Figure 1.
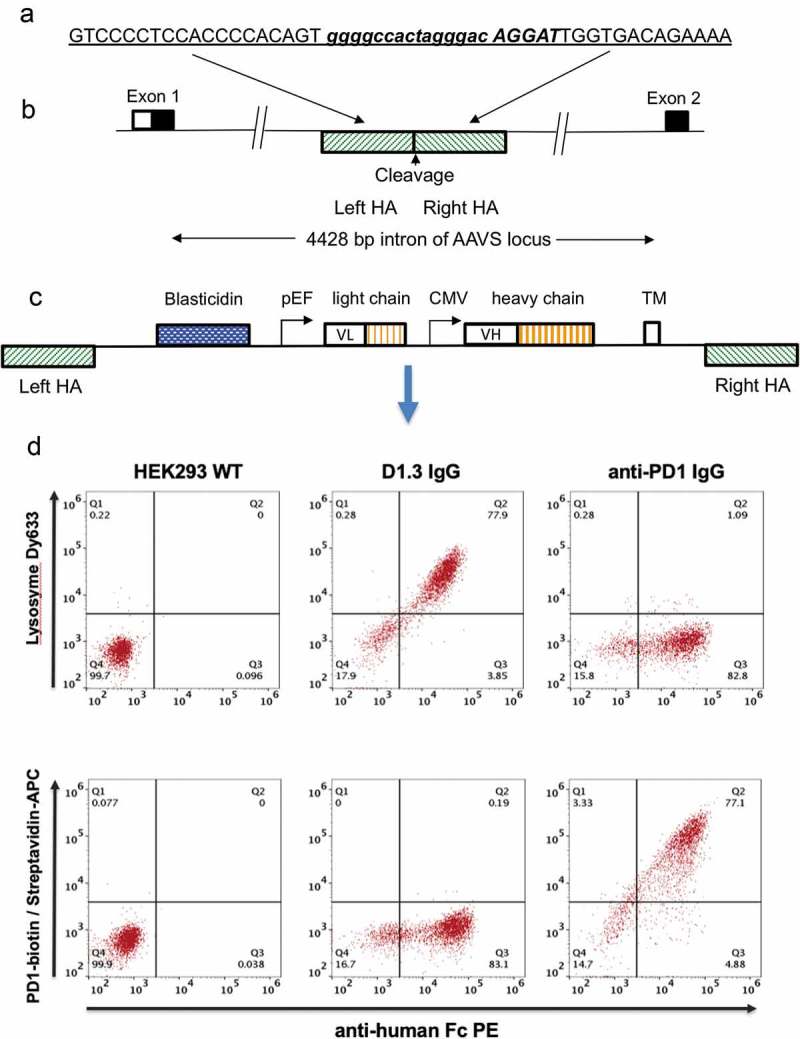
Antibody display cell line generation by nuclease mediated gene integration (a) Recognition sequence for left and right TALE nucleases are shown underlined and in upper case either side of the spacer region (shown in lower case). Emboldened and italicized sequence represents sequence of CRISPR target sequence (b) Representation of genomic AAVS locus. The AAVS cleavage site is located within a 4428 bp intron between the first and second exons of the gene encoding protein phosphatase 1, regulatory subunit 12C, PPP1R12C. TALE nucleases or CRISPR/Cas9 nucleases directed to this region are used to cleave the genome at this site. Hatched boxes on the 5ʹ and 3ʹ side of the cleavage site represent the left and right homology arms (HA), respectively (c) Representation of pD2 donor vector used to insert and display human IgG-formatted antibody genes. The transgene region is flanked by left and right homology arms (left HA, right HA) representing the sequences which flank the cleavage site in the AAVS locus. The vector encodes a promoter-less blasticidin gene, a dual promoter antibody expression cassette with antibody light chain and a human IgG2 heavy chain driven by pEF and CMV promoters, respectively. Expressed antibodies are anchored on the cell surface by a PDGFR transmembrane domain (TM) (d) Flow cytometric analysis of HEK293 cells displaying an anti-lysozyme or anti-PD1 antibody on the cell surface. Non-transfected HEK293 cells (left panel) and cells displaying either anti-lysozyme (middle panel) or anti-PD1 337_1_C08 (right panel) were stained with anti-Fc PE and either lysozyme conjugated with DyLight 633 (top row) or PD-1-biotin/streptavidin-APC.
