Abstract
Results from studies evaluating potential effects of prenatal exposure to radio-frequency electromagnetic fields from cell phones on birth outcomes have been inconsistent. Using data on 55,507 pregnant women and their children from Denmark (1996–2002), the Netherlands (2003–2004), Spain (2003–2008), and South Korea (2006–2011), we explored whether maternal cell-phone use was associated with pregnancy duration and fetal growth. On the basis of self-reported number of cell-phone calls per day, exposure was grouped as none, low (referent), intermediate, or high. We examined pregnancy duration (gestational age at birth, preterm/postterm birth), fetal growth (birth weight ratio, small/large size for gestational age), and birth weight variables (birth weight, low/high birth weight) and meta-analyzed cohort-specific estimates. The intermediate exposure group had a higher risk of giving birth at a lower gestational age (hazard ratio = 1.04, 95% confidence interval: 1.01, 1.07), and exposure-response relationships were found for shorter pregnancy duration (P < 0.001) and preterm birth (P = 0.003). We observed no association with fetal growth or birth weight. Maternal cell-phone use during pregnancy may be associated with shorter pregnancy duration and increased risk of preterm birth, but these results should be interpreted with caution, since they may reflect stress during pregnancy or other residual confounding rather than a direct effect of cell-phone exposure.
Keywords: birth outcomes, cell phones, exposure, preterm birth, radio-frequency electromagnetic fields
Cell-phone use has rapidly increased during the last several decades (1). Cell phones generate radio-frequency electromagnetic fields (RF-EMF), resulting in local exposure of the human body to RF-EMF. Concerns have been raised regarding the potential effects of RF-EMF exposure on human health. Short-term exposure in adults is regarded as safe; however, potential health effects of long-term exposure and exposure of fetuses and children are not well studied (2, 3).
The interpretation of results from epidemiologic studies evaluating health effects of RF-EMF exposure from cell-phone use is challenging. Because of the technical evolution of mobile communication systems, similar levels of cell-phone use do not necessarily induce similar levels of RF-EMF exposure. Users’ exposure to RF-EMF from third-generation (3G) devices is much lower than that from second-generation (2G) devices (4). More recent technologies (4G/5G) also differ from 3G devices, though less, in terms of users’ exposure.
Exposure to RF-EMF during pregnancy could affect the growth and development of the fetus and the duration of pregnancy, either directly due to radiation of the fetus and placenta or indirectly as a result of altered maternal physiology. Some animal studies have shown an association with steroidogenesis and lower birth weight in offspring (5, 6). However, not all animal studies support an association with adverse birth outcomes (7, 8). During cell-phone calling and texting, abdominal exposure is low and modeling studies estimate that the exposure levels of the human fetus are very low (9–12), although an experimental study in humans has shown that abdominal RF-EMF exposure may affect placental function (13). In addition, an association between RF-EMF exposure and thyroid dysfunction has been indicated in animal studies (14, 15).
Previous epidemiologic studies have given inconsistent results. In a cohort study from Turkey (n = 500), Col-Araz (16) retrospectively assessed cell-phone use and reported shorter pregnancy duration and increased risk for preterm birth. In a cohort study from Iran (n = 1,200), Mortazavi et al. (17) found no association with birth weight. In a much larger sample from Norway (n = 100,231), Baste et al. (18) found no association between cell-phone use and low birth weight, preterm birth, or small-for-gestational-age (SGA) birth.
Taking into account the ubiquity of cell-phone use, an effect on birth outcomes, even small, may have considerable public health impact. Our aim in this study was to explore the possible association of maternal cell-phone use with pregnancy duration and fetal growth in 4 birth cohorts from Denmark, the Netherlands, Spain, and South Korea.
METHODS
Study population
Our analysis was conducted within 4 population-based birth cohort studies participating in the Generalized EMF Research Using Novel Methods (GERoNiMO) Project (19)—namely, the Danish National Birth Cohort (DNBC) (20, 21), the Amsterdam Born Children and Their Development Study (ABCD) (22), the Spanish Environment and Childhood Project (INMA) (23), and the Korean Mothers and Children’s Environment Health Study (MOCEH) (24). In total, 113,319 pregnant women were enrolled in these cohorts from 1996 to 2011. All participants gave written informed consent, and each cohort study received ethical approval from local research ethics committees. Our inclusion criteria were the availability of information on frequency of cell-phone calls (both incoming and outgoing) during pregnancy, child’s birth weight, and gestational age at birth. Mother-child pairs were excluded in the case of multiple pregnancy, if a spontaneous abortion occurred (gestational age at birth <20 weeks; n = 39), or if records of birth outcomes were implausible (i.e., if gestational age at birth was ≥47 weeks (n = 27) and/or birth weight was more than 4 standard deviations away from the mean for gestational age, based on birth-weight reference curves (n = 126)). National sex-specific birth-weight reference curves relevant to the study period were available for the Netherlands (ABCD) (25), Spain (INMA) (26), and South Korea (MOCEH) (27). In DNBC, the Norwegian reference curves were used, because of unavailability of national Danish curves developed with similar methodology and relevant to the study period (28). In total, 55,507 mother-child pairs met our inclusion criteria (Table 1).
Table 1.
Availability of Data on Exposure and Outcomes and the Study Population for 4 Cohorts Included in an Analysis of Maternal Cell-Phone Use During Pregnancy and Birth Outcomes, 1996–2011
| Study Cohort | Location of Cohort | Enrollment | Cell-Phone Use During Pregnancy | Pregnancy Duration and Fetal Growth Outcomes | Study Population | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time Period | No. of Pairsa Enrolled (n = 113,319) | Time of Data Collection | No. of Pairs (n = 56,079) | % of Those Enrolled (49.5%) | No. of Pairs (n = 65,637) | % of Those Enrolled (57.9%) | No. of of Pairs Included in Analysis (n = 55,507) | % of Those Enrolled (49.0%) | ||
| DNBC | Denmark | 1996–2002 | 101,032 | 7 years postnatal | 50,040 | 49.5 | 54,498b | 53.9 | 49,668 | 49.2 |
| ABCD | The Netherlands | 2003–2004 | 8,266 | 7 years postnatal | 2,611 | 31.6 | 7,812 | 94.5 | 2,597 | 31.4 |
| INMA | Spain | 2003–2008 | 2,270 | Pregnancy | 1,993 | 87.8 | 1,975 | 87.0 | 1,934 | 85.2 |
| MOCEH | South Korea | 2006–2011 | 1,751 | Pregnancy | 1,435 | 82.0 | 1,352c | 77.2 | 1,308 | 74.7 |
Abbreviations: ABCD, Amsterdam Born Children and Their Development Study; DNBC, Danish National Birth Cohort; INMA, Spanish Environment and Childhood Project; MOCEH, Korean Mothers and Children’s Environment Health Study.
a Number of mother-child pairs.
b Out of 54,908 offspring whose mothers responded to the age 7 years questionnaire.
c Out of 1,481 offspring whose mothers responded to the cell-phone use questionnaire.
Maternal cell-phone use during pregnancy
The mothers from DNBC and ABCD reported their frequency of cell-phone calls during pregnancy 7 years postnatally. In INMA and MOCEH, similar questionnaires were given to the mothers during pregnancy. To be consistent with previous analyses within these cohorts (29), we classified exposure into 4 categories (none, low, intermediate, and high) based on available information regarding daily frequency of cell-phone calls during pregnancy (Table 2). During the enrollment period of DNBC, 2G devices were used; in the more recent cohorts, 3G and 2G devices were used alongside each other. Thus, RF-EMF exposure from similar cell-phone use should have been higher in DNBC, on average, and lower in the more recent cohorts.
Table 2.
Classification of Cell-Phone Exposure in 4 Cohorts Included in an Analysis of Maternal Cell-Phone Use During Pregnancy and Birth Outcomes, 1996–2011
| Exposure Classificationa | Study Cohort | Total (n = 55,507) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DNBC (n = 49,668) | ABCD (n = 2,597) | INMA (n = 1,934) | MOCEH (n = 1,308) | |||||||
| No. of Pairsb | % | No. of Pairs | % | No. of Pairs | % | No. of Pairs | % | No. of Pairs | % | |
| None | 30,185 | 60.8 | 180 | 6.9 | 53 | 2.7 | 15 | 1.2 | 30,433 | 54.8 |
| Low | 10,860 | 21.9 | 1,125 | 43.3 | 703 | 36.4 | 242 | 18.5 | 12,930 | 23.3 |
| Intermediate | 6,172 | 12.4 | 703 | 27.1 | 753 | 38.9 | 642 | 49.1 | 8,270 | 14.9 |
| High | 2,451 | 4.9 | 589 | 22.7 | 425 | 22.0 | 409 | 31.3 | 3,874 | 7.0 |
Abbreviations: ABCD, Amsterdam Born Children and Their Development Study; DNBC, Danish National Birth Cohort; INMA, Spanish Environment and Childhood Project; MOCEH, Korean Mothers and Children’s Environment Health Study.
a In the DNBC, ABCD, and INMA cohorts, no exposure corresponded to no cell-phone use, low exposure to ≤1 calls/day, intermediate exposure to 2–3 calls/day, and high exposure to ≥4 calls/day. In the MOCEH cohort, no exposure corresponded to no cell-phone use, low exposure to ≤2 calls/day, intermediate exposure to 3–5 calls/day, and high exposure to ≥6 calls/day.
b Number of mother-child pairs.
Pregnancy duration and fetal growth outcomes
We defined all outcomes of this study a priori. We examined pregnancy duration, using gestational age at birth, preterm birth (≤36 completed weeks), and postterm birth (>42 completed weeks); fetal growth, using birth weight ratio, SGA birth, and large-for-gestational-age (LGA) birth; and birth weight, low birth weight (≤2,499 g), and high birth weight (≥4,000 g), which reflect both pregnancy duration and fetal growth. Birth weight ratio was defined as the observed birth weight divided by the median birth weight from a national birth-weight reference curve (30), SGA birth as birth weight below the 10th percentile, and LGA birth as birth weight above the 90th percentile.
In DNBC, gestational age at birth was reported by midwives on the basis of the woman’s last menstrual cycle and ultrasound examinations. In INMA, the date of the last menstrual cycle was used, if this was consistent with the ultrasound-based estimate (≤7 days’ difference); otherwise, the ultrasound estimate was used. Women for whom this difference exceeded 3 weeks were removed from the study. In ABCD and MOCEH, gestational age at birth was defined on the basis of ultrasound examinations during pregnancy; if this information was not available, calculation of gestational age at birth was based on the last menstrual period.
Covariate data
We preselected the following covariates for the adjusted statistical models: maternal age at child’s birth (a natural spline term with 3 degrees of freedom), parity, active and passive smoking during pregnancy, alcohol consumption during pregnancy, prepregnancy body mass index (weight (kg)/height (m)2; a natural spline term with knots at cutoff values between underweight, normal, and overweight as appropriate for Caucasian and Asian populations) (31), height, educational level, socioeconomic position, and marital status. In addition, geographical region was a covariate for the analysis carried out within the multicenter cohorts (INMA and MOCEH) and maternal country of birth (European/non-European) for the analysis carried out within the DNBC, ABCD, and INMA cohorts, where this was heterogeneous. Data regarding the aforementioned variables were self-reported in questionnaires or telephone interviews during pregnancy or after birth. Definitions of covariates by cohort are provided in Web Tables 1–4 (available at https://academic.oup.com/aje).
Statistical analysis
Multiple imputation by chained equations was used for missing values in the covariates of the adjusted statistical models. It was performed in the study population and separately for each cohort. All covariates—apart from geographical region—and all study outcomes were used as predictors, and 20 complete data sets were obtained (32).
In each cohort, maternal characteristics and pregnancy duration and fetal growth outcomes were characterized by exposure group, using mean values and proportions as appropriate. Modified Wald (33), χ2, and Fischer exact tests were performed to detect any difference between the exposure groups (Web Tables 1–4).
For the analyses of birth weight and birth weight ratio, we used multiple linear regression models. To achieve normality of residuals, we excluded preterm neonates from the analysis of birth weight. Gestational age at birth was treated as time from conception to birth, and Cox proportional hazards models were used (34). To meet the assumption of proportional hazards, we used parity status, active and passive smoking, and alcohol consumption as stratifying variables. For the analyses of low and high birth weight, preterm and postterm birth, and SGA and LGA birth, we used logistic regression models. In all statistical models, the low exposure group was the reference group, because of the very low proportions of women reporting no use of cell phones during pregnancy in ABCD, INMA, and MOCEH (Table 2).
The calculated unadjusted and adjusted cohort-specific estimates were meta-analyzed using random-effects models. INMA was excluded from the meta-analysis of the odds ratios of the unexposed group for postterm birth, because there were no cases in that group (Web Table 3). Similarly, MOCEH was excluded from the meta-analysis of odds ratios of the unexposed group for postterm birth and low birth weight (Web Table 4). We refitted the adjusted statistical models described above with a continuous exposure variable and meta-analyzed the obtained estimates with a random-effects model. The corresponding P value is the reported statistical significance of the linear trend.
We performed the following sensitivity analysis: 1) complete-case analysis, to assess the influence of multiple imputation; 2) analysis with binary exposure (none/low vs. intermediate/high), to achieve maximum statistical power while including the unexposed and highly exposed mothers in the comparisons; 3) analysis of low birth weight restricted to nonpreterm neonates, to assess whether the results of primary analysis were driven by preterm births; 4) analysis of birth weight ratio, SGA, and LGA in DNBC using the observed birth weight percentiles per gestational age, to assess the impact of using the Norwegian reference curves in our primary analysis; 5) meta-analysis of results excluding one cohort at a time, to assess the influence of each cohort on our pooled estimates; and 6) meta-analysis of cohorts with retrospective exposure assessment (DNBC and ABCD) versus prospective exposure assessment (INMA and MOCEH), to assess the effect of recall error.
All analyses were performed using R statistical software (version 3.4.0; R Foundation for Statistical Computing, Vienna, Austria) (35) and the following software packages: “tableone” (36), “mice” (37), “miceadds” (38), “splines” (35), “survival” (39, 40), and “metafor” (41).
RESULTS
Descriptive statistics
In our study population of 55,507 mother-child pairs, mean birth weight was 3,578 (standard deviation (SD), 547) g; 1,448 (2.61%) children were born with low birth weight and 12,188 (21.96%) with high birth weight (Table 3). The average gestational age at birth was 39.98 (SD, 1.67) weeks; 2,271 (4.09%) children were born preterm and 3,170 (5.71%) postterm. The distribution of gestational age at birth was left-skewed and indicative of right-censoring, because of the cesarean deliveries and induced vaginal labors. The incidence of postterm birth varied between 6% in DNBC and 0.6% in MOCEH. Regarding fetal growth, the mean birth weight ratio was 1.01 (SD, 0.13); 3,535 children (6.37%) were born SGA and 8,287 (14.93%) LGA. Incidence of SGA and LGA was closer to the expected 10% in the ABCD, INMA, and MOCEH cohorts.
Table 3.
Pregnancy Duration and Fetal Growth Outcomes in an Analysis of Maternal Cell-Phone Use During Pregnancy and Birth Outcomes, 1996–2011
| Pregnancy Duration or Fetal Growth Outcome | Study Cohort | Total (n = 55,507) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DNBC (n = 49,668) | ABCD (n = 2,597) | INMA (n = 1,934) | MOCEH (n = 1,308) | |||||||
| No. of Pairsa | % | No. of Pairs | % | No. of Pairs | % | No. of Pairs | % | No. of Pairs | % | |
| Birth weight, gb | 3,602 (547) | 3,503 (521) | 3,262 (461) | 3,261 (441) | 3,578 (547) | |||||
| Low birth weight (≤2,499 g) | 1,248 | 2.51 | 77 | 2.96 | 89 | 4.60 | 34 | 2.60 | 1,448 | 2.61 |
| High birth weight (≥4,000 g) | 11,598 | 23.35 | 424 | 16.33 | 108 | 5.58 | 58 | 4.43 | 12,188 | 21.96 |
| Gestational age at birth, weeksb | 40.00 (1.67) | 39.93 (1.61) | 39.83 (1.60) | 39.19 (1.60) | 39.98 (1.67) | |||||
| Preterm birth (≤36 completed weeks) | 2,025 | 4.08 | 117 | 4.51 | 65 | 3.36 | 64 | 4.89 | 2,271 | 4.09 |
| Postterm birth (>42 completed weeks) | 2,982 | 6.00 | 108 | 4.16 | 72 | 3.72 | 8 | 0.61 | 3,170 | 5.71 |
| Birth weight ratiob,c | 1.01 (0.13) | 1.01 (0.12) | 1.01 (0.12) | 1.01 (0.12) | 1.01 (0.13) | |||||
| SGA birth (<10th percentile) | 3,030 | 6.10 | 206 | 7.93 | 182 | 9.41 | 117 | 8.94 | 3,535 | 6.37 |
| LGA birth (>90th percentile) | 7,660 | 15.42 | 276 | 10.63 | 205 | 10.60 | 146 | 11.16 | 8,287 | 14.93 |
Abbreviations: ABCD, Amsterdam Born Children and Their Development Study; DNBC, Danish National Birth Cohort; INMA, Spanish Environment and Childhood Project; LGA, large for gestational age; MOCEH, Korean Mothers and Children’s Environment Health Study; SD, standard deviation; SGA, small for gestational age.
a Number of mother-child pairs.
b Values are expressed as mean (standard deviation).
c Birth weight ratio was defined as observed birth weight divided by the median birth weight from a national birth-weight reference curve (30).
With respect to maternal cell-phone use during pregnancy, 55% of the mothers were classified in the unexposed group, 23% in the low-exposure group, 15% in the intermediate-exposure group, and 7% in the high-exposure group (Table 2). In the older cohort (DNBC), cell-phone use was less frequent (61% unexposed). In all 4 cohorts, mothers with higher cell-phone use during pregnancy were more often primiparous, were more likely to smoke during pregnancy, and were more likely to be exposed to secondhand smoke (Web Tables 1–4). In the ABCD, INMA, and MOCEH cohorts, higher maternal cell-phone use was associated with a higher educational level; however, the opposite was seen in the DNBC cohort.
Adjusted associations of maternal cell-phone use with pregnancy duration and fetal growth outcomes
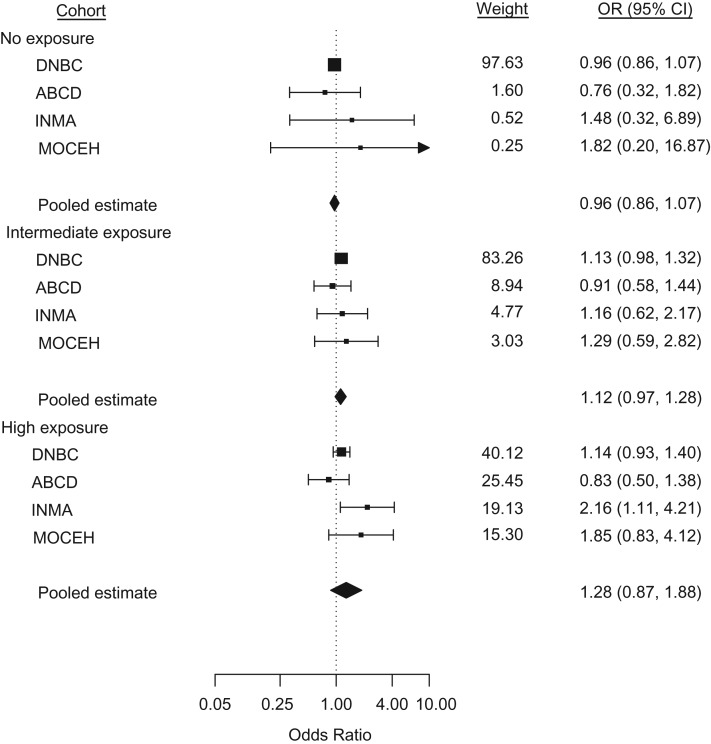
With respect to pregnancy duration, the intermediate-exposure group had a higher risk of giving birth at a lower gestational age compared with the low-exposure group (hazard ratio (HR) = 1.04, 95% confidence interval (CI): 1.01, 1.07) (Table 4, Web Figure 1). The hazard ratios for the other exposure groups were closer to unity (unexposed: HR = 0.99 (95% CI: 0.97, 1.01); highly exposed: HR = 1.02 (95% CI: 0.98, 1.06)), but a linear trend was observed (P < 0.001). In the analysis of preterm birth, a linear trend was observed (P = 0.003), though none of the odds ratios reached statistical significance (unexposed: odds ratio (OR) = 0.96 (95% CI: 0.86, 1.07); intermediate exposure: OR = 1.12 (95% CI: 0.97, 1.28); highly exposed: OR = 1.28 (95% CI: 0.87, 1.88)) (Table 4, Figure 1). For postterm birth, a significant odds ratio (OR = 0.85, 95% CI: 0.75, 0.97) was observed only for the intermediate-exposure group, but there was no linear trend in the results (P = 0.86) (Table 4, Web Figure 2). No association of maternal cell-phone use with fetal growth was detected in any of the examined outcomes (birth weight ratio, SGA, and LGA) (Table 4, Web Figures 3–5). Regarding birth weight, no association or linear trend was observed within the nonpreterm neonates (Web Figure 6); similarly, the odds of high birth weight did not differ from unity (Web Figure 7). In the analysis of low birth weight, we observed a significant decrease in the odds for the unexposed group (OR = 0.87, 95% CI: 0.76, 1.00) and a linear trend (P = 0.01) (Table 4, Web Figure 8). Note that 65% (n = 947) of the low-birth-weight cases were also born preterm. All cohort-specific unadjusted and adjusted estimates are shown in Web Tables 5 and 6.
Table 4.
Results From a Meta-Analysis of the Associations of Maternal Cell-Phone Use During Pregnancy With Pregnancy Duration and Fetal Growth Outcomes, 1996–2011
| Birth Outcomea and Category of Maternal Cell-Phone Useb | No. of Cases | Unadjusted Results | Adjusted Results | P for Trendc | ||
|---|---|---|---|---|---|---|
| MD | 95% CI | MDc | 95% CI | |||
| Birth weight in nonpreterm neonates, g | 0.093 | |||||
| None | 11.68 | 0.81, 22.54 | −11.15 | −53.24, 30.94 | ||
| Low | 0 | Referent | 0 | Referent | ||
| Intermediate | −16.20 | −35.13, 2.73 | −8.17 | −21.34, 5.00 | ||
| High | −11.84 | −29.88, 6.20 | −2.56 | −19.90, 14.78 | ||
| Birth weight ratio | 0.392 | |||||
| None | 0.00 | 0.00, 0.01 | 0.00 | −0.00, 0.00 | ||
| Low | 0 | Referent | 0 | Referent | ||
| Intermediate | −0.00 | −0.01, 0.01 | −0.00 | −0.00, 0.00 | ||
| High | −0.00 | −0.01, 0.00 | 0.00 | −0.00, 0.00 | ||
| OR | 95% CI | ORc | 95% CI | |||
| Low birth weight | 0.011 | |||||
| None | 684 | 0.81d | 0.71, 0.93 | 0.87d | 0.76, 1.00 | |
| Low | 373 | 1.00 | Referent | 1.00 | Referent | |
| Intermediate | 251 | 1.04 | 0.89, 1.23 | 0.95 | 0.81, 1.13 | |
| High | 140 | 1.23 | 1.01, 1.51 | 1.13 | 0.92, 1.40 | |
| High birth weight | 0.268 | |||||
| None | 7,244 | 1.02 | 0.87, 1.20 | 0.92 | 0.68, 1.24 | |
| Low | 2,739 | 1.00 | Referent | 1.00 | Referent | |
| Intermediate | 1,543 | 0.92 | 0.82, 1.03 | 0.96 | 0.83, 1.10 | |
| High | 662 | 0.89 | 0.77, 1.04 | 0.93 | 0.78, 1.11 | |
| Preterm birth | 0.003 | |||||
| None | 1,145 | 0.90 | 0.80, 1.00 | 0.96 | 0.86, 1.07 | |
| Low | 539 | 1.00 | Referent | 1.00 | Referent | |
| Intermediate | 393 | 1.17 | 1.02, 1.34 | 1.12 | 0.97, 1.28 | |
| High | 194 | 1.21 | 1.02, 1.44 | 1.28 | 0.87, 1.88 | |
| Postterm birth | 0.863 | |||||
| None | 1,799 | 0.92e | 0.84, 1.00 | 0.98e | 0.89, 1.07 | |
| Low | 770 | 1.00 | Referent | 1.00 | Referent | |
| Intermediate | 400 | 0.87 | 0.77, 0.98 | 0.85 | 0.75, 0.97 | |
| High | 201 | 1.06 | 0.82, 1.37 | 0.98 | 0.83, 1.16 | |
| SGA birth | 0.872 | |||||
| None | 1,779 | 0.90 | 0.82, 0.98 | 0.94 | 0.86, 1.03 | |
| Low | 877 | 1.00 | Referent | 1.00 | Referent | |
| Intermediate | 608 | 1.05 | 0.87, 1.26 | 1.03 | 0.88, 1.21 | |
| High | 272 | 0.95 | 0.82, 1.10 | 0.94 | 0.78, 1.13 | |
| LGA birth | 0.488 | |||||
| None | 4,773 | 1.01 | 0.95, 1.08 | 0.98 | 0.92, 1.04 | |
| Low | 1,916 | 1.00 | Referent | 1.00 | Referent | |
| Intermediate | 1,112 | 0.92 | 0.85, 1.00 | 0.97 | 0.89, 1.05 | |
| High | 490 | 0.89 | 0.80, 0.99 | 0.93 | 0.83, 1.04 | |
| HR | 95% CI | HRc | 95% CI | |||
| Gestational age at birth | <0.001 | |||||
| None | 1.00 | 0.98, 1.02 | 0.99 | 0.97, 1.01 | ||
| Low | 1.00 | Referent | 1.00 | Referent | ||
| Intermediate | 1.01 | 0.96, 1.06 | 1.04 | 1.01, 1.07 | ||
| High | 1.01 | 0.98, 1.05 | 1.02 | 0.98, 1.06 | ||
Abbreviations: ABCD, Amsterdam Born Children and Their Development Study; CI, confidence interval; DNBC, Danish National Birth Cohort; HR, hazard ratio; INMA, Spanish Environment and Childhood Project; LGA, large for gestational age; MD, mean difference; MOCEH, Korean Mothers and Children’s Environment Health Study; OR, odds ratio; SGA, small for gestational age.
a For definitions of birth outcomes, see Table 3.
b In the DNBC, ABCD, and INMA cohorts, no exposure corresponded to no cell-phone use, low exposure to ≤1 calls/day, intermediate exposure to 2–3 calls/day, and high exposure to ≥4 calls/day. In the MOCEH cohort, no exposure corresponded to no cell-phone use, low exposure to ≤2 calls/day, intermediate exposure to 3–5 calls/day, and high exposure to ≥6 calls/day.
c Adjusted for maternal age, parity, active and passive smoking, alcohol consumption, prepregnancy body mass index, educational level, socioeconomic position, marital status, and maternal height.
d Excluding MOCEH.
e Excluding MOCEH and INMA.
Figure 1.
Odds ratios (ORs) from a meta-analysis of the association of maternal cell-phone use during pregnancy with the odds of giving birth preterm. The meta-analysis included data on 55,507 pregnant women and their children from Denmark (1996–2002), the Netherlands (2003–2004), Spain (2003–2008), and South Korea (2006–2011). In the DNBC, ABCD, and INMA cohorts, no exposure corresponded to no cell-phone use, low exposure to ≤1 call/day, intermediate exposure to 2–3 calls/day, and high exposure to ≥4 calls/day. In the MOCEH cohort, no exposure corresponded to no cell-phone use, low exposure to ≤2 calls/day, intermediate exposure to 3–5 calls/day, and high exposure to ≥6 calls/day. Low exposure was the referent group for all cohorts. Squares show individual study estimates; diamonds show pooled estimates. Results were adjusted for maternal age, parity, active and passive smoking, alcohol consumption, prepregnancy body mass index (weight (kg)/height (m)2), educational level, socioeconomic position, marital status, and maternal height. For no exposure, Q = 0.90 (3 degrees of freedom (df)), P = 0.83, and I2 = 0.0%; for intermediate exposure, Q = 0.92 (3 df), P = 0.82, and I2 = 0.0%; and for high exposure, Q = 6.32 (3 df), P = 0.10, and I2 = 58.2%. Bars, 95% confidence intervals (CIs). ABCD, Amsterdam Born Children and Their Development Study; DNBC, Danish National Birth Cohort; INMA, Spanish Environment and Childhood Project; MOCEH, Korean Mothers and Children’s Environment Health Study.
Sensitivity analyses
In the complete-case analysis, all estimates lost statistical significance; however, the confidence intervals overlapped with the ones from the primary analysis, and the direction of the associations did not change for the outcomes related to pregnancy duration (Web Tables 7 and 8). When excluding one cohort at a time, similar results were obtained. However, the odds ratios for postterm birth were unstable (Web Tables 8 and 9). In the meta-analysis stratified by timing of cell-phone use data collection, we observed that the pooled odds ratios for postterm birth in the primary analysis were driven by the DNBC and ABCD studies, which were conducted earlier and had retrospective exposure assessment (Web Tables 8 and 10). In addition, the odds ratio for preterm birth gained statistical significance in the highly exposed group within the cohorts with prospective exposure assessment (OR = 2.03, 95% CI: 1.22, 3.39) (Web Tables 8 and 10). In the analysis with binary exposure, we observed an increased risk of giving birth at a lower gestational age (HR = 1.04, 95% CI: 1.02, 1.07) and increased odds of preterm birth (OR = 1.16, 95% CI: 1.05, 1.29) for the mothers who used their cell phones more often during pregnancy (Web Tables 8 and 11). The estimates for all of the other outcomes did not differ from unity (Web Table 11). No association of maternal cell-phone use during pregnancy with fetal growth or birth weight was detected in any of the sensitivity analyses (Web Tables 7, 9, 12, and 13). In particular, there was no association between maternal cell-phone use during pregnancy and low birth weight when the analysis was restricted to nonpreterm neonates (Web Table 13).
DISCUSSION
In this study, we examined the association of prenatal maternal cell-phone use with pregnancy duration and fetal growth outcomes in 4 general population birth cohorts. After adjusting for potential confounders, we found no association with fetal growth, but we observed an association with pregnancy duration. Women who reported more frequent calling had higher risk of giving birth at a lower gestational age compared with those reporting less frequent calling. This association was mainly driven by the preterm births; no association with postterm births was observed within the more recent cohorts (INMA and MOCEH), where postterm births were more rare. This association with pregnancy duration was reasonably stable across the cohorts, although in the Dutch cohort (ABCD) risk estimates were in the opposite direction. Notably, the association was more pronounced in the more recent cohorts (INMA and MOCEH), in which cell-phone use had been prospectively assessed during pregnancy, even though their RF-EMF exposure was expected to be lower than that in the older cohorts because of the increasing use of 3G devices.
To date, there have been few studies which examined the association of prenatal maternal cell-phone use with birth outcomes. Although our results for preterm birth are in line with those of a previous study from southern Turkey (16), an analysis of more than 100,000 births from Norway did not find such an association (18). However, unlike our study, those studies did not control for marital status, maternal educational level, or socioeconomic position. Maternal sociodemographic characteristics correlate with cell-phone use and birth outcomes, and the direction of the association with cell-phone use has been shown to differ between populations (42–45). Thus, residual confounding may contribute to the discrepancy between the results for preterm birth; however, it is not possible to determine the direction in which the results may have been affected.
In our study, we observed an association of maternal cell-phone use during pregnancy with pregnancy duration, but not with fetal growth. Since fetal exposure is very low during cell-phone calls (9–12), for the interpretation of these results we considered the potential effect of RF-EMF on maternal head and neck structures, as well as indirect pathways related to the use of cell phones rather than the radiation per se. Animal studies have suggested that RF-EMF exposure may result in minor thyroid gland dysfunction (14, 15). Additionally, higher preconception thyroid-stimulating hormone levels and subclinical hypothyroidism during pregnancy have been associated with higher risks of miscarriage and preterm birth (46–49). Thus, the increased risk for giving birth preterm among heavier users of cell phones that we observed could be mediated by mild thyroid dysfunction. However, the association of RF-EMF exposure from cell-phone use with thyroid function is not established, and large-scale epidemiologic studies on the topic are lacking. Increased oxidative stress has also been considered (50). However, it is not clear whether the elevation of radical oxygen species resulting from local RF-EMF exposure is of such an extent in humans that it could trigger systematic responses affecting birth outcomes. Causal pathways involving local radiation of parts of the human body other than the maternal head and neck were not considered, since this exposure would not be reflected in the number of cell-phone calls per day.
With regard to indirect pathways, stress may contribute to our results (51). Psychosocial stress—acute and chronic—has been associated with higher risk of preterm birth (52–54). Socioeconomic differences and behavioral risk factors (e.g., smoking, alcohol) contribute to this association, along with a direct biological effect (55–57). Maternal cortisol, levels of which increase under stress, stimulates the secretion of placental corticotrophin-releasing hormone during gestation, which participates in the cascade of events initiating labor (57, 58). The elevated levels of placental corticotrophin-releasing hormone among women under stress, and to a lesser extent other stress-related mechanisms, contribute to a higher risk for preterm initiation of spontaneous labor (57, 58). Although our results were adjusted for socioeconomic position, smoking, and alcohol consumption, the direct effect of stress on pregnancy duration was not controlled for. Personal dependency and demands from work and social networks are potential sources of psychosocial stress that were not captured in the covariates used in our analyses and may correlate with cell-phone use (51).
Our study had some important strengths. The large sample size allowed us to detect potential weak associations of maternal cell-phone use with birth outcomes. All of the examined outcomes are interrelated and reflect pregnancy duration, fetal growth, and birth weight. To reduce the probability of type I error, we proposed potential pathways for statistically significant associations only if they were robust across correlated outcomes and across different cohorts. We were also able to assess whether these associations persisted or became attenuated after the introduction of 3G devices, since our study window spanned the period during which 3G technology was introduced. Additionally, the availability of detailed information on maternal characteristics gave us the opportunity to adjust our results for confounders, which were not controlled for in previous studies.
Our study also had several limitations. The exposure variable was based only on the number of cell-phone calls per day; duration of calling was not taken into account, as that information was available only in MOCEH. Furthermore, the number of cell-phone calls per day during pregnancy was self-reported in all cohorts and was validated only in MOCEH (59). Thus, misclassification of exposure should have attenuated the observed association, under our assumption that misclassification was predominantly nondifferential (60–62). We expect that misclassification was much larger in the older cohorts (DNBC and ABCD), as the number of cell-phone calls per day was reported 7 years postnatal. Therefore, the estimates in the DNBC and ABCD cohorts should be more biased towards the null in comparison with the INMA and MOCEH cohorts. In addition, the etiology of the preterm births in our study population was not recorded. As a result, we could not determine whether the observed association with preterm birth was driven by spontaneous labor or labor that was induced because of pregnancy complications. Finally, maternal thyroid function during pregnancy was assessed only in a small subset of participants from the cohorts that were included in this analysis, and information about perceived stress levels during pregnancy was not consistently collected across the cohorts. Consequently, we could not explore or quantify the contributions of the proposed underlying mechanisms to the observed increase in the risk of preterm birth.
In conclusion, in our study, more frequent maternal cell-phone use during pregnancy was associated with shorter pregnancy duration, resulting in increased risk of preterm birth. No association with fetal growth or birth weight was observed. These results suggest that strong effects of cell-phone use on pregnancy duration and fetal growth are unlikely. The findings should be interpreted with caution, since they may reflect an effect of stress during pregnancy or other residual confounding rather than a direct effect of RF-EMF exposure.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Institute for Risk Assessment Sciences, Utrecht University, Utrecht, the Netherlands (Ermioni Tsarna, Marije Reedijk, Anke Huss, Roel Vermeulen); Julius Center for Health Sciences and Primary Care, University Medical Center, Utrecht, the Netherlands (Marije Reedijk, Roel Vermeulen); ISGlobal, Barcelona Institute for Global Health, Barcelona, Spain (Laura Ellen Birks, Mònica Guxens, Elisabeth Cardis, Martine Vrijheid); Department of Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain (Laura Ellen Birks, Mònica Guxens, Elisabeth Cardis, Martine Vrijheid); Spanish Consortium for Research on Epidemiology and Public Health, Instituto de Salud Carlos III, Madrid, Spain (Laura Ellen Birks, Mònica Guxens, Ferran Ballester, Aitana Lertxundi, Elisabeth Cardis, Martine Vrijheid); Department of Child and Adolescent Psychiatry/Psychology, Erasmus University Medical Centre–Sophia Children’s Hospital, Rotterdam, the Netherlands (Mònica Guxens); Epidemiology and Environmental Health Joint Research Unit, Foundation for the Promotion of Health and Biomedical Research of Valencia Region, Universitat Jaume I–Universitat de València, Valencia, Spain (Ferran Ballester, Llúcia González Safont); Department of Preventive Medicine, College of Medicine, Dankook University, Cheonan, South Korea (Mina Ha, Hyung-Ryul Lim); Environment Epidemiology and Child Development Area, BIODONOSTIA Health Research Institute, San Sebastian, Spain (Ana Jiménez-Zabala, Aitana Lertxundi); Public Health Division of Gipuzkoa, Basque Government, San Sebastian, Spain (Ana Jiménez-Zabala); Department of Epidemiology, School of Public Health, University of California, Los Angeles, Los Angeles, California (Leeka Kheifets, Madhuri Sudan); Department of Preventive Medicine and Public Health, Universidad del Pais Vasco/Euskal Herriko Unibertsitatea, Leioa, Spain (Aitana Lertxundi); Department of Clinical Epidemiology, Aarhus University, Aarhus, Denmark (Jorn Olsen, Madhuri Sudan); Unitat Predepartamental de Medicina, Universitat Jaume I, Castelló de la Plana, Spain (Llúcia González Safont); College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, California (Madhuri Sudan); and Department of Public Health, Amsterdam Public Research Institute, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands (Tanja Vrijkotte).
A.H. and R.V. contributed equally to this study.
The Generalized EMF Research Using Novel Methods (GERoNiMO) Project was supported by the European Union (grant 603794). The Amsterdam Born Children and Their Development Study (ABCD) was supported by the Netherlands Organization for Health Research and Development (grant 2100.0076) and the Electromagnetic Fields and Health Research program (grants 85600004 and 85800001). The Danish National Birth Cohort Study (DNBC) was supported by the Danish Epidemiology Science Centre, the Lundbeck Foundation (grant 195/04), the Egmont Foundation, the March of Dimes Birth Defect Foundation, the Augustinus Foundation, and the Medical Research Council (grant SSVF 0646). The Spanish Environment and Childhood Project (INMA) was supported by the European Union (grants FP7-ENV-2011, 282957, and HEALTH.2010.2.4.5-1); Instituto de Salud Carlos III (grants G03/176, CB06/02/0041, FIS-FEDER 03/1615, 04/1509, 04/1112, 04/1931, 05/1079, 05/1052, 06/1213, 07/0314, 09/02647, 11/01007, 11/02591, CP11/00178, FIS-PI06/0867, FIS-PS09/00090, FIS-PI041436, FIS-PI081151, FIS-PI042018, FIS-PI09/02311, FISPI13/1944, FIS-PI13/2429, FIS-PI14/0981, FIS-PI13/141687, CP13/00054 (including FEDER funds), and MS13/00054); the Conselleria de Sanitat Generalitat Valenciana; the Generalitat de Catalunya (grants CIRIT1999SGR and 00241); Obra Social Cajastur; the Universidad de Oviedo; the Department of Health of the Basque Government (grants 2005111093 and 2009111069); and the Provincial Government of Gipuzkoa (grants DFG06/004 and DFG08/001). The Korean Mothers and Children’s Environment Health Study (MOCEH) was supported by the National Institute of Environmental Research, the Ministry of the Environment, and the Information and Communication Technology (ICT) research and development program of the Ministry of Science and ICT (grants 2017-0-00961 and 2019-0-00102), South Korea.
Conflict of interest: none declared.
Abbreviations
- ABCD
Amsterdam Born Children and Their Development Study
- CI
confidence interval
- DNBC
Danish National Birth Cohort
- 2G
second-generation
- 3G
third-generation
- HR
hazard ratio
- INMA
Spanish Environment and Childhood Project
- LGA
large for gestational age
- MOCEH
Korean Mothers and Children’s Environment Health Study
- OR
odds ratio
- RF-EMF
radio-frequency electromagnetic fields
- SD
standard deviation
- SGA
small for gestational age
REFERENCES
- 1. World Bank Mobile cellular subscriptions (per 100 people). 2016. https://data.worldbank.org/indicator/IT.CEL.SETS.P2. Accessed February 9, 2018.
- 2. Health Protection Agency Health Effects From Radiofrequency Electromagnetic Fields. Report of the Independent Advisory Group on Non-Ionising Radiation (RCE-20). London, United Kingdom: Public Health England, United Kingdom Department of Health and Social Care; 2012. http://www.hpa.org.uk/webc/hpawebfile/hpaweb_c/1317133827077. Accessed February 27, 2018.
- 3. World Health Organization Electromagnetic fields and public health: mobile phones. 2014. http://www.who.int/mediacentre/factsheets/fs193/en/. Accessed February 27, 2018.
- 4. Lauer O, Frei P, Gosselin MC, et al. . Combining near- and far-field exposure for an organ-specific and whole-body RF-EMF proxy for epidemiological research: a reference case. Bioelectromagnetics. 2013;34(5):366–374. [DOI] [PubMed] [Google Scholar]
- 5. Daşdaǧ S, Akdaǧ MZ, Ayyildiz O, et al. . Do cellular phones alter blood parameters and birth weight of rats? Electro Magnetobiol. 2000;19(1):107–113. [Google Scholar]
- 6. Yüksel M, Nazıroğlu M, Özkaya MO. Long-term exposure to electromagnetic radiation from mobile phones and Wi-Fi devices decreases plasma prolactin, progesterone, and estrogen levels but increases uterine oxidative stress in pregnant rats and their offspring. Endocrine. 2016;52(2):352–362. [DOI] [PubMed] [Google Scholar]
- 7. Shirai T, Wang J, Kawabe M, et al. . No adverse effects detected for simultaneous whole-body exposure to multiple-frequency radiofrequency electromagnetic fields for rats in the intrauterine and pre- and post-weaning periods. J Radiat Res. 2017;58(1):48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sommer AM, Grote K, Reinhardt T, et al. . Effects of radiofrequency electromagnetic fields (UMTS) on reproduction and development of mice: a multi-generation study. Radiat Res. 2009;171(1):89–95. [DOI] [PubMed] [Google Scholar]
- 9. Cabot E, Christ A, Bühlmann B, et al. . Quantification of RF-exposure of the fetus using anatomical CAD-models in three different gestational stages. Health Phys. 2014;107(5):369–381. [DOI] [PubMed] [Google Scholar]
- 10. Nagaoka T, Togashi T, Saito K, et al. . An anatomically realistic whole-body pregnant-woman model and specific absorption rates for pregnant-woman exposure to electromagnetic plane waves from 10 MHz to 2 GHz. Phys Med Biol. 2007;52(22):6731–6745. [DOI] [PubMed] [Google Scholar]
- 11. Tateno A, Tanaka K, Nagaoka T, et al. . Specific absorption rates of pregnant females and their fetuses from simple and realistic electromagnetic sources. IEICE Commun Express. 2014;3(2):55–60. [Google Scholar]
- 12. Varsier N, Dahdouh S, Serrurier A, et al. . Influence of pregnancy stage and fetus position on the whole-body and local exposure of the fetus to RF-EMF. Phys Med Biol. 2014;59(17):4913–4926. [DOI] [PubMed] [Google Scholar]
- 13. Luo Q, Jiang Y, Jin M, et al. . Proteomic analysis on the alteration of protein expression in the early-stage placental villous tissue of electromagnetic fields associated with cell phone exposure. Reprod Sci. 2013;20(9):1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eşmekaya MA, Seyhan N, Ömeroǧlu S. Pulse modulated 900 MHz radiation induces hypothyroidism and apoptosis in thyroid cells: a light, electron microscopy and immunohistochemical study. Int J Radiat Biol. 2010;86(12):1106–1116. [DOI] [PubMed] [Google Scholar]
- 15. Misa-Agustiño MJ, Jorge-Mora T, Jorge-Barreiro FJ, et al. . Exposure to non-ionizing radiation provokes changes in rat thyroid morphology and expression of HSP-90. Exp Biol Med (Maywood). 2015;240(9):1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Col-Araz N. Evaluation of factors affecting birth weight and preterm birth in southern Turkey. J Pak Med Assoc. 2013;63(4):459–462. [PubMed] [Google Scholar]
- 17. Mortazavi SM, Shirazi KR, Mortazavi G. The study of the effects of ionizing and non-ionizing radiations on birth weight of newborns to exposed mothers. J Nat Sci Biol Med. 2013;4(1):213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baste V, Oftedal G, Møllerløkken OJ, et al. . Prospective study of pregnancy outcomes after parental cell phone exposure: the Norwegian Mother and Child Cohort Study. Epidemiology. 2015;26(4):613–621. [DOI] [PubMed] [Google Scholar]
- 19. Radiation Programme, ISGlobal Barcelona Institute for Global Health GERoNiMO: Generalized EMF Research Using Novel Methods. 2014. http://radiation.isglobal.org/index.php/geronimo-home. Accessed February 15, 2018.
- 20. Olsen J, Melbye M, Olsen SF, et al. . The Danish National Birth Cohort—its background, structure and aim. Scand J Public Health. 2001;29(4):300–307. [DOI] [PubMed] [Google Scholar]
- 21. Divan HA, Kheifets L, Obel C, et al. . Prenatal and postnatal exposure to cell phone use and behavioral problems in children. Epidemiology. 2008;19(4):523–529. [DOI] [PubMed] [Google Scholar]
- 22. van Eijsden M, Vrijkotte TG, Gemke RJ, et al. . Cohort profile: the Amsterdam Born Children and their Development (ABCD) study. Int J Epidemiol. 2011;40(5):1176–1186. [DOI] [PubMed] [Google Scholar]
- 23. Guxens M, Ballester F, Espada M, et al. . Cohort profile: the INMA–INfancia y Medio Ambiente–(Environment and Childhood) Project. Int J Epidemiol. 2012;41(4):930–940. [DOI] [PubMed] [Google Scholar]
- 24. Kim BM, Ha M, Park HS, et al. . The Mothers and Children’s Environmental Health (MOCEH) study. Eur J Epidemiol. 2009;24(9):573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Visser GH, Eilers PH, Elferink-Stinkens PM, et al. . New Dutch reference curves for birthweight by gestational age. Early Hum Dev. 2009;85(12):737–744. [DOI] [PubMed] [Google Scholar]
- 26. Carrascosa Lezcano A, Ferrández Longás A, Yeste Fernández D, et al. . Estudio transversal español de crecimiento 2008. Parte I: Valores de peso y longitud en recién nacidos de 26–42 semanas de edad gestacional. An Pediatr (Barc). 2008;68(6):544–551. [DOI] [PubMed] [Google Scholar]
- 27. Lim JS, Lim SW, Ahn JH, et al. . New Korean reference for birth weight by gestational age and sex: data from the Korean Statistical Information Service (2008–2012). Ann Pediatr Endocrinol Metab. 2014;19(3):146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand. 2000;79(6):440–449. [PubMed] [Google Scholar]
- 29. Birks L, Guxens M, Papadopoulou E, et al. . Maternal cell phone use during pregnancy and child behavioral problems in five birth cohorts. Environ Int. 2017;104:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Voskamp BJ, Kazemier BM, Schuit E, et al. . Birth weight ratio as an alternative to birth weight percentile to express infant weight in research and clinical practice: a nationwide cohort study. Obstet Gynecol Int. 2014;2014:749476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. [DOI] [PubMed] [Google Scholar]
- 32. Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206–213. [DOI] [PubMed] [Google Scholar]
- 33. Li KH, Meng XL, Raghunathan TE, et al. . Significance levels from repeated p-values with multiply-imputed data. Stat Sin. 1991;1:65–92. [Google Scholar]
- 34. Joseph KS, Kramer MS. The fetuses-at-risk approach: survival analysis from a fetal perspective. Acta Obstet Gynecol Scand. 2018;97(4):454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. R Core Team R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing; 2017. https://www.r-project.org/. Accessed October 12, 2017.
- 36. Yoshida K, Bohn J tableone: Create “Table 1” to describe baseline characteristics. 2015. https://cran.r-project.org/package=tableone. Accessed June 8, 2017.
- 37. van Buuren S, Groothuis-Oudshoorn K. Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. [Google Scholar]
- 38. Robitzsch A, Grund S, Henke T miceadds: Some additional multiple imputation functions, especially for “mice.” 2017. https://cran.r-project.org/package=miceadds. Accessed June 8, 2017.
- 39. Therneau TM. A Package for Survival Analysis in S Vienna, Austria: R Foundation for Statistical Computing; 2015. https://cran.r-project.org/package=survival. Accessed October 9, 2017.
- 40. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer-Verlag New York; 2000. [Google Scholar]
- 41. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 42. Bolte JF, Eikelboom T. Personal radiofrequency electromagnetic field measurements in the Netherlands: exposure level and variability for everyday activities, times of day and types of area. Environ Int. 2012;48:133–142. [DOI] [PubMed] [Google Scholar]
- 43. de Graaf JP, Steegers EA, Bonsel GJ. Inequalities in perinatal and maternal health. Curr Opin Obstet Gynecol. 2013;25(2):98–108. [DOI] [PubMed] [Google Scholar]
- 44. Lu X, Oda M, Ohba T, et al. . Association of excessive mobile phone use during pregnancy with birth weight: an adjunct study in Kumamoto of Japan Environment and Children’s Study. Environ Health Prev Med. 2017;22:Article 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morgen CS, Bjork C, Andersen PK, et al. . Socioeconomic position and the risk of preterm birth—a study within the Danish National Birth Cohort. Int J Epidemiol. 2008;37(5):1109–1120. [DOI] [PubMed] [Google Scholar]
- 46. Chen S, Zhou X, Zhu H, et al. . Preconception TSH and pregnancy outcomes: a population-based cohort study in 184 611 women. Clin Endocrinol (Oxf). 2017;86(6):816–824. [DOI] [PubMed] [Google Scholar]
- 47. Arbib N, Hadar E, Sneh-Arbib O, et al. . First trimester thyroid stimulating hormone as an independent risk factor for adverse pregnancy outcome. J Matern Fetal Neonatal Med. 2017;30(18):2174–2178. [DOI] [PubMed] [Google Scholar]
- 48. Schneuer FJ, Nassar N, Tasevski V, et al. . Association and predictive accuracy of high TSH serum levels in first trimester and adverse pregnancy outcomes. J Clin Endocrinol Metab. 2012;97(9):3115–3122. [DOI] [PubMed] [Google Scholar]
- 49. Casey BM, Dashe JS, Wells CE, et al. . Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105(2):239–245. [DOI] [PubMed] [Google Scholar]
- 50. Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR), European Commission Potential Health Effects of Exposure to Electromagnetic Fields (EMF) Brussels, Belgium: European Commission; 2015. http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_041.pdf. Accessed April 3, 2018.
- 51. Thomée S, Härenstam A, Hagberg M. Mobile phone use and stress, sleep disturbances, and symptoms of depression among young adults—a prospective cohort study. BMC Public Health. 2011;11:Article 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Medsker B, Forno E, Simhan H, et al. . Prenatal stress, prematurity, and asthma. Obstet Gynecol Surv. 2015;70(12):773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Beydoun H, Saftlas AF. Physical and mental health outcomes of prenatal maternal stress in human and animal studies: a review of recent evidence. Paediatr Perinat Epidemiol. 2008;22(5):438–466. [DOI] [PubMed] [Google Scholar]
- 54. Mulder EJ, Robles de Medina PG, Huizink AC, et al. . Prenatal maternal stress: effects on pregnancy and the (unborn) child. Early Hum Dev. 2002;70(1-2):3–14. [DOI] [PubMed] [Google Scholar]
- 55. Kristenson M, Eriksen HR, Sluiter JK, et al. . Psychobiological mechanisms of socioeconomic differences in health. Soc Sci Med. 2004;58(8):1511–1522. [DOI] [PubMed] [Google Scholar]
- 56. Lobel M, Cannella DL, Graham JE, et al. . Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27(5):604–615. [DOI] [PubMed] [Google Scholar]
- 57. Lockwood C, Ramin S, Barss V. Stress-associated preterm delivery: the role of corticotropin-releasing hormone. Am J Obstet Gynecol. 1999;180(1):S264–S266. [DOI] [PubMed] [Google Scholar]
- 58. Wadhwa PD, Entringer S, Buss C, et al. . The contribution of maternal stress to preterm birth: issues and considerations. Clin Perinatol. 2011;38(3):351–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Choi KH, Ha M, Burm E, et al. . Multiple assessment methods of prenatal exposure to radio frequency radiation from telecommunication in the Mothers and Children’s Environmental Health (MOCEH) study. Int J Occup Med Environ Health. 2016;29(6):959–972. [DOI] [PubMed] [Google Scholar]
- 60. Vrijheid M, Cardis E, Armstrong BK, et al. . Validation of short term recall of mobile phone use for the Interphone study. Occup Environ Med. 2006;63(4):237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goedhart G, Kromhout H, Wiart J, et al. . Validating self-reported mobile phone use in adults using a newly developed smartphone application. Occup Environ Med. 2015;72(11):812–818. [DOI] [PubMed] [Google Scholar]
- 62. Goedhart G, van Wel L, Langer CE, et al. . Recall of mobile phone usage and laterality in young people: the multinational Mobi-Expo study. Environ Res. 2018;165:150–157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.