Abstract
Background
Disability in activities of daily living (ADLs) is a dynamic process and transitions among different disability states are common. However, little is known about factors affecting recovery from disability. We examined the association between frailty and recovery from disability among nondisabled community-dwelling elders.
Methods
We studied 1,023 adults from the Cardiovascular Health Study (CHS) and 685 adults from the Health and Retirement Study (HRS), who were ≥65 years and had incident disability, defined as having difficulty in ≥1 ADL (dressing, eating, toileting, bathing, transferring, walking across a room). Disability recovery was defined as having no difficulty in any ADLs. Frailty was assessed by slowness, weakness, exhaustion, inactivity, and shrinking. Persons were classified as “nonfrail” (0 criteria), “prefrail” (1–2 criteria), or “frail” (3–5 criteria).
Results
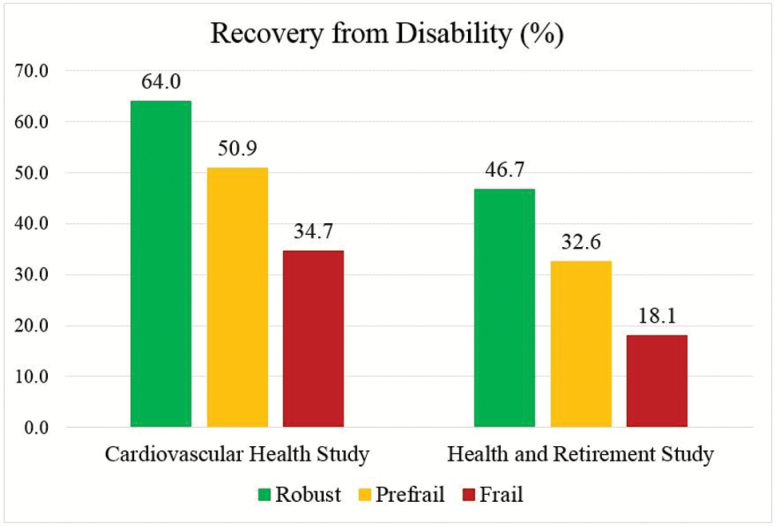
In total, 539 (52.7%) CHS participants recovered from disability within 1 year. Almost two-thirds of nonfrail persons recovered, while less than two-fifths of the frail recovered. In the HRS, 234 (34.2%) participants recovered from disability within 2 years. Approximately half of the nonfrail recovered, while less than one-fifth of the frail recovered. After adjustment, prefrail and frail CHS participants were 16% and 36% less likely to recover than the nonfrail, respectively. In the HRS, frail persons had a 41% lower likelihood of recovery than the nonfrail.
Conclusions
Frailty is an independent predictor of poor recovery from disability among nondisabled older adults. These findings validate frailty as a marker of decreased resilience and may offer opportunities for individualized interventions and geriatric care based on frailty assessment.
Keywords: Disablement process, Frailty, Resilience, Recovery
The prevalence of disability in activities of daily living (ADLs) has steadily declined among older Americans since early 1980s (1). This promising trend, however, appears to have slowed in the early 2000s (2–4). Recent prevalence estimates range from 13.1% in those aged 65–69 years to 36.4% in those aged ≥85 years among community-dwelling U.S. adults (5). ADL disability is associated with shorter survival and hospitalization, and higher health care expenditures, placing a substantial burden on older persons, their caregivers, and health care resources (6–9).
A growing body of research has demonstrated that disability is a dynamic process and transitions among different disability states are common, even among the oldest old or at the end of life (10–13). Knowledge of predictors for recovery from disability may offer new opportunities for interventions and geriatric care targeted at promoting recovery from disability, maintaining independence after recovery, and preventing recurrent disability. Prior studies have found that slow gait speed, low physical activity, and significant weight loss are associated with poor recovery of independence in ADLs (12,14,15). Although these are components of frailty, no prior study has examined frailty as a whole and its impact on recovery from disability among noninstitutionalized older adults.
We examined the association of frailty with recovery from disability among community-dwelling older adults without pre-existing disability from the Cardiovascular Health Study (CHS). Results were validated in the Health and Retirement Study (HRS). We postulated that frail older adults would have a compromised ability to cope with daily stressors, precluding them from recovering from incident disability.
Methods
Data and Participants
Cardiovascular Health Study (CHS)
The CHS is an ongoing cohort study of 5,888 community-dwelling men and women aged ≥65 years in the U.S. Participants were identified from a random sample of Medicare enrollees in four communities: Forsyth Country, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh, PA. A total of 5,201 participants were enrolled in 1989–1990 (original cohort). An additional sample of 687 African Americans were recruited in 1992–1993 (new cohort). All participants were requested to complete an interview, health questionnaire, and comprehensive physical examination, and to provide blood specimens at enrollment and annually through 1999–2000. Institutional review boards (IRB) at each site approved the study protocol; all participants signed informed consent. More details about the recruitment strategies and study design of the CHS have been published elsewhere (16).
Data on frailty were collected in 1989–1990, 1992–1993, and 1996–1997 examinations; data on ADL disability were collected annually from 1989 to 2000. Participants were included (n = 1,115) if they (a) had frailty assessment in one of the following time points: 1989–1990, 1992–1993, and 1996–1997, (b) had no ADL limitations at the time of frailty assessment, and (c) were disabled in at least one ADL task within 2 years. For participants who contributed more than one observation (eg, had frailty measure in 1989–1990 and disabled in 1991–1992, and had frailty measure in 1996–1997 and disabled in 1997–1998), only the first episode of disability was included.
Health and Retirement Study (HRS)
The HRS is an ongoing cohort study of a nationally representative sample of noninstitutionalized residents in the contiguous United States. The HRS is primarily funded by the U.S. National Institute on Aging and is designed, administered, and managed by the Institute for Social Research at the University of Michigan. In Wave 2006–2007, approximately half of the HRS participants were randomly selected to participate in an enhanced face-to-face interview, during which physical performance was assessed. The other half of the participants were assessed in Wave 2008–2009. Ethical approval was obtained from IRB at the University of Michigan; all participants signed informed consent. Further details about the recruitment strategies and study design of the HRS have been previously documented (17).
We used pooled data from the 2006–2007 and 2008–2009 waves, when gait speed and grip strength were measured. ADL disability was measured in these two waves and measured every 2 years thereafter. We included 705 persons who (i) were at least 65 years of age, (ii) had complete data on frailty measures (see below) in either wave, (iii) had no difficulty in any ADLs at the time of frailty assessment, and (iv) developed difficulty in at least one ADL task within 2 years.
Frailty
Frailty was assessed by the physical frailty phenotype (PFP) scale that is defined based on five criteria: slowness, weakness, exhaustion, inactivity, and shrinking (18). Operational definitions of the five criteria in the CHS cohort were adopted from the original publication described by Fried et al. (18). We modified the operational definitions for the HRS cohort because the measurements were not identical (Supplementary Table 1).
In the CHS, the slowness criterion was met when usual gait speed over a 15-feet (~4.6 m) walking course was less than or equal to the sex- and height-specific cut-points (worst quintile) (18). The weakness creation was met when handgrip strength was less than or equal to the sex- and body mass index-specific cut-points (worst quintile) (18). The exhaustion creation was met if the participants answered “A moderate amount of time; 3 to 4 days”, or “Most of the time; 5 to 7 days” when asked, “How often in the last week did you feel this way” to either of the two questions from Center for Epidemiologic Studies Depression (CES-D) scale (19) including, “I could not get going” and “I felt that everything I did was an effort.” The physical inactivity criterion was met for men who scored <383 kcals and women who scored <270 kcals on the Modified Minnesota Leisure Time Activities Questionnaire (20). For participants whose frailty was measured at enrollment—1989–1990 for the original cohort and 1992/1993 for the new cohort, the shrinking criterion was met if they reported losing ≥10 pounds (~4.5 kg) not due to diet or exercise in the last year. For participants whose frailty status was assessed at follow-up visit, the shrinking criterion was met if they lost ≥5% of body weight not due to diet or exercise in the past year.
In the HRS, slowness and weakness were defined using different sex- and body size-specific cut-points. The physical inactivity criterion was met for men who scored ≤5 and women who scored ≤4 on a physical activity scale, calculated based on the intensity and frequency of three physical activities. Mild, moderate, and vigorous activities were scored 2, 4, and 8 Metabolic Equivalent of Task, respectively (21). Weights were determined by the frequency; “Everyday”, “More than once a week”, “Once a week”, “1–3 times a month”, and “Hardly ever” was scored 7, 4, 1, 0.5, and 0, respectively (22). The exhaustion creation was met if participants answered “Yes” when asked, “Please tell me if each of the following was true for you much of the time during the past week” to either of the two CES-D questions described above. The shrinking criterion was met if participants lost ≥10% of body weight in the past 2 years (by direct measure; between prior; and current wave).
Frailty was identified by the number of criteria met. Persons who satisfied none of the criteria were considered “nonfrail”; those meeting one or two criteria were considered “prefrail”; and those with 3–5 criteria were defined as “frail”.
Outcomes
In the CHS, disability in each of the six ADLs—dressing, eating, toileting, bathing, transferring or getting out of bed, and walking across a room—was assessed by the question, “Do you have difficulty or are unable to” perform the task. In the HRS, participants were asked, “Because of a health or memory problem do you have any difficulty with” performing the task. Participants who responded “Yes” were considered having difficulty. CHS participants who answered “Could do it, but don’t for other reason” were considered not having difficulty. HRS participants who answered “Can’t do” or “Don’t do” were considered having difficulty; those who reported “Don’t know or not ascertained” or “Refused” were coded as missing. Participants who reported having difficulty in at least one ADL were considered disabled. Disability in one ADL was defined as mild and in ≥2 ADLs as severe.
Recovery from disability was defined as having no difficulty in any ADLs in the following visit after experiencing disability (within 1 year in the CHS and 2 years in the HRS). Persons who died before subsequent visit after being disabled were presumed not to have recovered. This is a commonly used strategy to deal with missing data due to death, justified by the fact that approximately 90% of older adults have disability within 1 year of death (23). Persons who were alive but not interviewed in the following visit after being disabled were considered missing and were therefore excluded (n = 92 for the CHS; n = 20 for the HRS).
Covariates
Clinic site (Bowman Gray, Johns Hopkins, Davis, and Pittsburgh) was included in the CHS. In both cohorts, age was calculated by the difference between the visit date of frailty assessment and a participant’s birth date; sex, education, and race/ethnicity were identified based on self-report. Education was categorized as <high school, high school or equivalent, or >high school. Race/ethnicity was dichotomized as white versus black or others in the CHS because <1% of the CHS participants were neither white nor black. In the HRS, race/ethnicity (non-Hispanic white, black, Hispanics, and others) was categorized as white versus others. Smoking status was categorized as current, former, and never smoker. Body mass index was calculated as body weight (kg) divided by height (m) squared, and was categorized as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–30.0 kg/m2), and obese (>30.0 kg/m2). Underweight and normal categories were collapsed due to small cell size in the underweight category. History of heart disease, hypertension, diabetes, cancer (excluding minor skin cancer), and arthritis was assessed based on self-reported physician diagnosis. Self-rated health was coded as excellent/very good versus good/fair/poor. Cognitive function was measured by the Modified Mini-Mental State Examination (3MS; range: 0–100) (24) in the CHS and by a modified Telephone Interview for Cognitive Status (TICS; range: 0–10) (25) in the HRS.
Analytic Approaches
We compared the characteristics between the two cohorts using a t-test assuming unequal variance for continuous variables and a χ2 test for categorical variables. Due to differences in design and measurements, the two cohorts were analyzed separately. We presented the numbers and proportions of persons who recovered from disability stratified by frailty status: nonfrail, prefrail, and frail. We also presented results for recovery from mild and severe disability (difficulty in ≥2 ADLs), respectively, for persons who were nonfrail, prefrail, and frail. Two studies were analyzed separately.
We used Poisson models with robust variance estimator to determine the unadjusted and adjusted association of frailty with recovery from disability. Compared with logistic model, Poisson model with nonfrail variance estimator could provide approximate estimate of relative risk—a measure that is more intuitive than an odds ratio (26). Frailty was modeled as a three-level categorical variable with nonfrail being the reference. Clinic site (only for the CHS), age, sex, race/ethnicity, education, smoking status, body mass index, history of chronic conditions, self-rated health, cognitive function (3MS in the CHS; TICS in the HRS), and severity of disability at onset (mild vs severe) were included in the multivariable adjusted models. All covariates were measured at the time of frailty assessment. We did not use the most recent assessment of covariates prior to the onset of incident disability because covariates measured after frailty may be in the causal pathway between frailty and recovery from disability. Adjustment for such mediators can lead to underestimation of the overall effect of frailty. We tested whether the association of frailty with recovery from disability differed by severity of disability by including a product term between frailty and disability levels. As a secondary analysis, we estimated the unadjusted association of each of five frailty criteria (slowness, weakness, exhaustion, inactivity, and shrinking) with recovery from disability.
All tests were two-sided with a significance level of 0.05. All statistical analyses were performed using Stata 15.
Results
Sample Description
A total of 1,023 CHS and 685 HRS participants were included in the primary analysis. Average age was 75.3 years (SD = 5.9) in the CHS cohort; HRS participants were slightly older, with an average age of 77.3 years (SD = 7.4; Table 1). Males comprised 36.5% of the CHS cohort and 40.3% of the HRS cohort. Whites consisted of 86.2% and 84.7% of the CHS and HRS cohorts, respectively. Compared with HRS participants, CHS participants were more educated, less likely to be obese, and had lower prevalence of chronic conditions. CHS participants were also less likely to be frail and experienced a lower rate of severe disability (difficulty in ≥2 ADLs) than HRS participants.
Table 1.
Characteristics of Study Participants
| Characteristics | CHS | HRS | p-value* |
|---|---|---|---|
| N = 1,023 | N = 685 | ||
| Age, y, mean (SD) | 75.3 (5.9) | 77.3 (7.4) | <.001 |
| Male, N (%) | 373 (36.5) | 276 (40.3) | .341 |
| Whites (vs others), N (%) | 882 (86.2) | 580 (84.7) | .254 |
| Education | |||
| <High school, N (%) | 272 (26.9) | 219 (32.0) | Ref. |
| =High school, N (%) | 268 (26.3) | 234 (34.1) | .661 |
| >High school, N (%) | 477 (46.8) | 232 (33.9) | <.001 |
| Smoking status | |||
| Never, N (%) | 473 (46.3) | 295 (43.4) | Ref. |
| Former, N (%) | 452 (44.3) | 327 (48.1) | .135 |
| Current, N (%) | 96 (9.4) | 58 (8.5) | .857 |
| Body mass index, kg/m2 | |||
| Underweight/Normal†, N (%) | 313 (30.8) | 169 (24.7) | Ref. |
| Overweight, N (%) | 443 (43.5) | 253 (36.9) | .661 |
| Obese, N (%) | 262 (25.7) | 263 (38.4) | <.001 |
| Heart disease‡, N (%) | 264 (25.8) | 277 (40.4) | <.001 |
| Hypertension, N (%) | 612 (59.8) | 466 (68.0) | <.001 |
| Diabetes, N (%) | 193 (19.0) | 181 (26.5) | <.001 |
| Cancer§, N (%) | 165 (16.1) | 144 (21.1) | .021 |
| Arthritis, N (%) | 676 (66.7) | 530 (77.4) | <.001 |
| Self-rated health||, N (%) | |||
| Excellent/very good | 300 (29.4) | 160 (23.4) | .007 |
| Cognitive function¶, mean (SD) | 91.2 (6.2) | 8.9 (1.6) | NA |
| Frailty, N (%) | |||
| Nonfrail | 289 (28.3) | 199 (28.2) | Ref. |
| Prefrail | 613 (59.9) | 384 (54.5) | <.001 |
| Frail | 121 (11.8) | 122 (17.3) | <.001 |
| Severe ADL disability#, N (%) | 272 (26.6) | 274 (40.0) | <.001 |
Notes: ADL = activities of daily living; CHS = Cardiovascular Health Study; HRS = Health and Retirement Study; SD = standard deviation.
*P-values were obtained from generalized linear regression with clustered sandwich estimator for comparison between CHS and HRS participants (HRS participants were clustered within households).
†Underweight and normal were collapsed due to small cell size in the underweight category.
‡Coronary heart disease and heart failure were included in the CHS; myocardial infarction, coronary heart disease, angina, heart failure, or other heart problems were included in the HRS.
§Nonmelanoma skin cancer was excluded.
||The reference was good/fair/poor.
¶Cognitive function was measured by the modified mini-mental status examination (range: 0–100) in the CHS and by the Telephone Interview for Cognitive Status (range: 0–10) in the HRS.
#Difficulty in two or more ADLs.
Association of Frailty with Recovery from Disability
Recovery from disability within 1 year was observed for 539 (52.7%) of the 1,023 newly disabled CHS participants. The likelihood of recovering from disability was lower in those with severe disability (37.5%) than in those with mild disability (58.2%), and it decreased steadily from nonfrail to frail (Figure 1). Almost two-thirds of nonfrail persons recovered, while less than two-fifths of the frail had recovery. We observed similar trends when stratifying the analyses by severity of disability (mild vs severe; Table 2). For persons who experienced mild disability, 68.9%, 55.8%, and 40.0% of the nonfrail, prefrail, and frail had recovery, respectively. For persons who had severe disability, 45.9%, 37.6%, and 26.1% of the nonfrail, prefrail, and frail recovered, respectively.
Figure 1.
Proportions of recovery from disability by frailty status. Notes: Participants who died in the following visit after the onset of incident disability were included and considered not to recover. Participants who were alive but not interviewed in the following visit after the onset of incident disability were excluded. Recovery from disability was defined as no difficulty in any of six activities of daily living (dressing, eating, toileting, bathing, transferring, and walking across a room) within 1 year in the Cardiovascular Health Study and within 2 years in the Health and Retirement Study after the onset of disability.
Table 2.
Frailty Status and Recovery From Disability by Severity of Disability
| Cardiovascular Health Study | Health and Retirement Study | |
|---|---|---|
| Recovery from Mild ADL Disability* Numbers (%) | ||
| Total | 437 (58.2) | 182 (44.3) |
| Frailty | ||
| Nonfrail | 157 (68.9) | 73 (53.3) |
| Prefrail | 250 (55.8) | 94 (43.1) |
| Frail | 30 (40.0) | 15 (26.8) |
| Recovery from severe ADL disability † Numbers (%) |
||
| Total | 102 (37.5) | 52 (19.0) |
| Frailty | ||
| Nonfrail | 28 (58.9) | 17 (30.9) |
| Prefrail | 62 (37.6) | 29 (18.3) |
| Frail | 12 (26.1) | 6 (10.0) |
Notes: Participants who died in the following visit after the onset of incident disability were included and considered not to recover. Participants who were alive but not interviewed in the following visit after the onset of incident disability were excluded. ADL = activities of daily living.
*Mild disability was defined as having difficulty in one ADL.
†Severe disability was defined as having difficulty in two or more ADL.
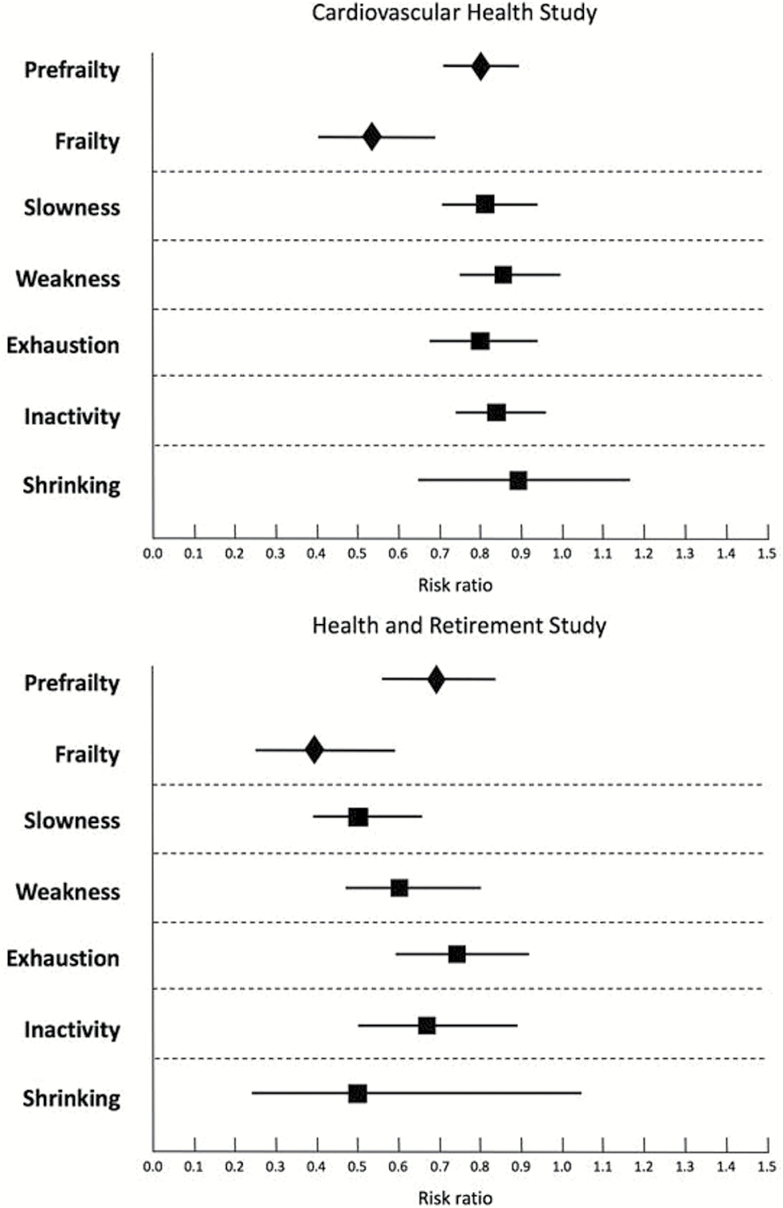
Of the 685 newly disabled HRS participants, 234 (34.2%) recovered from disability within 2 years, with the lower recovery rate in those with severe disability (19.0%) than with mild disability (44.3%; Table 2). There was a steep risk gradient for recovery from disability from nonfrail to frail (Figure 1). Approximately half of nonfrail persons recovered, while less than one-fifths of the frail had recovery. Nonfrail persons were two times more likely to recover from mild disability than those who were frail (53.3% vs 26.8%). When experiencing severe disability, nonfrail persons had an approximately 3-fold greater likelihood of having recovery than the frail (30.9% vs 10.0%; Table 2). In both cohorts, the association of frailty with recovery from disability was stronger compared with that for the individual components of the PFP scale (Figure 2).
Figure 2.
Association of frailty and components of frailty with recovery from disability. Notes: Points estimates (unadjusted) are accompanied by 95% confidence intervals. Prefrailty and frailty were modeled simultaneously with nonfrail persons being the reference. Each of five binary frailty components (slowness, weakness, exhaustion, inactivity, and shrinking) were estimated separately. Two study cohorts were analyzed independently.
Adjusted Association of Frailty with Recovery from Disability
After multivariable adjustment, prefrail, and frail CHS participants were 16% and 36% less likely to recover from disability within 1 year than the nonfrail, respectively (Table 3). The association of frailty and recovery did not differ between individuals with mild disability and those with severe disability (p = .671 for disability severity × prefrail vs nonfrail; p = .995 for disability severity × frail vs nonfrail). In the HRS, frail persons had a 41% lower likelihood of recovery from disability within 2 years than those who were nonfrail. Severity of disability did not modify the association of frailty with disability recovery (p = .271 for disability severity × prefrail vs nonfrail); p = .465 for disability severity × frail vs nonfrail).
Table 3.
Frailty Status and Recovery From Disability
| Recovery From ADL Disability* Relative Risk (95% CI) |
|||
|---|---|---|---|
| Cardiovascular Health Study N = 1,023 |
|||
| N (%) | Unadjusted | Adjusted† | |
| Frailty | |||
| Nonfrail | 289 (28.3) | Ref. | Ref. |
| Prefrail | 613 (59.9) | 0.80 (0.71, 0.89) | 0.84 (0.75, 0.95) |
| Frail | 121 (11.8) | 0.54 (0.42, 0.70) | 0.64 (0.49, 0.84) |
| Health and Retirement Study N = 685 |
|||
| Unadjusted | Adjusted c | ||
| Frailty | |||
| Nonfrail | 199 (28.2) | Ref. | Ref. |
| Prefrail | 384 (54.5) | 0.69 (0.56, 0.86) | 0.86 (0.69, 1.08) |
| Frail | 122 (17.3) | 0.39 (0.25, 0.59) | 0.59 (0.40, 0.88) |
Notes: ADL = activities of daily living; CI = confidence interval.
*No difficulty in any ADLs within 1 year (2 years for the HRS cohort) after the onset of incident disability.
bAdjusted for clinical site (Bowman Gray, Johns Hopkins, Davis, and Pittsburgh), age, sex, race (white, others), education (less than high school, high school or equivalent, more than high school), smoking status (current, former, never), body mass index (<25.0, 25.0–30.0, >30.0), history of heart disease (coronary heart disease and heart failure), hypertension, diabetes, cancer, and arthritis, self-rated health (excellent/very good, good/fair/poor), cognitive function measured by the modified mini-mental status examination, and severity of incident ADL disability (difficulty in one ADL vs difficulty in two or more ADLs). p-value for interactions disability severity × prefrail vs nonfrail=0.671, disability severity × frail vs nonfrail=0.995.
cAdjusted for age, sex, race (white, others), education (less than high school, high school or equivalent, more than high school), smoking status (current, former, never), body mass index (<25.0, 25.0–30.0, >30.0), history of heart disease (heart attack, coronary heart disease, angina, heart failure, or other heart problems), hypertension, diabetes, cancer, and arthritis, self-rated health (excellent/very good, good/fair/poor), cognitive function measured by the Telephone Interview for Cognitive Status, and severity of incident ADL disability (difficulty in one ADL vs difficulty in two or more ADLs). p-value for interactions disability severity × prefrail vs nonfrail=0.271, disability severity × frail vs nonfrail = 0.465.
Sensitivity analysis excluding persons who died prior to the follow-up interview after being disabled did not appreciably change the results (data not shown).
Discussion
The purpose of the present study was to understand the role of frailty, a clinical syndrome of decreased resilience to withstand acute stressors, in the recovery process of ADL disability among community-dwelling older adults without pre-existing disability. Using data from two large, population-based cohort studies, we showed that frailty was strongly associated with poor recovery from disability; once they developed disability, frail older adults were less likely to recover than the nonfrail. Our results highlight the importance of frailty in the pathway to poor recovery from disability among older persons.
Our study builds upon an earlier investigation conducted by Boyd et al. (27), showing that frailty, assessed by the PFP scale, was associated with decline in ADL function after hospitalization among 457 moderately to severely disabled older women. In addition to multi-component measures of frailty, prior studies have demonstrated the associations of the indicators of frailty—for example, gait speed, physical activity, and weight loss—with recovery from disability (12,14,15). Using data from 420 newly disabled persons from the Precipitating Events Project, a cohort study of 754 community-dwelling adults aged ≥70 years in greater New Haven, Connecticut, Hardy et al (12). found that individuals with mobility impairment, defined as requiring >10 seconds walking back and forth over a 10-foot course as quickly as possible, were less likely to recovery from ADL disability. Using data from the same cohort, Hardy and Gill showed that habitual physical activity was an independent risk factor for both time to and duration of recovery of ADL function (15). More recently, Gill et al. (14) found that significant weight loss, denoted as self-report of a 10-pound weight loss in the previous year, was associated with lower likelihood of recovery of prehospital ADL function among 292 elders newly admitted to a nursing home with disability after an acute hospitalization. Our study extends previous research in two important ways. First, instead of using single-item measures of frailty, we measured frailty using the PFP scale, a multi-component frailty instrument that has been well validated and widely used in research. In addition, independent validation of results, which is a routine practice of gene discovery studies, has been much less seen in epidemiological research. We replicated results in two independent samples, which may self-correct coincidental findings that happen to be statistically significant. Lastly, the use of two large, population-based cohorts enhances the generalizability of our findings.
The National Institute on Aging recently held a workshop emphasizing the needs to improve measures to assess resilience in human aging (28). Frailty, a clinical syndrome characterized by reduced resilience to stressors, is theoretically a good candidate assessment for lack of resilience. The present study provided empirical evidence demonstrating a strong association between frailty and recovery from one specific stressor—disability. In addition to disability, frail elders were also less likely to resist adverse effects of surgical procedures (29–31). These results, taken together, suggest that frailty is able to identify persons with diminished ability to resist or recover from multiple stressors. People who meet the criteria for the frailty phenotype may need to be evaluated for medical care management and receive interventions, such as exercise and nutritional and social support, as clinically indicated (32–35).
Our study has many strengths, including its prospective design, comprehensive set of measurements of potential confounders, large sample size, heterogeneity in demographic composition of study samples, and replication of results in an independent sample. To our knowledge, this study is the first to assess the association of a multicomponent measure of frailty with recovery of ADL function among community-dwelling older adults. Despite these strengths, we acknowledge several limitations. First, the PFP scale, which was developed in the CHS cohort, has been typically applied with adaptations (eg, moderately different items and different cut-points). Although the CHS and the HRS did not use the same PFP scale, the results were largely consistent between the two cohorts. In addition, numerous assessments of frailty have been proposed, but we only used one assessment—the PFP scale. Compared with many other frailty assessments, the PFP scale considers frailty a specific physiological state with its own definable phenotypic manifestation that is distinguishable from disability and comorbidity (36). Moreover, we did not include persons who were initially disabled because frailty status could have changed due to the onset of disability, which could lead to reverse causation. Furthermore, we only focused on the relationship between frailty and recovery from incident disability. Recovery from recurrent disability might have different risk factor profiles, which deserves consideration for future research. Lastly, frailty was only assessed once; there might be unobserved transitions between frailty states, especially in the HRS because of the longer interval between disability assessments.
In summary, this is the first study, to our knowledge, to examine the relationship between a multi-component measure of frailty and recovery from disability among community-dwelling older adults. We found that frailty, as assessed by the PFP scale, was an important risk factor for poor recovery from disability; frail elders without pre-existing disability had compromised ability to regain independence after being disabled. These findings validate frailty as a marker of decreased resilience and suggest that evaluation and intervention for frailty may not only prevent disability but also facilitate recovery from disability in nondisabled community-dwelling older persons.
Funding
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 (M.C.O.), R03AG048541 (Q.-L.X.), K08AG051187 (D.H.K.), P30CA006973 (R.V.), and P30AG013679 (D.H.K.) from the National Institute on Aging (NIA). D.H.K. is supported by the American Federation for Aging Research, the John A. Hartford Foundation, and the Atlantic Philanthropies.
Supplementary Material
Acknowledgements
A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
D.H.K. provides paid consultative services to Alosa Health, a nonprofit educational organization with no relationship to any drug or device manufacturers. Other authors have no conflicts of interest and no financial associations to disclose.
References
- 1. Freedman VA, Martin LG, Schoeni RF. Recent trends in disability and functioning among older adults in the United States: a systematic review. JAMA. 2002;288:3137–3146. doi:10.1001/jama.288.24.3137 [DOI] [PubMed] [Google Scholar]
- 2. Fuller-Thomson E, Yu B, Nuru-Jeter A, Guralnik JM, Minkler M. Basic ADL disability and functional limitation rates among older Americans from 2000–2005: the end of the decline?J Gerontol A Biol Sci Med Sci. 2009;64:1333–1336. doi:10.1093/gerona/glp130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin LG, Freedman VA, Schoeni RF, Andreski PM. Trends in disability and related chronic conditions among people ages fifty to sixty-four. Health Aff (Millwood). 2010;29:725–731. doi:10.1377/hlthaff.2008.0746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seeman TE, Merkin SS, Crimmins EM, Karlamangla AS. Disability trends among older Americans: national health and nutrition examination surveys, 1988–1994 and 1999–2004. Am J Public Health. 2010;100:100–107. doi:10.2105/AJPH.2008.157388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hung WW, Ross JS, Boockvar KS, Siu AL. Recent trends in chronic disease, impairment and disability among older adults in the United States. BMC Geriatr. 2011;11:47. doi:10.1186/1471-2318-11-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harrow BS, Tennstedt SL, McKinlay JB. How costly is it to care for disabled elders in a community setting?Gerontologist. 1995;35:803–813. doi:10.1093/geront/35.6.803 [DOI] [PubMed] [Google Scholar]
- 7. Manton KG. A longitudinal study of functional change and mortality in the United States. J Gerontol. 1988;43:S153–S161. doi:10.1093/geronj/43.5.S153 [DOI] [PubMed] [Google Scholar]
- 8. Gill TM, Robison JT, Tinetti ME. Difficulty and dependence: two components of the disability continuum among community-living older persons. Ann Intern Med. 1998;128:96–101. doi:10.7326/0003-4819-128-2-199801150-00004 [DOI] [PubMed] [Google Scholar]
- 9. Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi:10.1001/jama.279.8.585 [DOI] [PubMed] [Google Scholar]
- 10. Romoren TI, Blekeseaune M. Trajectories of disability among the oldest old. J Aging Health. 2003;15:548–566. doi:10.1177/0898264303253633 [DOI] [PubMed] [Google Scholar]
- 11. Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA. 2003;289:2387–2392. doi:10.1001/jama.289.18.2387 [DOI] [PubMed] [Google Scholar]
- 12. Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291:1596–1602. doi:10.1001/jama.291.13.1596 [DOI] [PubMed] [Google Scholar]
- 13. Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362:1173–1180. doi:10.1056/NEJMoa0909087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gill TM, Gahbauer EA, Han L, Allore HG. Factors associated with recovery of prehospital function among older persons admitted to a nursing home with disability after an acute hospitalization. J Gerontol A Biol Sci Med Sci. 2009;64:1296–1303. doi:10.1093/gerona/glp115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hardy SE, Gill TM. Factors associated with recovery of independence among newly disabled older persons. Arch Intern Med. 2005;165:106–112. doi:10.1001/archinte.165.1.106 [DOI] [PubMed] [Google Scholar]
- 16. Fried LP, Borhani NO, Enright P, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi:10.1016/1047-2797(91)90005-W [DOI] [PubMed] [Google Scholar]
- 17. Heeringa SG, Connor JH.. Technical Description of the Health and Retirement Survey Sample Design. Ann Arbor: University of Michigan; 1995. [Google Scholar]
- 18. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi:10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 19. Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. doi:10.1177/014662167700100306 [Google Scholar]
- 20. Taylor HL, Jacobs DR Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi:10.1016/0021-9681(78)90058-9 [DOI] [PubMed] [Google Scholar]
- 21. Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi:10.1249/00005768-199301000-00011 [DOI] [PubMed] [Google Scholar]
- 22. Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57:830–839. doi:10.1111/j.1532-5415.2009.02225.x [DOI] [PubMed] [Google Scholar]
- 23. Guralnik JM, LaCroix AZ, Branch LG, Kasl SV, Wallace RB. Morbidity and disability in older persons in the years prior to death. Am J Public Health. 1991;81:443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teng EL, Chui HC. The modified mini-mental state (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 25. Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatr Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 26. McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi:10.1093/aje/kwg074 [DOI] [PubMed] [Google Scholar]
- 27. Boyd CM, Ricks M, Fried LP, et al. Functional decline and recovery of activities of daily living in hospitalized, disabled older women: the Women’s Health and Aging Study I. J Am Geriatr Soc. 2009;57: 1757–1766. doi:10.1111/j.1532-5415.2009.02455.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hadley EC, Kuchel GA, Newman AB; Workshop Speakers and Participants Report: NIA workshop on measures of physiologic resiliencies in human aging. J Gerontol A Biol Sci Med Sci. 2017;72:980–990. doi:10.1093/gerona/glx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim DH, Kim CA, Placide S, Lipsitz LA, Marcantonio ER. Preoperative frailty assessment and outcomes at 6 months or later in older adults undergoing cardiac surgical procedures: a systematic review. Ann Intern Med. 2016;165:650–660. doi:10.7326/M16-0652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-Year postoperative mortality following major elective noncardiac surgery: a Population-Based Cohort Study. JAMA Surg. 2016;151:538–545. doi:10.1001/jamasurg.2015.5085 [DOI] [PubMed] [Google Scholar]
- 31. Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397–2404. doi:10.1161/CIRCULATIONAHA.111.025452 [DOI] [PubMed] [Google Scholar]
- 32. Luger E, Dorner TE, Haider S, Kapan A, Lackinger C, Schindler K. Effects of a home-based and volunteer-administered physical training, nutritional, and social support program on malnutrition and frailty in older persons: a randomized controlled trial. J Am Med Dir Assoc. 2016;17:671.e9–671.e16. doi:10.1016/j.jamda.2016.04.018 [DOI] [PubMed] [Google Scholar]
- 33. Tarazona-Santabalbina FJ, Gómez-Cabrera MC, Pérez-Ros P, et al. A Multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J Am Med Dir Assoc. 2016;17: 426–433. doi:10.1016/j.jamda.2016.01.019 [DOI] [PubMed] [Google Scholar]
- 34. Melis RJ, van Eijken MI, Teerenstra S, et al. A randomized study of a multidisciplinary program to intervene on geriatric syndromes in vulnerable older people who live at home (Dutch EASYcare Study). J Gerontol A Biol Sci Med Sci. 2008;63:283–290. doi:10.1093/gerona/63.3.283 [DOI] [PubMed] [Google Scholar]
- 35. Cameron ID, Fairhall N, Langron C, et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. doi:10.1186/1741-7015-11-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1–15. doi:10.1016/j.cger.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.