Abstract
Background
For localized prostate cancer (PCa), radical prostatectomy (RP) and radiotherapy (RT) are two standard interventions to decrease PCa mortality. Contemporary studies contained the elderly people; analyses focusing on patients over 75 years of age were still lacking.
Method
In the Surveillance Epidemiology and End Results (SEER) database (2004‐2015), people over 75 years of age with cT2 stage were selected in our research. Multivariable Cox proportional hazard models were used to analyze cancer‐specific mortality (CSM) and overall mortality (OM) after adjustment. The propensity score matching was performed to assume the randomization. An instrument variate (IVA) was used to calculate the unmeasured confounders.
Results
Radical prostatectomy is superior to RT in OM and CSM after adjustment for covariates (HR = 0.54, 95% CI = 0.47‐0.62, P < 0.001 and HR = 0.30, 95% CI = 0.20‐0.45, P < 0.001, respectively). The cox model after matching indicated similar consequence (OM: HR = 0.53, 95% CI = 0.46‐0.62, P < 0.001; CSM: HR = 0.27, 95% CI = 0.17‐0.43, P < 0.001). In the IVA‐adjusted model, the effect of treatment changed slightly (OM: HR = 0.65, 95% CI = 0.54‐0.78, P < 0.001; CSM: HR = 0.21, 95% CI = 0.12‐0.37, P < 0.001). Subgroup analyses showed that for patients with GS = 7, those received RP obtained the highest risk decline for overall death (HR = 0.41, 95% CI = 0.32‐0.52); and for patients with younger age, those received RP obtained the highest risk decline for CSM (HR = 0.11, 95% CI = 0.01‐0.52).
Conclusion
Patients over 75 years of age with cT2 stage will obtain more benefit from RP compared with RT, especially for patients with GS = 7 and younger age.
Keywords: cancer‐specific mortality, localized prostate cancer, radiation therapy, radical prostatectomy, the elderly
1. INTRODUCTION
Prostate cancer (PCa) is the second most common cancer in males, with an estimated 1.1 million confirmed cases worldwide in 2012, making up 15% deaths of all cancers diagnosed.1 This disease primarily encroaches on the elderly with age‐related increasing incidence rates.2 Autopsy studies also suggest that high‐grade intraepithelial neoplasia, the precursor of incidental PCa, are detected more easily among the old. Another European research showed that for men between 30 and 69 years of age, the prevalence of incidental PCa was 30% and sharply increased to 75% for those over 70 years of age.3 With the aging of population and the improvement of life expectancy, elderly people with PCa should be paid more concern.
For localized PCa, radical prostatectomy (RP) and radiotherapy (RT) are two standard interventions to decrease PCa mortality.4, 5 There is still controversy about the choice of treatment. Recently, the first randomized clinical trial, Prostate Testing for Cancer and Treatment, comparing RP, RT, and active monitoring showed that there was no significant difference of 10‐year cancer‐specific‐survival (CSS) in RP versus RT.6 However, several studies held the opposite opinion. Three retrospective studies indicated that people received RP was superior to RT in terms of biochemical recurrence, metastasis‐free survival, and CSS.7, 8, 9 These studies contained patients of all T stages and none of these focused on the elderly.
In consideration of worse physical conditions, advanced stage and higher pathological grade compared with the younger,10 the outcome of RP or RT among old people may differ from others. Two observational studies discussing the benefit of local treatment (RP or RT) for men ≥70 years of age of low risk and for men ≥75 years of age were performed.11, 12 Despite adjustment for covariates to control measured confounders, there remains residual confounding comparing RT with RP. In view of this, we aimed to provide risk estimates to inform contemporary treatment decision for PCa men aged ≥75 years.
2. METHOD
2.1. Study populations
In the Surveillance Epidemiology and End Results (SEER) database (2004‐2015), old men diagnosed with histological adenocarcinoma of the prostate [International Classification of Disease for Oncology (ICD‐O‐3) code 8140 of the prostate (site code C61.9)] over 75 years of age were selected in our research. Old people with T1 stage PCa mostly received RT, causing huge bias in the analysis. As a result, our research only included individuals with T2 stage. Further inclusion/exclusion criteria are depicted in Figure 1. Our study consisted of 10 563 patients in final, who were stratified based on different therapy type: RT group versus RP group. The total available covariates were listed in Table 1.
Figure 1.
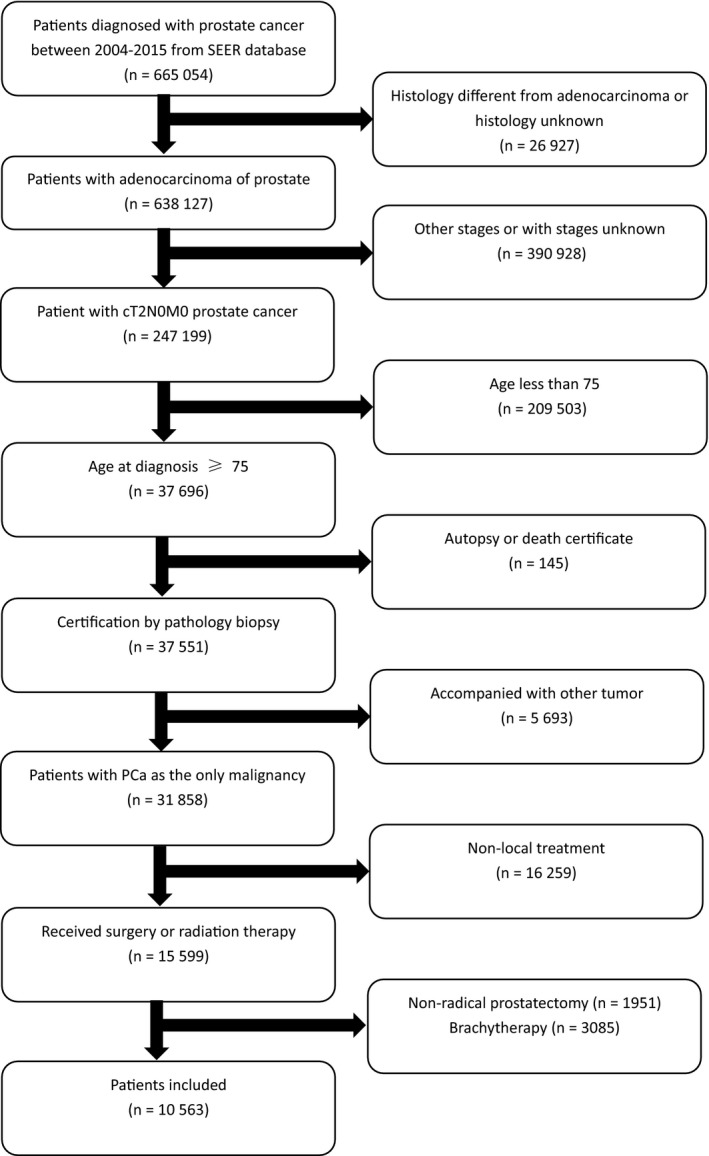
Flowchart of the patients selection
Table 1.
Baseline characteristics of 10 563 patients who received RT versus RP
| RT (N = 8447) | RP (N = 2116) | P value | |
|---|---|---|---|
| Age | 78.593 ± 3.184 | 76.894 ± 2.511 | <0.001 |
| PSA | 116.632 ± 137.769 | 74.947 ± 88.731 | <0.001 |
| Marital status | <0.001 | ||
| Married | 5878 (69.587%) | 1650 (77.977%) | |
| Single | 443 (5.244%) | 105 (4.962%) | |
| Divorced/widowed | 1265 (14.976%) | 252 (11.909%) | |
| Unknown | 861 (10.193%) | 109 (5.151%) | |
| Year of diagnosis | <0.001 | ||
| 2004 | 851 (10.075%) | 163 (7.703%) | |
| 2005 | 798 (9.447%) | 131 (6.191%) | |
| 2006 | 821 (9.719%) | 155 (7.325%) | |
| 2007 | 851 (10.075%) | 200 (9.452%) | |
| 2008 | 753 (8.914%) | 161 (7.609%) | |
| 2009 | 725 (8.583%) | 188 (8.885%) | |
| 2010 | 742 (8.784%) | 221 (10.444%) | |
| 2011 | 729 (8.630%) | 212 (10.019%) | |
| 2012 | 587 (6.949%) | 158 (7.467%) | |
| 2013 | 514 (6.085%) | 186 (8.790%) | |
| 2014 | 505 (5.978%) | 154 (7.278%) | |
| 2015 | 571 (6.760%) | 187 (8.837%) | |
| Race | <0.001 | ||
| White | 7014 (83.035%) | 1834 (86.673%) | |
| Black | 661 (7.825%) | 111 (5.246%) | |
| Other | 618 (7.316%) | 153 (7.231%) | |
| Unknown | 154 (1.823%) | 18 (0.851%) | |
| GS | <0.001 | ||
| ≤6 | 1195 (14.147%) | 343 (16.210%) | |
| 7 | 2009 (23.784%) | 513 (24.244%) | |
| ≥8 | 1324 (15.674%) | 111 (5.246%) | |
| Unknown | 3919 (46.395%) | 1149 (54.301%) | |
| Region | <0.001 | ||
| East | 2696 (31.917%) | 444 (20.983%) | |
| Pacific | 4233 (50.112%) | 1387 (65.548%) | |
| North | 1216 (14.396%) | 133 (6.285%) | |
| Other(Alaska and Southwest) | 302 (3.575%) | 152 (7.183%) |
Abbreviations: GS, Gleason score; PSA, prostate specific antigen; RP, radical prostatectomy; RT, radiation therapy.
2.2. Endpoint
Our main endpoint included: cancer‐specific mortality (CSM) defined as death caused by prostate malignancy (SEER code 28010); overall mortality (OM) defined as deaths from any reason reported by the SEER database. Survival time was defined as the duration from initial diagnosis to death from any cause or last follow‐up.
2.3. Statistical analysis
Firstly, we assessed the distribution of baseline characteristics with the use of a two‐sample t test and a chi‐square test to compare continuous and categorical variables, respectively. Data were presented as mean ± SD for continuous variables and as frequency (%) for categorical variables.
Secondly, a multivariable Cox proportional hazard model was used for analyses of OM and CSM after adjusting race, age, marital status, Gleason Score (GS), and prostate specific antigen (PSA).13
Thirdly, in consideration of baseline characteristics affecting the option of using different treatment methods, propensity score matching (PSM) (ratio 1:1, with a caliper set of 0.05) was performed to ensure that both the RT group and the RP group had similar baseline characteristics with the use of logistic regression to adjust for between‐group differences.14 The matching was conducted based on nearest‐neighbor matching principle. The matched process was considered as balanced with a P value >0.05.
Fourthly, considering the selection bias between patients who received RT versus RP, we additionally used an instrument variate (IVA) to calculate the unmeasured confounders. We selected yearly regional utilization rate as IVA to perform a two‐stage residual inclusion analysis.15, 16 This IVA was previously used in the literature 17, 18, 19, 20 and calculated for each of the four American regions as follows:
We calculated the F‐statistic to confirm its correlation with the option of therapy. And the residual, defined as the observed minus the predicted probability of receiving RP, was calculated. The second IVA assumption was verified that without the use of IVA, the correlation between exposure and outcome cannot be formally tested. Another multivariate Cox proportional hazard model including all covariates and residual was presented.
Sensitivity analyses were performed: (a) The analysis of CSM after adjusting propensity scores; (b) two additional models with inverse probability of treatment weighing (IPTW) and standardized mortality ratio weighting (SMRW) using the propensity score to evaluate the relationship between treatment types and outcomes including all eligible patients (entire cohort); (c) the analysis of CSM stratified by propensity scores; and (d) Competing‐risks regression assessing the CSM.
All analyses were performed with the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc, Boston, MA). A P value <0.05 was considered statistically significant.
3. RESULTS
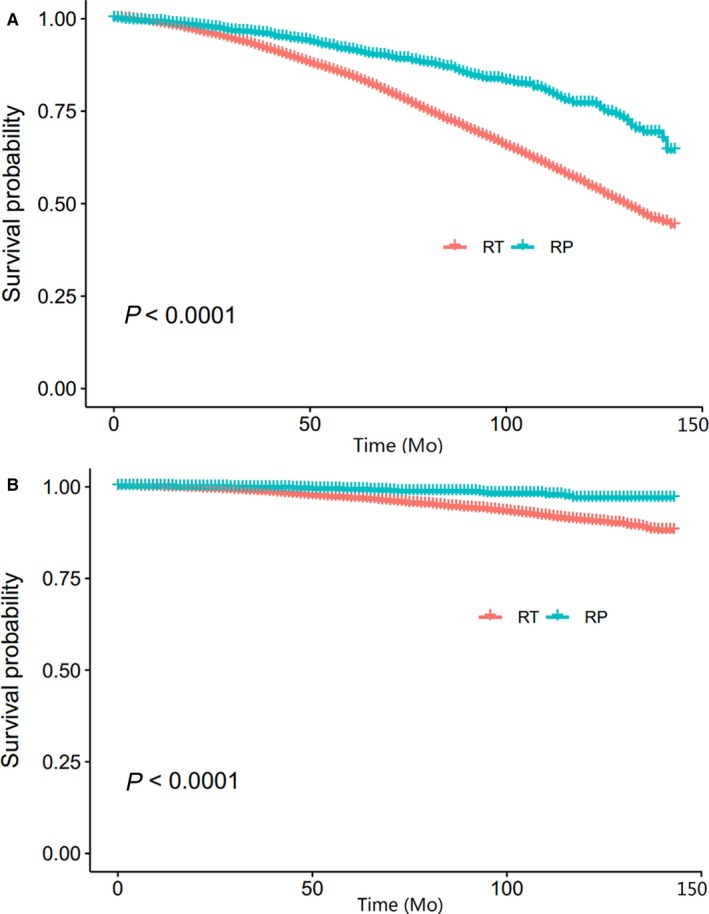
A total of 10,563 elderly men (age ≥75 years) were identified in our study; 8447 patients received RT while 2116 received RP. Details of the baseline characteristics stratified according to RT versus RP were reported in Table 1. The two treatment groups differed significantly in regard to most clinical indicators. Results of the multivariate Cox proportional hazard regression showed that RP could reduce more risks of OM and CSM after adjusted for race, age, marital status, GS and PSA (hazard ratio [HR]=0.54, 95% CI = 0.47‐0.62, P < 0.001 and HR = 0.30, 95% CI = 0.20‐0.45, P < 0.001, respectively). From the Kaplan‐Meier survival curve, the significant survival benefits were observed in the comparison of RP and RT (Figure 2).
Figure 2.
Subgroup analyses of CSM and OM (RT vs RP)
Following PSM, there were 2152 individuals in each treatment group (Table 2). The cox model after matching indicated that RP was still superior to RT (OM: HR = 0.53, 95% CI = 0.46‐0.62, P < 0.001; CSM: HR = 0.27, 95% CI = 0.17‐0.43, P < 0.001) (Table 3). In consideration of the differences after the matching, another cox model adjusted for propensity scores was conducted (Supplement Table S2). Moreover, the results of the sensitivity analyses showed similar outcome (Supplement Table S3). In the IVA‐adjusted model, the effect of treatment changed slightly (OM: HR = 0.65, 95% CI = 0.54‐0.78, P < 0.001; CSM: HR = 0.21, 95% CI = 0.12‐0.37, P < 0.001).
Table 2.
Baseline characteristics of 4304 patients after propensity score match according to treatment
| RT (N = 2152) | RP (N = 2152) | P value | |
|---|---|---|---|
| Age | 76.88 ± 2.30 | 76.89 ± 2.51 | 0.8791 |
| PSA | 79.51 ± 92.88 | 75.11 ± 88.92 | 0.1121 |
| Year of diagnosis | <0.0001 | ||
| 2004 | 226 (10.50%) | 165 (7.67%) | |
| 2005 | 186 (8.60%) | 132 (6.13%) | |
| 2006 | 189 (8.78%) | 156 (7.25) | |
| 2007 | 195 (9.06%) | 205 (9.53%) | |
| 2008 | 204 (9.48%) | 166 (7.71%) | |
| 2009 | 189 (8.78%) | 190 (8.83%) | |
| 2010 | 200 (9.29%) | 222 (10.32%) | |
| 2011 | 200 (9.29%) | 215 (9.99) | |
| 2012 | 139 (6.46%) | 161 (7.48%) | |
| 2013 | 148 (6.88%) | 193 (8.97%) | |
| 2014 | 127 (5.90%) | 158 (7.34%) | |
| 2015 | 149 (6.92%) | 189 (8.78%) | |
| Marital status | 0.0604 | ||
| Married | 1728 (80.3%) | 1679 (78%) | |
| Single | 103 (4.8%) | 106 (4.9%) | |
| Divorced/widowed | 202 (9.4%) | 256 (11.9%) | |
| Unknown | 119 (5.5%) | 111 (5.2%) | |
| Race | 0.0083 | ||
| White | 1892 (87.9%) | 1867 (86.8%) | |
| Black | 137 (6.4%) | 113 (5.3%) | |
| Other | 105 (4.9%) | 154 (7.2%) | |
| Unknown | 18 (0.8%) | 18 (0.8%) | |
| GS | <0.0001 | ||
| ≤6 | 324 (15.1%) | 346 (16.1%) | |
| 7 | 487 (22.6%) | 522 (24.3%) | |
| ≥8 | 277 (12.9%) | 115 (5.3%) | |
| Unknown | 1064 (49.4%) | 1169 (54.3%) | |
| Region | <0.0001 | ||
| East | 744 (34.6%) | 452 (21%) | |
| Pacific | 1006 (46.7%) | 1413 (65.7%) | |
| North | 319 (14.8%) | 134 (6.2%) | |
| Other(Alaska and Southwest) | 83 (3.9%) | 153 (7.1%) |
Abbreviations: GS, Gleason score; PSA, prostate specific antigen; RT, radiation therapy; RP, radical prostatectomy.
Table 3.
Multivariate cox regression analyses for OS and CSM in the total cohort and matched population
| Outcome | Treatment | Non‐adjusted model | Adjusted model | PSM model | IVA‐adjusted model |
|---|---|---|---|---|---|
| OM | RT | 1.0 | 1.0 | 1.0 | 1.0 |
| RP | 0.42 (0.37, 0.48) | 0.54 (0.47, 0.62) | 0.53 (0.46, 0.62) | 0.65 (0.54, 0.78) | |
| CSM | RT | 1.0 | 1.0 | 1.0 | 1.0 |
| RP | 0.21 (0.14, 0.32) | 0.30 (0.20, 0.45) | 0.27 (0.17, 0.43) | 0.21 (0.12, 0.37) |
Adjusted model: adjusted for race, age, marital status, Gleason score (GS) and prostate specific antigen (PSA). Propensity score matching (PSM) model: matched according to race, age, marital status, GS and PSA. Instrument variate (IV) adjusted model: adjusted for race, age, marital status, GS and PSA and residual
Abbreviations: CSM, cancer specific mortality; OM, overall mortality.
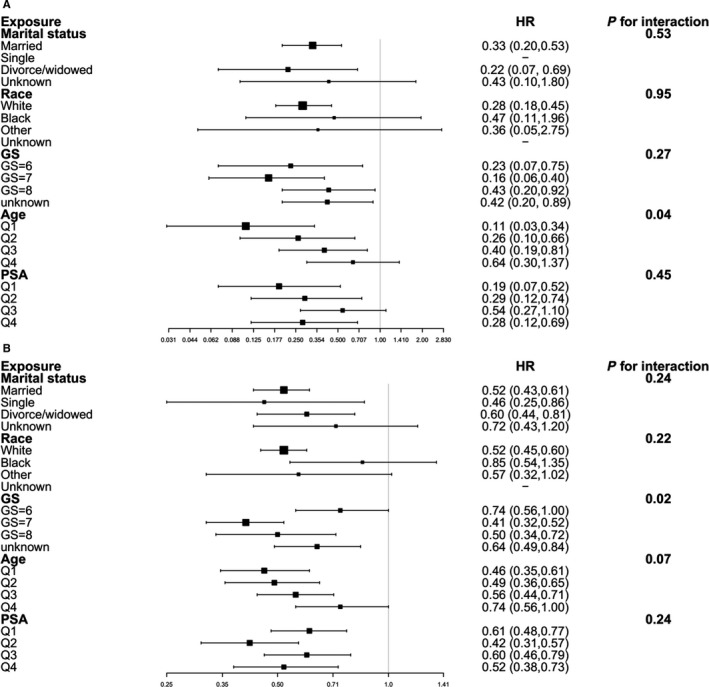
In the subgroup analyses (Figure 3), no significant interaction was observed between the effect of OS and marital status or race (P value of interaction, 0.24 and 0.22, respectively). We did observe a larger magnitude of association between GS and OS (P value for interaction, 0.02). The results indicated that for patients with GS = 7, those received RP obtained the highest risk decline for overall death (HR = 0.41, 95% CI = 0.32‐0.52). And no significant interaction was observed between the effect of OS and age or PSA (P value of interaction, 0.07 and 0.24, respectively). In the subgroup analyses of CSM, a significant relationship between CSM and age was observed (P value for interaction, 0.02), indicating that for patients with younger age, those received RP obtained the highest risk decline for CSM (HR = 0.11, 95% CI = 0.01‐0.52). Other covariates such as marital status, race, GS, and PSA showed no significant interaction effects (P value of interaction, 0.53, 0.95, 0.27 and 0.45, respectively).
Figure 3.
Kaplan‐Meier survival curve of CSM and OM (RT vs RP)
4. DISCUSSION
Among PCa people with the age ≥75, it still remains uncertain regarding the efficacy of modalities of local treatment. Several studies focusing on the role of RT or RP have excluded the old individuals.6, 21, 22 Based on the EAU guideline, both RT and RP are all radical treatments for patients with localized PCa. However, researches about old people aged over 75 years are lacking, doctors should take the poor conditions of the elderly into account when making the decision. Based on these considerations, we attempted to assess the efficacy of RT versus RP as the primary therapy in a contemporary cohort of US men diagnosed with cT2 PCa. We observed that, for PCa people aged over 75 years, those received RP showed less likeliness to die compared with those received RT. Besides, the patients in the RP group had lower risks of CSM than their counterparts who received RT.
In one observational study targeted to Swedish PCa patients of all ages and all clinical stages, men treated with RT are more likely to develop cancer‐specific death for the low‐ and intermediate‐risk cancer.23 In another observational study from SEER database, men treated with RP have higher cancer‐specific‐survival rate after metastasis than those treated with RT. The difference appears more significant among intermediate‐ and high‐risk cancer. 24 Although people with the age ≥75 were included in these studies, subgroup analyses of age and clinical T stage were not performed. According to Kevin R. Rice, et al, for low risk PCa in men ≥70 years of age, no significance was found in the comparison between RP and RT.11 Although baseline characteristics are different in the two groups, interestingly, the results from locally weighed regression (IPTW and SMRW models) suggested variations between the two therapies had little effect on the clinical outcome.
For lack of any published or ongoing randomized controlled trial testing the choice of curing cT2 PCa for men aged over 75 years, our observational study could provide important reference to the decision‐maker. Even more noteworthy is that our comprehensive assessment overcomes the limited ability to account for unmeasured confounders contrast to previous reports.
There are still some limitations of our study. Despite our efforts to overcome the selection bias and confounders in statistical analysis to the maximum extent, there are still indicators disturbing the accuracy of our results, which is natural in any observational study. Still another limitation is the lack of data about the details of androgen deprivation therapy using, which affects the oncological outcome. Last, although we believe the treatments accord with contemporary standards, treatments may be different due to constant evolution. In particular, the surgical procedures of RP and the radiation dose of RT would be considered inadequate by current standards.
5. CONCLUSION
Patients over 75 years of age with cT2 stage will obtain more benefit from RP compared with RT, especially for patients with GS = 7 and younger age. These findings may facilitate counseling regarding the standard treatments for localized cT2 PCa among old people.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
ACKNOWLEDGEMENTS
All authors contributed to the planning and design of the study. K.J. and S.Q. were involved in review of the raw data and directly involved in the analysis. J.K.L. and X.N.Z. provided analytical feedback based on aggregated results. X.T. and X.Y.L. drafted the manuscript, with input from all authors. Y.Y. was responsible for the chart making. All authors provided substantive review and commentary on multiple drafts and approved the final version. K.J. and S.Q. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Jin K, Qiu S, Li J, et al. How to choose proper local treatment in men aged ≥75 years with cT2 localized prostate cancer? Cancer Med. 2019;8:3370–3378. 10.1002/cam4.2221
Kun Jin and Shi Qiu contributed equally to this study.
Funding information
The authors acknowledge Ian Charles Tobias for reviewing the manuscript. Supported by the National key research and development program of China (Grant No. SQ2017YFSF090096), the Prostate Cancer Foundation Young Investigator Award 2013, the National Natural Science Foundation of China (Grant No. 81300627, 81370855, 81702536, 81770756), Programs from Science and Technology Department of Sichuan Province (Grant No. 2014JY0219 and 2017HH0063) and Young Investigator Award of Sichuan University 2017.
Contributor Information
Lu Yang, Email: wycleflue@163.com.
Qiang Wei, Email: weiqiang163163@163.com.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J. Clin. 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 3. Soos G, Tsakiris I, Szanto J, et al. The prevalence of prostate carcinoma and its precursor in Hungary: an autopsy study. Eur. Urol. 2005;48:739‐744. [DOI] [PubMed] [Google Scholar]
- 4. Shao YH, Albertsen PC, Roberts CB, et al. profiles and treatment patterns among men diagnosed as having prostate cancer and a prostate‐specific antigen level below 4.0 ng/ml. Arch Intern Med. 2010;170:1256‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawachi MH, Bahnson RR, Barry M, et al. NCCN clinical practice guidelines in oncology: prostate cancer early detection. J Natl Compr Canc Netw. 2010;8:240‐262. [DOI] [PubMed] [Google Scholar]
- 6. Hamdy FC, Donovan JL, Lane JA, et al. 10‐Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415‐1424. [DOI] [PubMed] [Google Scholar]
- 7. Kibel AS, Ciezki JP, Klein EA, et al. Survival among men with clinically localized prostate cancer treated with radical prostatectomy or radiation therapy in the prostate specific antigen era. J Urol. 2012;187:1259‐1265. [DOI] [PubMed] [Google Scholar]
- 8. Zelefsky MJ, Eastham JA, Cronin AM, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol. 2010;28:1508‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooperberg MR, Vickers AJ, Broering JM, Carroll PR. Comparative risk‐adjusted mortality outcomes after primary surgery, radiotherapy, or androgen‐deprivation therapy for localized prostate cancer. Cancer. 2010;116:5226‐5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delongchamps NB, Wang CY, Chandan V, et al. Pathological characteristics of prostate cancer in elderly men. J Urol. 2009;182(3):927‐930. [DOI] [PubMed] [Google Scholar]
- 11. Rice KR, Colombo ML, Wingate J, et al. Low risk prostate cancer in men ≥70 years old: to treat or not to treat. Urol Oncol. 2013;31:755‐760. [DOI] [PubMed] [Google Scholar]
- 12. Bandini M, Pompe RS, Marchioni M, et al. Radical prostatectomy or radiotherapy reduce prostate cancer mortality in elderly patients: a population–based propensity score adjusted analysis. World J Urol. 2018;36(1):7‐13. [DOI] [PubMed] [Google Scholar]
- 13. Vatandoust S, Kichenadasse G, O'Callaghan M, et al. Localized prostate cancer in elderly men aged 80–89, findings from a population‐based registry. BJU Int. 2018;121(3):48‐54. [DOI] [PubMed] [Google Scholar]
- 14. Rosenbaum PR, Rubin D. The central role of propensity score in observation studies for causal effects. Biometrika. 1983;70:41‐55. [Google Scholar]
- 15. Terza JV, Basu A, Rathouz PJ. Two‐stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008;27:531‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cai B, Small DS, Have T. Two‐stage instrumental variable methods for estimating the causal odds ratio: analysis of bias. Stat Med. 2011;30:1809‐1824. [DOI] [PubMed] [Google Scholar]
- 17. Hadley J, Yabroff KR, Barrett MJ, et al. Comparative effectiveness of prostate cancer treatments: evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst. 2010;102:1780‐1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hershman DL, Wright JD. Comparative effectiveness research in oncology methodology: observational data. J Clin Oncol. 2012;30:4215‐4222. [DOI] [PubMed] [Google Scholar]
- 19. Bekelman JE, Mitra N, Handorf EA, et al. Effectiveness of androgendeprivation therapy and radiotherapy for older men with locally advanced prostate cancer. J Clin Oncol. 2015;33:716‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wright JD, Huang Y, Burke WM, et al. Influence of lymphadenectomy on survival for early‐stage endometrial cancer. Obstet Gynecol. 2016;127:109‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate‐cancer screening trial. N Engl J Med. 2009;360:1310‐1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bill‐Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robinson D, Garmo H, Lissbrant IF, et al. Prostate cancer death after radiotherapy or radical prostatectomy: a nationwide population‐based observational study. Eur Urol. 2018;73(4):502‐511. [DOI] [PubMed] [Google Scholar]
- 24. Shao YH, Kim S, Moore DF, et al. Cancer‐specific survival after metastasis following primary radical prostatectomy compared with radiation therapy in prostate cancer patients: results of a population‐based, propensity score‐matched analysis. Eur Urol. 2014;65(4):693‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


