ABSTRACT
The Old World monkey, Rhesus macaque (Macaca mulatta, Mm), is frequently used as a primate model organism in the study of human disease and to test new vaccines/antibody treatments despite diverging before chimpanzees and orangutans. Mm and humans share 93% genome identity with substantial differences in the genes of the adaptive immune system that lead to different functional IgG subclass characteristics, Fcγ receptors expressed on innate immune cells, and biological interactions. These differences put limitations on Mm use as a primary animal model in the study of human disease and to test new vaccines/antibody treatments. Here, we comprehensively analyzed molecular properties of the Fc domain of the four IgG subclasses of Rhesus macaque to describe potential mechanisms for their interactions with effector cell Fc receptors. Our studies revealed less diversity in the overall structure among the Mm IgG Fc, with MmIgG1 Fc being the most structurally like human IgG3, although its CH2 loops and N297 glycan mobility are comparable to human IgG1. Furthermore, the Fcs of Mm IgG3 and 4 lack the structural properties typical for their human orthologues that determine IgG3’s reduced interaction with the neonatal receptor and IgG4’s ability for Fab-arm exchange and its weaker Fcγ receptor interactions. Taken together, our data indicate that MmIgG1-4 are less structurally divergent than the human IgGs, with only MmIgG1 matching the molecular properties of human IgG1 and 3, the most active IgGs in terms of Fcγ receptor binding and Fc-mediated functions. PDB accession numbers for deposited structures are 6D4E, 6D4I, 6D4M, and 6D4N for MmIgG1 Fc, MmIgG2 Fc, MmIgG3 Fc, and MmIgG4 Fc, respectively.
KEYWORDS: Rhesus macaque, Macaca mulatta, crystallizable fragment, Fc, IgG subclasses
Introduction
Immunoglobulins G (IgGs) constitute an important class of therapeutic agents for the treatment of cancer, inflammation, autoimmune, cardiovascular and infectious diseases. In addition to a serendipitous mechanism of action in which blocking or activating biochemical pathways occurs as a result of the interaction of the antigen binding fragment (Fab) arms of IgGs with biological targets, IgGs can recruit effector cells from the immune system through specific engagement of their crystallizable fragment (Fc) domain with Fc receptors (FcRs). FcRs can be either activating or inhibitory. Engagement of activating FcγRs leads to a variety of Fc receptor-dependent effector functions, including complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cell-mediated phagocytosis (ADCP). In the mechanism of ADCC, the effector cell expressing FcγRs recognizes and kills antibody-coated target cells expressing pathogen- or tumor-antigens on their surface.
The reaction with FcRs expressed at effector cell surfaces is mediated by the Fc of the IgG, the homodimeric assembly of the heavy chain constant (CH) regions 2 and 3 (e.g., CH2-CH3 domains), which are also modified by co-translational N-glycosylation of asparagine 297. There are four subclasses of IgGs in humans, IgG1, IgG2, IgG3, and IgG4, which differ in their Fc regions. The unique amino acid composition and glycosylation status, which may be one of ~36 different glycan signatures, modulate the overall Fc structure and has been linked to the variable ability of human IgG1-4 to engage FcRs and mediate effector functions.1
The best-known species of Old World monkeys, the Rhesus macaque, Macaca mulatta (Mm), shares about 93% of its genome with Homo sapiens (Hs). The genetic, physiologic and metabolic similarity to humans makes this monkey a valuable non-human primate (NHP) model to explore research protocols related to various diseases, including infectious diseases. The best example of the latter are studies related to the development of a human immunodeficiency virus (HIV) vaccine or passively administered anti-HIV monoclonal antibodies where macaques infected with simian immunodeficiency virus (SIV) or simian/human immunodeficiency virus (SHIV) are used to test new vaccines or passive transfer regiments.2 With regard to HIV vaccine development, many recent NHP studies, similar to those in humans, have implicated antibody Fc-effector activities in reduced viremia or a reduced risk of infection,3–6 pointing toward the association of Fc-mediated effector functions with protection. Although in many cases these associations were reported for experiments performed in humans and macaques with the same vaccine regiment, the accurate interpretation of these studies is complicated by the inter-species differences in the functional characteristics of the IgG subclasses, FcγRs expressed on innate immune cells, and their biological interplay. Recent studies clearly indicate that, despite evolutionary proximity, there are significant immunological differences between the two species that is manifest in different functional profiles between IgG subclasses,7 significant differences in FcγR expression profiles,7,8 and significantly greater allotypic diversity in FcγRs and IgG subclasses in Rhesus macaques.7–9 In addition, recently performed detailed analysis of the specific interaction of Mm and Homo sapiens (Hs) IgG subclasses with species matched or mismatched FcγR variants indicated significant differences in affinity and glycan sensitivity pointing toward an evolutionary divergence between the functional properties of IgGs.10,11 Given that Fc-mediated effector functions have been implicated in protection in HIV vaccine studies, these interspecies functional differences may put limitations on the use of Mm as an appropriate animal model.
Here, we present crystal structures of the Fc domains of all four Macaca mulatta IgG subclasses, compare them to their human counterparts, and evaluate their molecular properties in the context of possible interactions with FcγRs. We also examined the glycosylation patterns and nuclear magnetic resonance (NMR) spectra for the Fcs of MmIgG1-4 and HsIgG1 for indications of differences in mobility of the N297 glycan. Our studies reveal significant variability in the overall Fc structures of macaques and humans, and differences in sugar composition and movement that all indicate unexpected structural diversification that may serve as an explanation for the different functional characteristics of macaque and human IgG antibodies.
Results
IgG1 is distinct among the mm IgG subclasses in regard to overall Fc structure and CH2 loop conformation
To gain insight into the overall Fc architecture of the four IgG subclasses of Macaca mulatta, we solved crystal structures of the Fc from IgG1-4 (Figure 1, Table 1). All four macaque Fcs crystallized with one Fc homodimer in the asymmetric unit with comparable resolutions ranging from 2.8 to 3.5 Å. MmIgG1 Fc crystallized in space group P21, while the MmIgG2, MmIgG3, and MmIgG4 Fcs crystallized in space group P32. MmIgG3 Fc crystals were merohedrally twinned with a twin fraction of 0.308 as determined by Xtriage.12 The final models consisted of almost complete sequences of the two CH2-CH3 domains that assemble into the Fc dimer. Protein Data Bank (PDB) accession numbers for the deposited structures are 6D4E, 6D4I, 6D4M, and 6D4N for MmIgG1 Fc, MmIgG2 Fc, MmIgG3 Fc, and MmIgG4 Fc, respectively. Complete data collection and refinement statistics are shown in Table 1.
Figure 1.
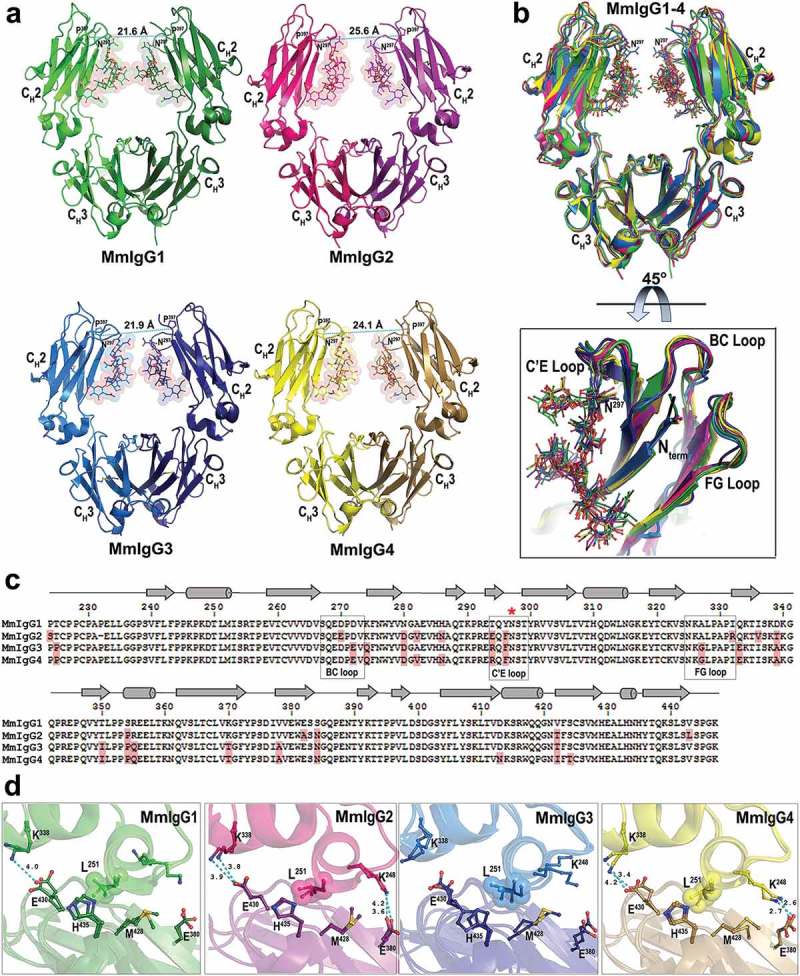
Crystal structures of the Rhesus macaque IgG1-4 Fc. (a) The overall structures are shown in a ribbon diagram with the two heavy chains (CH2-CH3 domains) in lighter and darker shades of green (MmIgG1), pink (MmIgG2), blue (MmIgG3) and yellow (MmIgG4). The sugars attached to N297 are shown as spheres colored by atom type (backbone color for carbon; red for oxygen and blue for nitrogen). The distances between Cα carbons of P238 are shown to indicate the differences in the distances between CH2 domains. (b) The structures of MmIgG1-4 Fcs were superimposed based on CH3-CH3 homodimer to show differences in the conformation and distances of CH2 domains in the Fc dimer. A 45° view shows the conformation of C’E, BC, and FG loops among CH2 domains of the Fcs. (c) Sequence alignment of the four subtypes of MmIgG Fc. The sequence identity among the four sequences is 86%. Residues of MmIgG2-4 different than MmIgG1 sequence are shaded in pink. The secondary elements as determined by the structures are shown above the sequence with arrows for β–strands, cylinders for α-helix and solid lines for random coil. Residue N297 is indicated with a red star and the C’E, BC, and FG loops are in boxes. (d) CH2−CH3 interface. Residues contributing to the interface through salt bridges/hydrogen bonds and residues of the hydrophobic “ball-in socket” joint are shown as sticks. Distances for hydrogen bonds/electrostatic interactions are as shown.
Table 1.
Data collection and refinement statistics.
| MmIgG1 Fc | MmIgG2 Fc | MmIgG3 Fc | MmIgG4 Fc | |
|---|---|---|---|---|
|
Data collection Wavelength, Ǻ Space group Cell parameters a, b, c, Å α, β, γ, ° Complexes/a.u. Resolution, (Å) # of reflections Total Unique Rmergeb, % Rpim, % CC1/2 I/σ Completeness, % Redundancy |
0.979 P21 57.6, 64.5, 80.6 90, 104.5, 90 1 50–2.8 (2.85–2.8) 40,046 13,809 16.6 (73.9) 11.3 (57.9) 0.99 (0.62) 9.7 (0.8) 95.2 (81.7) 2.9 (2.0) |
0.979 P32 66.9, 66.9, 87.5 90, 90, 120 1 50–2.95 (3.0–2.95) 23,189 8,919 8.7 (65.7) 6.4 (48.5) 0.99 (0.75) 13.6 (1.29) 96.1 (97.7) 2.6 (2.7) |
0.979 P32a 65.9, 65.9, 89.1 90, 90, 120 1 50–3.45 (3.5–3.45) 13,835 5,321 24.2 (80.2) 17.2 (57.0) 0.99 (0.30) 7.4 (1.3) 94.9 (98.0) 2.6 (2.6) |
0.979 P32 66.6, 66.6, 90.7 90, 90, 120 1 50–3.25 (3.3–3.25) 35,792 7,018 9.0 (97.1) 4.4 (49.1) 0.99 (0.65) 31.3 (1.2) 98.9 (99.7) 5.1 (4.8) |
|
Refinement statistics Resolution, Å Rc % Rfree, % chain A res. range chain B res. range # of atoms Protein Water Ligand/Ion Overall B value (Å)2 Protein Water Ligand/Ion RMSDd Bond lengths, Å Bond angles, ° Ramachandrane favored, % allowed, % outliers, % PDB ID |
50.0–2.8 22.1 27.2 [236–444] [236–444] 3,331 7 199 77 51 112 0.006 1.1 90.1 5.8 4.1 6D4E |
50.0–2.95 20.7 26.1 [237–445] [236–444] 3,337 6 198 76 52 95 0.008 1.2 95.7 3.1 1.2 6D4I |
50.0–3.45 24.9 27.0 [237–444] [236–444] 3,320 – 198 108 – 103 0.006 1.0 73.6 20.6 5.8 6D4M |
50.0–3.25 21.3 26.5 [237–444] [237–444] 3,310 – 198 151 – 151 0.008 1.3 89.8 9.0 1.2 6D4N |
Values in parentheses are for highest-resolution shell.
aTwinned crystal with a twin fraction of 0.308 and twin law of (h,-h-k,-l) as determined by Xtriage12
b Rmerge = ∑│I − <I>│/∑I, where I is the observed intensity and <I> is the average intensity obtained from multiple observations of symmetry-related reflections after rejections.
c R = ∑║Fo│ − │ Fc║/∑│Fo│, where Fo and Fc are the observed and calculated structure factors, respectively.
dRMSD = Root mean square deviation.
eCalculated with MolProbity.
The overall topologies of the MmIgG1-4 Fc domains closely resemble that of human, Homo sapiens (Hs), IgG1 (HsIgG1) with a classical immunoglobulin fold for the CH2 and CH3 domains (Figure 1(a)). The distributions of secondary elements are the same among the CH2-CH3 monomers of MmIgG1-4 and overlap with the secondary element distribution in HsIgG1 Fc (Figure 1(b,c)). As expected based on human IgG Fc, homodimers are largely formed by noncovalent interactions between CH3 domains with the CH2 domains held in place by association of their N-termini and contacts between glycans linked to N297 on each monomer. The same overall dimeric assembly of CH2-CH3 building blocks is preserved in macaque Fcs, with glycans attached to N297 occupying the space between two CH2 domains, and contacts (as defined by a 10 Å cutoff) between the N297-linked glycan on each monomer forming the Fc dimer (Figure 1). The closest point of approach between the ordered part of the monomer glycan that can be modeled in the crystal structure varies between Fc structures from Man2-Man2 on the 3-arms in MmIgG1 Fc (6 Å), Fuc-Man2 in MmIgG3 (6 Å) and MmIgG4 (9 Å), and Fuc-Man3 on the 6-arm in MmIgG2 (9 Å). The observed differences may reflect the inherent disorder and heterogeneity of the glycan, limitations of the crystal structure resolution, and conformational differences in the Fc dimer, but may also be due, as discussed below, to differences in how the glycan is stabilized in each Fc, which can influence the conformation of the C’E loop, which makes important contributions to the Fcγ receptor binding site. Structural alignments of the individual CH2 and CH3 domains of the four macaque IgG subclasses confirm the very close similarity of their overall structures with an average Root Square Mean Deviation (RMSD) values for main chain atoms of 1.0 Å (with a range of 0.34–1.5 Å for CH2 domains and 0.75 Å with a range of 0.36–1.0 Å for CH3). In contrast, analysis of the CH2-CH3 monomers that assemble into the Fc and the Fc dimer indicate more deviation in the relative domain orientations in the Fc and Fc dimer, as shown by an average RMSD among the CH2-CH3 monomers of 1.1 Å (with a range of 0.35–1.6 Å) and 1.4 Å (with a range of 0.9–1.8 Å) for CH2-CH3 dimers. In addition, when the overall assembly of each Fc is analyzed in pairwise comparisons, the MmIgG1 Fc appeared to be the most different of the remaining macaque IgG subclasses with an average RMSD of 1.6–1.8 Å. In comparison, the RMSDs for the other macaque Fc dimers fall in a range of 0.9–1.1 Å.
In human Fcs, the BC loop, the C’E loop (with glycan attached to N297), and the FG loop of both CH2-CH3 domains forming the Fc dimer form much of the binding interface in the asymmetric interaction with high-affinity receptor FcγRI and low-affinity receptors FcγRII and III.13,14 Although in the receptor-free state the regions corresponding to the BC, C′E and FG loops are mobile, causing difficulties in model building, we were able to successfully define backbones and side chains for these regions in all the macaque Fc structures. Structural alignments of these regions among MmIgG1-4 revealed very close similarity of the conformation of the backbone forming the BC, C′E and FG loops (Figure 1(b)). The average RMSD value for main chain atoms of residues 267–273 (BC loop), 294–299 (C′E loop) and 325–332 (FG loop) are 1.1 Å, 0.52 Å, and 1.0 Å, respectively. In addition, similar to the pattern seen for the macaque IgG Fc dimers, the BC and C′E loop regions of MmIgG1 Fc were consistently the most structurally divergent of the macaque Fcs in these regions with the exception of the FG loop, where MmIgG3 Fc was the most divergent.
IgG1 and 3 of Mm show the greatest CH2 domain mobility and Fc dimer asymmetry in their crystal structures
The variability of the overall Fc structures of MmIgG1 through 4 revealed by X-ray crystallography results mostly from the changes in the relative orientations of CH2 and CH3 domains in the CH2−CH3 monomer that cannot be solely attributed to crystal packing (MmIgG2, 3 and 4 share the same space group), and therefore potentially reflect differences in the CH2−CH3 domain interface. The CH3 domains in the Fc form a dimer that appears to be invariant in Fc structures, while the relative orientations of individual CH2 domains to the plane of assembled CH3−CH3 dimer varies between structures (Table 2). Therefore, the macaque Fcs assemble to form slightly asymmetric structures, as do their human counterparts, with noticeable differences among the IgG subclasses that can be quantified by various metrics, such as (1) the dimer width measured by the distances between selected residues at the top of CH2 domain; and (2) the level of asymmetry measured by the inter domain angle between the CH2 domain and the CH3-CH3 plane (Table 2). Analysis of the dimer width indicates that the distance between CH2 domains is closest for MmIgG1 and 3 [21.6 Å and 21.9 Å] when measured by the α-carbon (Cα) of P238 on each monomer, and furthest for MmIgG2 and 4 [25.6 Å and 24.1 Å]. A similar trend is also seen when other CH2 residues are used to measure dimer width and overall Fc conformation, e.g., F241, R301, and P32915 (Table 2), indicating that it is the entire CH2 domain that shifts, and not just a local conformational difference between residues.
Table 2.
Comparison of the conformational parameters of the Rhesus macaque and human IgG1-4 Fc. The parameters (distances and angles) describing properties of the dimeric assembly of Fcs were calculated from the structures of Fc of macaque and human IgG1 through 4. For human, only the fucosylated N297glycan core apo Fc structures of IgG1 and all apo Fc structures of IgG2-4 available in the Protein Data Bank (PDB, codes as shown) were selected. The top panel shows a graphic definition of each conformational parameter and include distances between selected residues for CH2 domain separation measurements; three-point angles to describe the relative orientations of the CH2 and CH3 domains in each monomer (CH2/CH3 angle) and each CH2 domain relative to the CH3-CH3 homodimer (CH2/CH3-CH3 angle), and the Cα atom angle bend (the angle between i-1, i, and i + 1 Cα atoms) of residues 339 to 343 of the heavy chain to describe the CH2-CH3 hinge.
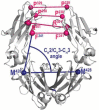 |
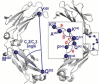 |
||||||||||||
| |
|
P238 (Å) |
F241 (Å) |
R301 (Å) |
P329 (Å) |
Chain |
CH2/CH3-CH3 angle (º) |
CH2/CH3 angle (º) |
a (º) |
b (º) |
c (º) |
d (º) |
e (º) |
| IgG1 | Mm | 21.6 | 24.6 | 33.6 | 29.3 | A | 48.7 | 79.4 | 110.4 | 118.6 | 142.7 | 108.7 | 111.6 |
| B | 50.0 | 78.0 | 112.3 | 122.0 | 143.8 | 108.1 | 112.6 | ||||||
| 3AVE | 19.3 | 21.8 | 32.3 | 25.1 | A | 50.6 | 76.8 | 112.9 | 120.5 | 140.6 | 113.7 | 115.5 | |
| B | 52.8 | 74.5 | 110.0 | 122.9 | 144.8 | 107.3 | 115.1 | ||||||
| 4DZ8 | 19.5 | 22.3 | 32.9 | 24.2 | A | 49.1 | 77.7 | 111.9 | 121.2 | 142.5 | 113.6 | 117.0 | |
| B | 53.2 | 74.6 | 110.0 | 126.1 | 144.3 | 111.9 | 114.5 | ||||||
| 4W4N | 19.5 | 22.0 | 32.6 | 23.0 | A | 52.9 | 75.1 | 108.1 | 125.9 | 143.5 | 111.8 | 111.2 | |
| B | 50.3 | 77.0 | 109.8 | 117.3 | 141.6 | 110.0 | 116.4 | ||||||
| 1H3Y | 16.7 | 22.3 | 25.9 | 29.6 | A | 50.0 | 75.9 | 128.4 | 123.1 | 148.5 | 105.9 | 110.4 | |
| B | 49.6 | 75.8 | 109.3 | 110.5 | 132.0 | 97.0 | 122.8 | ||||||
| 1H3V | 19.3 | 23.2 | 32.9 | 26.9 | A | 50.0 | 78.6 | 115.6 | 116.2 | 137.7 | 112.0 | 118.4 | |
| B | 52.2 | 76.4 | 112.9 | 124.0 | 145.6 | 107.3 | 120.4 | ||||||
| IgG2 | Mm | 25.6 | 27.4 | 32.1 | 38.0 | A | 48.0 | 82.3 | 103.0 | 121.1 | 134.6 | 107.4 | 114.9 |
| B | 48.0 | 81.5 | 107.9 | 126.2 | 128.6 | 107.8 | 112.9 | ||||||
| 4HAF | 27.7 | 27.8 | 39.5 | 32.0 | A | 49.7 | 83.6 | 108.3 | 127.4 | 138.5 | 109.3 | 120.2 | |
| B | 53.9 | 79.9 | 106.2 | 127.0 | 142.7 | 109.2 | 119.6 | ||||||
| 4HAG | 21.4 | 25.1 | 32.7 | 32.3 | A | 50.2 | 79.2 | 115.3 | 111.6 | 145.8 | 110.5 | 112.9 | |
| B* | – | – | – | – | – | – | – | ||||||
| IgG3 | Mm | 21.9 | 26.2 | 30.9 | 36.5 | A | 46.9 | 81.5 | 111.1 | 122.5 | 138.1 | 91.8 | 125.7 |
| B | 49.7 | 81.6 | 93.7 | 122.2 | 137.6 | 112.7 | 115.2 | ||||||
| 5W38 | 23.5 | 26.3 | 35.5 | 29.9 | A | 48.1 | 80.8 | 109.2 | 120.2 | 139.6 | 110.7 | 119.2 | |
| B | 48.2 | 80.1 | 108.6 | 119.0 | 137.8 | 112.0 | 120.1 | ||||||
| IgG4 | Mm | 24.1 | 27.1 | 33.1 | 36.9 | A | 47.5 | 81.8 | 112.9 | 118.0 | 140.0 | 104.9 | 117.8 |
| B | 47.5 | 82.1 | 116.0 | 117.9 | 138.7 | 102.2 | 115.1 | ||||||
| 4C54 | 19.8 | 22.2 | 33.7 | 36.1 | A | 52.2 | 76.3 | 114.5 | 124.7 | 140.1 | 107.1 | 115.7 | |
| B | 53.3 | 74.5 | 111.7 | 125.8 | 142.7 | 111.4 | 114.5 | ||||||
| 4C55 | 19.6 | 22.3 | 34.0 | 36.9 | A | 51.5 | 77.6 | 112.5 | 121.1 | 145.2 | 107.1 | 116.8 | |
| B | 54.7 | 74.0 | 115.4 | 122.6 | 138.9 | 110.3 | 112.9 | ||||||
| 5LG1 | 20.7 | 23.3 | 35.4 | 30.9 | A | 51.4 | 80.0 | 112.0 | 118.5 | 142.5 | 111.0 | 112.5 | |
| B | 55.2 | 73.1 | 108.8 | 115.1 | 145.2 | 109.2 | 114.4 | ||||||
*In 4HAG the dimer is created by crystallographic symmetry resulting in identical parameters for monomers A and B in the dimer.
When the differences in the Fc asymmetry among the MmIgG1-4 Fcs is analyzed using a 3-point angle to describe the relative orientation of each CH2 domain relative to the CH3-CH3 homodimer [CH2/CH3-CH3 angle, Table 2], the Fcs of MmIgG1 and 3 show the highest levels of asymmetry with the differences between the CH2/CH3-CH3 angles for monomers A and B of 1.3º and 2.8º, respectively. The observed increased CH2 mobility of MmIgG1 and 3 results directly from the changes at the CH2-CH3 interface, which in both monomers are stabilized by a fewer number of interdomain interactions (Figure 1(d)). In humans, the CH2−CH3 interface is stabilized through a network of a salt bridges/hydrogen bonds formed between K248 – E380 and K338 – E430, and a hydrophobic “ball-in socket”, where L251 packs against the side chains of E430, H435, and M428.16 Whereas interactions mediated by K248 – E380 and K338 – E430 are well-preserved in MmIgG2 and 4, they all are lost in the monomers of MmIgG3, and all but one (K338 – E430 in one monomer) are lost in MmIgG1 (Figure 1(d)). Also, the hydrophobic “ball-in socket” interactions are loosened in MmIgG1 and 3 as indicated by larger distances between contributing carbon atoms. These changes are accompanied by increased mobility and less restrained conformation of the backbone of the hinge region connecting the CH2 and CH3 domains (residues 339 to 343). As shown in Table 2, the angle bend (the angle between i-1, i, and i + 1 Cα atoms) of residues forming the hinge region is more variable in MmIgG1 and 3 compared to the other subclasses (the Cα atom angle bend for the hinge is in the range of 91.8–143.8º for MmgG1 and 3 and in the range of 102.2–134.6º for MmIgG2 and 4). Taken together, these data indicate that the Fc of MmIgG1 and 3 show greater variability of their CH2 domains and greater CH2 domain asymmetry in the Fc dimer relative to orientation of the CH3−CH3 dimer.
IgG1 through 4 of Mm show less diversity in their Fc structure as compared to their human counterparts. MmIgG1 displays the highest structural similarity to HsIgG3
We performed pairwise main chain atom RMSD comparisons of macaque and human Fc structures to examine both the structural similarity of the individual CH2 and CH3 domains and the relative domain organization in CH2-CH3 monomers and CH2-CH3 dimers (full Fc) (Figure 2). Of the data available in the PDB for human Fcs, we selected only structures of Fcs in an unbound state that span a range of resolutions and space groups. In addition, for human IgG1 Fc, only structures with fucose linked to GlcNAc1 were chosen. When the structural similarity between IgG subclasses is analyzed (Figure 2(a)), the macaque IgGs show significantly decreased diversity of the overall structure of individual Fc components: CH2 (0.34–1.5 Å), CH3 (0.36–1.0 Å), CH2-CH3 monomer (0.35–1.6 Å) and assembled CH2-CH3 dimer (Fc) (0.88–1.8 Å) compared to human (0.16–2.4 Å for CH2, 0.15–0.76 Å for CH3, 0.23–2.9 Å for the CH2-CH3 monomer, and 0.45–3.7 for the CH2-CH3 dimer, respectively). In pairwise macaque-human comparisons, MmIgG1 Fc shows the highest structural similarity to HsIgG3 Fc with main chain atom RMSD values of 0.8 Å, 0.9 Å and 1.1 Å for its CH2 domain, the CH2-CH3 monomer and the Fc dimer, respectively. These values are the lowest compared to any other Fc, macaque or human. Furthermore, the macaque Fcs of IgG2-4 are most similar in structure to HsIgG2 Fc for their CH2 domains (0.5–0.6 Å) and for their CH2-CH3 monomers (1.2–1.5 Å), whereas their dimers are slightly more similar to HsIgG3 (1.7–1.9 Å versus 1.7–2.0 Å for HsIgG2). In general, there was a much higher degree of structural similarity in the CH3 domains among subclasses both from the same species and between species than in the CH2 domain, the monomer, or the Fc dimer. This remains true even though there are many more examples of human Fc structures at varying resolutions and from different space groups. For example, the human main chain atom RMSD for the CH3 domain is relatively low with a range of 0.4–0.5 Å. Differences in the CH2 domain are more pronounced and may reflect differences in the glycan linked to N297 or in the Fcγ receptor binding BC, C′E and FG loops, although differences in resolution may also contribute to the RMSD range; a resolution difference of 1 Å between two structures has been estimated to contribute as much as 0.5 Å difference to the calculated RMSD.17 Overall the structural comparisons identify the closest similarity between CH2-CH3 dimers (Fc) of MmIgG1 to HsIgG3 (1.1 Å), MmIgG2 to HsIgG2 and HsIgG3 (1.9 Å), MmIgG3 to HsIgG3 (1.9 Å) and MmIgG4 to HsIgG2 and HsIgG4 (1.7 Å) (Figure 2).
Figure 2.
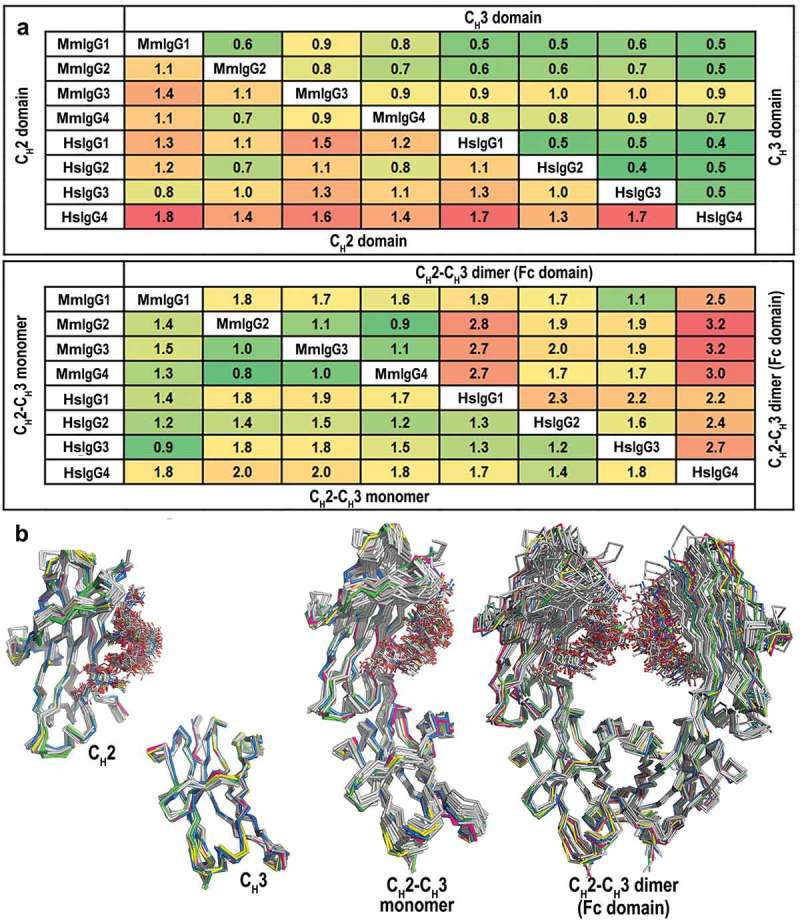
Comparison of the overall structures of the Rhesus macaque and human IgG1-4 Fc. (a) Average RMSD values for main chain atoms for pairwise comparisons of CH2, CH3, CH2-CH3 monomers and CH2-CH3 dimers (Fc domain). The structures of the Fcs of human IgG1-4 used in the alignments include: IgG1, PDB codes: 3AVE, 4DZ8, 4W4N, 1H3Y and 1H3V; IgG2, PDB codes: 4HAF, 4HAG; IgG3, PDB code: 5W38 and IgG4, PDB codes: 4C54, 4C55, 5LG1. (b) Structural alignment of CH2, CH3, CH2-CH3 monomers, and CH2-CH3 dimers. MmFcs are colored in green for IgG1, pink for IgG2, blue for IgG3 and yellow for IgG4. Human Fcs are colored in grey. CH2-CH3 dimers are aligned by superimposing the CH3 domains.
Structural comparisons of the Fc assembly as defined by the dimer width and levels of asymmetry measured by inter-domain angles between the CH2 domain and the CH3-CH3 plane of human and macaque IgG subclass orthologs reveal noticeable differences (Table 2). The macaque Fc of IgG1 has a greater dimer width compared to its human counterpart as measured by the distances between all four residues used to measure Fc dimer width (in macaque α-carbon distances between P238, F241, R301, P329 of 21.6 Å, 24.6 Å, 33.6 Å and 29.3 Å, respectively, compared to the distances between equivalent residues in the human ortholog of 16.7–19.5 Å, 21.8–23.2 Å, 25.9–32.9 Å, 23.0–29.3 Å, respectively). Also distances between P238 and F241 are significantly greater in MmIgG4 as compared to HsIgG4 (24.1 Å and 27.1 Å in Mm compared to 19.6–20.7 Å and 22.2–23.3 Å in Hs, Table 2). In addition, the Fc dimer asymmetry as measured by the CH2/CH3-CH3 angle is significantly greater for the human orthologs than the macaque, with the exception of MmIgG3. The difference between monomers for Fc of MmIgG1, 2 and 4 is in a range of 0–1.3º versus an average difference for equivalent IgGs in human are 2.1º- 2.7º. MmIgG3 Fc, the exception, has a difference of 2.8º versus the human IgG3 Fc difference of 0.1º. One caveat, however, is the lower resolution of the MmIgG3 structure of 3.45 Å, which adds a greater level of uncertainty to the MmIgG3 comparisons, and the crystal packing for the human Fcs differs from that seen for the macaque Fcs, which may influence relative CH2-CH3 domain orientation. Altogether, these data indicate that the human Fc structures show more asymmetry than macaque for IgGs1, 2, and 4.
IgG3 and 4 of Mm lack the structural properties of their human counterpart
Figure 3(a,b) shows the structural alignments of the macaque and human Fcs with residues highlighted that differ between macaque and human amino acid sequence. Pairwise sequence comparisons show MmIgG1 and MmIgG4 to be most similar to their human counterparts, with a sequence identity of 92.4% and 90.2% (residues 224–447), respectively. Interestingly, MmIgG2 and MmIgG3 are more similar in amino acid sequence to HsIgG1 Fc (91.5% and 91.4% sequence identity, respectively) than to their human counterpart (89.1% and 87.9% sequence identity, respectively). However, there is more sequence variability among the Fcs of macaque IgG1-4 (sequence identity in a range of 90.2–93.3%) compared to the Fcs of human IgG1-4 (sequence identity in a range of 92–95.9%). The majority of these differences occur within the CH2 domain for MmIgG1 and 2 and in the CH3 domain for MmIgG3 and 4.
Figure 3.

Structural comparisons of the Rhesus macaque and human Fcs. (a) Pairwise comparisons of the overall structures shown in a ribbon diagram with the two heavy chains (CH2-CH3 domains) in lighter and darker shades of green (MmIgG1), pink (MmIgG2), blue (MmIgG3) and yellow (MmIgG4) overlaid on their human counterpart in grey. The sugars attached to N297 are shown as sticks and side chains for residues that differ between macaque and human shown as balls and sticks colored by atom type (backbone color for carbon; red for oxygen and blue for nitrogen). Residues in the BC, C’E, and FG loops known in human to contribute to the Fcγ receptor binding are highlighted by color-matched circles. The same color scheme is used in remaining panels. (b) The conformation of C’E, BC, and FG loops among CH2 domains of the Fcs. (c) Pairwise sequence alignment of the four subtypes of macaque and human IgG Fc. Residues that are different between macaque and human are shaded in pink. The C’E, BC, and FG loops are in boxes. Residue N297 is indicated with a red star and residues at positions 405, 410 and 435 are indicated by a red arrow.
None of the macaque Fcs are identical in the amino acid sequence with their human counterparts in all their Fcγ receptor binding loops (Figure 3(c)). While some of these changes are charge reversing, e.g., the 294 E to R difference at the start of the C′E loop in macaques in MmIgG3 and MmIgG4, most are conservative, e.g., the 268 H to Q difference in MmIgG1–3 or the 272 E to D difference in MmIgG1-2 within the BC loop. Although the macaque IgGs can bind to human Fcγ receptors with largely similar subclass affinities, in contrast to the broader range of affinities for the human IgGs, and human IgGs can bind macaque Fcγ receptors,10,11 these sequence differences complicate the comparison of counterparts due to a lack of receptor complex structures for all but the human IgG1. The CH2 loop conformations of the MmIgG2 BC, C′E and FG loop backbones are most similar to HsIgG1 with main chain atom RMSD values of 1.08 Å, 1.02 Å, 1.20 Å, and 1.15 Å for MmIgG1-4 Fc, respectively. When similarities between individual loops are analyzed the BC and C’E loops of MmIgG2 and FG loop of MmIgG1 are closest in overall conformation to the equivalent loops of HsIgG1 (Figure 3(b)).
In addition, superimpositions of the CH2 loop regions of Mm to human subclass counterparts (Figure 3(b)) indicate that MmIgG4 does not have the unique ‘flipped’ FG loop conformation seen in some HsIgG4 structures.18 All but one monomer in a HsIgG4 Fc structure measured at room temperature adopt ‘the flipped’ FG loop conformation, as compared to the ‘typical’ conformation adopted by CH2 domains of HsIgG1 and the other human IgGs. It has been suggested that the HsIgG4 equilibrium does not favor ‘the typical’ conformation, which is preferentially recognized by the low-affinity Fcγ receptors: FcγRII, FcγRIII and C1q.18,19 In addition, sequence analysis indicates that the Fc of MmIgG4 lacks Ser and Arg at positions 228 and 409, respectively (Figure 3(c)), which are residue changes known to facilitate the Fab-arm exchange in human IgG4s.18 As in human/macaque IgG1-3, lysine is present at position 409; however, Leu replaces Phe at position 405, which destabilizes the CH3-CH3 dimer in an analogous manner.20 Finally, MmIgG3 lacks the H435R mutation present in human IgG3 that interferes with neonatal receptor (FcRn) interactions and shortens the HsIgG3 half-life.21 MmIgG3 and the other macaque IgGs preserve His at 445, likely allowing the known pattern of interactions with human FcRn. However, a structure of a macaque Fc in complex with MmFcRn is required to confirm this for macaque FcRn. Altogether, the structural comparison of macaque and human Fcs indicates that there is less structural diversity among the macaque IgG subclasses, with MmIgG3 and 4 lacking the features known to be unique to their human counterparts.
IgG1 of Mm shows the closest similarity to human IgG1 in regard to N297 glycan behavior as revealed by NMR spectroscopy
Next, we employed solution NMR spectroscopy to analyze the N297 glycan of MmIgG1-4 Fcs, and compared them to the spectra of HsIgG1 Fc. Though structures determined by x-ray crystallography reveal extensive detail, the sampling of multiple conformations is potentially eliminated by crystal contacts or the cryogenic temperatures used for data collection, including motion of the IgG Fc N-glycans.22 MmIgG Fcs expressed in cultures supplemented with [13CU]-glucose showed [13C] labeling of the carbohydrate resonances in a 2d 1H-13C heteronuclear single quantum coherence (HSQC) experiment (Figure 4). The spectra of the MmIgG Fcs revealed superior intensity and resolution when compared to HsIgG1 Fc, which showed very broad lines and limited resolution between 70 and 80 ppm (13C), in a spectrum mostly containing carbohydrate CH correlations (this difference is also evident by comparing the peaks highlighted with dashed boxes in Figure 4). MmIgG3 Fc revealed the highest-quality spectrum, followed by MmIgG4, MmIgG2, MmIgG1, and HsIgG1 in order of decreasing quality.
Figure 4.
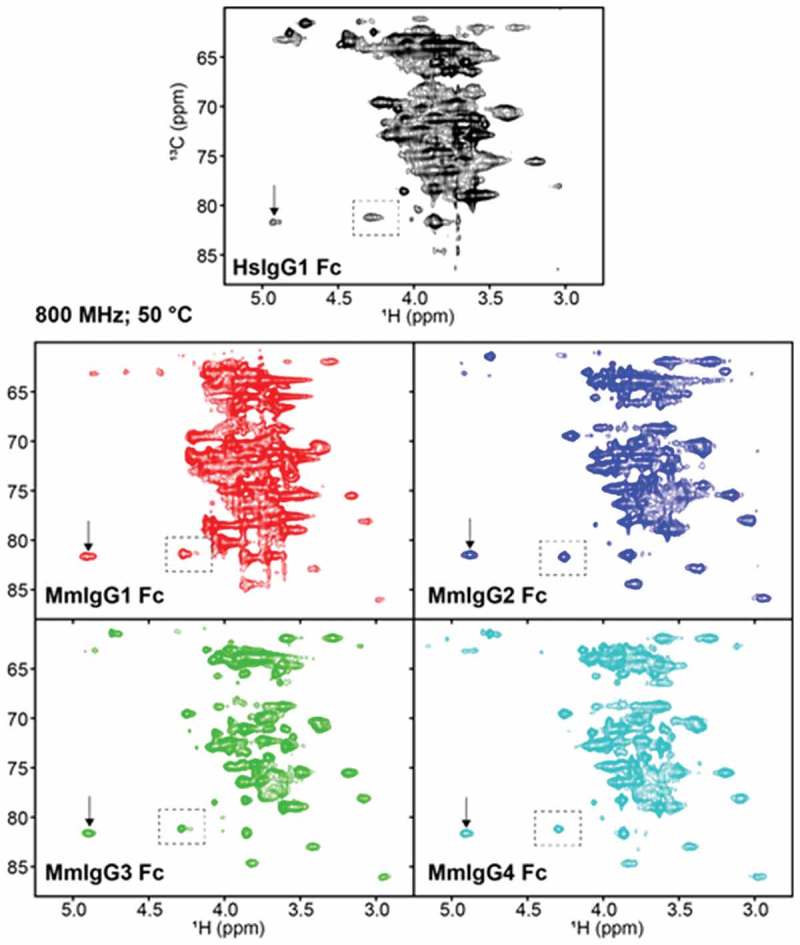
Differences in spectral quality in 1H-13C HSQC spectra of [13CU-glycan]-Fcs collected at 18.8 T and 50ºC. These spectra were processed with only a sine-squared line-broadening function in the direct dimension. The vertical arrow highlights the peak which corresponds to the GlcNAc1 H6-C1 correlation. Peaks within the dashed boxes are used for comparisons of spectra in the main text.
The identity of each peak in these spectra is unknown due to an inability to perform through-bond experiments on samples with only 60% labeling of the [13CU]-glycan, as well as the availability of few options to generate inter-residue correlations within the glycan. One peak, however, is unique. The anomeric H1-C1 correlation on the GlcNAc1 residue is the only anomeric carbon linked through an N atom to the N297 sidechain. These correlations are shown with a vertical arrow in the HSQC spectra and are well separated from other peaks (Figure 4). The GlcNAc1 H1-C1 peak from HsIgG1 Fc reveals two states, a major peak corresponding to a binding-competent form and a single minor peak on the left-hand side of the spectrum as shown in Figure 5(a). Only the minor peak is observed in spectra of HsIgG1 Fc mutants that cannot bind FcγRIIIa.23 Furthermore, the positions of comparable peaks from mouse IgG2b and 2c Fc also correlate with FcγRIV binding affinity.24 Thus, this H1-C1 correlation represents a well studied and valuable reporter of IgG Fc structure. The GlcNAc1 H1-C1 correlations appeared at comparable resonance frequencies for all IgG Fcs tested (Figure 5(a)). Analysis of the GlcNAc1 H1-C1 correlations revealed trends similar to the HSQC spectra, with broader lines for the HsIgG1 and MmIgG1 Fc (125 and 102 Hz) compared to MmIgG2-4 at 18.8T (66–83 Hz; Figure 5(a)). The presence of two peaks was evident for HsIgG1 Fc at both 14.1T and 18.8T (Figure 5(a,b)). Of the macaque Fcs, only MmIgG1 Fc clearly exhibited similar features to HsIgG1 Fc with two peaks identified at 14.1T (Figure 5(b)), though the weaker peak was only visible at low contours in the spectrum collected at 18.8T (data not shown). The spectrum of MmIgG4 potentially contains a similar feature, but MmIgG2 and 3 Fc do not (Figure 5(a)).
Figure 5.
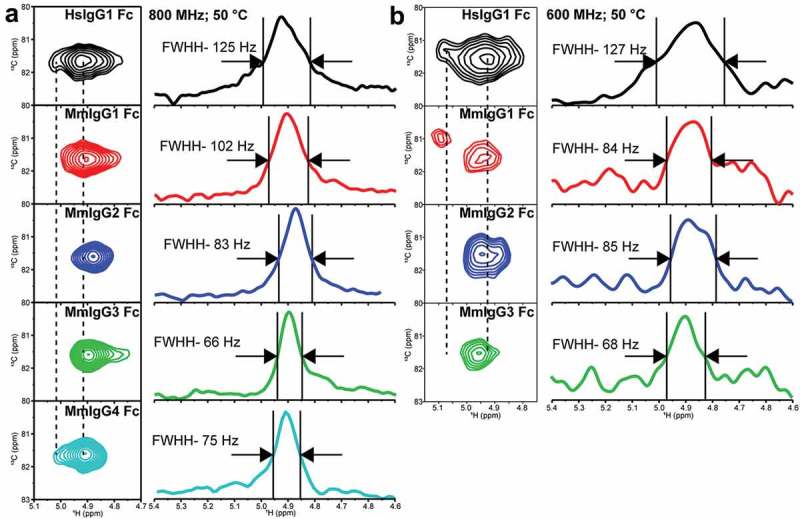
Differences in the anomeric 1H1-13C1 correlation in the N297-linked GlcNAc1 residue (a) at 18.8 T and 50ºC and (b) at 14.1 T and 50ºC. These spectra were processed with a combination of sine-squared and 20 Hz exponential multiplier line-broadening functions in the direct dimension.
The N297 glycan of IgG1 of Mm shows the greatest number of CH2 contacts stabilizing the glycan and the least processing compared to other IgGs
A complex-type biantennary glycan was visible for each Fc X-ray structure of MmIgG1-4 connected to N297. Ordered electron density was observed up to the terminal N-acetylglucosamine (GlcNAc) on both the 3, (α1-3), and 6, (α1–6), -arms of the N-linked glycan, as found in human Fc structures (Figure S1(a,b)). Density was also seen for fucose linked to the first GlcNAc, indicating that the predominant glycan in the crystal structures contained a core fucose residue. The glycan is stabilized through interactions with the CH2 domain, and these interactions differ slightly among the macaque Fcs, which has potential implications both in glycan processing in the Golgi and in the glycan’s contribution to C’E loop conformation and receptor binding. In all Fc structures, the glycan is stabilized primarily through CH-π interactions to F241 and F243 (Figure S1(a)). In all but the MmIgG3 Fc, the glycan is further stabilized by hydrogen bonds to D265 and R301; MmIgG3 Fc uses Q295 in place of D265 and lacks any contacts with R301. In addition, MmIgG1 and MmIgG2 make hydrogen bonds to the terminal GlcNAc via K246, and N297 is stabilized in MmIgG4 by a hydrogen bond to T299. In total, MmIgG1 makes the most CH2 contacts stabilizing the glycan and most closely resembles the glycan-CH2 interaction network of HsIgG1. In contrast, MmIgG3 makes the fewest numbers of contacts with features largely identical between monomers in the dimer. The nature of these interactions is supported by the quality of the N-glycan spectra as noted above, with MmIgG1 Fc revealing broader lines originating from greater contacts and an increased contribution from intermediate exchange on the NMR timescale. As noted, the MmIgG3 Fc N-glycan forms the fewest stabilizing contacts and provided the sharpest lines among those Fcs analyzed here, implying that it has the least restricted movement in solution.
To further characterize configurational variability of the N297 glycan in Mm IgG1-4, we analyzed sugar composition in Fc preparations used in crystallographic studies and obtained by recombinant expression in HEK293F cells. In addition to simply identifying sugar composition, these analyses provide insight into N-glycan motion since motion and accessibility directly correlate to IgG1 Fc N-glycan processing during secretion. N-glycans were liberated by PNGaseF from peptides obtained by digestion of the Fc of each of four macaque IgGs, modified with procainamide and separated by hydrophilic interaction liquid chromatography (HILIC) prior to mass analysis using HILIC-electrospray ionization mass spectrometry (ESI-MS)/MS. This approach identified 16 unique N-glycan species with 86% complex-type biantennary structures shown in Figure 6(a) and Table S1. Each macaque IgG Fc showed high levels of processing, including galactosylation (68–84%), sialylation (11–56%) and core fucosylation (74–86%). MmIgG1 Fc N-glycans showed the greatest degree of galactosylation and sialylation among the macaque Fcs at 85% and 56%, respectively, with 56% and 13% containing two of each modification, respectively. The IgG3 and 4 Fc N-glycans appeared to be the least processed, with higher levels of oligomannose forms (19–23% vs. 5–11% for IgG1 and 2), lower sialylation (11–29% vs. 46–56%) and less galactose incorporation (68–70% vs. 80–84%). These results indicate the MmIgG1 Fc N-glycan is more highly processed, followed by MmIgG2 Fc, MmIgG4 Fc, and MmIgG3 Fc in decreasing order. This processing is likely due to greater accessibility of the MmIgG1 N-glycan to the fucosyltransferase, sialyltransferase and galactosyltransferase enzymes during expression, although it is possible MmIgG1 transits through the Golgi at a slower rate than other MmIgG Fcs.
Figure 6.
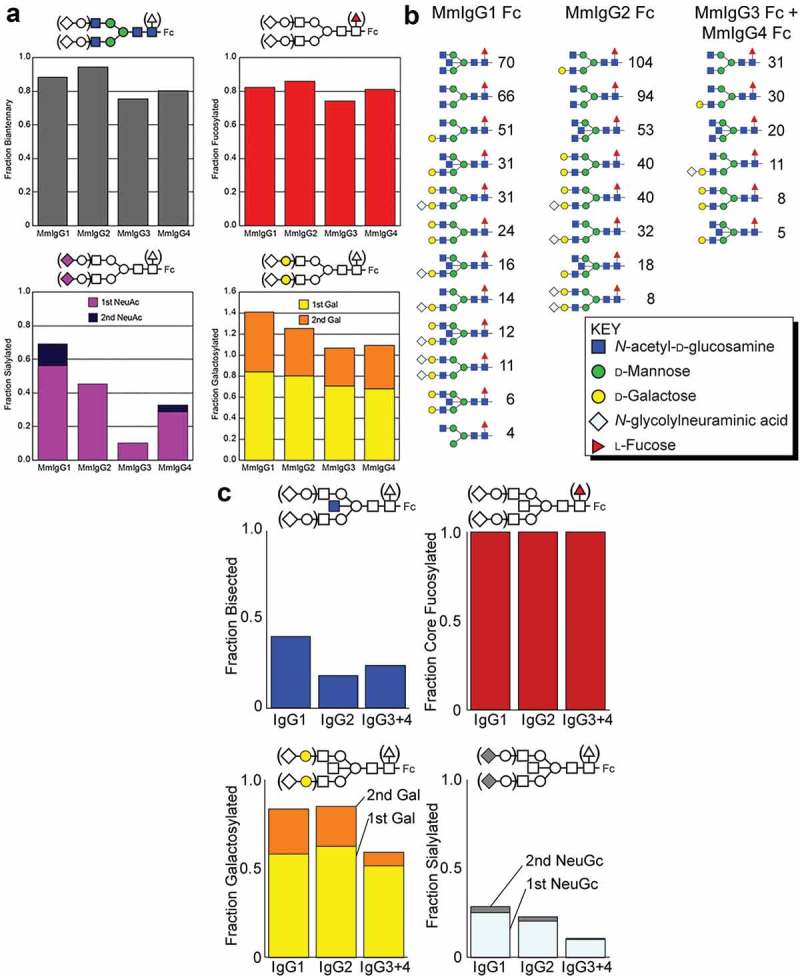
Analysis of the N-glycan composition of the Fcs of MmIgG1-4 expressed in HEK293F cells and present in RM sera. (a) N-glycan composition of the Fcs of MmIgG1-4 expressed in HEK293F is shown with NeuAc = N-acetylneuraminic acid; Gal = galactose. (a) left panel shows the percentage of complex-type N-glycans. The upper right panel shows the percent fucosylated, the lower left shows the percentage with one or two sialic acid residues and the lower right indicates galactosylation. (b) and (c) Composition and configuration of the Fc N-glycan of MmIgG1-4 present in RM sera. Comparable results from a different animal are shown in Figure S3. Fc N-glycans identified by LC-ESI-MS/MS are ranked according to spectral counts (panel B). Incidence of each N-glycan modification observed. NeuGc = N-glycolylneuraminic acid; Gal = galactose (panel C).
IgG1 shows most extensive glycan processing as compared to other IgGs present in macaque sera
Macaque sera was characterized by ESI-MS/MS through identification of subclass-specific IgG glycopeptide fragments that contain amino acid sequence and N-glycan composition information. Trypsin digestion of macaque IgG generates three unique N-glycopeptides: one for IgG1, one for IgG2, and a third for both IgG3 and 4. Analysis of IgG glycopeptides from two animals resulted in two datasets with 37 and 39 unique glycopeptides, respectively, from 1 μg of proteolyzed, protein G-purified IgG. The datasets contain an absolute mass error of 0.0033 Da and 0.0035 Da, respectively (Table S2). As expected, the predominant macaque IgG N-glycan was a fucosylated complex-type with two antennae. No afucosylated glycopeptides reached sufficient intensity to trigger collection of an MS2 spectrum by the mass spectrometer. Analyzing MS1 data identified peaks corresponding to glycopeptides lacking a fucose reside, but these appeared at an intensity reduced by 100–1000-fold when compared to the similar fucosylated form (G1 vs. G1F). We observed similar intensity differences between glycopeptides with and without a fucose reside in an analysis of the same mixture using a hydrophilic interaction column before ESI-MS/MS (data not shown); therefore, the majority (greater than or equal to 99%) of macaque IgG Fc from these two serum samples of glycopeptides contains a fucose residue. Sialylated glycopeptides contained only N-glycolylneuraminic acid with no evidence of N-acetylneuraminic acid; N-glycolylneuraminic acid is not made by humans due to a mutation that likely occurred at or following separation of the Pan and Homo lineages.25
Specific features in the MS2 spectra provide strong evidence regarding N-glycan configuration. We identified the presence of a bisecting GlcNAc residue from a single peptide ion that contains three N-acetyl-hexosamine residues and a single hexose. This ion permits the differentiation of a glycan with a bisecting GlcNAc from a glycan with an additional GlcNAc residue attached to the branched (α1-3) mannose or (α1-6) mannose residues. The presence of core fucosylation is similarly evident from an ion containing the peptide with a single N-acetyl-hexosamine residue and a deoxyhexose (Figure S2). Every N-glycan species analyzed with MS2 spectra contained a core fucose with no evidence of branch fucosylation (Figure 6(b,c)). N297-glycans of MmIgG1 experienced more processing, including greater levels of bisecting GlcNAc, galactose and sialylation, than MmIgG2-4 (Figures 6(b,c) and S3). MmIgG2 showed the lowest levels of bisecting GlcNAc (18% and 14%, for datasets one and two, respectively). Both MmIgG1 and MmIgG2 showed higher levels of galactose and sialic acid incorporation compared to MmIgG3 and 4, which was consistent with the recombinant HEK293 cell-expressed macaque IgG Fcs (Figure 6(b,c)). These results are consistent with a previous study using capillary electrophoresis that did not differentiate IgG subclasses.26
Discussion
Recent evidence from natural infection, vaccination, and studies in animal models indicates the key roles of Fc-mediated effector activities in anti-HIV antibody function in vivo. In the Fc-mediated effector mechanism, FcRs expressed on the surface of the effector cell are specifically engaged to form a functional complex with the Fc region of IgG. Multiple variables contribute to the effectiveness of this process at the basic level, among which the receptor type and IgG subclass are the most important. Human IgG subclasses differ in amino acid composition and the overall architecture of the IgG molecule, and therefore exhibit different affinities for FcγRs and potencies in immune cell activation and their capacity to activate complement.27
In contrast to humans, less is known about Fc-mediated effector functions in macaques, including Rhesus macaque, Macaca mulatta, an animal commonly used in disease models to test new vaccine concepts and antibody therapeutics. Recent sequence and Fc receptor binding analyses have identified significant differences between rhesus and human IgG subclasses in their affinities for species matched or mismatched FcRs.10,11 In humans, IgG1 and 3 were found to be roughly equivalent in affinities for human Fcγ receptors, and to dominate the Fc effector function response against HIV, with substantially weaker responses from HsIgG2 and 4. Interestingly, in humans, IgG1 and IgG3 are structurally very different with IgG3, which has an extended hinge region comprising of 62 amino acids and six disulfide bridges.28 It was suggested that the longer hinge and higher flexibility of human IgG3 molecules could be beneficial in the Fc-effector mechanism in situations of poor accessibility or sparse distribution of antigen, which is particularly relevant in light of the correlation of protective IgG3 responses in the RV144 vaccine trial.6 Mm IgG3 lacks this unusual property, having a short hinge similar to the hinges of other macaque IgGs and that of human IgG1. When tested for binding to macaque FcγRs, MmIgG1 was found to display the highest affinity; MmIgG3 and the other MmIgGs displayed similar and significantly weaker affinities to the same receptors.10 In both rhesus and humans, IgG1 is the predominant subclass found in sera. Levels of IgG3 are similar. It remains to be answered what the exact role the enhanced Fc-effector activities of HsIgG3 play in protection in humans and if/what macaque mechanism compensates for decreased potency of MmIgG3. Interestingly, significantly greater allotypic diversity in FcγRs is found in macaques as compared to humans,7–9,29,30 suggesting that low diversity among macaque IgG molecules is potentially compensated by a greater allotypic diversity in FcγRs.
Here, we analyzed for the first time the X-ray structures of the Fc domain of four IgG subclasses of Rhesus macaque, Macaca mulatta, and compared them to their human counterparts. We coupled our studies with solution NMR spectroscopy for additional insight into the N297-glycan conformation, structural features of glycoproteins that cannot be easily accessed by crystallography.
Our crystallographic studies indicate that the Fc domains across the macaque IgG subclasses are less varied structurally than human Fcs with main chain atom RMSD values for the Fc backbone below 1.8 Å (compared to 2.7 Å in humans). The Fc of MmIgG1 is the most divergent of the macaque Fcs with the highest RMSD values of pairwise comparisons of individual CH2 and CH3 domains, the CH2-CH3 monomer, and the dimer (Figure 2). MmIgG1 and MmIgG3 show the greatest CH2 domain mobility as indicated by increased Fc dimer asymmetry in the crystal structure. In contrast, the structure of MmIgG1 Fc was resolved to 2.8 Å resolution, allowing more precise atom positions and more reliable interpretations. Indeed, MmIgG1 shows increased CH2 mobility that results from the changes at the CH2-CH3 interface with fewer interdomain interactions. The CH2-CH3 interface in MmIgG1 is ‘looser’ compared to interfaces stabilizing the CH2-CH3 monomers of human Fcs. This increases the mobility of CH2 domain in the CH2-CH3 dimer, and likely contributes to the ability of IgG1 to interact with Fcγ receptors. In humans, the Fc-Fcγ interface of the intact IgG involves the lower hinge region between the CH2 domain and the CH2 BC, C′E, and FG loops. It has also been shown that CH2 loop motion and relative domain orientation influence the Fc−FcγR interaction in human and mice.16,24
Interestingly, although the macaque IgG Fcs are very similar in their crystallographic structures and MmIgG1 shows the most structural similarity to HsIgG3, solution NMR spectroscopy detected the same or greater motion of the N297 glycan among the MmIgG Fcs as observed for HsIgG1 Fc. In addition, MmIgG1 spectra exhibited GlcNAc1 H1-C1 peak features most similar to that of HsIgG1. The Barb group showed previously for the Fc of HsIgG1 that the GlcNAc1 H1-C1 peak reveals two states, a major peak corresponding to a binding-competent form and a single minor peak observed in spectra of HsIgG1 Fc mutants that cannot bind FcγRIIIa.23 The close similarities of GlcNAc1 H1-C1 peaks between MmIgG1 and HsIgG1 suggest that MmIgG1 effectively samples the receptor binding-competent conformation. Interestingly, the spectrum of MmIgG4 potentially contains a similar feature, but MmIgG2 and 3 Fc do not. Furthermore, the observed decreased linewidths and increased spectral intensities for the Fcs of MmIgG1-4 indicated that their nuclei experience the same or greater motion on the nanosecond (ns) timescale or less line broadening originating from relatively slower microsecond-millisecond (μs-ms) motions than the HsIgG1 Fc N-glycan. Based on previous studies of HsIgG1 Fc, we believe the broader lines are due to slow conformational exchange on the µs-ms timescale.22,31,32 Of the MmIgG Fcs, MmIgG3 experienced the least contribution from slow motions, followed by MmIgG4, MmIgG2, and MmIgG1 in increasing order. Of these antibody fragments, spectra of the MmIgG1 Fc’s N-glycan appeared most similar to HsIgG1. The broad lines for the IgG1 Fc GlcNAc1 H1-C1 peaks indicate slow conformational interconversion proximal to the point of attachment at N297. The impact of slow conformational exchange is less evident in MmIgG2-4. These conclusions are supported by the definition of the interface formed between N-glycan and polypeptide residues observed by X-ray crystallography with more glycan stabilizing contacts made in MmIgG1 than in MmIgG2-4. Though these NMR measurements provide insight into local motion, they do not provide information regarding the amplitude of motion, which is an important factor for the accessibility of Fc N-glycans to glycan-modifying enzymes that alter the receptor-binding affinity of the antibody.1,33
The Fc receptor affinity of IgGs is modulated by the N-linked glycosylation of N297. The N297-glycans are partially buried at the interface of the CH2-CH3 dimer, but their compositions and mobility have been shown to contribute to affinity of Fc for Fc receptors. Human Fc structures lacking glycans display an altered conformation in which the CH2 domains collapse to form a more compact dimer that is largely devoid of binding to most Fc receptors, emphasizing the importance of the glycan structure on Fc conformation.34 The N297 glycan also effects the conformation of the C’E loop, which is directly involved in Fcγ receptor binding. Deletion or truncation of the glycan leads to C’E loop disorder and reduced Fcγ receptor affinity.23,32 Terminal sialylation of either or both arms of the glycan has also been linked to reduced Fcγ receptor-induced activity, but only for fucosylated N-linked glycans in the case of FcγRIIIA.15 Whether this reduced activity is due to changes in Fc glycan structure and Fc/Fcγ receptor affinity or is mediated by a sialic acid receptor such as DC-SIGN is still a matter of debate.35 We performed analyses of N297 glycan composition of recombinantly expressed Fcs of MmIgG1-4 used in crystallographic and NMR analyses and IgGs isolated from sera of Macaca mulatta. Interestingly in both cases, the N297-glycan of MmIgG1 Fc was found to be the most highly processed. For recombinantly prepared Fcs, the processing was the greatest in MmIgG1, followed by MmIgG2, MmIgG4, and MmIgG3 in decreasing order. As to the IgGs isolated from sera, the N-glycans of IgG1 experienced more processing, including greater levels of bisecting GlcNAc, galactose, and sialylation, than those of IgG2-4, with MmIgG2 showing the lowest levels of bisecting GlcNAc. These data indicate greater accessibility of the MmIgG1 N-glycan to the fucosyltransferase, sialyltransferase and galactosyltransferase enzymes during expression, suggesting greater mobility of the CH2 domain, although another explanation could be that MmIgG1 transits through the Golgi at a slower rate than other MmIgGs. Greater processing increases the heterogeneity among sugars, and since glycan composition can modulate Fc receptor binding, increases the range of activities that can be given to a single IgG molecule.
To summarize, our studies indicate that the Fc domains of the macaque IgG subclasses are less structurally variable than their human counterparts. Many unusual characteristics present in the human IgG subclasses are absent in macaques. For example, the long variable hinge region in human IgG3 between CH1 and CH2 is replaced by a hinge resembling that of human IgG1 and the other IgGs. The MmIgG3 Fc is also more similar to human IgG1 and other Mm IgGs, the H345R mutation present in HsIgG3 that decreases affinity human FcRn and shortens its half-life21 is absent. Furthermore, the MmIgG4 lacks the two residues S228 and R409 mutation that in human facilitates Fab-arm exchange of IgG4.18 Interestingly, the ability of Fab-arm exchange in IgG4 in rhesus depends on the animal origin. Rhesus of both Indian and Chinese origin have lysine at position 409 of IgG4 similar to human/macaque IgG1-3, but have a Phe to Leu change at residue 405 that is thought to destabilize the CH3-CH3 dimer in an analogous manner.20 However, Indian Rhesus macaques do not contain a destabilizing mutation at P228 in the hinge and do not undergo Fab-arm exchange.20 In contrast, Chinese Rhesus macaques contain A228 at position 228 and are capable of IgG4 Fab-arm exchange.20 Our studies also indicate that MmIgG1 Fc is the most unique among the macaque IgG subclasses, showing the closest similarity of overall structure to human IgG3 Fc and a N297 glycan motion similar to HsIgG1. By chance macaque IgG1 and human IgG1 and 3 are the most active IgGs in terms of Fcγ receptor binding. MmIgG2 through 4 appear more similar to the less active HsIgG2, although differences between them are apparent.
The conclusions drawn here for Rhesus macaque are likely to hold for other macaque species as well. For example, the IgG1 of Macaca fascicularis (cynomolgus monkey), another commonly used animal in HIV-1 research, is 100% identical in sequence to MmIgG1 in the Fc region.30 Sequence identity for MfIgG2, 3, and 4 for the Fc region are 99.5%, 98.6%, and 96.7%, respectively, to their MmIgG counterparts showing a similar high degree of conservation with no changes to the sequence in the loops directly involved in receptor binding (Figure S4). The perfect conservation of the MfIgG1 Fc to MmIgG1 Fc likely implies that MfIgG1 plays a similar role in M. fascicularis with its structural similarity to the more active HsIgG1 and 3.
This raises the question as to the function of the IgG subclasses and class switching. In humans, the IgG subclasses can provide a spectrum of different responses to the same epitope, some strongly activating such as IgGs 1 and 3, and others less so, i.e., IgGs 2 and 4. This may give the host the ability to fine-tune the ADCC response to the stimulus. For example, in humans, repeated or long-term exposure to antigens may lead to IgG4 becoming the dominant subclass in certain settings, such as for long-term beekeepers or individuals who have undergone immune therapy for allergies.27 Although less pronounced than in humans, the IgG subclasses in macaques also display a range of activities as evidenced in their range of binding affinities to the Fcγ receptors and their structural differences. As with modification of the core glycan on N297,36 the duplication of IgGs into subclasses affords the organism a means to fine-tune Fc effector function.37,38 In macaques as well as in humans, these differences are evident both in structure and function. The question as to what stimuli govern selection of a particular subclass remains open for both.
To conclude, the choice of Rhesus macaques as the primary nonhuman primate model is not solely determined by their similarity to human. Other primates are more closely related to humans, but are no longer used in research for humanitarian reasons. The similarities between human and macaque IgG1 support their use in comparative studies. The similarities become fewer for the other IgGs. There is no good macaque equivalent for human IgG3 both in composition (with the longer hinge region) and in activity as measured by receptor affinity. Similarly, there is no good equivalent for diminished activity of human IgG4, with the macaque IgG4 having comparable composition and activity to the MmIgG2 and 3. These differences may present a problem in recapitulating results in macaques, such as the protective effect of non-neutralizing IgG3 responses seen in the RV144 vaccine trial. They may also present a problem in recapitulating immune tolerance responses or responses to chronic infections or repeated immune activation. The unique characteristics of IgG3 in combination with IgG1 in humans may make the human immune system more activating in the initial immune response than that in macaques, but less active in repeated immune stimulation due to the diminished activity of IgG2 and 4 in humans relative to 2 and 4 in macaques. Although an imperfect model at best, the macaque NHP model may be sufficient for most purposes given these caveats and serve as a good surrogate until trials in humans are warranted.
Materials and methods
Fc domain expression and purification
The genes coding for crystallizable fragments of Indian Rhesus macaque, Macaca mulatta (Mm) IgG1-4 (MmIgG1 Fc, MmIgG2 Fc, MmIgG3 Fc and MmIgG4 Fc),29 spanning residues below the upper hinge of the immunoglobulin heavy chain (residues 237–447) were synthesized with the macaque FcγRIIIA leader sequence8 by Blue Heron Biotech, LLC and cloned into the pCMV6-A-Puro expression vector. For crystallization experiments, Fc proteins were prepared by transfection of HEK 293F Freestyle cells (Life Technologies) with 0.5 mg of plasmid/liter of culture and expressed in Freestyle 293 medium (Life Technologies) for 6 days. Fc proteins were purified from medium passed over a HiTrap protein A column (GE Healthcare) equilibrated with phosphate-buffered saline (PBS) pH 7.2. The column was washed with PBS and the Fc eluted with 0.1 M glycine pH 3.0. Eluted fractions were immediately diluted 10:1 with 1 M Tris-HCl pH 8.5 to raise the pH. Eluted protein was concentrated, and the buffer exchanged for PBS pH 7.2. Fcs were further purified by size exclusion chromatography over a Superdex 200 gel filtration column (GE Healthcare) equilibrated with 25 mM Tris-HCl pH 8.5 and 150 mM sodium chloride. Elution fractions were collected and concentrated to approximately 10 mg/ml for use in crystallization trials. For NMR experiments, the [13CU-glycan]-variants Fcs were prepared by adding 3 g/L [13CU]-glucose to Freestyle293 medium (Life Technologies) as previously described.39 Purification was the same as for protein used for crystallization except the final gel filtration step was omitted. [13CU-glycan]-HsIgG1 Fc was expressed as previously described.23
Crystallization and data collection
Crystals were initially grown from commercial crystallization screens and later optimized to produce crystals suitable for data collection. MmIgG1 Fc crystals grew from 20% polyethylene glycol (PEG) average molecular weight 4000 and 0.1 M sodium citrate pH 4.5, MmIgG2 Fc crystals grew from 3 M sodium chloride and 0.1 M sodium acetate pH 4.5, MmIgG3 Fc crystals from 35% tacsimate pH 7.0, and MmIgG4 Fc from 1.34% PEG 8000 and 3.35 M ammonium citrate pH 8.5. Prior to data collection, crystals were transferred to crystallization condition plus cryoprotectant, 20% MPD or 20% glycerol, and then flash frozen in liquid nitrogen.
Diffraction data were collected at the Stanford Synchrotron Radiation Light Source (SSRL) BL12-2 beamline on a Dectris Pilatus 6M area detector. All data were processed and reduced with HKL2000.40 Structures were solved by molecular replacement with PHASER from the CCP4 suite41 based on the coordinates of the human IgG1, IgG2, or IgG4 Fc (PDB: 3AVE for MmIgG1 Fc and MmIgG3 Fc, 4HAF for MmIgG2 Fc, and 3C54 for MmIgG4 Fc). Refinement was carried out with Refmac41 and/or Phenix,12 and model building was done with COOT.41 Data collection and refinement statistics are shown in Table 1. Ramachandran statistics were calculated with MolProbity and illustrations were prepared with Pymol Molecular graphics (http://pymol.org).
Solution NMR spectroscopy
NMR experiments were performed on Bruker systems operating at 14.1T or 18.8T, equipped with an Avance II or Avance III console, respectively, and 5 mm cryogenically cooled probes. [13CU-glycan]-IgG Fc was exchanged into a buffer containing 20 mM sodium phosphate, 100 mM sodium chloride, 0.5 mM 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS), 100% 2H2O, pH 7.4 using a 10 kDa-cutoff centrifugal concentration device (Millipore) and placed in a 4 mm Shigemi tube. NMR spectra were collected at 50ºC sample temperature that was externally calibrated with ethylene glycol. NMR spectra were referenced to the internal DSS standard at 0.07 ppm 1H. Standard 2d 1H-13C heteronuclear single quantum coherence pulse sequences, without sensitivity enhancement, were obtained from the Bruker pulse sequence library. NMR spectra were processed with NMRPIPE and analyzed in NMRVIEWJ.
Mass spectrometry
Preparation of samples and HILIC-ESI-MS/MS-based analysis of procainamide-derivatized N-glycans were performed as previously described.42 Sera obtained from two healthy rhesus macaques of Indian origin that were housed in the Division of Animal Models at the Institute of Human Virology. Total IgG was purified with protein G agarose resin (Sigma) using standard protocols, except samples were eluted with 100 mM formic acid and neutralized with 1M ammonium carbonate.43 Approximately 10 μg serum antibody was diluted to 100 ng/μl with 50 mM ammonium carbonate buffer pH 8.0 and denatured at 90°C for 5 min. Samples were incubated overnight at 37°C with 1:80 (antibody: trypsin by mass) sequencing grade trypsin (Promega). Resulting peptides were reduced with 5 mM dithiothreitol (Sigma) at 37°C for 1 h and alkylated with 15 mM iodoacetamide (VWR) at 25°C for 1 h. Precipitates were removed by centrifuging the samples at 10,000 × g for 3 min and the supernatant was lyophilized. The peptides were resuspended in 10 μl 0.1% formic acid, 0.01% trifluoroacetic acid in double-distilled water, then diluted with 40 μl 0.1% formic acid, 5% acetonitrile in double distilled water. Five μl was injected onto a glass capillary C18 column (prepared at ISU). The C18 chromatography was performed using the 75 μm ID × 200 mm glass capillary C18 column (5 μm particle size) connected to a nanoLC 1200 system (Thermo Scientific) in line with a Q-Exactive Orbitrap mass spectrometer. The C18 column was pre-equilibrated with 95% Solvent A (0.1% formic acid in double-distilled water) and 5% Solvent B (0.1% formic acid in acetonitrile) before sample injection. Peptides and glycopeptides were eluted with a gradient of 5–35% Buffer B over 60 min at a flow rate of 3 μl/min. Top 20 eluted ions over a 120 ms elution window within scan range of 600–2500 m/z were subjected to higher-energy collisional dissociation fragmentation at a normalized collision energy of 27 eV. A blank run was performed in between each sample run by injecting 5% Buffer B to prevent carryover from the previous sample. Raw data were analyzed with BYONIC (Protein Metrics). Each glycopeptide ion was validated manually. Ion abundance was estimated by counting the number of MS2 spectra for each ion collected by the instrument.
Funding Statement
This work was supported by the National Institutes of Health [AI116274]; National Institutes of Health [GM115489]; National Institutes of Health [AI120756]; National Institutes of Health [AI129769].
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- ADCP
antibody-dependent cell-mediated phagocytosis
- CDC
complement-dependent cytotoxicity
- CH2
heavy chain constant region 2
- CH3
heavy chain constant region 3
- ESI-MS
electrospray ionization mass spectrometry
- Fab
antigen-binding fragment
- Fc
crystallizible fragment
- FcR
Fc receptor
- FcγR
Fcγ receptor
- HEK
human embryonic kidney
- HILIC
hydrophilic interaction liquid chromatography
- HIV
human immunodeficiency virus
- Hs
Homo sapiens
- IgG
immunoglobulin G
- Mf
Macaca fascicularis
- Mm
Macaca mulatta
- MS
mass spectrometry
- NHP
non-human primate
- NMR
nuclear magnetic resonance
- PBS
phosphate buffered saline
- PDB
Protein Data Bank
- RMSD
root mean square deviation
- SHIV
simian human immunodeficiency virus
- SIV
simian immunodeficiency virus.
Acknowledgments
We thank our IHV colleagues for outstanding support of the studies leading to the ideas presented above, specifically Dr. Krishanu Ray for his critical insights. This work was supported by NIH grants: NIAID R01 AI116274 and R01 AI129769 to MP, NIAID P01 AI120756 to GT, and NIGMS R01 GM115489 to AB. We thank Mr. Joel Nott of the ISU Protein Facility for assistance with the LC-ESI/MS/MS data collection, Dr. D. Bruce Fulton and Dr. Shu Xu (ISU) for help with NMR instrumentation. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript. The authors have no conflicts of interest to report.
Author contributions
WDT, NG, and MP designed, performed research and analyzed all the data; GPS, KRP and AWB performed NMR studies and analyzed the data, GKL provided macaque sera samples and analyzed the data; WDT, AWB and MP wrote the paper with all authors providing comments or revisions.
Conflict of interest
There is no conflict of interest.
Disclaimer
The views expressed in this presentation are those of the authors and do not reflect the official policy or position of the Uniformed Services University, US Army, the Department of Defense, or the US Government.
Supplemental material
Supplemental data for this article can be accessed on the here.
References
- 1.Dekkers G, Treffers L, Plomp R, Bentlage AEH, de Boer M, Koeleman CAM, Lissenberg-Thunnissen SN, Visser R, Brouwer M, JY Mok, et al. Decoding the human immunoglobulin G-glycan repertoire reveals a spectrum of Fc-receptor- and complement-mediated-effector activities. Front Immunol. 2017;8:877. doi: 10.3389/fimmu.2017.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch VM, Lifson JD.. Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment, and prevention. Adv Pharmacol. 2000;49:437–77. [DOI] [PubMed] [Google Scholar]
- 3.Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, Liu J, Abbink P, Maxfield LF, Seaman MS, et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 2013;155:531–39. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-Roman VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, Robert-Guroff M. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174:2185–89. [DOI] [PubMed] [Google Scholar]
- 5.Lewis GK, Pazgier M, Evans D, Ferrari G, Bournazos S, Parsons MS, Bernard NF, Finzi A. Beyond viral neutralization. AIDS Res Hum Retroviruses. 2017;33:60–64. doi: 10.1089/aid.2016.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yates NL, Liao HX, Fong Y, Decamp A, Vandergrift NA, Williams WT, SM Alam, Ferrari G, ZY Yang, KE Seaton, PW Berman et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6:228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warncke M, Calzascia T, Coulot M, Balke N, Touil R, Kolbinger F, Heusser C. Different adaptations of IgG effector function in human and nonhuman primates and implications for therapeutic antibody treatment. J Immunol. 2012;188:4405–11. doi: 10.4049/jimmunol.1200090. [DOI] [PubMed] [Google Scholar]
- 8.Rogers KA, Scinicariello F, Attanasio R. IgG Fc receptor III homologues in nonhuman primate species: genetic characterization and ligand interactions. J Immunol. 2006;177:3848–56. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen DC, Scinicariello F, Attanasio R. Characterization and allelic polymorphisms of rhesus macaque (Macaca mulatta) IgG Fc receptor genes. Immunogenetics. 2011;63:351–62. doi: 10.1007/s00251-011-0514-z. [DOI] [PubMed] [Google Scholar]
- 10.Chan YN, Boesch AW, Osei-Owusu NY, Emileh A, Crowley AR, Cocklin SL, Finstad SL, Linde CH, Howell RA, Zentner I, et al. IgG binding characteristics of Rhesus macaque FcgammaR. J Immunol. 2016;197:2936–47. doi: 10.4049/jimmunol.1502252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boesch AW, Osei-Owusu NY, Crowley AR, Chu TH, Chan YN, Weiner JA, Bharadwaj P, Hards R, Adamo ME, Gerber SA, et al. Biophysical and functional characterization of Rhesus macaque IgG subclasses. Front Immunol. 2016;7:589. doi: 10.3389/fimmu.2016.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;D66:213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406:267–73. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- 14.Radaev S, Motyka S, Fridman WH, Sautes-Fridman C, Sun PD. The structure of a human type III Fcgamma receptor in complex with Fc. J Biol Chem. 2001;276:16469–77. doi: 10.1074/jbc.M100350200. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed AA, Giddens J, Pincetic A, Lomino JV, Ravetch JV, Wang LX, Bjorkman PJ. Structural characterization of anti-inflammatory immunoglobulin G Fc proteins. J Mol Biol. 2014;426:3166–79. doi: 10.1016/j.jmb.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank M, Walker RC, Lanzilotta WN, Prestegard JH, Barb AW. Immunoglobulin G1 Fc domain motions: implications for Fc engineering. J Mol Biol. 2014;426:1799–811. doi: 10.1016/j.jmb.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carugo O. How root-mean-square distance (r.m.s.d.) values depend on the resolution of protein structures that are compared. J Appl Crystallogr. 2003;36:125–28. doi: 10.1107/S0021889802020502. [DOI] [Google Scholar]
- 18.Davies AM, Rispens T, Ooijevaar-de Heer P, Gould HJ, Jefferis R, Aalberse RC, Sutton BJ. Structural determinants of unique properties of human IgG4-Fc. J Mol Biol. 2014;426:630–44. doi: 10.1016/j.jmb.2013.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao MH, Smith RI, Morrison SL. Structural features of human immunoglobulin G that determine isotype-specific differences in complement activation. J Exp Med. 1993;178:661–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labrijn AF, Rispens T, Meesters J, Rose RJ, Den Bleker TH, Loverix S, van den Bremer ET, Neijssen J, Vink T, Lasters I, RC Aalberse, et al. Species-specific determinants in the IgG CH3 domain enable Fab-arm exchange by affecting the noncovalent CH3-CH3 interaction strength. J Immunol. 2011;187:3238–46. doi: 10.4049/jimmunol.1003336. [DOI] [PubMed] [Google Scholar]
- 21.Stapleton NM, Andersen JT, Stemerding AM, Bjarnarson SP, Verheul RC, Gerritsen J, Zhao Y, Kleijer M, Sandlie I, de Haas M, et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat Commun. 2011;2:599. doi: 10.1038/ncomms1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barb AW, Prestegard JH. NMR analysis demonstrates immunoglobulin G N-glycans are accessible and dynamic. Nat Chem Biol. 2011;7:147–53. doi: 10.1038/nchembio.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subedi GP, Barb AW. The structural role of antibody N-glycosylation in receptor interactions. Structure. 2015;23:1573–83. doi: 10.1016/j.str.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falconer DJ, Barb AW, Permyakov EA. Mouse IgG2c Fc loop residues promote greater receptor-binding affinity than mouse IgG2b or human IgG1. PLoS One. 2018;13:e0192123. doi: 10.1371/journal.pone.0192123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. A mutation in human CMP-sialic acid hydroxylase occurred after the homo-pan divergence. Proc Natl Acad Sci U S A. 1998;95:11751–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahan AE, Tedesco J, Dionne K, Baruah K, Cheng HD, De Jager PL, Barouch DH, Suscovich T, Ackerman M, Crispin M, et al. A method for high-throughput, sensitive analysis of IgG Fc and Fab glycosylation by capillary electrophoresis. J Immunol Methods. 2015;417:34–44. doi: 10.1016/j.jim.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton DR, Gregory L, Jefferis R. Aspects of the molecular structure of IgG subclasses. Monogr Allergy. 1986;19:7–35. [PubMed] [Google Scholar]
- 29.Scinicariello F, Engleman CN, Jayashankar L, McClure HM, Attanasio R. Rhesus macaque antibody molecules: sequences and heterogeneity of alpha and gamma constant regions. Immunology. 2004;111:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen DC, Sanghvi R, Scinicariello F, Pulit-Penaloza J, Hill N, Attanasio R. Cynomolgus and pigtail macaque IgG subclasses: characterization of IGHG genes and computational analysis of IgG/Fc receptor binding affinity. Immunogenetics. 2014. doi: 10.1007/s00251-014-0775-4. [DOI] [PubMed] [Google Scholar]
- 31.Barb AW, Meng L, Gao Z, Johnson RW, Moremen KW, Prestegard JH. NMR characterization of immunoglobulin G Fc glycan motion on enzymatic sialylation. Biochemistry. 2012;51:4618–26. doi: 10.1021/bi300319q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subedi GP, Hanson QM, Barb AW. Restricted motion of the conserved immunoglobulin G1 N-glycan is essential for efficient FcgammaRIIIa binding. Structure. 2014;22:1478–88. doi: 10.1016/j.str.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subedi GP, Barb AW. The immunoglobulin G1 N-glycan composition affects binding to each low affinity Fc gamma receptor. MAbs. 2016;8:1512–24. doi: 10.1080/19420862.2016.1218586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies AM, Jefferis R, Sutton BJ. Crystal structure of deglycosylated human IgG4-Fc. Mol Immunol. 2014;62:46–53. doi: 10.1016/j.molimm.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohm S, Schwab I, Lux A, Nimmerjahn F. The role of sialic acid as a modulator of the anti-inflammatory activity of IgG. Semin Immunopathol. 2012;34:443–53. doi: 10.1007/s00281-012-0308-x. [DOI] [PubMed] [Google Scholar]
- 36.Mahan AE, Jennewein MF, Suscovich T, Dionne K, Tedesco J, Chung AW, Streeck H, Pau M, Schuitemaker H, Francis D, et al. Antigen-specific antibody glycosylation is regulated via vaccination. PLoS Pathog. 2016;12:e1005456. doi: 10.1371/journal.ppat.1005456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast A-S, Schoen MK, Rolland M, Suscovich TJ, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6:228ra38. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 38.Ackerman ME, Mikhailova A, Brown EP, Dowell KG, Walker BD, Bailey-Kellogg C, Suscovich TJ, Alter G, Douek DC. Polyfunctional HIV-specific antibody responses are associated with spontaneous HIV control. PLoS Pathog. 2016;12:e1005315. doi: 10.1371/journal.ppat.1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subedi GP, Johnson RW, Moniz HA, Moremen KW, Barb A. High yield expression of recombinant human proteins with the transient transfection of HEK293 cells in suspension. J Vis Exp. 2015;106:e53568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otwinowski Z, Minor W, Charles W, Carter JR. Processing of X-ray diffraction data collected in oscillation mode. Meth Enzymol. 1997;276:307–26. Academic Press [DOI] [PubMed] [Google Scholar]
- 41.Collaborative Computational Project N The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–63. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 42.Patel KR, Roberts JT, Subedi GP, Barb AW. Restricted processing of CD16a/Fc gamma receptor IIIa N-glycans from primary human NK cells impacts structure and function. J Biol Chem. 2018;293:3477–89. doi: 10.1074/jbc.RA117.001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pucic M, Knezevic A, Vidic J, Adamczyk B, Novokmet M, Polasek O, Gornik O, Šupraha-Goreta S, Wormald MR, Redžić I, et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol Cell Proteomics. 2011;10:M111 010090. doi: 10.1074/mcp.M111.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varki A. Essentials of glycobiology. New York, NY: Cold Spring Harbor Laboratory Press; 2017. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


