g., dosage and timing), types of assessment tools, methods of data synthesis, and length of follow-up. For studies with multiple publications, we extracted data from the publication with the most mature data.
We assessed study quality, limitations, and potential bias using the Cochrane Risk of bias tool29 and used the GRADE approach30 to provide an overall assessment of evidence related to study outcomes (for RCT the initial quality of evidence is rated as high and this rating can be lowered depending on risk of bias, inconsistency, indirectness, imprecision, or publication bias, or raised depending on effect size, dose response, and handling of plausible confounding). We aimed to perform meta-analyses using the Cochrane Review Manager software31 where a sufficient number of homogeneous studies were available, or to perform a narrative review.
Results
We identified 1199 unique studies. After screening titles and abstracts, we reviewed 14 full-text articles. No trials of caffeine or ramelteon were identified. Three trials using melatonin (n = 32 subjects) and one trial using tasimelteon (n = 84 subjects) were included (Supplementary File S3). Their findings are summarized in Table 2.
Table 2.
Quality Rating of the Included Trials
| Source |
Sample Size |
Intervention |
Duration of Treatment |
Primary Efficacy Measure |
| Sack et al.9 | 7 (for entrainment), 6 (for all other measures) | Crossover trial of 10 mg melatonin vs. placebo | 0.5 circadian beatsa | Entrainment |
| Fischer et al.32 | 12 | Crossover trial of 5 mg melatonin vs. placebo | 1 night | Total sleep time |
| Roth et al.33 | 13 | RCT of 2 mg melatonin vs. placebo | 6 weeks | Change in total sleep time from baseline to end of treatment |
| Lockley et al.34 | 84 | RCT of 20 mg tasimelteon vs. placebo | 2.5 circadian beatsa or 6 mo (whichever is shorter). | Proportion of entrained patients (aMT6s) |
The time taken to complete one full 24-hour cycle of circadian phase (e.g., the time taken for aMT6s' peak to cycle from 4 AM to 4 AM).
P value not specified.
Table 2.
Extended
| Source |
Findings |
Quality Rating |
| Sack et al.9 | Entrainment: melatonin 6/7; placebo 0/7 (P < 0.001). | 1B |
| Mean circadian period: melatonin phase 24.0 ± 0.1 h; placebo phase 24.4 ± 0.2 h (P < 0.01) | ||
| Total sleep time: melatonin phase 382.6 ± 60.0 min placebo phase 309.4 ± 91.6 minb | ||
| Sleep latency: melatonin phase 10.5 ± 6.6 min; placebo phase 13.7 ± 11.0 minb | ||
| Sleep efficiency: melatonin phase 79.5% ± 12.5; placebo phase 62.8% ± 16.7 (P = 0.06). | ||
| Time spent awake after the onset of sleep: melatonin phase 88.4 ± 61.2 min; placebo phase 165.9 ± 71.8 min (P = 0.05). | ||
| Fischer et al.32 | Total sleep time: melatonin 403.77 ± 13.10 min; placebo 313.95 ± 31.99 min (P < 0.05) | 1B |
| Sleep efficiency: melatonin 85% ± 2.0; placebo 68 ± 7.0 (P < 0.05) | ||
| Time spent awake after sleep onset: melatonin 41.14 ± 10.32 min; placebo 96.05 ± 26.89 min (P < 0.05) | ||
| Roth et al.33 | Change in total sleep time: melatonin 0.72 ± 0.699 h; placebo 0.27 ± 0.449 h (P = 0.18) | 1B |
| Change in sleep latency: melatonin −0.48 ± 0.765 h; placebo −0.001 ± 0.314 h (Cohen's d = 0.82, P = 0.13) | ||
| Change in sleep latency: melatonin −0.48 ± 0.765 h; placebo −0.001 ± 0.314 h (P = 0.13) | ||
| Change in duration of daytime naps: melatonin −0.217 h; placebo 0.033 h (Cohen's d = 0.82)b | ||
| Change in sleep onset: melatonin −0.133 h; placebo 0.000 hb | ||
| Change in sleep offset: melatonin −0.617 h; placebo 0.133 hb | ||
| Lockley et al.34 | Entrainment: tasimelteon 8/40; placebo 1/38 (P = 0.017). | 1B |
| LQ-nTST: tasimelteon 56.80; placebo 17.08 (P = 0.006). | ||
| UQ-dTST: tasimelteon −46.68; placebo −17.87 (P = 0.005). | ||
| MoST: tasimelteon 35; placebo 14.48 (P = 0.012). | ||
| CGI-C: tasimelteon 2.6; placebo 3.4 (P = 0.009). |
GRADE Assessment
Using the GRADE approach (Tables 3, 4) the quality of evidence for each of the outcomes for melatonin was considered low. The quality of evidence for tasimelteon was considered either low or moderate. It should be noted that none of the trials included in this review used any validated sleep questionnaires.
Table 3.
Summary of the GRADE Evidence Ratings of the Melatonin Trials
| Melatonin compared with Placebo for CRSD related to visual impairment | |||
| Patient or Population: Perceptively Blind Patients With CRSD | |||
| Intervention: Melatonin | |||
| Comparison: Placebo | |||
| Outcomes |
N of Participants (studies) |
Quality of the Evidence (GRADE) |
Comments |
| Entrainment of circadian rhythm (melatonin) | 7 (1) | ⊕⊕⊖⊖ low |
One small crossover trial9 with inadequately described masking and allocation and poorly defined outcome. |
| Total sleep time | 32 (3) | ⊕⊕⊖⊖ low |
One small RCT33 and two crossover trials9,32 all with inadequately described masking and allocation. All trials showed general agreement. |
| Sleep efficiency | 19 (2) | ⊕⊕⊖⊖ low |
One small RCT33 and one crossover trial9 with masking and allocation problems. Both showed general agreement. |
| Sleep latency | 32 (3) | ⊕⊕⊖⊖ low |
One small RCT33 and two crossover trials9,32 with masking and allocation problems. All showed general agreement. |
| Wake | 19 (2) | ⊕⊕⊖⊖ low |
One small RCT33 and one small crossover trial9 with masking and allocation problems. Both showed general agreement. |
Table 4.
Summary of the GRADE Evidence Ratings of the Tasimelteon Trial
| Tasimelteon compared with Placebo for CRSD related to visual impairment | |||
| Patient or Population: Perceptively Blind Patients With CRSD | |||
| Intervention: Tasimelteon34 | |||
| Comparison: Placebo | |||
| Outcomes |
N of Participants (studies) |
Quality of the Evidence (GRADE) |
Comments |
| Entrainment of circadian rhythm (melatonin) | 84 (1) | ⊕⊕⊖⊖ Low |
One RCT with suitable masking and allocation. Endpoint was changed after some participants had been enrolled. Precision may be an issue due to sample size. |
| Entrainment of circadian rhythm (cortisol) | 84 (1) | ⊕⊕⊖⊖ Low |
One RCT with suitable masking and allocation. Endpoint was changed after some participants had been enrolled. Precision may be an issue due to sample size. |
| LQ-nTST | 84 (1) | ⊕⊕⊕⊖ Moderate |
One RCT with suitable masking and allocation. Precision may be an issue due to sample size. |
| UQ-dTST | 84 (1) | ⊕⊕⊕⊖ Moderate |
One RCT with suitable masking and allocation. Precision may be an issue due to sample size. |
| MoST | 84 (1) | ⊕⊕⊕⊖ Moderate |
One RCT with suitable masking and allocation. Precision may be an issue due to sample size. |
| CGI-C | 84 (1) | ⊕⊕⊖⊖ Low |
One RCT with suitable masking and allocation. Endpoint was changed after all participants had been enrolled. Precision may be an issue due to sample size. |
Bias
The risk of bias in all three melatonin studies was generally unclear due to incomplete reporting of randomization and masking procedures for assessors and masking procedures for participants and personnel in Roth 201533 and Fischer 2003.32 Lockley 201534 was considered to have a high risk of bias due to selective reporting, as they enrolled participants between October 25, 2010 and October 29, 2012 then modifying their primary endpoint in May 2012 from total sleep time (TST) to entrainment of circadian rhythms, but was otherwise considered to have low risk of bias (Fig.).
Figure.
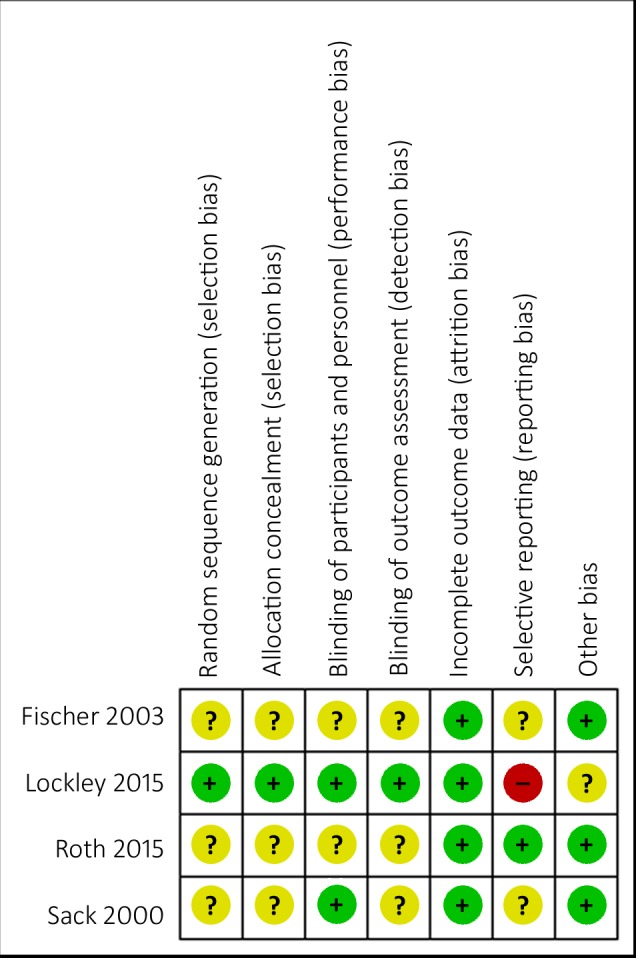
Risk of bias summary: review author's judgments about methodologic quality for each included study.
Melatonin
All three melatonin studies excluded patients with perception of light. Two were crossover trials where, due to the same participants being used in both treatment groups, the results in each treatment arm are related to each other. Therefore, inclusion of these trials in a meta-analysis (using RevMan) would result in a biased estimate of the standard error of the summary statistic.35 There was not sufficient information reported to extract data from before the crossover period; therefore, we reported results as a narrative synthesis.
Sack et al.9 compared 10 mg melatonin with placebo in a crossover trial. Due to incomplete data collection in the placebo phase, one of seven participants was excluded from all analysis other than entrainment. Despite the lack of an objective definition of entrainment their conclusion of entrainment in six of seven participants is reasonable as the mean τ of 24.0 ± 0.1 hours during the melatonin treatment was significantly different (P < 0.01) from the mean τ during the placebo treatment (24.4 ± 0.2 hours) and was not significantly different from 24.0 hours (P = 0.12). Secondary outcomes were measured by polysomnography (PSG). Nonstatistically significant improvements in TST (382.6 ± 60.0 vs. 309.4 ± 91.6 minutes) and sleep latency (10.5 ± 6.6 vs. 13.7 ±11.0 minutes) were found in the melatonin phase. The increase in sleep efficiency (TST divided by total time in bed) in the melatonin phase was approaching significance (79.5 ± 12.5% vs. 62.8 ± 16.7%, P = 0.06) and wake (the time spent awake after sleep onset) was significantly lower in the melatonin phase (88.4 ± 61.2 vs. 165.9 ± 71.8 minutes, P = 0.05).
Fischer et al.32 compared 5 mg melatonin with placebo in a crossover trial. All 12 participants had self-reported recurrent daytime sleepiness and insomnia and no perception of light (NPL; defined as no clinically elicitable perception of light using the normal clinical process of shining a flashlight toward the eye from 4 directions of a quadrant) due to damage of the retina and/or the tractus retinohypothalamicus. A single dose of the study treatment was taken at 10 PM for a single night with a washout period of at least 1 week between treatments. Participants were allowed to sleep between 11 PM and 6:30 AM. PSG was used to measure outcomes. Melatonin significantly improved TST (403.77 ± 13.10 vs. 313.95 ± 31.99 minutes), sleep efficiency (85 ± 2.0% vs. 68 ± 7.0%), and reduced wake (41.14 ± 10.32 vs. 96.05 ± 26.89 minutes).
Roth et al.33 compared 2 mg melatonin with placebo in a RCT. All 13 participants self-reported delay of sleep onset and offset and fewer than 6 hours sleep per night for at least 6 weeks. Total loss of outer retinal photosensitivity was confirmed by electroretinography. Treatment (5 melatonin, 8 placebo) was taken between 9 PM and 10 PM for 6 weeks. Sleep was allowed between 11 PM and 7 AM. Sleep measures were self-reported via interactive voice reporting system (IVRS) each morning. Despite large effect sizes of melatonin reported for ΔTST (Cohen's d = 0.82) and sleep latency (d = 0.92), the change from baseline to end of treatment (ΔTST: 43.2 ± 41.9 minutes [95% CI: −8.4 to 95.4] vs. 16.2 ± 26.9 minutes [95% CI: −6.6 to 38.4], latency −28.8 ± 45.9 minutes [95% CI: −85.8 to 27.6] vs. −0.06 ± 18.8 minutes [95% CI: −15.6 to 15.6], onset [8 vs. 0 minutes, SD not reported] / offset time [−37 vs. 8 minutes, SD not reported], duration of daytime naps [−13 vs. 2 minutes, SD not reported]) were not statistically significant.
All three trials investigated different doses of melatonin (10, 5, and 2 mg). Due to small sample sizes (n = 7–13), all three studies were most likely underpowered to detect significant effects on sleep characteristics. While all three studies found a clinically significant (>20 minutes33) increase in mean TST with melatonin (43 minutes, Roth et al.33; 73 minutes, Sack et al.,9 and 90 minutes, Fischer et al.32) this was only statistically significant in Fischer et al.32
Sleep efficiency was significantly improved in Fischer et al.,32 approaching significance in Sack et al.9 (P = 0.06) and was not investigated in Roth et al.33 The baseline sleep latency was not abnormally delayed (defined as >39 minutes32) in Fischer et al.32 or Sack et al.,9 which may explain why they did not find a clinically or statistically significant change. Roth et al.33 did not find a statistically significant change but found a large effect size (d = 0.92). Wake was significantly decreased in Fischer et al.32 and Sack et al.9 but was not measured as an outcome in Roth et al.33
Tasimelteon
One RCT comparing 20 mg tasimelteon with placebo of tasimelteon34 met the inclusion criteria. All 84 participants (42 placebo, 42 tasimelteon) had free-running circadian rhythms defined as a τ above 24.25 hours as determined by urinary aMT6s. Total loss of visual function was not confirmed with electrophysiology. Treatment was initiated when participants' urinary melatonin was predicted to peak (by extrapolation of τ) 3.5 hours before the end of their 9-hour sleep window. Circadian periods were calculated from urine samples collected sequentially at home every 4 hours (8–10 hours overnight) for 48 hours weekly for a minimum of 4 weeks. Participants took the study drug 1 hour before their chosen bedtime (between 9 PM and 1 AM) and kept a fixed 9-hour window for potential sleep. Night- and daytime logs were completed via IVRS.
Tasimelteon had a significant effect on entrainment of circadian rhythm, assessed from urinary melatonin rhythms as follows: eight of 40 (20%) of the tasimelteon group entrained compared with one of 38 (3%) of the placebo group (P = 0.0171; 95% CI = 3.2%–31.6%). Tasimelteon caused a significant improvement in measures of sleep architecture as follows: lower quartile of treatment nights (LQ-nTST), upper quartile of treatment days (UQ-dTST), midpoint of sleep timing (MoST), as well as Clinical Global Impression of Change (CGI-C; a Likert scale from 1 [very much improved] to 7 [very much worse]) with mean change (95% CI) of 39.71 (12.16–67.27), −28.61 (−48.26 to 8.97), 20.52 (4.61–36.42) and −0.8 (−1.4 to −0.2), respectively.
Adverse Events
Neither Sack et al.9 nor Fischer et al.32 reported any AEs. Roth et al.33 reported one mild AE in the melatonin group and two mild AEs in the placebo group (the exact nature of AEs was not reported), no serious AEs and no clinically significant changes in vital signs, physical examinations, hematology, blood biochemistry, or urinalysis parameters. Lockley et al.34 reported more frequent AEs in tasimelteon compared with placebo as follows: headaches 17% versus 7%, increased alanine amino transferase concentration 10% versus 5%, abnormal dreams 10% versus 0%, urinary tract infection 7% versus 2%, and upper respiratory tract infection 7% versus 0%.
Discussion
To our knowledge, this is the first systematic review of pharmacological treatments for CRSD related to ocular disease. Ramelteon has been shown to advance the phase of endogenous circadian rhythm in sighted participants,15 however, we did not identify any trials of ramelteon in a population with visual impairment. No RCT of caffeine were identified. The quality of evidence for each of the outcomes for melatonin was considered low. The quality of evidence for tasimelteon outcomes were considered either low or moderate. No validated sleep questionnaires were used in any of the included trials.
Circadian phase may remain ‘reset' after discontinuing melatonin,36 raising the possibility of crossover effects in the two crossover trials of melatonin.9,32 Sack et al.9 found no significant crossover effect using analysis of variance. Fischer et al.32 did not test for crossover effect.
Crossover trials must also account for mismatching of melatonin phase. For example, a patient with a τ of 24.5 hours with a melatonin onset at 10 PM would have shifted to a melatonin onset at 1:30 AM after a week, and would therefore have reduced TST and sleep efficiency. Sack et al.9 accounted for this by initiating treatment when the participants' plasma melatonin concentration rose above 10 pg/mL at 9 PM. Fischer et al.32 did not account for matching of melatonin phase.
Entrainment may be strongly affected by the phase at treatment initiation.12,37 Sack et al.9 and Lockley et al.34 both initiated treatment in the advance phase. Neither Fischer et al.32 nor Roth et al.33 had entrainment as an outcome.
Treatment duration is similarly vital.13 A patient on placebo for 2.5 beats whose acrophase at treatment initiation was 3:30 AM would have their acrophase at 3:30 PM at census leading to worse characteristics of sleep. Since Lockley et al.34 treated for 2.5 beats or 6 months (whichever is shorter) duration may be imbalanced between treatment groups. Roth et al.33 was at risk of confounding as their treatment duration of 2 weeks did not account for circadian period. Sack et al.9 continued treatment for half a beat but as it was a crossover trial the measurements were consistent between treatment phases. As Fischer et al.32 used a single dose of IMP there was no risk of confounding due to treatment duration.
The nonvisual pRGC may still be functional, even when ocular disease leads to blindness, which can still allow circadian entrainment.38 Indeed, the impact of the different ocular diseases on pRGC integrity and subsequent sleep quality or circadian biology is unclear. Ideally, the impact of ocular diseases on the function of pRGC should be directly measured and correlated to the level of sleep disruption. More research is needed to understand both the causes and possible treatments for CRSD related to ocular disease.
The evidence for melatonin treatment for CRSD is weak due to small sample sizes of current studies and methodologic issues. The outcome of entrainment was only measured in one trial involving seven patients, although entrainment was not specifically defined, the entrainment of six of seven participants is consistent with nonrandomized trials, where all participants who initiated treatment in the advance phase entrained.10,12 As entrainment is an objective measure it is less likely to be biased by potential inadequacies in randomization and masking. All three melatonin studies included measures of total sleep time and sleep latency. Sack et al.9 and Fischer et al.32 both used the most robust measure of PSG, whereas, Roth et al.33 used self-reported measures that are more prone to bias from inadequacies in randomization and masking. However, the general trend for increased total sleep and reduced sleep latency supports the findings of a nonrandomized trial of 10 participants with NPL.12
Despite the low quality of evidence, given the minimal side effects of melatonin, it seems reasonable to conclude that it could be a cost-effective choice for free-running CRSD related to visual impairment due to its potential to increase the total sleep time, sleep efficiency, and ultimately cause entrainment.
Tasimelteon is a selective melatonin agonist. However, taking into account the high cost point of tasimelteon, and given that the potential for melatonin to entrain blind participants with free-running circadian rhythms was first reported in a clinical trial in 200010 it is perhaps surprising that tasimelteon gained FDA16 approval without direct comparison to melatonin. As non–24-hour sleep–wake disorder (i.e., CRSD related to visual impairment) was considered a novel indication FDA approval34 was granted based exclusively on the SET and RESET34 trials (both of which compared tasimelteon with placebo). However, the endpoint of entrainment was not accepted by the FDA as it was considered an unvalidated surrogate. Instead their decision was based on the endpoints of nTST, LQ-nTST, dTSD, UQ-DTSD, CGI-C, and MoST,39 which were viewed as clinically important measures of the duration of night-time sleep and daytime naps. Despite a high risk of reporting bias (Fig.), due to a change in outcomes after participants had been recruited, the overall study design was robust enough to provide low-quality evidence to show the effectiveness of tasimelteon to entrain free-running circadian rhythms.34 However, these participants self-reported as totally blind, and thus may have had functioning retina.
Strengths
To our knowledge this is the first systematic review to specifically target the relatively unexplored area of CRSD related to visual impairment. Following the prespecified protocol (Supplementary File S4), we comprehensively searched multiple journal databases, gray literature databases, and hand-searched citations using clear inclusion and exclusion criteria. We used the Cochrane risk of bias assessment and GRADE to assess the quality of studies.
Limitations
The decision to exclude clinical trials that were not reported in English may have introduced bias and reduced the precision of estimates of treatment effects. However, the effect of excluding trials not reported in English is generally minimal.40
Only small sample sizes were reported in these studies. Therefore, they may have been underpowered to find a significant treatment effect. The only trial to report a sample size calculation ran their trial as a pilot study due to lack of available participants.
Conclusions
There are no current NICE guidelines regarding the treatment of CRSD in visual impairment. This group of patients have not only visual loss but CRSD, which has a significant impact on quality of life. This review shows that melatonin and tasimelteon may be effective in improving subjective measures of sleep and ultimately causing entrainment. Despite no head to head trial with melatonin, tasimelteon has already received FDA and EMA approval for the treatment of non–24-hour sleep–wake disorder but at US$102,000 per patient per year is prohibitively expensive. As melatonin may be as effective, with fewer side effects and, at £185 per patient per year,41 a far more financially viable alternative, melatonin should be considered as the treatment of choice but further trials are required to establish this.
Supplementary Material
Acknowledgments
This work was conducted as part of the MSc in Evidence-Based Health Care at the University of Oxford.
We gratefully acknowledge funding from Wellcome Trust grant 090684/Z/09/A and we acknowledge support from the National Institute for Health Research (NIHR) Clinical Research Network (CRN) Thames Valley and South Midlands.
C. Andrews reports grants from Wellcome Trust and the NIHR CRN Thames Valley and South Midlands.
C. Heneghan has received expenses and fees for his media work including British Broadcasting Corporation Inside Health. He holds grant funding from the NIHR, the NIHR School of Primary Care Research, The NIHR Oxford Biomedical Research Centre, and he is a NIHR Senior Investigator. On occasion, he receives expenses for teaching Evidence-Based Medicine and is also paid for his general practitioner work in National Health Service (NHS) out of hours (contract with Oxford Health NHS Foundation Trust).
A. Plüddemann reports grants from NIHR, grants from NIHR School of Primary Care Research, during the conduct of the study; and occasionally receives expenses for teaching Evidence-Based Medicine.
Disclosure: C.D. Andrews, None; R.G. Foster, Director Circadian Therapeutics (S); I. Alexander, None; S. Vasudevan, None; S.M. Downes, Circadian Therapeutics (C); C. Heneghan, None; A. Plüddemann, None
References
- 1.Leger D, Guilleminault C, Defrance R, Domont A, Paillard M. Prevalence of sleep/wake disorders in persons with blindness. Clin Sci. 1999;97:193–199. [PubMed] [Google Scholar]
- 2.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 Suppl):S7–S10. [PMC free article] [PubMed] [Google Scholar]
- 3.Lockley SW, Arendt J, Skene DJ. Visual impairment and circadian rhythm disorders. Dialogues Clin Neurosci. 2007;9:301–314. doi: 10.31887/DCNS.2007.9.3/slockley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buxton OM, Cain SW, O'Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masri S, Kinouchi K, Sassone-Corsi P. Circadian clocks, epigenetics, and cancer. Curr Opin Oncol. 2015;27:50–56. doi: 10.1097/CCO.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 7.Hillman DR, Murphy AS, Antic R, Pezzullo L. The economic cost of sleep disorders. Sleep. 2006;29:299–305. doi: 10.1093/sleep/29.3.299. [DOI] [PubMed] [Google Scholar]
- 8.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 9.Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 10.Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol. 2000;164:R1–R6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- 11.Lewy AJ, Bauer VK, Hasler BP, Kendall AR, Pires MLN, Sack RL. Capturing the circadian rhythms of free-running blind people with 0.5 mg melatonin. Brain Res. 2001;918:96–100. doi: 10.1016/s0006-8993(01)02964-x. [DOI] [PubMed] [Google Scholar]
- 12.Hack LM, Lockley SW, Arendt J, Skene DJ. The effects of low-dose 0.5-mg melatonin on the free-running circadian rhythms of blind subjects. J Biol Rhythms. 2003;18:420–429. doi: 10.1177/0748730403256796. [DOI] [PubMed] [Google Scholar]
- 13.Skene DJ, Arendt J. Circadian rhythm sleep disorders in the blind and their treatment with melatonin. Sleep Med. 2007;8:651–655. doi: 10.1016/j.sleep.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Borja NL, Daniel KL. Ramelteon for the treatment of insomnia. Clin Ther. 2006;28:1540–1555. doi: 10.1016/j.clinthera.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Richardson GS, Zee PC, Wang-Weigand S, Rodriguez L, Peng X. Circadian phase-shifting effects of repeated ramelteon administration in healthy adults. J Clin Sleep Med. 2008;4:456–461. [PMC free article] [PubMed] [Google Scholar]
- 16.Dhillon S, Clarke M. Tasimelteon: first global approval. Drugs. 2014;74:505–511. doi: 10.1007/s40265-014-0200-1. [DOI] [PubMed] [Google Scholar]
- 17.European Medicines Agency. EMEA/H/C/003870. Accessed September 2, 2017. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003870/human_med_001873.jsp&mid=WC0b01ac058001d124.
- 18.Burke TM, Markwald RR, McHill AW, et al. Effects of caffeine on the human circadian clock in vivo and in vitro. Sci Transl Med. 2015 Sep 16;7:305ra146. doi: 10.1126/scitranslmed.aac5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antle MC, Steen NM, Mistlberger RE. Adenosine and caffeine modulate circadian rhythms in the Syrian hamster. Neuroreport. 2001;12:2901–2905. doi: 10.1097/00001756-200109170-00029. [DOI] [PubMed] [Google Scholar]
- 20.Mayer W, Scherer I. Phase Shifting Effect of Caffeine in the Circadian Rhythm of Phaseolus coccineus L. Z Für Naturforschung C. 2014;30:855–856. [Google Scholar]
- 21.Mistlberger RE, Skene DJ. Nonphotic entrainment in humans? J Biol Rhythms. 2005;20:339–352. doi: 10.1177/0748730405277982. [DOI] [PubMed] [Google Scholar]
- 22.Sack RL, Lewy AJ, Blood ML, Stevenson J, Keith LD. Melatonin administration to blind people: phase advances and entrainment. J Biol Rhythms. 1991;6:249–261. doi: 10.1177/074873049100600305. [DOI] [PubMed] [Google Scholar]
- 23.Roth T, Seiden D, Weigand S, Zhang J, Rieckhoff H, Sainati S., III Phase III study to determine the efficacy of ramelteon in elderly patients with chronic insomnia. Proc New Clin Drug Eval Unit. 2005. pp. 6–9.
- 24.Seiden D, Zee P, Weigand S, et al. Double-blind, placebo-controlled outpatient clinical trial of ramelteon for the treatment of chronic insomnia in an elderly population. Sleep. 2005. p. A228.
- 25.Zammit G, Erman M, Wang-Weigand S, Sainati S, Zhang J, Roth T. Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia. J Clin Sleep Med. 2007;3(5):495–504. [PMC free article] [PubMed] [Google Scholar]
- 26.Erman M, Seiden D, Zammit G, Sainati S, Zhang J. An efficacy, safety, and dose–response study of ramelteon in patients with chronic primary insomnia. Sleep Med. 2006;7:17–24. doi: 10.1016/j.sleep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 27.St. Hilaire MA, Lockley SW. Caffeine does not entrain the circadian clock but improves daytime alertness in blind patients with non-24-hour rhythms. Sleep Med. 2015;16:800–804. doi: 10.1016/j.sleep.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928–d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 32.Fischer S, Smolnik R, Herms M, Born J, Fehm HL. Melatonin acutely improves the neuroendocrine architecture of sleep in blind individuals. J Clin Endocrinol Metab. 2003;88:5315–5320. doi: 10.1210/jc.2003-030540. [DOI] [PubMed] [Google Scholar]
- 33.Roth T, Nir T, Zisapel N. Prolonged release melatonin for improving sleep in totally blind subjects: a pilot placebo-controlled multicenter trial. Nat Sci Sleep. 2015. pp. 13–23. [DOI] [PMC free article] [PubMed]
- 34.Lockley SW, Dressman MA, Licamele L, et al. Tasimelteon for non-24-hour sleep–wake disorder in totally blind people (SET and RESET): two multicentre, randomised, double-masked, placebo-controlled phase 3 trials. Lancet. 2015;386:1754–1764. doi: 10.1016/S0140-6736(15)60031-9. [DOI] [PubMed] [Google Scholar]
- 35.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Cochrane Handbook for Systematic Reviews of Interventions. Accessed August 30, 2017. Available at: http://handbook-5-1.cochrane.org/index.htm#chapter_16/16_4_7_issues_in_the_incorporation_of_cross_over_trials.htm. [Google Scholar]
- 36.Jan J, Espezel H, Appleton R. The treatment of sleep disorders with melatonin. Dev Med Child Neurol. 1994;36:97–107. doi: 10.1111/j.1469-8749.1994.tb11818.x. [DOI] [PubMed] [Google Scholar]
- 37.Lewy AJ, Emens JS, Bernert RA, Lefler BJ. Eventual entrainment of the human circadian pacemaker by melatonin is independent of the circadian phase of treatment initiation: clinical implications. J Biol Rhythms. 2004;19:68–75. doi: 10.1177/0748730403259670. [DOI] [PubMed] [Google Scholar]
- 38.Zaidi FH, Hull JT, Peirson SN, et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17:2122–2128. doi: 10.1016/j.cub.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Center for Drug Evaluation and Research Summary Review. Accessed August 27, 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205677Orig1s000SumR.pdf.
- 40.Jüni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol. 2002;31:115–123. doi: 10.1093/ije/31.1.115. [DOI] [PubMed] [Google Scholar]
- 41.Drug Tariff | NHSBSA. Accessed September 17, 2017. Available at: https://www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/drug-tariff.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


