Abstract
Antibody–drug conjugates are monoclonal antibodies conjugated to cytotoxic agents. They use antibodies that are specific to tumour cell-surface proteins and, thus, have tumour specificity and potency not achievable with traditional drugs. Design of effective antibody–drug conjugates for cancer therapy requires selection of an appropriate target, a monoclonal antibody against the target, potent cytotoxic effector molecules, and conjugation of the monoclonal antibody to cytotoxic agents. Substantial advances in all these aspects in the past decade have resulted in regulatory approval of ado-trastuzumab emtansine and brentuximab vedotin for clinical use. Several promising antibody–drug conjugates are now in late-phase clinical testing. Ongoing efforts are focused on identifying better targets, more effective cytotoxic payloads, and further improvements in antibody–drug linker technology. Improved understanding of the mechanistic basis of antibody–drug conjugate activity will enable design of rational combination therapies with other agents, including immunotherapy.
Introduction
Most monoclonal antibodies by themselves have little antitumour activity, even after binding to the target antigen. Some notable exceptions include monoclonal antibodies to HER2, EGFR, and CD20, which have remarkable activity against tumours expressing these antigens. However, despite scant antitumour activity of monoclonal antibodies, their specificity for the target antigen makes them useful cancer therapeutic agents. Antitumour activity has been accomplished by conjugating antibodies with different effector molecules that accomplish cell death after antibody binding and internalisation. Such effector molecules include cytotoxic agents, bacterial or plant protein toxins (immunotoxins), and radiopharmaceutical agents.
Immunotoxins are recombinant proteins consisting of an antibody or antibody fragment targeting the tumour antigen, linked to protein toxins such as diphtheria toxin or pseudomonas exotoxin A.1 Up to now, the only immunotoxin approved by the US Food and Drug Administration (FDA) is denileukin diftitox for treatment of CD25-positive cutaneous T-cell lymphoma.2 Another immunotoxin, moxetumomab pasudotox, targeting CD22 has shown substantial activity in patients with hairy cell leukaemia and is now being assessed in a multicentre trial in patients with relapsed or refractory hairy cell leukaemia (ClinicalTrials.gov number, NCT01829711).3 In the case of solid tumours, immunotoxins have been less effective mainly because they induce an immune response restricting their activity. However, major tumour regressions were reported with an anti-mesothelin immunotoxin, SS1P, in patients with treatmentrefractory mesothelioma when it was given in combination with pentostatin and cyclophosphamide.4 Advances in developing immuno toxins that are inherently less immunogenic show promise in preclinical studies and are now being evaluated in the clinic,5 but are outside the scope of this Review.
Antibody–drug conjugates make use of antibodies that are specific to tumour cell-surface proteins6 and have tumour specificity and potency not achievable with traditional drugs7,8 (figure 1). Although the idea of linking drugs to tumour-targeted antibodies was clear, development of therapeutic antibody–drug conjugates needed several technological advancements (figure 2). Early antibody–drug conjugates were mouse monoclonal antibodies covalently linked to anticancer drugs such as doxorubicin, vinblastine, and methotrexate. These conjugates had little success in clinical trials because of immunogenicity, scant potency, suboptimum target selection, and insufficient selectivity for tumour versus normal tissue. The lessons from these early efforts led to improvements in technology and renewed interest in antibody–drug conjugates.9 Replacing murine antibodies with humanised or fully human antibodies prevented immunogenicity. Potency was improved by using drugs that were 100–1000 times more potent. Careful target and antibody selection improved selectivity and efficiency of internalisation.
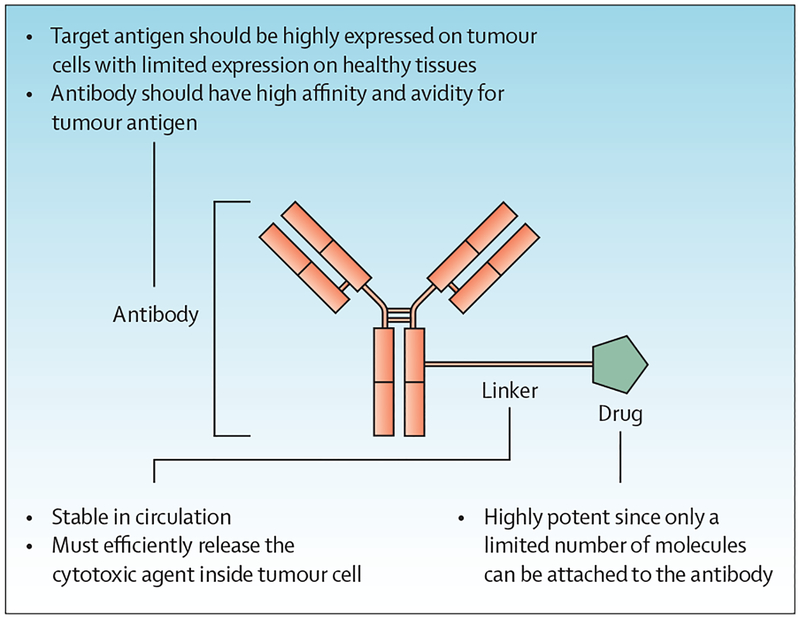
Figure 1: Structure of an antibody–drug conjugate.
An antibody–drug conjugate consists of a monoclonal antibody conjugated to a cytotoxic agent via a linker. The antibody is specific to tumour cell surface proteins, thereby providing the specificity and potency not achievable with traditional drugs. The linker is the short chemical spacer that binds the drug to the antibody, which must be stable in circulation. In the cell, most linkers are labile; however, some are stable, requiring degradation of the antibody and linker to release the cytotoxic agent. The cytotoxic drug used in antibody–drug conjugates is usually highly potent, with IC50 values in the subnanomolar range in cell culture.
Figure 2: Contrast between early-generation and new-generation antibody–drug conjugates.
Early antibody–drug conjugates (left) were mouse monoclonal antibodies linked covalently to anticancer drugs such as doxorubicin, vinblastine, and methotrexate, and had several limitations. Technological advances have enabled design of antibody–drug conjugates that comprise humanised antibodies (right), which are less immunogenic than earlier antibody–drug conjugates, with several favourable pharmacokinetic properties. IC50=concentration needed to achieve 50% inhibition. DM1=emtansine. DM4=ravtansine. MMAE=monomethyl auristatin E. MMAF=monomethyl auristatin F.
As a result of this work, gemtuzumab ozogamicin was granted accelerated FDA approval for the treatment of acute myeloid leukaemia in 2000; however, this conjugate was withdrawn from the market in 2010 because it failed to meet efficacy targets in post-marketing clinical trials.10,11 Two antibody–drug conjugates have since achieved FDA approval: trastuzumab emtansine was approved in 2013 for the treatment of metastatic breast cancer, and brentuximab vedotin was approved in 2011 for the treatment of refractory Hodgkin’s lymphoma and systemic anaplastic large-cell lymphoma. More than 40 antibody–drug conjugates are now in or nearing clinical trials.
In this Review, we discuss the key considerations in the development of antibody–drug conjugates—including target selection, cytotoxic payload, linkers, and conjugation—and antibody–drug conjugates currently in clinical use and in development.
Development of effective antibody–drug conjugates
There are important considerations to be made in antibody–drug conjugate design (table 1). A major consideration is the selection of the target. Arguably, the target is the most important contributor to antitumour activity and tolerability of an antibody–drug conjugate. Targets for antibody–drug conjugates can be present either on tumour cells, tumour-associated cells (eg, tumour endothelial cells), or in the tumour microenvironment. The target antigen should be expressed preferentially on the surface of a tumour compared with normal cells.12–16 For example, the antigens targeted by antibody–drug conjugates approved for use by regulatory agencies are expressed strongly on tumour cells, but have low expression elsewhere. Uniformly high expression of CD30 is seen in anaplastic large-cell lymphoma, whereas amplification and overexpression of HER2 happens in about 15–20% of breast cancers. In addition to differential expression on cancer cells, antibody–drug conjugate targets must have an extracellular epitope amenable to specific antibody binding and be capable of internalisation into target cells where the drug can be released.
Table 1:
Important considerations in antibody–drug conjugate design
| Description | Importance | |
|---|---|---|
| Target expression | Tumour-specific antigen against which the antibody is directed | Target should be expressed preferentially on tumour cells over non-malignant cells; high-level expression is advantageous, although not necessary |
| Target internalisation | Intracellular trafficking of the target antigen and the bound antibody-drug conjugate via receptor-mediated endocytosis | Rate and extent of internalisation affects drug uptake and release in tumour and non-malignant cells |
| Linker stability | Covalent coupling of the cytotoxic drug to the antibody | Stable linkers prevent premature release of cytotoxic drug to non-target tissue; to maximise drug exposure in tumour tissue and minimise toxic effects, drug release should happen intracellularly |
| Conjugation | Specific method of attachment of the cytotoxic drug and linker to the antibody | The method of conjugation, number of drugs per antibody (drug: antibody ratio), and drug position have an effect on the physical properties of the antibody-drug conjugate, thereby affecting aggregation, antigen binding, and clearance of the conjugate from the circulation |
| Cytotoxic payload | Type of cytotoxic drug that is conjugated to the antibody | The mechanism of action and potency of cytotoxic agents has implications in terms of frequency of dosing and toxic effects |
Targets of antibody–drug conjugates are mostly unmutated proteins; however, as mutations in cancer cell-surface proteins are discovered, development of antibody–drug conjugates with greater selectivity might be possible. Technologies for antibody discovery, including phage display libraries and humanised mice, can produce fully human antibodies, and mouse antibody humanisation can result in highly specific non-immunogenic antibodies. The antibody–drug conjugate–target complex must also internalise into the target cells where the drug can be released.
Most drugs used in antibody–drug conjugates are highly potent cytotoxic agents targeting tubulin or DNA, with IC50 values (ie, the concentration needed to achieve 50% inhibition) in the subnanomolar range in cell culture. Although antibody–drug conjugates are highly selective, only a small fraction of the drug reaches the intracellular target.
Maytansinoid and dolastatin analogues target tubulin and fragment microtubules.17 Dolastatin 10, the parent molecule of the auristatins, did not progress past a phase 2 trial in patients with prostate cancer because of toxic effects on non-malignant tissue that prevented dose escalation.18 Maytansine was assessed in clinical trials in the late 1970s but results of phase 2 clinical trials were disappointing, with little evidence of response.19
Duocarmycins, pyrrolobenzodiazepines and calicheamicins are agents that target the minor groove of DNA causing irreversible alkylation leading to cell death. Duocarmycins are antibiotics that alkylate DNA in A–T-rich regions of the double-helix minor groove; these agents were assessed in clinical trials and dose-limiting toxic effects arose at doses too low to achieve antitumour activity.20,21 Calicheamicins bind in the DNA minor groove and induce double-strand breaks but have narrow therapeutic indices and serious late toxic effects.22,23 Amatoxin analogues are cytotoxic cyclopeptides that inhibit RNA polymerase II and III, and SN-38 targets topoisomerase I, resulting in breaks in double-strand DNA.24 SN-38, the active metabolite of irinotecan, is poorly bioavailable and has a narrow therapeutic index.25 Amatoxins are produced by poisonous mushrooms, and toxic effects on non-malignant tissue precluded their clinical investigation as small molecules.26 All these molecules are highly potent but are non-tumour selective.
In addition to potency, several other properties of the cytotoxic payload—including molecular structure, chemical structure, and sensitivity to drug-resistance mechanisms—are key determinants of the safety and clinical activity of antibody–drug conjugates. The molecular structure of the cytotoxic payload should allow conjugation to the linker. Sufficient water solubility and prolonged stability in blood are important, because antibody–drug conjugates are prepared in aqueous solution and administered intravenously. Furthermore, antibody–drug conjugates are typically scheduled in a similar way to cytotoxic chemotherapy, with dosing typically once every 3 weeks.27–29
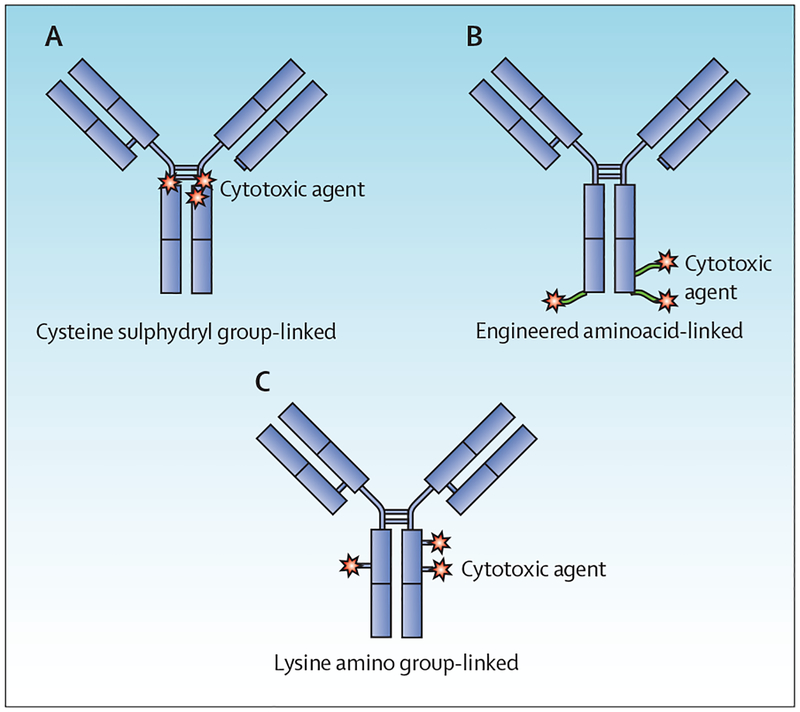
The linker connects the cytotoxic payload to the monoclonal antibody and maintains the antibody–drug conjugate in a fairly stable state in circulation. Commonly, linkers react with lysine sidechains on the antibody or with sulphydryl groups in the hinge regions of the antibody (figure 3). Most linkers are labile in the intracellular environment, resulting in the release of the cytotoxic payload after internalisation.30,31 For example, acid-labile hydrazones are degraded in the lysosome under low pH conditions, whereas disulphide linkers are cleaved selectively in the reductive cytosolic cellular milieu. Other linkers need intracellular enzymatic cleavage for the release of the cytotoxic payload. For example, peptide linkers—such as citrulline–valine—are degraded by lysosomal proteases. Non-cleavable thioether linkers depend on degradation of the antibody in the lysosome for release of the cytotoxic payload. Linkers with polyethylene glycol spacers increase solubility of antibody–drug conjugates.32,33 A stable linker minimises non-specific systemic release of the cytotoxic drug and is, therefore, an important determinant of antibody–drug conjugate safety.
Figure 3: Ways to link drugs in antibody–drug conjugates.
(A) Drug is linked through intra-strand sulphydryl linkages. (B) Drug is linked through genetically engineered unnatural aminoacids to provide specific binding sites and, thus, one chemical species. (C) Drug is linked through the epsilon amino groups of lysine.
Drug-loading stoichiometry and molecular homogeneity are important determinants of the safety and efficacy of antibody–drug conjugates. The manufacturing goal is for conjugates to be one chemical species. An antibody that is under-conjugated cuts the potency of the antibody–drug conjugate, and one that is highly conjugated decreases the circulating half-life and impairs binding of the antibody–drug conjugate to the target, reducing its potency and efficacy.34 Usually, three or four drug molecules per antibody molecule is optimum. Site-specific conjugation approaches are being investigated to achieve antibody–drug conjugates that are one chemical species. Antibodies with non-native aminoacid linker sites incorporated site-specifically can be produced efficiently.35
Antibody–drug conjugates in the clinic
Currently, two antibody–drug conjugates have been approved for use in the USA and Europe: brentuximab vedotin and ado-trastuzumab emtansine. In general, these antibody–drug conjugates are well tolerated, with toxic effects consistent with the known mechanism of action of the cytotoxic payloads—eg, neutropenia and neuropathy with brentuximab vedotin, and rises in hepatic aminotransferase levels with ado-trastuzumab emtansine. However, the exact mechanisms of toxic effects that are attributable to the antibody–drug conjugates are complex, with contributions from every component of the conjugate—ie, the monoclonal antibody, the linker, and the cytotoxic payload. Mechanisms include non-specific systemic release of the cytotoxic drug because of premature lysis of the linker, and internalisation of the antibody–drug conjugate by cells not expressing the target. For example, thrombo cytopenia, a common toxic effect of ado-trastuzumab emtansine, is thought to be due to the internalisation of the antibody–drug conjugate by megakaryocyte precursor cells via an Fc receptor-mediated process, after which the cytotoxic DM1 component of ado-trastuzumab emtansine impedes differentiation to mature megakaryocytes and subsequent platelet formation.36 Toxic effects can also result from a bystander effect, which refers to non-selective cytotoxicity of target-negative cells that are located in close proximity to target-positive cells.
Brentuximab vedotin
In 2011, brentuximab vedotin became the first antibody–drug conjugate to be approved by the FDA under its accelerated approval regulations.37 This agent is a CD30-directed antibody–drug conjugate consisting of the chimeric anti-CD30 IgG1 antibody, the micro tubuledisrupting agent monomethyl auristatin E, and a protease-cleavable linker that attaches the cytotoxic agent covalently to the antibody. After binding CD30, the antibody–drug conjugate is internalised rapidly and transported to lysosomes, where the peptide linker is cleaved selectively. Monomethyl auristatin E is then released into the cell, binds tubulin, and prompts arrest of the cell cycle between the gap 2 phase and mitosis, causing cell apoptosis.38 Brentuximab vedotin is approved for patients with relapsed or refractory CD30-positive Hodgkin’s lymphoma after autologous stem-cell transplantation (ASCT) or after at least two previous therapies when ASCT or multiagent chemotherapy is not a treatment option. Moreover, brentuximab vedotin is approved for use in patients with relapsed or refractory systemic anaplastic large-cell lymphoma. It is given intravenously at a dose of 1·8 mg/kg over 30 min every 3 weeks. Approval of brentuximab vedotin for treatment of these two disorders was based on data from two single-arm phase 2 trials.39,40 In 77 patients with Hodgkin’s lymphoma who relapsed after ASCT and were subsequently treated with brentuximab vedotin,40 the number of patients who achieved an objective response was 58 (75%; 95% CI 65–83) and the number of patients with a complete response was 26 (34%; 25–44). The median duration of the objective response was 6·7 months and 20·5 months for those 58 patients who achieved a complete response. For 58 patients with relapsed or refractory anaplastic large-cell lymphoma treated with brentuximab vedotin,39 the number of patients achieving an objective response was 50 (86%; 95% CI 77–95), and 33 achieved a complete response (57%; 44–70). The median duration of the objective response was 12·6 months (95% CI 5·7–not estimable [NE]) and 13·2 months (10·8–NE) for those 50 patients who achieved a complete response. Among all patients treated in both trials, the most common adverse events were neutropenia, peripheral sensory neuropathy, fatigue, nausea, anaemia, upper respiratory infection, diarrhoea, pyrexia, rash, thrombocytopenia, cough, and vomiting.41 Important serious adverse reactions reported were Stevens–Johnson syndrome, tumour lysis syndrome, and progressive multifocal leuko enceph alopathy. Phase 3 trials of brentuximab vedotin as monotherapy and in combination with chemotherapeutic agents are ongoing in patients with CD30-positive cutaneous T-cell lymphoma ( NCT01578499), Hodgkin’s lymphoma ( NCT02166463), and CD30-positive mature T-cell lymphoma ( NCT01777152).
Ado-trastuzumab emtansine
Ado-trastuzumab emtansine is an antibody–drug conjugate composed of trastuzumab and DM1, a maytansine derivative that is conjugated covalently to the antibody via a stable thioether linker.42 On binding to HER2, ado-trastuzumab emtansine undergoes receptor-mediated internalisation and subsequent proteolytic digestion, releasing the cytotoxic anti-microtubule agent within the target cells. Furthermore, it blocks HER2-mediated signal transduction, facilitates antibodydependent cell-mediated cytotoxicity, and inhibits shedding of the HER2 extracellular domain.43 Ado-trastuzumab emtansine was approved in 2013 as a single agent for the treatment of patients with HER2-positive metastatic breast cancer who had previously received trastuzumab and a taxane, separately or in combination. The recom mended dose of ado-trastuzumab emtansine is 3·6 mg/kg, administered as an intravenous infusion every 3 weeks.44
Approval of ado-trastuzumab emtansine was based on results of a phase 3 trial in 991 patients with HER2-positive metastatic breast cancer.45 In that trial, patients were randomly allocated either ado-trastuzumab emtansine (n=495) or lapatinib plus capecitabine (n=496). The co-primary endpoints were progression-free survival and overall survival. Significant improvements in these endpoints were recorded in patients assigned ado-trastuzumab emtansine compared with those allocated lapatinib plus capecitabine. Median progression-free survival was 9·6 months in the ado-trastuzumab emtansine group, and 6·4 months for the lapatinib plus capecitabine group (hazard ratio [HR] 0·65, 95% CI 0·55–0·77; p<0·001). Median overall survival was 30·9 months and 25·1 months, in the ado-trastuzumab emtansine group and the lapatinib plus capecitabine group, respectively (0·68, 0·55–0·85; p<0·001). The most common adverse drug reactions were fatigue, nausea, musculoskeletal pain, thrombocytopenia, headache, increased aminotransferase levels, and constipation. Adverse events occurring more frequently in the ado-trastuzumab emtansine group than in the lapatinib plus capecitabine group included thrombo-cytopenia (31% vs 3%), constipation (27% vs 11%), increased amino transferase levels (29% vs 14%), headache (28% vs 15%), epistaxis (23% vs 8%), arthralgia (19% vs 8%), pyrexia (19% vs 8%), dry mouth (17% vs 5%), and myalgia (14% vs 4%). Rare but serious adverse events included hepatotoxicity, which can potentially lead to liver failure, and reduced left-ventricular ejection fraction.
Ado-trastuzumab emtansine was also assessed in 404 patients with advanced HER2-positive breast cancer who had been treated previously with trastuzumab-based and lapatinib-based therapy and a taxane.46 In this phase 3 trial, progression-free survival was improved significantly with ado-trastuzumab emtansine compared with clinician’s choice (median 6·2 months [95% CI 5·6–6·9] vs 3·3 months [2·9–4·1]; stratified HR 0·53, 95% CI 0·42–0·66; p<0·0001). Findings of an interim analysis of this trial on overall survival favoured ado-trastuzumab emtansine (HR 0·55, 95% CI 0·37–0·83; p=0·0034). A phase 3 trial comparing ado-trastuzumab emtansine monotherapy with trastuzumab plus docetaxel in first-line treatment of HER2-positive metastatic breast cancer is ongoing ( NCT02144012). Additional phase 3 clinical trials are ongoing in the adjuvant setting in breast cancer ( NCT01772472), and in advanced HER2-positive gastric cancer ( NCT01641939).
Selected antibody–drug conjugates in clinical development
More than 40 antibody–drug conjugates are in clinical development for various haematological malignant diseases and solid tumours. Table 2 summarises selected ongoing trials of antibody–drug conjugates, the targets used, cytotoxic payloads, areas of focus for clinical development, and stages of development. We provide a brief overview of three of these antibody–drug conjugates that are in late-phase clinical trials: anetumab ravtansine, inotuzumab ozogamicin, and sacituzumab govitecan. Moreover, we discuss two promising conjugates that are in early phases of clinical testing: rovalpituzumab tesirine and glembatumumab vedotin.
Table 2:
Selected antibody–drug conjugates in clinical development
| Target | Cytotoxic payload | Main indication | Phase |
ClinicalTrials.gov identifier |
|
|---|---|---|---|---|---|
| Sacituzumab govitecan (IMMU-132)47 | TROP2 | SN-38 | Triple-negative breast cancer | 3 | NCT02574455; NCT02161679 |
| Inotuzumab ozogamicin (CMC-544)48 | CD22 | Calicheamicin | Acute lymphoblastic leukaemia | 3 | NCT01564784 |
| Anetumab ravtansine (BAY 94–9343)49 | Mesothelin | DM4 | Mesothelioma | 2 | NCT02610140 |
| Gemtuzumab ozogamicin50 | CD33 | Calicheamicin | Acute myeloid leukaemia, acute promyelocytic leukaemia | 2 | NCT01409161; NCT01869803 |
| Depatuxizumab mafodotin (ABT-414)51 | EGFR | MMAF | Glioblastoma | 2 | NCT02573324; NCT02343406 |
| Glembatumumab vedotin (CDX-011)52 | GPNMB | MMAE | Osteosarcoma, melanoma, triple-negative breast cancer | 2 | NCT02487979; NCT02302339 |
| Denintuzumab mafodotin (SGN-CD19A) | CD19 | MMAF | Diffuse large B-cell lymphoma | 2 | NCT02592876 |
| Mirvetuximab soravtansine (IMGN-853)53 | Folate receptor α | DM4 | Folate receptor α-positive epithelial ovarian cancer | 2 | NCT02631876 |
| AGS-16C3F54 | ENPP3 | MMAF | Renal cell carcinoma | 2 | NCT02639182 |
| Rovalpituzumab tesirine (Rova-T; SC16LD6.5)55,56 | DLL3 | Pyrrolobenzodiazepine | Small-cell lung cancer | 1/2 | NCT01901653 |
| Labetuzumab govitecan (IMMU-130)57 | CEACAM5 | SN-38 | Colorectal cancer, epithelial cancers | 1/2 | NCT01605318 |
| BMS-986148 | Mesothelin | ND | Mesothelin-expressing cancers | 1/2 | NCT02341625 |
| Humax-TF-ADC | Tissue factor | MMAE | Tissue factor expressing tumours | 1/2 | NCT02001623 |
| Vadastuximab talirine (SGN-CD33A) | CD33 | Pyrrolobenzodiazepine | Acute myeloid leukaemia | 1/2 | NCT02614560 |
| TAK-264 (MLN-0264)58 | Guanylyl cyclase C | MMAE | Gastrointestinal cancers | 1/2 | NCT02391038 |
| Milatuzumab-doxorubicin (CD74-DOX) | CD74 | Doxorubicin | Chronic lymphocytic leukaemia, non-Hodgkin lymphoma, multiple myeloma | 1/2 | NCT01101594 |
SN-38=active metabolite of irinotecan. DM4=ravtansine. MMAF=monomethyl auristatin F. MMAE=monomethyl auristatin E. ND=not disclosed.
Anetumab ravtansine is an antibody–drug conjugate that targets the tumour differentiation antigen mesothelin. Mesothelin is highly expressed in several malignant diseases, including epitheloid mesotheliomas, pancreatic cancer, biliary adenocarcinomas, gastric and ovarian cancers, and non-small-cell lung cancer.59 Because expression of mesothelin in healthy human tissue is restricted to mesothelial cells lining the pleura, peritoneum, and pericardium, this target is attractive for antibody–drug conjugate therapy. Anetumab ravtansine is composed of the human anti-mesothelin monoclonal antibody BAY 86–1903 conjugated to the tubulin inhibitor DM4 by a disulphide linker.49 It binds to human mesothelin with high affinity and selectivity, thereby inducing efficient antigen internalisation.
Findings of preclinical studies of anetumab ravtansine showed potent and selective killing of mesothelin-expressing tumours, with a correlation noted between the amount of mesothelin expression and antitumour activity.49 Moreover, anetumab ravtansine induced a bystander effect on neighbouring mesothelin-negative tumour cells. In the phase 1 clinical trial,60 which included tumour types with known high expression of mesothelin, the maximum tolerated dose was defined as 6·5 mg/kg, administered intravenously every 3 weeks. Dose-limiting toxic effects were keratitis and neuropathy. Preliminary results suggest clinical activity, with partial responses reported in seven (18%) of 38 patients treated at the maximum tolerated dose.60 Phase 2 investigations are ongoing in mesothelin-expressing cancers (eg, NCT02610140).
Inotuzumab ozogamicin is composed of an anti-CD22 monoclonal antibody attached covalently to calicheamicin, a cytotoxic antibiotic.61,62 CD22 is a B-cell lineage-restricted type I transmembrane protein and a member of the SIGLEC (sialic acid-binding immuno globulin-like lectins) family of cell-surface receptors. CD22 interacts with diverse sialic acid-bearing molecules present on various cell types—eg, B cells and T cells, neutrophils, and monocytes—to regulate signal transduction of surface immunoglobulin receptors on B cells, B-cell migration, and maintenance of peripheral B-cell tolerance. CD22 is expressed in most B-lymphoid malignant diseases, including non-Hodgkin lymphoma, chronic lymphocytic leukaemia, and acute lymphocytic leukaemia.63 Inotuzumab ozogamicin has subnanomolar binding affinity and is internalised rapidly to deliver the calecheamicin payload intracellularly.
Findings of phase 1 studies of inotuzumab ozogamicin showed encouraging activity in indolent and aggressive lymphoma.64 The recommended phase 2 dose was 1·8 mg/m2, administered intravenously every 3–4 weeks. Thrombocytopenia was a dose-limiting toxic effect. Abnormalities in liver function were reported but were generally mild to moderate and reversible. In a phase 2 trial, nine (18%) of 49 patients with refractory and relapsed acute lymphocytic leukaemia had complete responses and 19 (39%) patients had complete responses in bone marrow.65 Final results of a phase 3 trial comparing inotuzumab ozogamicin with a pre defined investigator’s choice in patients with relapsed or refractory CD22-positive acute lymphoblastic leukaemia are awaited ( NCT01564784).
Sacituzumab govitecan is an antibody–drug conjugate that is potent at low nanomolar concentrations and active in various human epithelial tumour xenografts at non-toxic doses.47 It targets trophoblast antigen 2 (TROP2; also known as TACSTD2), a transmembrane glycoprotein that is usually expressed in trophoblasts—cells that can invade uterine decidua during the process of placental implantation.66 It is also expressed in healthy tissues, including the epidermis, exocervix, oesophagus, tongue, urothelium, kidney, pancreas, and breast. TROP2 is overexpressed in many epithelial cancers—including breast, cervical, and colorectal— and in haematological malignant diseases such as extranodal nasal-type lymphoma and non-Hodgkin lymphoma. Unlike currently approved antibody–drug conjugates, which contain highly potent cytotoxic drugs, the cytotoxic payload in sacituzumab govitecan is SN-38, a topoisomerase I inhibitor that is the active metabolite of irinotecan. The linker CL2A lends intermediate conjugate stability in serum, is attached to the hydroxyl group on the lactone ring of SN-38, and contains a short polyethylene glycol moiety to enhance solubility. In the low pH environment of lysosomes, and in the tumour microenvironment, the carbonate bond between the linker and SN-38 is cleaved, releasing the active form of SN-38.
In a phase 1 trial of sacituzumab govitecan, in which patients with various solid tumours were not selected by TROP2 expression, the maximum tolerated dose was 12 mg/kg, administered intravenously on days 1 and 8 of a 21-day cycle.25 The dose-limiting toxic effect was neutropenia. Although the maximum tolerated dose was 12 mg/kg, doses of 8·0 mg/kg and 10·0 mg/kg were selected for further expansion, because patients were most likely to tolerate additional cycles at these levels with minimum supportive care, and responses were recorded at these levels. Findings of phase 1/2 trials of sacituzumab govitecan have shown promising antitumour activity in patients with triple-negative breast cancer and in those with platinum-resistant urothelial carcinoma.67,68 Interim results in 34 patients with refractory or relapsed triple-negative breast cancer showed an objective response in seven (21%).68 A phase 3 trial is planned in patients with refractory or relapsed triple-negative breast cancer ( NCT02574455).
Rovalpituzumab tesirine is an antibody–drug conjugate that targets delta-like 3 (DLL3) and is comprised of a humanised monoclonal antibody, dipeptide linker, and pyrrolobenzodiazepine, a cell cycle-independent, DNA-damaging agent.55 DLL3 belongs to the delta protein ligand family, members of which function as NOTCH ligands and are characterised by a DSL domain, EGF repeats, and a transmembrane domain. DLL3 does not bind to NOTCH receptors in trans and acts instead as a cis inhibitor; it inhibits signalling when expressed in the same cell as the NOTCH receptor.69 Expression of DLL3 in healthy tissue is highest in the fetal brain, and the protein has a key role in somitogenesis during embryonic development. However, it is not expressed at detectable levels in healthy adult tissues. DLL3 is highly expressed on the surface of human neuroendocrine tumours and their tumour-initiating cells, including roughly two-thirds of small-cell lung cancer.
Rovalpituzumab tesirine induced durable tumour regression in vivo, with activity correlating with DLL3 expression.55 In a phase 1 trial of patients with recurrent small-cell lung cancer who were not selected on the basis of DLL3 expression,56 the maximum tolerated dose was identified as 0·2 mg/kg administered intravenously every 3 weeks, and 0·3 mg/kg administered intra venously every 6 weeks. Dose-limiting toxic effects were thrombocytopenia and capillary leak syndrome. Interim data56 showed evidence of clinical activity, with seven (22%) partial responses among 32 patients who were treated at the maximum tolerated dose (objective response achieved by all seven patients). An ongoing phase 2 trial is evaluating rovalpituzumab tesirine in patients with recurrent small-cell lung cancer ( NCT01901653).
Glembatumumab vedotin is an antibody–drug conjugate that targets glycoprotein NMB (GPNMB). It combines a fully human monoclonal antibody against GPNMB with monomethyl auristatin E, via a proteasesensitive valine–citrulline peptide linker. GPNMB is expressed at higher levels in several human malignant diseases, relative to corresponding non-malignant tissue, and is a negative prognostic marker.
Various dosing schedules for glembatumumab vedotin have been assessed in phase 1/2 trials in patients with advanced melanoma and breast cancer.52,70 In the melanoma study,70 the recommended phase 2 dose was 1·88 mg/kg, administered intravenously once every 3 weeks. Modest clinical activity was recorded at this dose, with five (15%) of 34 patients achieving an objective response (all were partial responses). In the breast cancer study,52 this dose resulted in four (12%) of 33 patients achieving an objective response (all were partial responses), with a suggestion of increased benefit in women with triple-negative breast cancer (objective response in two [20%] of ten patients). However, in a phase 2 randomised trial of patients with advanced breast cancer expressing GPNMB (in ≥5% of epithelial or stromal cells, by immuno histochemistry),71 who were randomly allocated either glembatumumab vedotin or standard chemo therapy, the primary endpoint of an objective response in at least 10% of patients was not met. Ongoing trials are investi gating glembatumumab vedotin in osteosarcoma ( NCT02487979), melanoma ( NCT02302339), and triple-negative breast cancer overexpressing GPNMB ( NCT01997333).
Conclusion
Despite approval of only two agents to date, antibody–drug conjugates have potential as targeted treatments for cancer. Antibody–drug conjugates targeting mesothelin, CD22, TROP2, DLL3, and GPNMB are in advanced stages of clinical testing. Moreover, improved understanding of the mechanistic basis of antibody–drug conjugate activity will enable rational design of combinations with other classes of drugs. Findings of studies indicate that, in addition to intracellular release of the cytotoxic payload, antibody–drug conjugates can induce antitumour immune activity.72 For example, ado-trastuzumab emtansine increased the amount of tumour-infiltrating lymphocytes in human primary breast cancers and induced infiltration by effector T cells in murine breast tumours.72 In mouse models, the combination of ado-trastuzumab emtansine with blockade of the PD-1/CTLA-4 inhibitory pathway greatly enhanced T-cell responses and overcame primary resistance to immune checkpoint-blocking antibodies.72 Activation of the innate immune system, and initiation of an antitumour immune response, also accompany brentuximab vedotin-mediated tumour cell death.73 Recruitment of tumour-infiltrating lymphocytes by antibody–drug conjugates offers potential synergies with immunotherapeutic strategies—eg, antibodies blocking immune checkpoints, including CTLA-4 or PD-1—that are more effective in the context of an inflamed tumour microenvironment. Several trials combining immune checkpoint inhibitors with antibody–drug conjugates are ongoing in different tumour types (eg, NCT02318901, NCT02605915, NCT01896999, NCT02581631, NCT02684292, NCT02572167). Future developments in the area of antibody–drug conjugates will possibly address identification of better targets, use of novel and effective cytotoxic payloads, and further improve linker technologies to provide more potent and safer drugs.
Search strategy and selection criteria.
We did a systematic search of MEDLINE between January, 2003, and September, 2015, with the following terms: “antibody drug conjugate”, “immunoconjugates”, “chemotherapy”, “cancer”, and “monoclonal antibodies”. We restricted our search to reports written in English and selected peer-reviewed clinical studies and other studies of clinical significance. Bibliographies of identified articles, guidelines, and conference proceedings of professional societies were reviewed for additional references. The final reference list was generated on the basis of originality and relevance to the broad scope of this Review.
Acknowledgments
We thank the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (USA) for support.
Footnotes
Declaration of interests
We declare no competing interests.
References
- 1.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer 2006; 6: 559–65. [DOI] [PubMed] [Google Scholar]
- 2.Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol 2001; 19: 376–88. [DOI] [PubMed] [Google Scholar]
- 3.Kreitman RJ, Tallman MS, Robak T, et al. Ph ase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol 2012; 30: 1822–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassan R, Miller AC, Sharon E, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med 2013; 5: 208ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassan R, Alewine C, Pastan I. New life for immunotoxin cancer therapy. Clin Cancer Res 2016; 22: 1055–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science 2013; 341: 1192–98. [DOI] [PubMed] [Google Scholar]
- 7.Sievers EL, Senter PD. Antibody–drug conjugates in cancer therapy. Annu Rev Med 2013; 64: 15–29. [DOI] [PubMed] [Google Scholar]
- 8.Teicher BA, Chari RV. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res 2011; 17: 6389–97. [DOI] [PubMed] [Google Scholar]
- 9.Chari RV. Targeted delivery of chemotherapeutics: tumor-activated prodrug therapy. Adv Drug Deliv Rev 1998; 31: 89–104. [DOI] [PubMed] [Google Scholar]
- 10.Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood 2016; 127: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013; 121: 4854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teicher BA. Antibody-drug conjugate targets. Curr Cancer Drug Targets 2009; 9: 982–1004. [DOI] [PubMed] [Google Scholar]
- 13.Chari RV. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc Chem Res 2008; 41: 98–107. [DOI] [PubMed] [Google Scholar]
- 14.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov 2006; 5: 147–59. [DOI] [PubMed] [Google Scholar]
- 15.Carter PJ, Senter PD. Antibody-drug conjugates for cancer therapy. Cancer J 2008; 14: 154–69. [DOI] [PubMed] [Google Scholar]
- 16.Beck A, Haeuw JF, Wurch T, Goetsch L, Bailly C, Corvaia N. The next generation of antibody-drug conjugates comes of age. Discov Med 2010; 10: 329–39. [PubMed] [Google Scholar]
- 17.Lopus M, Oroudjev E, Wilson L, et al. Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules. Mol Cancer Ther 2010; 9: 2689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaishampayan U, Glode M, Du W, et al. Phase II study of dolastatin-10 in patients with hormone-refractory metastatic prostate adenocarcinoma. Clin Cancer Res 2000; 6: 4205–08. [PubMed] [Google Scholar]
- 19.Issell BF, Crooke ST. Maytansine. Cancer Treat Rev 1978; 5: 199–207. [DOI] [PubMed] [Google Scholar]
- 20.MacMillan KS, Boger DL. Fundamental relationships between structure, reactivity, and biological activity for the duocarmycins and CC-1065. J Med Chem 2009; 52: 5771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Small EJ, Figlin R, Petrylak D, et al. A phase II pilot study of KW-2189 in patients with advanced renal cell carcinoma. Invest New Drugs 2000; 18: 193–97. [DOI] [PubMed] [Google Scholar]
- 22.Thorson JS, Sievers EL, Ahlert J, et al. Understanding and exploiting nature’s chemical arsenal: the past, present and future of calicheamicin research. Curr Pharm Des 2000; 6: 1841–79. [DOI] [PubMed] [Google Scholar]
- 23.Damle NK, Frost P. Antibody-targeted chemotherapy with immunoconjugates of calicheamicin. Curr Opin Pharmacol 2003; 3: 386–90. [DOI] [PubMed] [Google Scholar]
- 24.Anderl J, Faulstich H, Hechler T, Kulke M. Antibody–drug conjugate payloads. Methods Mol Biol 2013; 1045: 51–70. [DOI] [PubMed] [Google Scholar]
- 25.Starodub AN, Ocean AJ, Shah MA, et al. First-in-human trial of a novel anti-Trop-2 antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res 2015; 21: 3870–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letschert K, Faulstich H, Keller D, Keppler D. Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol Sci 2006; 91: 140–49. [DOI] [PubMed] [Google Scholar]
- 27.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 2010; 363: 1812–21. [DOI] [PubMed] [Google Scholar]
- 28.Ansell SM. Brentuximab vedotin. Blood 2014; 124: 3197–200. [DOI] [PubMed] [Google Scholar]
- 29.Burris HAIII, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol 2011; 29: 398–405. [DOI] [PubMed] [Google Scholar]
- 30.Polson AG, Ho WY, Ramakrishnan V. Investigational antibody-drug conjugates for hematological malignancies. Expert Opin Investig Drugs 2011; 20: 75–85. [DOI] [PubMed] [Google Scholar]
- 31.Kellogg BA, Garrett L, Kovtun Y, et al. Disulfide-linked antibody-maytansinoid conjugates: optimization of in vivo activity by varying the steric hindrance at carbon atoms adjacent to the disulfide linkage. Bioconjug Chem 2011; 22: 717–27. [DOI] [PubMed] [Google Scholar]
- 32.Zhao RY, Wilhelm SD, Audette C, et al. Synthesis and evaluation of hydrophilic linkers for antibody-maytansinoid conjugates. J Med Chem 2011; 54: 3606–23. [DOI] [PubMed] [Google Scholar]
- 33.Kovtun YV, Audette CA, Mayo MF, et al. Antibody–maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res 2010; 70: 2528–37. [DOI] [PubMed] [Google Scholar]
- 34.Hamblett KJ, Senter PD, Chace DF, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res 2004; 10: 7063–70. [DOI] [PubMed] [Google Scholar]
- 35.Tian F, Lu Y, Manibusan A, et al. A general approach to site-specific antibody drug conjugates. Proc Natl Acad Sci USA 2014; 111: 1766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahapatra K, Darbonne W, Bumbaca D, et al. Abstract A135: T-DM1-induced thrombocytopenia results from impaired platelet production in a HER2-independent manner. Mol Cancer Ther 2011; 10 (11 suppl): A135. [Google Scholar]
- 37.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 2010; 363: 1812–21. [DOI] [PubMed] [Google Scholar]
- 38.Katz J, Janik JE, Younes A. Brentuximab vedotin (SGN-35). Clin Cancer Res 2011; 17: 6428–36. [DOI] [PubMed] [Google Scholar]
- 39.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol 2012; 30: 2190–96. [DOI] [PubMed] [Google Scholar]
- 40.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 2012; 30: 2183–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Claro RA, McGinn K, Kwitkowski V, et al. US Food and Drug Administration approval summary: brentuximab vedotin for the treatment of relapsed Hodgkin lymphoma or relapsed systemic anaplastic large-cell lymphoma. Clin Cancer Res 2012; 18: 5845–49. [DOI] [PubMed] [Google Scholar]
- 42.LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res 2011; 17: 6437–47. [DOI] [PubMed] [Google Scholar]
- 43.Lambert JM, Chari RV. Ado-trastuzumab emtansine (T-DM1): an antibody-drug conjugate (ADC) for HER2-positive breast cancer. J Med Chem 2014; 57: 6949–64. [DOI] [PubMed] [Google Scholar]
- 44.Amiri-Kordestani L, Blumenthal GM, Xu QC, et al. FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin Cancer Res 2014; 20: 4436–41. [DOI] [PubMed] [Google Scholar]
- 45.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012; 367: 1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krop IE, Kim SB, Gonzalez-Martin A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15: 689–99. [DOI] [PubMed] [Google Scholar]
- 47.Cardillo TM, Govindan SV, Sharkey RM, Trisal P, Goldenberg DM. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res 2011; 17: 3157–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner-Johnston ND, Goy A, Rodriguez MA, et al. A phase 2 study of inotuzumab ozogamicin and rituximab, followed by autologous stem cell transplant in patients with relapsed/refractory diffuse large B-cell lymphoma. Leuk Lymphoma 2015; 56: 2863–69. [DOI] [PubMed] [Google Scholar]
- 49.Golfier S, Kopitz C, Kahnert A, et al. Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther 2014; 13: 1537–48. [DOI] [PubMed] [Google Scholar]
- 50.Daver N, Kantarjian H, Ravandi F, et al. A phase II study of decitabine and gemtuzumab ozogamicin in newly diagnosed and relapsed acute myeloid leukemia and high-risk myelodysplastic syndrome. Leukemia 2016; 30: 268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gan HK, Fichtel L, Lassman AB, et al. A phase 1 study evaluating ABT-414 in combination with temozolomide (TMZ) for subjects with recurrent or unresectable glioblastoma (GBM). Proc Am Soc Clin Oncol 2014; 32 (5 suppl): abstr 2021. [Google Scholar]
- 52.Bendell J, Saleh M, Rose AA, et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with locally advanced or metastatic breast cancer. J Clin Oncol 2014; 32: 3619–25. [DOI] [PubMed] [Google Scholar]
- 53.Borghaei H, O’Malley DM, Seward SM, et al. Phase 1 study of IMGN853, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC) in patients (Pts) with epithelial ovarian cancer (EOC) and other FRA-positive solid tumors. Proc Am Soc Clin Oncol 2015; 33 (suppl): abstr 5558. [Google Scholar]
- 54.Thompson JA, Motzer R, Molina AM, et al. Phase I studies of anti-ENPP3 antibody drug conjugates (ADCs) in advanced refractory renal cell carcinomas (RRCC). Proc Am Soc Clin Oncol 2015; 33 (suppl): abstr 2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saunders LR, Bankovich AJ, Anderson WC, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015; 7: 302ra136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rudin CM, Pietanza MC, Spigel DR, et al. A DLL3-targeted ADC, rovalpituzumab tesirine, demonstrates substantial activity in a phase I study in relapsed and refractory SCLC. J Thorac Oncol 2015; 10 (9 suppl 2): oral 10.1. [Google Scholar]
- 57.Dotan E, Berlin J, Starodub A, et al. Activity of IMMU-130 anti-CEACAM5-SN-38 antibody-drug conjugate (ADC) on metastatic colorectal cancer (mCRC) having relapsed after CPT-11: phase I study. Proc Am Soc Clin Oncol 2014; 32 (5 suppl): abstr 3106. [Google Scholar]
- 58.Almhanna K, Messersmith WA, Ahnert JR, et al. MLN0264, an investigational, first-in-class antibody-drug conjugate (ADC) targeting guanylyl cyclase C (GCC): phase I, first-in-human study in patients (pts) with advanced gastrointestinal (GI) malignancies expressing GCC. Proc Am Soc Clin Oncol 2013; 31 (suppl): abstr TPS3646. [Google Scholar]
- 59.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancer s. Proc Natl Acad Sci USA 1996; 93: 136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hassan R, Bendell JC, Blumenschein G, et al. Phase I study of anti-mesothelin antibody drug conjugate anetumab ravtansine. J Thorac Oncol 2015; 10 (9 suppl 2): oral 11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DiJoseph JF, Armellino DC, Boghaert ER, et al. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calichea micin for the treatment of B-lymphoid malignancies. Blood 2004; 103: 1807–14. [DOI] [PubMed] [Google Scholar]
- 62.Shor B, Gerber HP, Sapra P. Preclinical and clinical development of inotuzumab-ozogamicin in hematological malignancies. Mol Immunol 2015; 67: 107–16. [DOI] [PubMed] [Google Scholar]
- 63.Vaickus L, Ball ED, Foon KA. Immune markers in hematologic malignancies. Crit Rev Oncol Hematol 1991; 11: 267–97. [DOI] [PubMed] [Google Scholar]
- 64.Advani A, Coiffer B, Czuczman MS, et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin’s lymphoma: results of a phase I study. J Clin Oncol 2010; 28: 2085–93. [DOI] [PubMed] [Google Scholar]
- 65.Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed ac ute lymphocytic leukaemia: a phase 2 study. Lancet Oncol 2012; 13: 403–11. [DOI] [PubMed] [Google Scholar]
- 66.Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer 2015; 6: 84–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Faltas B, Goldenberg DM, Ocean AJ, et al. Sacituzumab govitecan, a novel antibody–drug conjugate, in patients with metastatic platinum-resistant urothelial carcinoma. Clin Genitourin Cancer 2016; 14: e75–79. [DOI] [PubMed] [Google Scholar]
- 68.Bardia A, Vahdat LT, Robinson Diamond J, et al. Therapy of refractory/relapsed metastatic triple-negative breast cancer (TNBC) with an anti-Trop-2-SN-38 antibody-drug conjugate (ADC), sacituzumab govitecan (IMMU-132): phase I/II clinical experience. Proc Am Soc Clin Oncol 2015; 33 (suppl): abstr 1016. [Google Scholar]
- 69.Geffers I, Serth K, Chapman G, et al. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. J Cell Biol 2007; 178: 465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ott PA, Hamid O, Pavlick AC, et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with advanced mel anoma. J Clin Oncol 2014; 32: 3659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yardley DA, Weaver R, Melisko ME, et al. EMERGE: a randomized phase II study of the antibody-drug conjugate glembatumumab vedotin in ad vanced glycoprotein NMB-expressing breast cancer. J Clin Oncol 2015; 33: 1609–19. [DOI] [PubMed] [Google Scholar]
- 72.Muller P, Kreuzaler M, Khan T, et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med 2015; 7: 315ra188. [DOI] [PubMed] [Google Scholar]
- 73.Gardai SJ, Epp A, Law C-L. Abstract 2469: brentuximab vedotin-mediated immunogenic cell death. Cancer Res 2015; 75 (15 suppl): 2469. [Google Scholar]