Abstract
Background:
Previous studies have investigated α1GABAA and α5GABAA receptor mechanisms in the behavioral effects of ethanol in monkeys. However, genetic studies in humans and preclinical studies with mutant mice suggest a role for α2GABAA and/or α3GABAA receptors in the effects of ethanol. The development of novel positive allosteric modulators (PAMs) with functional selectivity (i.e., selective efficacy) at α2GABAA and α3GABAA receptors allows for probing of these subtypes in preclinical models of the discriminative stimulus and reinforcing effects of ethanol in rhesus macaques.
Methods:
In discrimination studies, subjects were trained to discriminate ethanol (2 g/kg, i.g.) from water under a fixed-ratio schedule of food delivery. In oral self-administration studies, subjects were trained to self-administer ethanol (2% w/v) or sucrose (0.3–1% w/v) under a fixed-ratio schedule of solution availability.
Results:
In discrimination studies, functionally selective PAMs at α2GABAA and α3GABAA (HZ-166) or α3GABAA (YT-III-31) receptors substituted fully (maximum percentage of ethanol-lever responding ≥80%) for the discriminative stimulus effects of ethanol without altering response rates. Full substitution for ethanol also was engendered by a nonselective PAM (triazolam), an α5GABAA-preferring PAM (QH-ii-066) and a PAM at α2GABAA, α3GABAA, and α5GABAA receptors (L-838,417). A partial (MRK-696) or an α1GABAA-preferring (zolpidem) PAM only engendered partial substitution (i.e., ~50–60% ethanol-lever responding). In self-administration studies, pretreatments with the functionally selective PAMs at α2GABAA and α3GABAA (XHe-II-053 and HZ-166) or α3GABAA (YT-III-31 and YT-III-271) receptors increased ethanol, but not sucrose, drinking at doses that had few, or no, observable sedative-motor effects.
Conclusions:
Our results confirm prior findings regarding the respective roles of α1GABAA and α5GABAA receptors in the discriminative stimulus effects of ethanol and, further, suggest a key facilitatory role for α3GABAA and potentially α2GABAA receptors in several abuse-related effects of ethanol in monkeys. Moreover, they reveal a potential role for these latter subtypes in ethanol’s sedative effects.
Keywords: ethanol, GABAA, alpha, drug discrimination, self-administration
Introduction
Alcohol (ethanol) use disorder (AUD) and the health consequences of alcohol abuse are a major global public health concern. Alcohol causes or negatively affects many health conditions, and represents the 5th leading risk factor for premature death and disability (World Health Organization, 2015). Despite decades of research on treatment strategies, AUD is still prevalent across the globe, and currently available treatments are only partially effective (Mason, 2017). Discovery and development of improved treatments requires advances in our understanding of the neuropharmacological mechanisms underlying the behavioral effects of ethanol related to its abuse.
Ethanol induces its abuse-related effects, in part, by potentiating the activity of γ-aminobutyric acid (GABA) at GABAA receptors (Stephens et al., 2017). GABAA receptors are heteromeric protein complexes composed of five subunits that form a ligand gated ion channel (Rudolph and Möhler, 2004; Olsen and Sieghart, 2008). Nineteen distinct subunits have been identified in mammals, with α, β, and γ subunits being the most abundant forms in the mammalian brain (Sieghart and Sperk, 2002). Several of the families have multiple isoforms, including α (1–6), β (1–3) and γ (1–3) (Olsen and Sieghart, 2008). Importantly, distinct α subunits have been shown to contribute differentially to the abuse-related effects of ethanol. For example, previous studies from our laboratory have demonstrated that α5, but not α1, subunit-containing GABAA receptors (α5GABAA and α1GABAA receptors, respectively) play a prominent role in the reinforcing and discriminative stimulus effects of ethanol in nonhuman primates (Platt et al., 2005; Rüedi-Bettschen et al., 2013; Sawyer et al., 2014). Much remains to be learned, however, regarding the role of other α-subunit-containing receptors in ethanol-induced behavioral and abuse-related effects.
Several studies have confirmed a link between variants of the GABRA2 gene, which encodes the α2 subunit of GABAA receptors (i.e., α2GABAA), and alcoholism (Edenberg et al., 2004; Enoch et al., 2008; Syoka et al., 2008). Moreover, specific polymorphisms in the GABRA2 gene have been shown to mediate the expression of the subjective effects of ethanol (Covault et al. 2004; Pierucci-Lagha et al. 2005). Given the evidence implicating GABRA2 in AUD, there has been considerable interest in understanding the role of α2GABAA receptors in the reinforcing effects of ethanol; results by-and-large showing contradictory findings. In this regard, mice containing a mutant α2 subunit that was insensitive to potentiation by ethanol yet retained normal GABA sensitivity consumed smaller or larger amounts of ethanol compared to wild type (WT) controls depending on the nature of the test (Blednov et al., 2011). On the other hand, α2 knockout mice showed similar levels of ethanol self-administration compared to WT animals (Dixon et al., 2012). These studies suggest that α2GABAA receptors may play a role in the reinforcing effects of ethanol, but the exact nature of that role remains poorly understood.
Research also has shown a possible genetic linkage between the gene encoding the α3 subunit of GABAA receptors (i.e., α3GABAA) and alcoholism (Parsian and Cloninger, 1997). Although the evidence is limited at present, recent studies have pointed to a role of α3GABAA receptors in the behavioral effects of ethanol. For example, α3GABAA knockout mice showed greater ethanol-induced hyperlocomotion and less sensitivity to the hypolocomotor effects of relatively high ethanol doses compared to WT mice (Blednov et al., 2013). Deletion of α3GABAA receptors also slowed recovery from ethanol-induced motor incoordination (Blednov et al., 2013).
Although the various studies described above are suggestive of α2GABAA and/or α3GABAA receptor involvement in the effects of ethanol, to date no direct pharmacological investigations into these subtypes have been performed likely due to the difficulty in targeting the two subtypes because they possess a high degree of molecular/structural homology in both the extracellular and the transmembrane domains (Whiting et al., 1999). The recent availability of novel compounds that vary with respect to intrinsic effcacy at distinct α subunit-containing GABAA receptors has allowed investigations of their differential roles in the abuse-related effects of ethanol (Cook et al., 2009, 2017; Fischer et al., 2010; Namjoshi et al., 2013). Therefore, the aim of the present study was to investigate the effects of functionally selective positive allosteric modulators (PAMs) at α2GABAA and α3GABAA receptors in rhesus monkey models of intra-gastric ethanol discrimination and ethanol drinking (Rüedi-Bettschen et al., 2013; Sawyer et al., 2014). “Functional selectivity” refers to relatively higher intrinsic efficacy at a distinct subtype (or subtypes) compared to other subtypes, based on in vitro experiments using GABA-potentiated Cl- conductance as the defining measure. Quantitative behavioral observations were conducted following ethanol- or sucrose-drinking sessions in order to assess the behavioral effects of the different test compounds per se and on ethanol-induced behaviors. To facilitate comparison with previous findings, additional nonselective and selective PAMs were evaluated in some experiments.
Materials and Methods
Animals
Fourteen adult male rhesus macaques (Macaca mulatta) weighing 7–14 kg served as subjects. Four monkeys participated in the ethanol discrimination studies, and a separate cohort of 10 monkeys participated in the sucrose (N=5) or ethanol (N=5) self-administration studies. Monkeys were individually-housed in a colony room with a 12:12 hour light/dark cycle. All subjects were fed a diet of monkey chow (Teklad 25% Monkey Diet, Harlan/Teklad, Madison, WI) supplemented with fresh and dried fruit and seeds; water was available ad lib. Subjects in the ethanol discrimination studies were maintained at 90–95% of their free-feeding weights by adjusting their access to food. All subjects in the ethanol discrimination studies were experimentally naïve. Animals in the self-administration studies had been previously trained to self-administer ethanol or sucrose using an operant panel (Vallender et al., 2010; Ruedi-Bettschen et al., 2013; Sawyer et al., 2014). The sole exception was one sucrose drinker who had prior experience in operant intravenous cocaine self-administration procedures and was naıve to the oral self-administration procedures. All protocols and animal care and handling followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, revised 2011) and the recommendations of the American Association for Accreditation of Laboratory Animal Care, and were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center and Harvard Medical School. Blood ethanol concentration (BEC) and ethanol discrimination studies were conducted at the New England Primate Research Center, Harvard Medical School. Self-Administration studies were conducted at the University of Mississippi Medical Center.
Drugs and compounds
Ethanol (95%; Pharmco Products, Brookfield, CT) was diluted to the appropriate concentration (as determined by the specific study) using tap water. Sucrose solutions also were prepared using tap water. Table 1 summarizes the pharmacological compounds used in the present study and their putative mechanisms of action. Triazolam and zolpidem were obtained from Sigma/RBI (St. Louis, MO). L-838,417 was obtained from Merck, Sharp, and Dohme Research Laboratories (Harlow Essex, UK). All three compounds were prepared in a vehicle of 50% propylene glycol (Fisher Scientific; Suwanee, GA) and 50% saline. MRK-696 also was obtained from Merck, Sharp, and Dohme Research Laboratories and was prepared in 100% propylene glycol and diluted using 50–80% propylene glycol and water solutions. QH-ii-066 (Huang et al., 2003), HZ-166 (Cook et al., 2009), XHe-II-053 (Cook et al., 2009), YT-III-31 and YT-III-271 (Cook et al., 2017) were synthesized at the Department of Chemistry and Biochemistry, University of Wisconsin-Milwaukee, and dissolved in 20% ethanol, 60% propylene glycol and 20% sterile water. Vehicle and experimental compounds were administered intramuscularly (i.m.; ethanol or sucrose self-administration studies) or intra-gastrically (i.g., ethanol discrimination studies). All drugs were injected in a volume of 0.1–1.0 ml/kg, depending on the dose, solubility and route of administration, and were prepared the day of a test session. Doses for triazolam (0.03 to 0.3 mg/kg), MRK-696 (0.3 to 10 mg/kg), zolpidem (3 to 30 mg/kg), QH-ii-066 (1 to 10 mg/kg), L-838,417 (0.03 to 1 mg/kg), HZ-166 (1 to 30 mg/kg), XHe-II-053 (0.3 to 3 mg/kg), YT-III-31 (0.1 to 3 mg/kg) and YT-III-271 (0.1 to 1 mg/kg) were chosen based on the preliminary dose-effect curves as determined in the present or previous studies (Rowlett et al., 2005; Licata et al., 2010; Fischer et al., 2010; 2011; 2013; Shinday et al., 2013; Sawyer et al., 2014).
Table 1.
Putative mechanisms of action for positive allosteric modulators (PAMs) targeting specific GABAA receptor subunits
| Compound | Mechanism of action |
|---|---|
| Triazolam1 | Nonselective benzodiazepine PAM |
| MRK-6962 | Nonselective benzodiazepine partial PAM |
| QH-ii-0663 | α5GABAA receptor-preferring PAM |
| Zolpidem1 | ɑ1GABAA receptor-preferring PAM |
| L-838,4172 | Functionally selective partial PAM at α2/3/5GABAA receptors that lacks efficacy at α1GABAA receptors (α1-sparing compound) |
| YT-III-314,5 | Functionally selective PAM at α3GABAA receptors |
| YT-III-2714 | Functionally selective PAM at α3GABAA receptors |
| HZ-1666 | Functionally selective PAM at α2GABAA and α3GABAA receptors |
| XHe-II-0536 | Functionally selective PAM at α2GABAA and α3GABAA receptors |
Huang et al., 2003
α1/2/3/5GABAA receptors = GABAA receptors containing α1, α2, α3, and α5 subunits, respectively.
Blood Ethanol Concentration
BECs were determined for monkeys in the ethanol discrimination studies (n=4) after the administration of a bolus dose of ethanol (2 g/kg). Monkeys were lightly sedated with ketamine (10 mg/kg, i.m.) prior to insertion of a lubricated orogastric tube (5 French) down one nostril, through the esophagus and into the stomach. Accurate placement was determined by observation of the animal and by placing a small amount of tap water into the catheter through a syringe. For blood collection, monkeys were sedated with ketamine if necessary and 2 to 3 ml of blood were collected in a sterile 10-ml tube (BD Vacutainer, sodium heparin 158 USP; BD, Franklin Lakes, NJ) from the femoral or saphenous veins. For the determination of the time course of BECs, blood was collected at time points 0, 15, 30, 60, 120 and 240 min post administration of ethanol. Samples were then centrifuged at 1,150Xg for 8 to 12 minutes. The plasma was transferred to polypropylene tubes and frozen at 80°C for later analysis. Analysis was conducted using a rapid high performance plasma ethanol analysis using ethanol oxidase with an AM1 series analyzer and Analox Kit GMRD-113 (Analox Instruments USA, Lunenburg, MA). This process reliably detects BECs ranging from 0 to 350 mg/dl with an internal standard of 100 mg/dl. BECs were determined in triplicate.
Surgery
Subjects in the ethanol discrimination studies were prepared with a chronic intra-gastric catheter manufactured to custom dimensions (silicone, i.d.: 1.98 mm; o.d.: 3.17 mm; 60 cm total length, SAI Infusion Technologies, Lake Villa, IL). Intra-gastric ethanol administration is advantageous as it ensures precise drug delivery (unlike drinking) and mimics to some extent the route of administration used by humans. Intra-gastric administration also presumably minimizes smell and taste cues that could confound discrimination studies. Briefly, monkeys were sedated initially with 10–20 mg/kg i.m. of ketamine. Throughout surgery, anesthesia was maintained by an isoflurane/oxygen mixture. Under aseptic conditions, an intra-gastric catheter was implanted in the middle of the fundus of the stomach (area chosen as the site of implantation because it is minimally vascularized) and inserted and sutured to the stomach via a butterfly patch. The distal end of the catheter was passed lateral to the incision through the muscle layers, then guided subcutaneously using a probe and exited in the mid-scapular region. The external end of the catheter was fed through a fitted jacket and tether system (Lomir Biomedical, Toronto, Canada), attached to a fluid swivel mounted to the animal’s cage and closed-ended with a slit/burp valve to prevent clogging, leaking and backflow. To maintain catheter patency, catheters were flushed daily with water.
Ethanol Discrimination
Ethanol discrimination sessions occurred 5 d/wk in the animal’s home cage. Each monkey’s cage was fitted with a custom-made operant panel (MetalSmiths, Boston, MA) containing stimulus lights, levers and a food pellet dispenser with food hopper (Med Associates, Inc., Georgia, VT). Monkeys (n=4) were trained to discriminate intra-gastrically administered ethanol (2 g/kg) from vehicle (water) under a 30-response fixed ratio (FR 30) schedule of food reinforcement. The ethanol dose was chosen based on previous ethanol discrimination studies in cynomolgus monkeys (Helms et al., 2008; 2009). After an intra-gastric infusion of ethanol, 30 consecutive responses on one lever (counterbalanced across monkeys) produced a food pellet (1 g sucrose pellet, Bio-serve, Flemington, NJ), whereas after an intra-gastric infusion of vehicle, 30 consecutive responses on the other lever produced a food pellet. Each response on the incorrect lever (e.g., the vehicle-appropriate lever after ethanol injection) reset the FR requirement to 30. Delivery of each food pellet was followed by a 10-s timeout period, during which the lights were off and responses had no scheduled consequences.
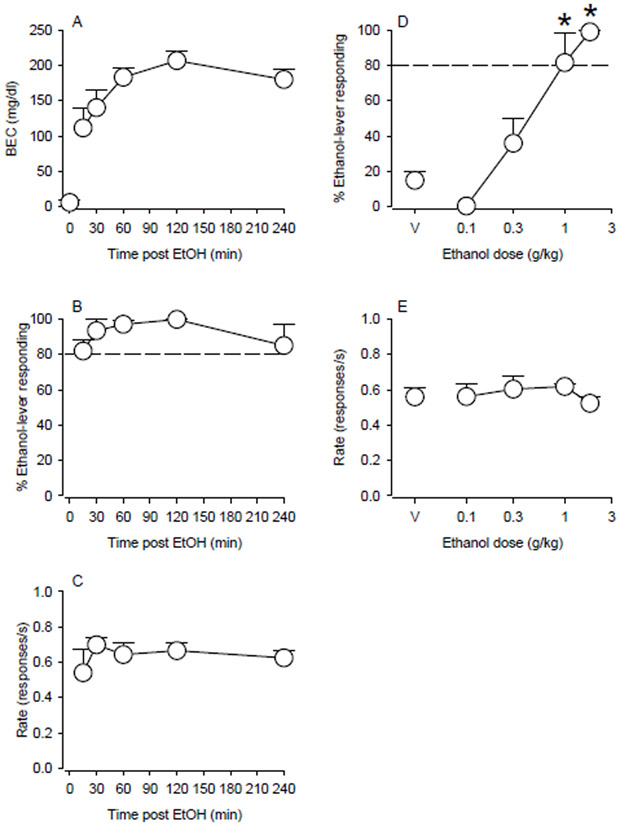
Training sessions consisted of an intra-gastric administration of either ethanol or vehicle followed by a 120-min pretreatment period during which responding had no programmed consequences. The pretreatment time was established based on the results obtained for the time course of BECs (Fig. 1A), which showed that BECs peak at 120 min post oro-gastric administration of 2 g/kg ethanol (dose used in the ethanol discrimination studies). Infusions of ethanol or vehicle were administered from outside the homecage via a catheter extension. Each infusion was followed by a 2-ml injection of water to flush the catheter of any residual ethanol solution. At the beginning of a session, a set of two white stimulus lights above the response levers was illuminated. The session ended after the completion of the 30th FR 30 or after 30 min had elapsed, whichever occurred first.
Figure 1.
Discriminative stimulus effects of ethanol in monkeys trained to discriminate intragastrically-delivered ethanol (2 g/kg) from water. (A) Blood ethanol concentrations (BECs) induced by oral administration of a bolus dose of ethanol (2 g/kg), and (B) the discriminative stimulus and (C) rate effects of that dose in ethanol discrimination as a function of pretreatment time. (D) Ethanol fully substitutes for itself across a range of doses and (E) does not alter response rate compared to vehicle (“V”). Points represent means and SEM in N=4 monkeys. *Indicates p<0.05 compared with respective vehicle baseline.
Once consistent stimulus control was achieved (i.e., ≥80% of the responses in the first ratio as well as across the entire session occur on the condition-appropriate lever for at least four of the preceding five training sessions), test sessions were conducted once or twice per week with training sessions on intervening days. At the beginning of the test sessions, vehicle, ethanol (0.1–2 g/kg, i.g.), triazolam (0.03–0.3 mg/kg, i.g.), MRK-696 (0.3–10 mg/kg, i.g.), QH-ii-066 (1–10 mg/kg, i.g.), zolpidem (3–30 mg/kg, i.g.), L-838,417 (0.03–1 mg/kg, i.g.), YT-III-31 (0.1–1 mg/kg, i.g.) or HZ-166 (1–17.8 mg/kg, i.g.) were administered. After a pretreatment period (30-min for YT-III-31; 60-min for all other test compounds), the lights were illuminated, and the session continued until the completion of the 30th FR 30 or after 30 min had elapsed, whichever occurred first. A single dose of vehicle, ethanol or test compound was administered in a given test session. The effects of all doses were determined twice, once after a vehicle training day and once after an ethanol training day.
Ethanol or Sucrose Self-Administration
Drinking sessions occurred 5 d/wk in the animal’s home cage, as previously described (Sawyer et al., 2014). Each session lasted 3 hours. Animals were trained to drink either ethanol (2%, w/v; n=5) or sucrose solution (0.3 or 1%, w/v, depending on the animal; n=5) using an operant drinking panel mounted on the side of the home cage. The ethanol concentration was chosen because it maintained intake significantly above water levels and is on the ascending limb of the concentration-effect curve (see Ruedi-Bettschen et al., 2013), thus allowing us to detect either increases or decreases in drinking after pretreatment administration. The sucrose concentrations were chosen because they maintained approximately equivalent levels of intake to ethanol (EtOH) under baseline conditions (Ruedi-Bettschen et al., 2013; Sawyer et al., 2014).
The drinking panel contained two retractable sippers (Med Associates) connected with tygon tubing to stainless steel reservoirs mounted outside of the cage. A response lever (Med Associates) was positioned below each sipper, and a set of colored lights were positioned above. Each lever press resulted in an audible click and served as a response. In these experiments, only 1 side of the panel was active. Illumination of white lights signaled the start of the session and ethanol or sucrose availability. Every 10 responses (FR 10) resulted in a switch from illumination of the white light to illumination of a red light and extension of the drinking spout for 30 seconds. Depression of the spout during extension resulted in fluid delivery, continuing as long as the sipper was both depressed and extended. Thus, both the actual duration (up to 30 seconds) and volume of intake were controlled by the subject. A brief (1 second) timeout followed each spout extension, in which all stimulus lights were dark and responding had no programmed consequences. Responses were recorded and outputs controlled by a software program (MedPC; Med Associates). At the end of each session, reservoirs were drained and the amount of liquid consumed (ml) measured.
XHe-II-053 (0.3–3 mg/kg), HZ-166 (0.3–3 mg/kg), YT-III-31 (0.1–3 mg/kg) or YT-III-271 (0.1–1 mg/kg) were administered intramuscularly 10 minutes before the start of a self-administration session. Each dose of each compound was studied for a minimum of 5 consecutive sessions and until intake was stable, which was defined as no upward or downward trend in amount consumed (ml) over 3 consecutive days (i.e., for each 3 day period, intake could not be consistently increasing or decreasing across the consecutive days). Following evaluation of each dose, monkeys were returned to baseline self-administration conditions (i.e., with no pretreatment injection) until intake stabilized again. Doses were randomized within each treatment condition, and all doses of a particular compound were generally completed before beginning a new compound.
Observable Behavior
The behavior of each monkey was recorded for 5 minutes each day immediately following the conclusion of the day’s self-administration session, using a focal animal approach as described in Platt and colleagues (2000; 2002) and modified for rhesus monkeys (see Rüedi-Bettschen et al., 2013; Sawyer et al., 2014; Duke et al., 2018). Briefly, a trained observer blind to the drug treatments observed a specific monkey for 5 minutes and recorded each instance that a particular behavior occurred during 15-second intervals. Scores for each behavior were calculated as the number of 15-second bins in which the behavior occurred (e.g., a maximum score would be 20). For sedation measures, structured exposure to stimuli were included in the observation sessions (Duke et al., 2018). When a monkey was observed to have closed eyes, an assessment of the animal’s responsiveness to the stimuli was determined. Specifically, the observer presented three stimuli: 1) walked at a normal pace towards the cage, 2) spoke the animal’s name, and 3) moved the lock used to secure the door of the cage. If the monkey responded immediately (i.e., opened eyes and oriented to the observer), rest/sleep posture was scored. If the monkey attended more slowly (i.e., > 3 seconds following stimuli) and was observed to be assuming an atypical posture that differed from the characteristic rest/sleep posture (e.g., unable to keep an upright posture), the observer scored moderate sedation. If the monkey did not open eyes across/throughout the 15-s interval after all three stimuli, the observer noted the loss of ability to respond to external stimuli and made as assessment of deep sedation. The assessment of sedation was initiated during the 5-min sampling period if the animal presented, at any time during that period, with its eyes closed. The result of this assessment was recorded for each remaining 15-sec interval of the 60-sec epoch unless eyes opened. Afterwards, eyes closing again initiated the assessment. If eyes remained closed, then the assessment was repeated at the beginning of the next 60-sec epoch. The order in which animals were observed and the observer performing the scoring each day was randomized. Twelve observers participated in the scoring throughout the duration of the study; each observer underwent a minimum of 20 hours of training and met an inter-observer reliability criterion of ≥ 90% agreement with all other observers.
Statistical Analysis
The alpha level for all statistical analyses was set at 0.05. For the ethanol discrimination studies, percentage of ethanol-lever responding was computed for individual subjects throughout a test session by dividing the number of responses on the ethanol lever by the total number of responses on both levers and multiplying by 100. Mean percentage of ethanol-lever responding and S.E.M. were then calculated for the group of monkeys at each dose. A drug was considered to substitute fully for ethanol if the maximum percentage of drug-lever responding was ≥80%. The overall rate of responding throughout the session was computed by dividing the total number of responses (regardless of lever) by the total duration. Mean response rate and S.E.M. were then calculated for the group at each dose. The effect of each drug on the percentage of ethanol-lever responding and response rate was analyzed by separate one-way repeated measures analyses of variance (RM ANOVAs). Further analysis was performed using Bonferroni t-tests comparing the effects of different doses of each drug with vehicle control.
For the ethanol or sucrose self-administration studies, daily volumes (ml) served as the measure of intake for individual subjects. For ethanol self-administration, dose was calculated as follows: volume consumed (ml) x ethanol concentration (g/ml)]/weight (kg). Data are expressed as mean intake over 3 sessions. To compare the effects of the test compounds on ethanol and sucrose self-administration, intakes were converted to percent baseline intake. Baseline intake was considered to be the mean amount of ethanol or sucrose consumed across the 3 days immediately prior to beginning pretreatment tests with a given dose of a compound. For each drug, a mixed-design ANOVA (between subjects variable: group; within subjects variable: dose) was used to assess differential effects of the PAMs in the two groups. Significant interactions were followed by Bonferroni t-tests.
For the behavioral observation studies, for each drug, frequency scores for each observed behavior were averaged separately for the ethanol and sucrose groups. We have determined previously that modified frequency data are normally-distributed for both baseline and drug-induced behavior (Duke et al., 2018), therefore these data were analyzed using separate one-way RM ANOVAs (within group factor: dose) for each behavior. Bonferroni t-tests were used where appropriate.
Results
Ethanol Discrimination
In order to determine the pretreatment time that would result in maximum BECs after the administration of 2 g/kg ethanol (training dose), the time course of BECs after the orogastric administration of a bolus dose of ethanol was established (Fig. 1A). Because the highest BECs were observed at 120 min, this pretreatment time was used in the ethanol discrimination studies. Using a training dose of 2 g/kg ethanol and a 120 min pretreatment, monkeys acquired intra-gastric ethanol discrimination after 152 to 174 sessions. While this number of training sessions exceeds that which has been reported previously for male monkeys trained at 2 g/kg ethanol (cf. Grant et al., 2000), there were a number of differences between that study and the current study including species (M. fascicularis vs. M. mulatta), pretreatment time (30 min vs. 120 min.) and testing environment (sound-attenuated chambers vs. home cage). Any of these factors could have affected training time. During training sessions on days immediately before test sessions, individual monkeys made an average of 93 ± 1% responses on the ethanol-associated lever after ethanol infusions and 7 ± 2% responses on the ethanol-associated lever after water infusions. Rates of responding during training sessions were 0.476 ± 0.047 responses/s after ethanol infusions and 0.482 ± 0.037 responses/s after water infusions.
Under test conditions, the training dose of ethanol (2 g/kg) engendered >80% responding on the ethanol-associated lever across a range of pretreatment times (30 to 240 min, Fig. 1B) and did not reliably alter response rate at any time point (Fig. 1C). At the 120-min pretreatment time, increasing doses of ethanol (0.1–2 g/kg) engendered dose-dependent increases in the percentage of responses on the ethanol-associated lever (Fig. 1D). Low doses of ethanol (0.1– 0.3 g/kg) engendered low-to-modest levels of responding on the ethanol-associated lever, whereas doses of ethanol ≥ 1.0 g/kg elicited virtually exclusive responding on the ethanol-associated lever that was significantly higher than responding after vehicle [F(4,9) = 9.41, p<0.005; Bonferroni t-tests, p<0.05 vs. vehicle]. No significant differences were observed in average rates of responding after ethanol administration at all doses compared with average rates of responding following vehicle (Fig. 1E).
Effects of Nonselective Benzodiazepine Receptor Ligands
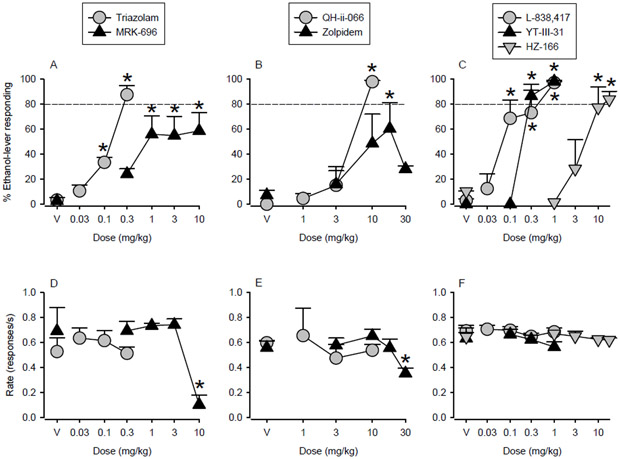
Significant and dose-related increases in ethanol-lever responding compared to vehicle were observed after pretreatment with the nonselective benzodiazepine receptor high efficacy PAM triazolam (Fig. 2A; F(3,6) = 44.09, p<0.001; Bonferroni t-tests, p<0.05 vs. vehicle). Over the dose range tested, triazolam engendered a maximum of 88% ethanol-lever responding (i.e., full substitution) while exerting no significant effects on mean rates of responding (Fig. 2D). Increasing doses of the nonselective benzodiazepine receptor partial PAM MRK-696 also engendered dose-related increases in the percentage of responses on the ethanol-associated lever. These increases differed significantly from the percentage of ethanol-lever responding engendered by vehicle, with partial substitution, i.e., average maximum of 60% responses on the ethanol-associated lever (≥1 mg/kg; Fig. 2A; F(4,12) = 5.14, p<0.05; Bonferroni t-tests, p<0.05 vs. vehicle). These changes were observed at doses that also significantly reduced average rates of responding compared with those engendered by vehicle injections at the highest dose tested (Fig. 2D; F(4,12) = 13.80, p<0.001; Bonferroni t-tests, p<0.05 vs. vehicle).
Figure 2.
Effects of GABAA receptor positive allosteric modulators (PAMs) with distinct efficacy at α subunits in monkeys trained to discriminate intragastrically-delivered ethanol (2 g/kg) from water. Ethanol-like discriminative stimulus and rate effects of (A, D) nonselective benzodiazepine PAMs (triazolam and MRK-696), (B, E) α5- (QH-ii-066) and α1- (zolpidem) preferring GABAA receptor PAMs and (C, F) functionally selective PAMs at α2/3/5GABAA (L-838,417), α2/3GABAA (HZ-166) or α3GABAA (YT-III-31) receptors. Points represent means and SEM in N=4 monkeys. *Indicates p<0.05 compared with respective vehicle baseline.
Effects of the α5GABAA Receptor-Preferring PAM QH-ii-066 and the α1GABAA Receptor-Preferring PAM Zolpidem
The α5GABAA receptor PAM QH-ii-066 had discriminative stimulus effects that were qualitatively similar to those of ethanol (Fig. 2B). Increasing doses of QH-ii-066 engendered significant and dose-dependent increases in the percentage of responses on the ethanol-associated lever [F(3,5) = 25.87, p<0.005; Bonferroni t-tests, p<0.05 vs. vehicle]. Full substitution for ethanol was observed in each subject at a dose (10.0 mg/kg) that did not significantly reduce average rates of responding compared with levels following vehicle administration (Fig. 2E). Although the α1GABAA receptor PAM zolpidem also produced ethanol-lever responding that differed significantly from that engendered by vehicle [Fig. 2B; F(4,12) = 4.77, p<0.05; Bonferroni t-tests, p<0.05 vs. vehicle], over the dose range tested the average maximum percent ethanol-lever responding engendered by this drug was 59%. Administration of a higher dose (30 mg/kg) of zolpidem resulted in a lower percentage of responses made to the ethanol-paired lever and a significant reduction in average rates of responding compared with average rates of responding after vehicle administration (Fig. 2E; F(4,12) = 5.55, p<0.01; Bonferroni t-tests, p<0.05 vs. vehicle).
Effects of α2GABAA and α3GABAA Receptor-Preferring PAMs
All α2/α3GABAA receptor ligands reproduced the discriminative stimulus effects of ethanol (Fig. 2C), engendering dose-dependent increases in ethanol-lever responding that were significantly different from that engendered by vehicle [L-838, 417: F(4,12) = 14.68, p<0.001; Bonferroni t-tests, p<0.05 vs. vehicle; HZ-166: F(4,9) = 14.47, p<0.001; Bonferroni t-tests, p<0.05 vs. vehicle; YT-III-31: F(3,9) = 120.45, p<0.001; Bonferroni t-tests, p<0.05 vs. vehicle]. L-838,417 (functionally selective PAM at α2GABAA, α3GABAA, and α5GABAA receptors), HZ-166 (functionally selective PAM at α2GABAA and α3GABAA receptors) as well as YT-III-31 (functionally selective PAM at α3GABAA receptors) induced almost exclusive responding on the ethanol-paired lever over the dose ranges tested, generating 97%, 84% and 99% ethanol-lever responding, respectively. None of these compounds significantly altered average rates of responding when compared to average rates following vehicle administration (Fig. 2F).
Ethanol or Sucrose Self-Administration
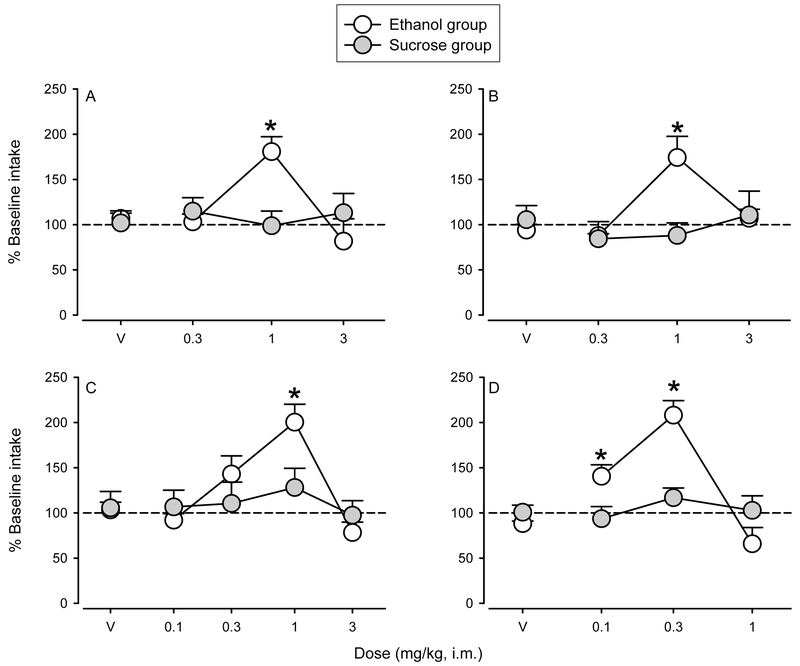
All animals reliably self-administered consistent amounts of ethanol or sucrose during the baseline periods preceding each drug test, with no significant differences in intake across the different treatment baseline periods within individuals (Table 2). These levels of intake produce BECs over 80 mg/dl (cf. Ruedi-Bettschen et al., 2013; Sawyer et al., 2014). In addition, there were no significant differences in the quantity consumed between the ethanol and sucrose groups (Table 2). The effects of pretreatments with different doses of α2GABAA and α3GABAA receptor-preferring compounds on ethanol and sucrose intake are shown in Fig. 3. Daily pretreatment with the functionally selective PAMs at α2GABAA and α3GABAA receptors XHe-II-053 [group X dose: F(3,24) = 7.20, p<0.005] and HZ-166 [group X dose: F(3,24) = 5.39, p<0.01] resulted in significant increases in ethanol intake at 1 mg/kg without affecting sucrose intake (Bonferroni t-tests, p<0.05; Fig. 3A and Fig. 3B). Pretreatments with the functionally selective α3GABAA PAMs YT-III-31 [group X dose: F(4,32) = 2.67, p<0.05] and YT-III-271 [group X dose: F(3,24) = 15.57, p<0.0001] also induced significant increases in ethanol, but not sucrose, intake compared with vehicle for the doses of 1 mg/kg and 0.1 and 0.3 mg/kg, respectively (Bonferroni t-tests, p<0.05; Fig. 3C and Fig. 3D).
Table 2.
Average baseline intake for each monkey across the duration of the study.
| Monkey | Group | Average intakea ml |
Average intakea g/kg |
|---|---|---|---|
| MM-33 | Ethanol | 801.8 (56.4) | 1.4 (0.06) |
| MM-71 | 605.5 (40.9) | 1.3 (0.05) | |
| MM-162 | 611.3 (42.6) | 1.4 (0.03) | |
| MM-267 | 952.5 (41.1) | 1.6 (0.05) | |
| MM-374 | 615.3 (32.3) | 1.4 (0.06) | |
| MM-106 | Sucrose | 1628.8 (88.0) | N/A |
| MM-167 | 912.8 (25.2) | N/A | |
| MM-201 | 799.0 (62.6) | N/A | |
| MM-354 | 1004.8 (50.22) | N/A | |
| MM-488 | 505.0 (38.3) | N/A | |
| Ethanol group total | 717.3 (69.5) | ||
| Sucrose group total | 970.1 (184.9) | ||
Values are presented as mean (SEM).
Figure 3.
Effects of positive allosteric modulators (PAMs) with functional selectivity at α2GABAA and/or α3GABAA receptors on ethanol (open symbols) or sucrose (closed symbols) drinking. The functionally selective PAMs at α2/3GABAA receptors (A) XHe-II-053 and (B) HZ-166 and at α3GABAA receptors (C) YT-III-31 and (D) YT-III-271 increase ethanol, but not sucrose, drinking. Data are expressed as percent baseline intake. Points represent the mean intake over 3 sessions ± SEM. *Indicates p<0.05 compared with respective vehicle baseline.
Observable Behavior
Of the behaviors scored, six demonstrated significant effects for at least one compound at one dose. Significant effects of pretreatments with α2GABAA and α3GABAA receptor-preferring compounds are summarized in Table 3. Notably, rest/sleep posture, a behavior associated with relatively mild sedative effects (Duke et al., 2018) was induced by all of the test compounds in the monkeys self-administering ethanol (Table 3, RM ANOVAs, F’s>18.0; note that ethanol did not induce rest/sleep posture under vehicle conditions). For most compounds, this significant effect was observed at the highest dose tested, the exception being YT-III-31, which engendered rest/sleep posture at 1.0 and 3.0 mg/kg (Bonferroni t-tests, p<0.05 vs. vehicle). In the sucrose self-administration group, increases in rest/sleep posture occurred for the highest doses tested for XHe-II-053 and YT-III-31, but not HZ-166 and YT-III-271 (RM ANOVAs F’s>7.0, Bonferroni t-tests, p’s<0.05). Because HZ-166 administration has been shown to result in rest/sleep posture at doses higher than those studied herein (Duke et al., 2018) and ethanol alone appears to lack this effect (present study, Rüedi-Bettschen et al., 2013; Sawyer et al., 2014), these data suggest that ethanol may potentiate this measure of mild sedation over the dose ranges tested.
Table 3.
Summary of drug effects on selected observable behaviors
| Behavior | Group | XHe-II-053 | HZ-166 | YT-III-31 | YT-III-271 |
|---|---|---|---|---|---|
| Rest/Sleep Posture | Ethanol | ↑ (3.0) | ↑ (3.0) | ↑ (1.0, 3.0) | ↑ (1.0) |
| Passive Visual | = | = | ↑ (3.0) | ↓ (0.1) | |
| Locomotion | = | = | ↓ (3.0) | ↑ (0.1) | |
| Forage | = | = | = | = | |
| Tactile/Oral Exploration | = | = | = | ↑ (0.1) | |
| Self-groom | = | = | = | ↑ (0.3, 1.0) | |
| Rest/Sleep Posture | Sucrose | ↑ (3.0) | = | ↑ (3.0) | = |
| Passive Visual | = | = | = | = | |
| Locomotion | = | = | ↓ (3.0) | ↑ (0.1) | |
| Forage | = | = | ↓ (3.0) | = | |
| Tactile/Oral Exploration | ↓ (0.3, 1.0, 3.0) | = | = | = | |
| Self-groom | = | = | = | = |
Direction of significant effects is indicated by ↓ (decrease), ↑ (increase), or = (no change). Doses at which these effects occurred in comparison to vehicle are indicated in parentheses and reported in milligram per kilogram.
Other behavioral effects associated with the subtype-selective compounds were altered in a less systematic fashion (Table 3). For example, YT-III-31 and YT-III-271 both altered locomotion in the ethanol and sucrose drinking groups (RM ANOVAs F’s>3.0, Bonferroni t-tests, p’s<0.05), whereas XHe-II-053 and HZ-166 were ineffective. Interestingly, YT-III-31 and YT-III-271 had opposite effects on locomotion, with YT-III-31 decreasing and YT-III-271 increasing this measure, albeit at high versus low doses respectively. Of note, YT-III-271 induced changes in a larger number of behaviors scored in the ethanol group, increasing more active behaviors such as locomotion, tactile/oral exploration and self-groom at low and intermediate doses. Other behaviors (e.g., Passive Visual, Tactile/Oral Exploration) showed similar effects, i.e., non-systematic effects for a subset (or a single) compound only (summarized in Table 3).
Discussion
In the present study, we initiated novel investigations into the contribution of α2GABAA and α3GABAA receptors to the discriminative stimulus and reinforcing effects of ethanol. The most significant findings of the present study were that: (1) functionally selective α2GABAA/α3GABAA and α3GABAA receptor PAMs share discriminative stimulus effects with ethanol; and (2) functionally selective α2GABAA/α3GABAA and α3GABAA receptor PAMs augment the reinforcing effects of ethanol at doses that have few, or no, observable sedative effects. Together, these findings indicate that α2GABAA/α3GABAA receptors play an important role in key abuse-related effects of ethanol. Because the two functionally selective α3GABAA PAMs had effects similar to the α2GABAA/α3GABAA PAMs, our findings suggest that modulation of the α3GABAA receptor subtype may be the more important contributor to the observed effects.
Compelling evidence suggests that drug effects serving as discriminative stimuli in nonhuman primates can be similar to those reported as subjective effects in humans (Schuster and Johanson, 1988). Previous studies in squirrel monkeys and cynomolgus macaques indicate that, while α1GABAA receptors may play a limited role in ethanol’s discriminative stimulus effects under some conditions (Platt et al., 2005; Helms et al., 2008), α5GABAA receptor mechanisms are more likely to be involved in the discriminative stimulus effects of ethanol and in the ethanol-like discriminative stimulus effects of benzodiazepines (Platt et al., 2005; Helms et al., 2009). Accordingly, in the present study we show that an α5GABAA, but not an α1GABAA, receptor-preferring PAM fully substituted for the discriminative stimulus effects of ethanol in rhesus monkeys. Of note, as in Platt et al., (2005), the α1GABAA-preferring PAM zolpidem partially substituted for the discriminative stimulus effects of ethanol. In our previous study in squirrel monkeys, we had shown that this partial effect was not altered by a relatively high dose of an α1GABAA-preferring antagonist (Platt et al., 2005). Although zolpidem has selectivity for α1GABAA receptors, it also binds with lower affinity to α2GABAA and α3GABAA receptors and not at all to α5GABAA receptors (Sanger, 2004), raising the possibility that zolpidem’s ethanol-like discriminative stimulus effects reflected its binding to the α2GABAA and/or α3GABAA receptor subtypes. The present findings also corroborate previous findings with ethanol self-administration in rhesus monkeys supporting different contributions of α1GABAA and α5GABAA receptor subtypes to the reinforcing effects of ethanol (Rüedi-Bettschen et al., 2013; Sawyer et al., 2014).
Perhaps most importantly, the α1-sparing compound L-838,417 also fully substituted for ethanol, whereas the nonselective benzodiazepine partial PAM MRK-696 only engendered partial substitution under our experimental conditions. Both compounds have similar levels of partial modulation at all GABAA receptor subunits, except for α1 subunits, with L-838,417 lacking efficacy at α1GABAA receptors. Thus, the results with L-838,417 confirm results of previous studies (e.g., Platt et al., 2005) that modulation of α1-containing GABAA receptors is not necessary to reproduce the discriminative stimulus effects of ethanol, and the results with MRK-696 suggest that positive modulation at α1 subunits may in fact attenuate ethanol discrimination. Furthermore, our findings showing that L-838,417 fully substituted for ethanol also suggest that α2GABAA and α3GABAA, in addition to α5GABAA receptors, can be key facilitators of the discriminative stimulus effects of ethanol. A hypothesis that corroborates, in part, human laboratory studies (Covault et al. 2004, Pierucci-Lagha et al. 2005) and is supported by the observation that the functionally selective PAM at α2GABAA and α3GABAA receptors HZ-166 and the functionally selective PAM at α3GABAA receptors YT-III-31 also fully substituted for ethanol. Together, the current and previous findings suggest a role for α3GABAA and α5GABAA, and potentially α2GABAA, receptors in the subjective effects of ethanol. Of note, though, the potency of test drugs to substitute for ethanol (Grant et al., 2000) and the role of different neurotransmitters systems, including GABA, on the discriminative stimulus effects of ethanol (Grant, 1999) have been shown to vary with ethanol training dose. Therefore, the conclusions of the present study potentially are applicable only to the training conditions used in the present study.
Convergent evidence suggests that α5GABAA receptors are involved in the reinforcing effects of ethanol, while preclinical evidence for the involvement of α1GABAA receptors in ethanol reinforcement remains equivocal (for review, see Chandler et al. 2017). Importantly, our current findings also point to a key role for α2GABAA and/or α3GABAA receptors in the reinforcing effects of ethanol, with a series of functionally selective PAMs at α2GABAA and α3GABAA receptors (HZ-166, XHe-II-053, YT-III-31, YT-III-271) enhancing ethanol intake at doses that did not alter sucrose intake. Importantly, the ethanol concentration used in the self-administration studies is on the ascending limb of the ethanol concentration-effect function (cf. Ruedi-Bettschen et al., 2013). Therefore, by increasing ethanol consumption at the dose tested in the present study, we can infer that the subtype-selective compounds shifted the ethanol dose-effect function to the left, indicating that functionally selective PAMs at α2GABAA and/or α3GABAA receptors have enhanced the potency of ethanol to function as a reinforcer. Because the novel PAMs YT-III-31 and YT-III-271 have greater intrinsic efficacy at α3GABAA than other receptors, increasing GABA currents at α3GABAA subtypes by ~900–1000%, compared with only ~200–350% increase for other subtypes (Namjoshi et al., 2013; Cook et al., 2017), there is the possibility that the former subtype is critical for the reinforcing effects of GABAA-modulating compounds, a notion that has been raised previously for the reinforcing effects of the benzodiazepine midazolam compared to cocaine (Shinday et al. 2013).
Consistent with the idea that α3GABAA, more so than α2GABAA, receptors are important for ethanol self-administration is the previous observation that deletion of α2GABAA receptors in mice does not alter ethanol self-administration compared to WT animals (Boehm et al., 2004; Dixon et al., 2012). Moreover, a study with α2 “knock-in” mice, which express functional GABAA receptors with a benzodiazepine-insensitive subunit instead of α2 subunits, demonstrated inconsistent results with respect to ethanol drinking (Blednov et al., 2011). Unfortunately, no studies have examined ethanol drinking in α3GABAA knockout mice (Mayfield et al., 2016; Chandler et al., 2017) precluding a direct test of the hypothesis. While our data are clearly suggestive of a role for α3GABAA receptor subtypes in ethanol self-administration, the lack of pharmacological tools (e.g., α2GABAA selective PAMs) and key results in knockout mice, combined with other potentially relevant factors (e.g., species differences, methodological factors), does not allow us to make firm conclusions about α2 vs. α3 subunits at this time.
Pretreatment with two of the α2GABAA and/or α3GABAA receptor ligands, XHe-II-053 and YT-III-31, increased the frequency of rest/sleep posture when administered before sucrose self-administration sessions. Those findings are in agreement with and extend previous studies showing that α2GABAA and/or α3GABAA receptors are likely involved in the sedative effects of GABAA receptor ligands (Fischer et al., 2011; Duke et al., 2018). Interestingly, all four compounds (XHe-II-053, YT-III-31, HZ-166 and YT-III-271) demonstrated rest/sleep posture in ethanol-drinking animals, in one case at a dose that was lower than that needed to engender this behavior in sucrose animals, suggesting that the effects of these subtype-selective compounds on rest/sleep posture were potentiated in ethanol-drinking animals. The increase in this form of mild sedation may have contributed to the inverted U-shaped dose-response functions observed during the pretreatment studies and ethanol self-administration (i.e., rest/sleep posture occurred at the highest doses, and this sedative effect may have contributed to drinking levels returning to baseline). With respect to the extant literature, our findings that stimulation of α2GABAA and/or α3GABAA receptors may contribute to the sedative-like effects of ethanol are in agreement with previous studies in rodents showing a reduced ethanol-induced loss of righting reflex in α2GABAA receptor knockout animals (Boehm et al. 2004), as well as a loss of righting reflex induced by the combination of subthreshold doses of the benzodiazepine diazepam and ethanol in α3GABAA receptor knock-in mice (Tauber et al. 2003).
In summary, our results provide evidence that α3GABAA receptors play key roles in the discriminative stimulus and reinforcing effects of ethanol in rhesus monkeys, with a potential role for α2GABAA receptors. These findings corroborate evidence from clinical genetic association studies indicating a positive association of both GABRA2 and GABRA3 with an increased risk for developing alcoholism across varied populations (Parsian and Cloninger, 1997; Covault et al. 2004; Edenberg et al., 2004; Pierucci-Lagha et al. 2005; Enoch et al., 2008; Syoka et al., 2008). Previously, critical involvement has been shown for the α5GABAA receptor subtype in the reinforcing (Rüedi-Bettschen et al., 2013) and discriminative stimulus effects (Platt et al., 2005; Helms et al., 2009) of ethanol in nonhuman primates, with the present study substantiating those findings. In contrast, for the α1GABAA receptor, the present study as well as the preponderance of findings from previous studies suggest a limited role for this subtype, although some primate and most rodent studies have not yielded similar results and conclusions (for review, see Chandler et al., 2017).
Acknowledgments
The authors thank Laura Teixeira, Casey Boyer, Zhiqiang Meng, and Madelynn Sirbu for their assistance with data collection. We also would like to thank the Shimadzu Analytical Laboratory of Southeastern Wisconsin.
Sources of support: This work was supported by AA016179 (DMP), DA011792 (JKR), DA033795 (JKR), DA043204 (JKR), MH096463 (JMC), NS076517 (JMC), and HL118561 (JMC).
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Atack JR. GABAA receptor subtype-selective modulators. I. α2/α3-selective agonists as non-sedating anxiolytics. Curr Top Med Chem. 2011;11: 1176–202. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Chandra D, Homanics GE, Rudolph U, Harris RA (2013) Linking GABAA receptor subunits to alcohol-induced conditioned taste aversion and recovery from acute alcohol intoxication. Neuropharmacology 67: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Borghese CM, McCracken ML, Benavidez JM, Geil CR, Osterndorff-Kahanek E, Werner DF, Iyer S, Swihart A, Harrison NL, Homanics GE, Harris RA (2011) Loss of ethanol conditioned taste aversion and motor stimulation in knockin mice with ethanol-insensitive α2-containing GABA(A) receptors. J Pharmacol Exp Ther 336: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Alva H, Creech K, Findlay G, Harris RA (2003) GABAA receptor alpha 1 and beta 2 subunit null mutant mice: behavioral responses to ethanol. J Pharmacol Exp Ther 305: 854–863. [DOI] [PubMed] [Google Scholar]
- Boehm SL 2nd, Ponomarev I, Jennings AW, Whiting PJ, Rosahl TW, Garrett EM, et al. Harris RA (2004) gamma-Aminobutyric acid A receptor subunit mutant mice: new perspectives on alcohol actions. Biochem Pharmacol 68: 1581–1602. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR (2004) Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet 129B:104–109. [DOI] [PubMed] [Google Scholar]
- Cook JM, Clayton TS, Jain HD, Johnson YT, Yang J, Rallipalli SK, Wang Z, Namjoshi OA, Poe MM (2017) GABAergic receptor subtype selective ligands and their uses. US Patent. 9,597,342 Issued March 21, 2017.
- Cook JM, Zhou H, Huang S, Srirama Sarma PVV, Zhang C (2009) Stereospecific anxiolytic and anticonvulsant agents with reduced muscle-relaxant, sedative-hypnotic and ataxic effects. US Patent. 7,618,958 B2 Issued November 17, 2009.
- Dixon CI, Walker SE, King SL, Stephens DN (2012) Deletion of the gabra2 gene results in hypersensitivity to the acute effects of ethanol but does not alter ethanol self-administration. PLoS One 7: e47135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke AN, Meng Z, Platt DM, Atack JR, Dawson GR, Reynolds DS, Tiruveedhula VVNPB, Li G, Stephen MR, Sieghart W, Cook JM, Rowlett JK (2018) Evidence that sedative effects of benzodiazepines involve unexpected GABAA receptor subtypes: Quantitative observation studies in rhesus monkeys. J Pharmacol Exp Ther 366: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI Jr, O’Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H (2004) Variations in GABRA2, encoding the alpha 2 subunit of the GABAA receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet 74: 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M (2008) The role of GABAA receptors in the development of alcoholism. Pharmacol Biochem Behav 90: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BD, Atack JR, Platt DM, Reynolds DS, Dawson GR, Rowlett JK (2011) Contribution of GABAA receptors containing α3 subunits to the therapeutic-related and side effects of benzodiazepine-type drugs in monkeys. Psychopharmacology (Berl) 215: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BD, Licata SC, Edwankar RV, Wang ZJ, Huang S, He X, Yu J, Zhou H, Johnson EM Jr, Cook JM, Furtmüller R, Ramerstorfer J, Sieghart W, Roth BL, Majumder S, Rowlett JK (2010) Anxiolytic-like effects of 8-acetylene imidazobenzodiazepines in a rhesus monkey conflict procedure. Neuropharmacology 59: 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BD, Teixeira LP, van Linn ML, Namjoshi OA, Cook JM, Rowlett JK (2013) Role of gamma-aminobutyric acid type A (GABAA) receptor subtypes in acute benzodiazepine physical dependence-like effects: evidence from squirrel monkeys responding under a schedule of food presentation. Psychopharmacology (Berl) 227: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA (2004) Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Ther 311: 1071–1079. [DOI] [PubMed] [Google Scholar]
- Gallegos RA, Lee RS, Criado JR, Henriksen SJ, Steffensen SC (1999) Adaptive responses of gamma-aminobutyric acid neurons in the ventral tegmental area to chronic ethanol. J Pharmacol Exp Ther 291: 1045–1053. [PubMed] [Google Scholar]
- Grant KA (1999) Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav 64: 261–267. [DOI] [PubMed] [Google Scholar]
- Grant KA, Waters CA, Green-Jordan K, Azarov A, Szeliga KT (2000) Characterization of the discriminative stimulus effects of GABAA receptor ligands in Macaca fascicularis monkeys under different ethanol training conditions. Psychopharmacology 152: 181–188. [DOI] [PubMed] [Google Scholar]
- Helms CM, Grant KA (2011) The effect of age on the discriminative stimulus effects of ethanol and its GABAA receptor mediation in cynomolgus monkeys. Psychopharmacology (Berl) 216: 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Rogers LS, Grant KA (2008) Gamma-hydroxybutyric acid in male and female cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol. Behav Pharmacol 19: 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Rogers LS, Grant KA (2009) Antagonism of the ethanol-like discriminative stimulus effects of ethanol, pentobarbital, and midazolam in cynomolgus monkeys reveals involvement of specific GABAA receptor subtypes. J Pharmacol Exp Ther 331: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, He X, Ma C, Liu R, Yu S, Dayer CA, Wenger GR, McKernan R, Cook JM (2000) Pharmacophore/receptor models for GABAA/BzR subtypes (α1β3γ2, γ5γ3γ2, and α6β3γ2) via a comprehensive ligand mapping approach. J Med Chem 43: 71–95. [DOI] [PubMed] [Google Scholar]
- Huang Q, He X, Ma C, Liu R, Yu S, Dayer CA, Wenger GR, McKernan R, Cook JM (2000) Pharmacophore/receptor models for GABAA/BzR subtypes (alpha1beta3gamma2, alpha5beta3gamma2, and alpha6beta3gamma2) via a comprehensive ligand-mapping approach. J Med Chem 43: 71–95. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Thuras P, Hanson KL, Brekke M, Sletten S (2005) Follow-up study of anxiety disorder and alcohol dependence in comorbid alcoholism treatment patients. Alcohol Clin Exp Res 29: 1432–1443. [DOI] [PubMed] [Google Scholar]
- Licata SC, Platt DM, Rüedi-Bettschen D, Atack JR, Dawson GR, Van Linn ML, Cook JM, Rowlett JK (2010) Discriminative stimulus effects of L-838,417 (7-tert-butyl-3-(2,5-difluoro-phenyl)-6-(2-methyl-2H-[1,2,4]triazol-3-ylmethoxy)-[1,2,4]triazolo[4,3-b]pyridazine): role of GABA(A) receptor subtypes. Neuropharmacology 58: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ (2017) Emerging pharmacotherapies for alcohol use disorder. Neuropharmacology 122: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J, Arends MA, Harris RA, Blednov YA (2016) Genes and alcohol consumption: Studies with mutant mice. Int Rev Neurobiol 126: 293–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Matsushita N, Kobayashi K, Kobayashi K (2004) Identification of GABAA receptor subunit variants in midbrain dopaminergic neurons. J Neurochem 89: 7–14. [DOI] [PubMed] [Google Scholar]
- Namjoshi OA, Wang ZJ, Rallapalli SK, Johnson EM Jr., Johnson YT, Ng H, Ramerstorfer J, Varagic Z, Sieghart W, Majumder S, Roth BL, Rowlett JK, Cook JM (2013). Search for α3/α2 subtype selective ligands that are stable on human liver microsomes. Bioorganic & Medicinal Chemistry 21: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W (2008) International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev 60: 243–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsian A, Cloninger CR (1997) Human GABA receptor α1 and α3 subunits genes and alcoholism. Alcohol Clin Exp Res 21:430–433. [DOI] [PubMed] [Google Scholar]
- Platt DM, Duggan A, Spealman RD, Cook JM, Li X, Yin W, Rowlett JK (2005) Contribution of alpha 1GABAA and alpha 5GABAA receptor subtypes to the discriminative stimulus effects of ethanol in squirrel monkeys. J Pharmacol Exp Ther 313: 658–667. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR (2005) GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology 30:1193–1203. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR (2005) Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci U S A 102: 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Möhler H (2004) Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol 44: 475–498. [DOI] [PubMed] [Google Scholar]
- Rüedi-Bettschen D, Rowlett JK, Rallapalli S, Clayton T, Cook JM, Platt DM (2013) Modulation of α5 subunit-containing GABAA receptors alters alcohol drinking by rhesus monkeys. Alcohol Clin Exp Res 37: 624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger DJ (2004) The pharmacology and mechanisms of action of new generation, non-benzodiazepine hypnotic agents. CNS Drugs 18 (S1): 9–15. [DOI] [PubMed] [Google Scholar]
- Sawyer EK, Moran C, Sirbu MH, Szafir M, Van Linn M, Namjoshi O, Phani Babu Tiruveedhula VV, Cook JM, Platt DM (2014) Little evidence of a role for the α1GABAA subunit-containing receptor in a rhesus monkey model of alcohol drinking. Alcohol Clin Exp Res 38: 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CR, Johanson CE (1988) Relationship between the discriminative stimulus properties and subjective effects of drugs. Psychopharmacol Ser 4: 161–175. [DOI] [PubMed] [Google Scholar]
- Shinday NM, Sawyer EK, Fischer BD, Platt DM, Licata SC, Atack JR, Dawson GR, Reynolds DS, Rowlett JK (2013) Reinforcing effects of compounds lacking intrinsic efficacy at α1 subunit-containing GABAA receptor subtypes in midazolam- but not cocaine-experienced rhesus monkeys. Neuropsychopharmacology 38: 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Sperk G (2002) Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem 2: 795–816. [DOI] [PubMed] [Google Scholar]
- Soyka M, Preuss UW, Hesselbrock V, Zill P, Koller G, Bondy B (2008) GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. J Psychiatr Res 42: 184–191. [DOI] [PubMed] [Google Scholar]
- Stephens DN, King SL, Lambert JJ, Belelli D, Duka T (2017) GABAA receptor subtype involvement in addictive behaviour. Genes Brain Behav 16: 149–184. [DOI] [PubMed] [Google Scholar]
- Sur C, Wafford KA, Reynolds DS, Hadingham KL, Bromidge F, Macaulay A, Collinson N, O’Meara G, Howell O, Newman R, Myers J, Atack JR, Dawson GR, McKernan RM, Whiting PJ, Rosahl TW (2001) Loss of the major GABAA receptor subtype in the brain is not lethal in mice. J Neurosci 21: 3409–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Täuber M, Calame-Droz E, Prut L, Rudolph U, Crestani F (2003) alpha2-gamma-Aminobutyric acid (GABA)A receptors are the molecular substrates mediating precipitation of narcosis but not of sedation by the combined use of diazepam and alcohol in vivo. Eur J Neurosci 18: 2599–2604. [DOI] [PubMed] [Google Scholar]
- Vallender EJ, Rüedi-Bettschen D, Miller GM, Platt DM (2010) A pharmacogenetic model of naltrexone-induced attenuation of alcohol consumption in rhesus monkeys. Drug Alcohol Depend 109: 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA (1999) Molecular and functional diversity of the expanding GABAA receptor gene family. Ann NY Acad Sci 868: 645–653 [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2015. Alcohol. [Google Scholar]