Abstract
Accelerated neurological disorders are increasingly prominent among the HIV-infected population and are likely driven by the toxicity from long-term use of antiretroviral drugs. We explored potential side-effects of antiretroviral drugs in HIV-infected primary human astrocytes and whether opioid co-exposure exacerbates the response. HIV-infected human astrocytes were exposed to the reverse transcriptase inhibitor, emtricitabine alone or in combination with two protease inhibitors ritonavir and atazanavir (ERA) with and without morphine co-exposure. The effect of the protease inhibitor, lopinavir alone or in combination with the protease inhibitor, abacavir and the integrase inhibitor, raltegravir (LAR) with and without morphine co-exposure was also explored. Exposure with emtricitabine alone or ERA in HIV-infected astrocytes caused a significant decrease in viral replication and attenuated HIV-induced inflammatory molecules, while co-exposure with morphine negated the inhibitory effects of ERA, leading to increased viral replication and inflammatory molecules. Exposure of FTC alone or in combination with morphine caused a significant disruption of mitochondrial membrane integrity. Genetic analysis revealed a significant increase in the expression of p62/SQSTM1 which correlated with an increase in the histone-modifying enzyme, ESCO2, after exposure with ERA alone or in combination with morphine. Furthermore, several histone-modifying enzymes such as CIITA, PRMT8 and HDAC10 were also increased with LAR exposure alone or in combination with morphine. Accumulation of p62/SQSTM1 is indicative of dysfunctional lysosomal fusion. Together with the loss of mitochondrial integrity and epigenetic changes, these effects may lead to enhanced viral titer and inflammatory molecules contributing to the neuropathology associated with HIV.
Keywords: antiretroviral drugs, opioid, autophagy, scaffold protein, arginine methyl transferase
Introduction
The introduction of combined antiretroviral drugs (ARVs) more than 20 years ago has resulted in the immediate prevention of Human Immunodeficiency Virus (HIV) disease progression and has led to a significant increase in the life expectancy of infected people (Samji et al., 2013). Within the brain, residing glia (astrocytes and microglia) act mostly as a reservoir for HIV, maintaining a low level of HIV replication in the presence of antiretroviral drugs (Palmer et al., 2008). However, since antiretroviral drugs are not able to completely eradicate HIV from infected individuals, the increased lifespan has resulted in continuous brain exposure to virions and viral proteins, leading to a chronic state of inflammation and accumulation of neurological damage (Canizares et al., 2014; Rao et al., 2014). Although dispersion of many antiviral drugs into the central nervous system (CNS) is limited by poor penetration of the blood brain barrier (BBB) (Bertrand and Toborek, 2015; Decloedt et al., 2015; Ene et al., 2011), long-term antiretroviral-related toxicity in the CNS is considered another likely contributor to neurodegeneration and in accelerated aging associated with HIV (Akay et al., 2014; Kranick and Nath, 2012; Shah et al., 2016).
As patients are living longer, chronic pain has become a common manifestation affecting as much as 39 to 85% of people living with HIV/AIDS (Merlin et al., 2016). Co-morbidity with opiates are not only associated with recreational use but also with medicinal use related to opioid addiction and for the relief of pain in HIV-infected individuals (Al-Hasani and Bruchas, 2011). Several researchers have shown that opioids can have significant adverse interactive effects with many antiretroviral drugs that can contribute to nonadherence and poor clinical outcomes in this high-risk population (Iribarne et al., 1998; Kumar et al., 1996). Some studies have indicated that even low concentrations of ARVs that penetrate the BBB could have toxic effects (Robertson et al., 2012; Robertson et al., 2010) which can be further exacerbated by co-exposure to opioid drugs. Few studies have examined the adverse effects of opiates (morphine) on the use of ARVs in attenuating HIV replication and viral-induced inflammatory molecules in HIV-infected glial cells. Furthermore, limited information is available about the underlying mechanisms regulating drug-drug interactions between opioids and ARVs potentially leading to neuronal dysfunction (Hauser and Knapp, 2014; Maubert et al., 2015). In the current study, we explore the potential interactive effects of various antiretroviral drugs when combined with morphine. With changes in inflammatory molecule secretion and the influence of mitotoxicity, we propose a potential role of the adaptor protein p62/SQSTM1 and the autophagy pathway in mediating ARV neurodegeneration in the HIV population and drug abusing population.
Material and Methodology
HIV infection and treatments of human astrocytes
Primary human astrocytes (catalog #: 1800, ScienCell, Carlsbad, CA, USA) were grown to ∼80% confluency and infected with HIV-1SF162 (p24 = 1 ng/mL; from Dr. Jay Levy through the NIH AIDS Research and Reference Reagent Program; Germantown, MD, USA) followed by exposure with 10 μM each of emtricitabine, ritonavir, atazanavir (ERA) or lopinavir, abacavir, raltegravir (LAR) alone or in combination with 500 nM of morphine sulfate (Sigma-Aldrich, St. Louis, MO, USA) for 3, 5, and 7 days. HIV infection was quantified by p24 levels using a p24 antigen ELISA (ZeptoMetrix, Buffalo, NY, USA) and by RT-PCR using the long terminal repeat (LTR) primers (F-3): 5'-TTTGTTATATTTTGTGAGTTTGTAT-3' (nucleotide position: 200 to 224, 9285 to 9309), reverse primer (R-1), 5'-CAAAAAACTCCCAAACTCAAATCTA-3' (nucleotide position: 496 to 472, 9581 to 9557). Concentration of the antiretroviral drugs are based on previously published literature by others (Cohen et al., 2017; Ene et al., 2011; Kravcik et al., 1999; Nooka and Ghorpade, 2017) and cell viability assay. Morphine at a concentration of 500 nM has been previously shown to fully activate the u-opioid receptor as well as to enhance HIV mediated neurotoxicity (El-Hage et al., 2005; Gurwell et al., 2001; Hauser et al., 1996; Stiene-Martin and Hauser, 1991).
ELISA
Levels of interleukin (IL)-6, monocyte chemotactic protein-1 (MCP-1), regulated upon activation normal T-cell expressed and secreted (RANTES), and tumor necrosis factor alpha (TNF-α) were measured by ELISA at 3, 5, and 7 days post-treatment (R&D Systems, Minneapolis, MN, USA). The O.D. was read at A450 on a Synergy HTX plate reader (BioTek, Winooski, VT, USA).
Real Time Polymerase chain reaction (PCR)
Relative abundance of mRNA was assessed by SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) in 20 μL real-time PCR reactions with gene specific primers using a Bio-Rad CFX96 real time system. All data were normalized to GAPDH and presented as 2^-ΔΔCt for fold-change values.
Cellular Membrane Integrity
Intracellular energy balance was evaluated using a mitochondrial ToxGlo assay (Promega, Madison, WI, USA). Briefly, a fluorogenic peptide substrate (bis-AAF-R110) was added to the cells and fluorescence was measured. Then, an ATP detection reagent was added to the cells and ATP levels were determined by luminescence. Fluorescence and luminescence were measured using a Synergy HTX plate reader (BioTek).
Reactive oxygen species (ROS)
ROS production were measured using the indicator 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2 DCFDA; Invitrogen, Carlsbad, CA, USA), which is de-acetylated to dichlorofluorescein (DCF). After treatments, fluorescence was measured at λex = 485 nm and λem = 520 nm using a Synergy HTX plate reader (BioTek).
Cell viability assay
Viability was assessed using a live/dead fluorescence assay (ScienCell) which yields two-color discrimination of the population of live cells (green) from the dead cells (red). Cells were imaged using an inverted fluorescence microscope (Zeiss, Germany), manually quantified and reported as percent of viability.
RNA Expression profiling
Human Cellular Stress Responses (catalog #: PAHS-019Z, Qiagen; Valencia, CA, USA) and Human Epigenetic Chromatin Modification Enzymes (catalog #: PAHS-085Z, Qiagen) RT2 Profiler PCR Arrays were used for RNA profiling. Ct data were interpreted from the manufacturer’s website (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php). Relative expression was calculated using the ΔΔCT method with five housekeeping genes and compared with the expression in control cells.
Western blotting
Membranes were probed with primary antibodies against p62/SQSTM1, LC3B, Beclin1, and β-actin as internal control. Primary antibodies were followed by incubation with the respective secondary antibody conjugated to horseradish peroxidase. Immunoblots were exposed to SuperSignal West Femto Substrate (Thermo Scientific, Waltham, MA, USA) and visualized using a ChemiDoc imaging system (Bio-Rad). Protein expression was calculated using ImageJ software (National Institutes of Health (NIH), Bethesda, MD, USA).
Statistical analysis
Data were analyzed using analysis of variance (ANOVA) techniques followed by Bonferonni’s post hoc test for multiple comparisons (GraphPad Software, Inc., La Jolla, CA, USA). Alpha level of p < 0.05 was considered significant.
Results
Antiviral effects of emtricitabine (FTC) and lopinavir (LPV) in HIV-infected astrocytes co-exposed with morphine.
It is accepted that despite having poor penetration into the CNS and providing incomplete protection against HIV replication in brain reservoirs, combination antiretroviral therapies (cART) can improve cognition and reduce the prevalence of HIV-associated neurological complications. Despite those successes, long-term use of antivirals can have detrimental effects leading to enhanced neurotoxicity in HIV-infected individuals. We assessed the effectiveness of the reverse transcriptase inhibitor, emtricitabine (FTC; low CNS penetrance (Ene et al., 2011; Lahiri et al., 2016)) with and without morphine co-exposure (Fig. 1 A). Exposure to FTC significantly reduced HIV infection; however, co-exposure with morphine reverted this inhibition by three days post treatment. Similar effects were detected on days 5 and 7, albeit not significantly. Release of inflammatory molecules were detected by ELISA and interestingly, although viral titer was attenuated, expression of MCP-1 was enhanced with FTC alone and with morphine by days 3, 5 and 7 of post-treatment (Fig. 1B). Separately, HIV-infected primary human astrocytes were exposed to the protease inhibitor, lopinavir (LPV; high CNS penetrance (Ene et al., 2011; Lahiri et al., 2016)) with and without morphine co-exposure. Exposure to LPV had no inhibitory effect on HIV titer (Fig. 1A) yet enhanced HIV-induced MCP-1 secretion only on day 3 (Fig. 1B). Co-exposure with morphine showed minimal interactive effect on viral titer (Fig. 1A) and showed minor, albeit significant, decrease in IL-6 secretion (Fig. 1B). Overall the data shows reduced efficacy in the antiviral response of FTC in HIV-infected astrocytes when co-exposed with morphine.
Figure 1.
HIV replication and secretion of inflammatory molecules in HIV-infected astrocytes exposed to emtricitabine (FTC) and lopinavir (LPV) alone or in combination with morphine. HIV replication in human astrocytes was measured using HIV p24 Gag protein ELISA (a). Values were determined from standard curves and are presented as the mean ± the standard error of mean (S.E.M.) of three independent experiments. Corresponding cell culture supernatants were used to detect the levels of MCP-1, TNF-α, IL-6, and RANTES by ELISA (b). Values were determined from standard curves and are presented as the mean ± the S.E.M. of three independent experiments (p < 0.05 * vs. HIV, $ vs. HIV+FTC, & vs. HIV+FTC+Mor, % vs. HIV+LPV)
Enhanced mitochondrial damage and cytotoxicity with emtricitabine (FTC) and lopinavir (LPV) in HIV-infected astrocytes co-exposed with morphine.
Antiretroviral toxicity has been partly attributed to mitochondrial damage and oxidative stress molecule release (Chandra et al., 2009; Kline et al., 2009; Manda et al., 2011; Papparella et al., 2007). In addition, mitochondrial dysfunction has been confirmed as a great contributor to the occurrence of neurodegenerative diseases (Baloh, 2008; Choi et al., 2011; Dodson and Guo, 2007; Squitieri et al., 2006; Wang et al., 2008). To this end, we assessed effects of FTC and LPV on mitochondrial membrane integrity in HIV-infected astrocytes and determined whether co-exposure with morphine alters these responses. To assess cellular toxicity, we used the bis-AAF- R110 substrate which cannot cross intact membranes of live cells, giving reduced signal in viable cells. HIV infection exerted high levels of cytotoxicity compared to uninfected cells, whereas exposure with FTC alone or combined with morphine significantly enhanced cytotoxicity when compared to HIV-infected or uninfected cells, suggesting damage to the membrane (Fig. 2A). In contrast, significant toxicity with LPV alone or combined with morphine was undetectable. To discern whether the cytotoxicity observed was correlated with mitochondrial toxicity or primary necrosis, ATP levels were determined by measuring luminescence which was proportional to ATP. Levels of ATP remained unchanged, despite a significant increase of 35% in cytotoxicity suggesting that the increase may not necessarily be due to mitochondrial toxicity but rather to necrosis (Fig. 2A). This data correlates with published literature stating LPV toxicity has been linked more closely to the macrophage/microglia cell type (Chen et al., 2009; Lagathu et al., 2007; Zhang et al., 2014). Since mitochondria harbor the bulk of oxidative pathways (Andreyev et al., 2005; Murphy, 2009), we also assessed whether FTC and LPV cause changes to ROS in HIV-infected astrocytes and whether co-exposure with morphine alters the responses. HIV infection caused significant increases in ROS production when compared to uninfected cells, while exposure with FTC alone or with morphine further enhanced, albeit not significantly, the production of ROS in HIV-infected astrocytes (Fig. 2B). The overall data shows significant damage in membrane integrity with FTC and LPV alone or in combination with morphine. We also found that the damage to membrane integrity with FTC or LPV treatment did not correlate with viral titer levels (Fig. 1 A), suggesting that other factor(s) may be responsible for the damage.
Figure 2.
Mitochondrial damage and cytotoxicity measurements in HIV-infected astrocytes exposed to emtricitabine (FTC) and lopinavir (LPV) in viral-infected astrocytes co-exposed with morphine. Mitochondrial damage in human astrocytes was measured using a mitochondrial fluorescence and luminescence assay (a). Data are presented as percent of increased compared to untreated control cells. ROS production in human astrocytes was assessed by DCF fluorescence at the indicated time points after indicated treatments (b). Error bars show the S.E.M. of three independent experiments. Cellular viability was assessed by live/dead cell fluorescence assay. Data are presented as percent viability compared to untreated control cells (c). Error bars show the S.E.M. of three independent experiments (p < 0.05 * vs. HIV, $ vs. HIV+FTC, & vs. HIV+FTC+Mor, % vs. HIV+LPV)
Suppressive effect of cART was reverted by co-exposure with morphine in HIV-infected astrocytes.
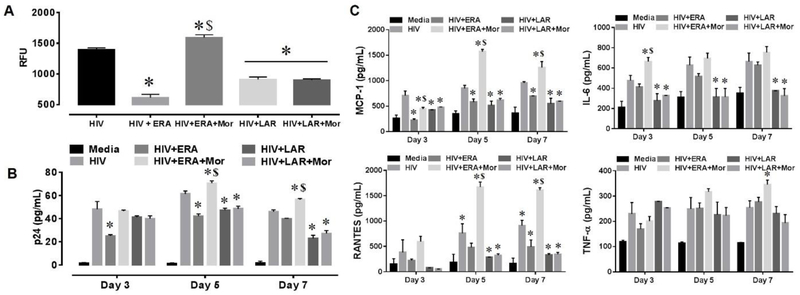
Since ARVs are not prescribed as a monotherapy but rather in combination, we combined 3 different types of antiretrovirals in two sets. The first consisted of the reverse transcriptase inhibitor, emtricitabine, in combination with two protease inhibitors, ritonavir and atazanavir (ERA). The second set consisted of the protease inhibitor, lopinavir, in combination with the protease inhibitor, abacavir and the integrase inhibitor, raltegravir (LAR). HIV-infected astrocytes were exposed for 3, 5, and 7 days to the ERA or LAR, with or without morphine co-exposure. Exposure to ERA showed a ∼2-fold attenuation in viral replication by LTR RT-PCR (Fig. 3 A), while co-exposure with morphine reverted the antiviral response and heightened viral replication by 3.5-fold when compared to ERA-treated cells. Likewise, exposure with LAR attenuated viral replication by 1.5-fold (Fig. 3 A); however, co-exposure with morphine did not alter the antiviral response of LAR. Assessment by p24 ELISA showed similar patterns (Fig. 3B). Next, the release of inflammatory molecules were detected by ELISA and showed a time-dependent increases in the release of the chemokines MCP-1, RANTES and the cytokines IL-6 and TNF-α by HIV-infected astrocytes (Fig. 3C). Although these secretions were attenuated with ERA, co-exposure with morphine negated the response leading to a significant increase of 3-fold and 3.6-fold in MCP-1, RANTES, respectively after 5 days post-treatment (Fig. 3C). HIV-induced secretion of IL-6 and TNF-α were not significantly attenuated with ERA, while co-exposure with morphine further enhanced cytokine release at 5 and 7 days post-treatment (Fig. 3C). Similar results were seen with LAR; however, co-exposure with morphine showed no further changes in inflammatory responses (Fig. 3C). Overall, the data shows a drug-drug interactive effect between morphine and cART in astrocytes leading to failure in the attenuation of viral replication and inflammatory molecule secretion by ERA but not LAR, potentially contributing to HIV-associated neuropathology.
Figure 3.
HIV replication and secretion of inflammatory molecules in HIV-infected astrocytes exposed to ERA and LAR alone or in combination with morphine. HIV replication in human astrocytes was measured using qRT-PCR- and LTR-specific primers (a). Data are represented as relative fluorescence units (RFU); GAPDH was used as internal control. Error bars show S.E.M. for three independent experiments. HIV replication in human astrocytes was measured using HIV p24 Gag protein ELISA (b). Values were determined from standard curves and are presented as the mean ± the S.E.M. of three independent experiments. Corresponding cell culture supernatants used for p24 ELISA were used to detect the levels of MCP-1, TNF-α, IL-6, and RANTES by ELISA (c). Values were determined from standard curves and are presented as the mean ± the S.E.M. of three independent experiments (p < 0.05 * vs. HIV, $ vs. HIV+ERA, & vs. HIV+ERA+Mor, % vs. HIV+LAR)
Increased levels of the ubiquitin-binding scaffold protein, p62/SQSTM1 by exposure with ERA and morphine in HIV-infected astrocytes.
Cytokines can contribute to viral control by upregulating the antiviral immune response (Chevalier et al., 2011; Muller et al., 1994). However, chemokines such as RANTES and MCP-1 can increase the target cell pool for HIV by recruiting the primary target cells, CD4+ T cells and monocytes, respectively. Alternately, pro-inflammatory cytokines such as IL-6 and TNF-α produced during HIV infection can increase antiviral immunity but can also induce the transcription factor, nuclear factor kappa B (NF-κB) which enhances HIV replication (Alcami et al., 1995; Osborn et al., 1989; Vallabhapurapu and Karin, 2009). Since sustained inflammatory molecule release and viral replication may induce stress and cytotoxicity, potentially contributing to HIV-associated neuropathology, RNA from HIV-infected astrocytes exposed for 3 and 7 days with ERA and LAR, with and without morphine co-exposure were used to explore cellular stress responses. We identified a significant increase in the mRNA levels of the ubiquitin-binding scaffold protein, p62/SQSTM1, in HIV-infected astrocytes exposed with ERA alone which was further upregulated in combination with morphine at 7 days post-treatment (Fig. 4A). The data was confirmed with RT-PCR using primers specific for p62/SQSTM1 and showed an approximate 2-fold increase with ERA and a synergistic increase of about 8-fold when combined with morphine (Fig. 4B). Upon protein analysis, we detected a significant upregulation in the expression of p62/SQSTM1 in HIV-infected astrocytes exposed to ERA that was further increased with morphine (Fig. 4C, D). Interestingly, the upregulation in p62/SQSTM1 was not detected in HIV-infected astrocytes exposed to LAR alone or in combination with morphine (Fig. 4). Given p62/SQSTM1 is involved in autophagosome maturation and lysosomal clearance, we measured expression levels of two autophagy related proteins Beclin1 and LC3 (Fig. 4). Since p62/SQSTM1 is a multifunctional, stress-induced protein involved in the regulation of inflammatory responses and redox homeostasis, we believe that the high level of p62/SQSTM1 in HIV-infected astrocytes exposed to ERA and morphine could be the consequence of increased toxicity with ERA (Supplemental data 1). This data shows that co-exposure with ERA and morphine increases p62/SQSTM1 at RNA and protein levels which correlate with the increased release of inflammatory molecules and increased toxicity in HIV-infected astrocytes.
Figure 4.
Expression of human cellular stress genes and autophagy-related genes in HIV-infected astrocytes exposed with ERA and LAR alone or in combination with morphine. Relative mRNA expression of selected genes was measured using the human cellular stress responses RT2 profiler PCR array following the indicated treatments for 7 days (a). Data are presented as expression relative to untreated control cells. mRNA levels of p62/SQSTM1 were assessed by qRT-PCR following indicated treatments for 3 days and 7 days (b). Values were determined by the 2^-ΔΔCT method and normalized to GAPDH. Whole cell lysates from human astrocytes following 7 days of the indicated treatments were subjected to immunoblotting with antibodies against p62/SQSTM1, LC3, and Beclin1 protein (c, d). Densitometry was performed for quantification, and the ratio of each protein to β-actin are presented graphically. Error bars show the S.E.M. of three independent experiments (p < 0.05 * vs. HIV, $ vs. HIV+ERA, & vs. HIV+ERA+Mor, % vs. HIV+LAR)
Increased levels of histone-modifying enzymes and G-protein coupled receptors by exposure of LAR and morphine in HIV-infected astrocytes.
Studies have shown a relationship between modulation of DNA methylation and the subsequent expression of the histone-modifying enzymes and antiretroviral therapy (Rosea et al., 2017; Trejbalova et al., 2016). RNA from HIV-infected astrocytes exposed for 3 and 7 days with ERA and LAR, with and without morphine co-exposure were used to explore epigenetic changes. The 84-gene array revealed a 3-fold increase in the mRNA expression of the histone-modifying enzyme ESC02 in HIV-infected astrocytes exposed with ERA alone and a 4-fold increase when combined with morphine at day 7. Separately, exposure with LAR alone caused about a 5-fold increase in the histone-modifying enzymes HDAC10 and KAT2A, about 8-fold increase in the histone-modifying enzymes AURKC and CIITA, and about a 12-fold increase the histone-modifying enzyme PRMT8 (Fig. 5A) at day 7. The expression of several of these genes including HDAC10, KAT2A, SETD6, and PRMT8 were further increased by co-exposure with morphine at day 7. The data was confirmed with RT-PCR using primers specific for ESC02, HDAC10, KAT2A, AURKC, CIITA, PRMT8 and SETD6, and showed a similar trend (Fig. 5B-H). While some of these enzymes and changes in their expression have been linked to HIV infectivity (Boulanger et al., 2005; Invernizzi et al., 2006; Kanazawa et al., 2000; Ran et al., 2017), a strong link has yet to be reported in conjunction with morphine. Of note, these epigenetics changes were also examined at day 3; however, differences in gene expression were minimal for both sets of ARV with or without morphine (Supplementary data 2).
Figure 5.
Expression of histone-modifying enzymes and G-protein coupled receptors in HIV-infected astrocytes exposed with ERA and LAR alone or in combination with morphine. Relative mRNA expression of selected genes was measured using the human epigenetic chromatin modification enzymes array following the indicated treatments for 7 days (a). Data are presented as expression relative to untreated control cells. mRNA levels of the indicated genes were assessed by qRT-PCR following indicated treatments for 7 days (b–h). Values were determined by the 2^-ΔΔCT method and normalized to GAPDH. Error bars show S.E.M. for three independent experiments (p < 0.05 * vs. HIV, $ vs. HIV+ERA, & vs. HIV+ERA+Mor, % vs. HIV+LAR)
Evidence has suggested a direct role of the μ-opioid receptor-1 (MOR-1) in HIV replication and pathogenesis (Dever et al., 2012) and given that astrocytes express opioid receptors as well as HIV co-receptors (Dever et al., 2012; El-Hage et al., 2005), we explore the possible interaction between cART and the G-protein coupled receptors utilized by both HIV and morphine. We detected significant increases in expression of the MOR-1 variants (MOR-1A, MOR-1K, and MOR-1X) after exposure with LAR alone that were further increased by co-exposure with morphine at day 7 but not detected at day 3 (Supplemental data 2). Gene expression changes of the MOR variants upon exposure to ERA alone or in combination with morphine were insignificant. CCR5 expression, an HIV co-receptor, was increased with both sets of cART alone and in combination with morphine.
Overall, the data shows increased gene expression of several histone-modifying enzymes after exposure with LAR and morphine, which could be involved with LAR-induced suppression of HIV titer and attenuation in the release of inflammatory molecules in HIV-infected astrocytes. The changes in the expression of the opioid receptor variants and CCR5 may or may not be directly correlated with the changes in the epigenetic genes but still warrant further investigation.
Discussion and Conclusion
In this study we explored the effects of antiretroviral drugs and the possible interaction with the opiate morphine in HIV-infected human astrocytes. Despite the great success of antiretrovirals in increasing the lifespan of HIV-infected individuals, the life expectancy of treated patients remains 10–30 years shorter than the normal population (2008; Lohse et al., 2007). There have been numerous reports demonstrating that antiretroviral drugs, such as zidovudine, lamivudine, indinavir, and abacavir can induce oxidative stress, modulate the mechanisms of phagocytosis, and impact mitochondrial function and DNA replication (Apostolova et al., 2011; Bertrand and Toborek, 2015; Blas-Garcia et al., 2010; Brinkman et al., 1999; Giunta et al., 2011; Manda et al., 2011). In addition, therapeutic dosages of nelfinavir and saquinavir have been shown to cause an inhibition of normal proteasome function (Piccinini et al., 2005). Furthermore, antiretroviral-induced neurotoxicity was recently linked to the autophagy pathway as shown by the dysregulation of ER stress and the inhibition of autophagosome formation by blocking the activity of the Beclin-1/Atg14/PI3KIII complex in regard to synthesis of phosphatidylinositol 3-phosphate by the non-nucleoside reverse transcriptase inhibitor, efavirenz (Bertrand and Toborek, 2015). Although effective in reducing viral load and preventing severe forms of neurological disorders in HIV populations, milder forms of HAND remain prevalent regardless of treatment (Ellis et al., 2007; Harezlak et al., 2011). We observed that co-exposure to morphine reverted the reduction of viral titer by emtricitabine, ritonavir and atazanavir (ERA), which correlated with significantly increased production of RANTES, MCP-1, and TNF-α in HIV-infected astrocytes (Fig. 3). These observations agree with a previous study published by Vaidya et al., 2016. Accordingly, they used mathematical and experimental data from simian immunodeficiency virus (SIV)-infected macaques to calculate the susceptibility rate to SIV infection and reduced antiretroviral efficacy (with both early and late ART initiations) in cells co-exposed to morphine. They showed that with intermediate levels of drug efficacy, ART failed to control the viral load in morphine-dependent animals (animals injected intramuscularly with morphine for approximately 18 weeks) but not in morphine non-dependent animals (Vaidya et al., 2016). They suggested that the effect of morphine on HIV infectivity, despite the presence of ARV, may be due to an increase in HIV co-receptors CCR5 and CXCR4 in the periphery. As shown by others, heterologous cross desensitization between the MOR-1 and the CCR5 receptor may lead to downstream signaling interactions that could directly affect HIV infectivity and immune responses against HIV (Chen et al., 2004; Melik Parsadaniantz et al., 2015). In our studies, LAR exposure in combination with morphine increased the mRNA expression levels of different MOR variants and the CCR5 co-receptor (Supplementary data 2); however, these modulations did not significantly change HIV infectivity or secretion of inflammatory molecules (Fig. 3). These observations may be attributed to the potential involvement of modulations to viral latency by LAR at transcriptional levels. As shown previously, epigenetic variations of proviral and cellular DNA may be associated with the establishment of HIV latency (Kumar et al., 2015). In terms of epigenetic modulations, exposure to LAR and morphine caused a significant increase in several histone-modifying enzymes (Fig. 5). We speculate that these epigenetic changes potentially correlate with a decrease in viral titer and a decrease in the secretion of inflammatory responses in HIV-infected astrocytes. For example, the increased mRNA levels of methyltransferase SET Domain Containing 6 (SETD6) detected in Fig.5, was shown by others to repress the NF-κB system (Chang et al., 2011; Levy et al., 2011), and to regulate both inflammatory gene expression and HIV replication (Fiume et al., 2012; Gangwani and Kumar, 2015; Pitha, 2011; Swingler et al., 1994). Another gene in the methyltransferase family, SET domain bifurcated 1 (SETDB1) was shown to methylate the HIV Tat protein and to recruit inhibitory proteins complex to the HIV-1 genome causing a repression of HIV-1 transcription (Van Duyne et al., 2008). The protein arginine methyltransferases (PRMT)-8, a plasma membrane-associated protein mainly expressed in brain tissues, was also increased with co-exposure of LAR and morphine (Fig. 5). Although PRMT8 has not been shown to be associated with HIV replication, the overexpression of PRMT6, another protein within the family, was linked with the reduction of Tat transactivation in HIV-1 production and viral replication (Xie et al., 2007). Low expression levels of PRMT6 in peripheral blood mononuclear cells derived from chronically infected and non-infected HIV individuals correlated with enhanced CD4+ T-cell exhaustion due to a permanent state of cell activation, even under antiretroviral drug treatments (Bogoi et al., 2018). Although HIV individuals receiving cART exhibited a slightly higher expression of PRMT6 compared with HIV patients not receiving cART, PRMT6 protein levels were lower than the expression observed in non-HIV infected individuals (Bogoi et al., 2018). Significant expression levels of the histone deacetylase 10 (HDAC10) was also detected with LAR alone and in combination with morphine (Fig. 5). HDAC10 has been reported to be downregulated by HIV-1 virus-associated envelope glycoprotein (vEnv) which lead to enhanced infectivity of progeny viruses (Ran et al., 2017). A study by Ran et al. confirmed the role of HDAC10 in HIV infectivity by generating HDAC10 knockdown in Jurkat T-cells followed by infection with HIV. Both viral integrated DNA level and the amount of produced p24 in the supernatant from the HDAC10 knockdown Jurkat T-cells were significantly higher than in control cells. This suggest that reduced HDAC10 expression level facilitated the early steps towards to the establishment of HIV infection. Despite numerous evidence by others showing epigenetic as a mechanism for regulating HIV, we still don’t know how epigenetic changes modulate viral infection and inflammatory molecules in a morphine-induced system, and further studies to examine the possible association between LAR and morphine within an HIV latency model are necessary to confirm whether these results are due to epigenetic changes.
Since we did not observe a strong link between ERA exposure and epigenetic changes, and based on previous investigations (Lapierre et al., 2018; Rodriguez et al., 2017), we suspected that increases in HIV titer and inflammatory molecules by ERA in the presence of morphine could be mediated through other mechanisms, such as the autophagy pathway. Autophagy is a dynamic process of degrading and clearing intracellular proteins and organelles (Moreau et al., 2010; Wang and Hill, 2015; Zhou and Spector, 2008). Although autophagy is considered a cytoprotective response to viral factors, it plays a complex role in HIV infection which can either induce or inhibit autophagy in permissive cells (Dever et al., 2015; El-Hage et al., 2015; Rodriguez et al., 2017; Wang et al., 2012; Zhou and Spector, 2008). Our group has reported that HIV infection in microglia triggers increased autophagosome formation but blocks fusion with the lysosome, thereby halting the autophagic process and leaving engulfed virions intact (El-Hage et al., 2015). HIV-induced neuronal damage and astrocytic toxicity have been shown to involve suppression of autophagy, supporting its role in HIV-induced neuropathology (Ellis et al., 2007). Evidence shows that exploitation of autophagy by viruses may be induced through a MOR stimulated signaling pathway (Zhao et al., 2010). Despite autophagy clearing intracellular pathogens including viruses, autophagosome formation, in many cases, promotes viral replication and assembly (Jackson, 2015) while also inhibiting the fusion of autophagosomes with lysosomes (El-Hage et al., 2015). p62/SQSTM1 can facilitate the clearance of non-ubiquitinated proteins and protein aggregates by autophagy. Additionally, either an accumulation or a reduction of p62/SQSTM1 can result in the impaired degradation of proteasome substrates (Seibenhener et al., 2004). We identified significant increases in mRNA and protein levels of p62/SQSTM1 in HIV-infected astrocytes exposed to ERA alone which increased upon co-exposure with morphine. High levels of p62/SQSTM1 are indicative of dysregulation in autophagic maturation/lysosomal fusion which could be related to the increased viral production and increased inflammatory molecule production by the host (Fig. 3). Altered function of p62/SQSTM1 may ultimately lead to neurodegenerative disorders associated with HIV (Du et al., 2009; Kuusisto et al., 2001, 2002). This supports the concept that induction of autophagy is the primary mechanism for increased viral production in cells co-treated with morphine and cART.
In conclusion, we report that the combination of the protease inhibitor, lopinavir with the protease inhibitor, abacavir and the integrase inhibitor, raltegravir (LAR) modulate several histone-modifying enzymes that have been associated with decreased HIV infection. Concurring with our observations that LAR decreases HIV titer and cytokine and chemokine release, even in the presence of morphine. On the other hand, the combination of the reverse transcriptase inhibitor, emtricitabine with the protease inhibitors ritonavir and atazanavir (ERA) in the presence of morphine reversed the reduction of HIV by the ARVs alone. These observations together with accumulation of p62/SQSTM1 and possible modulation of the autophagy pathway could be the triggering cause of enhanced neurotoxicity by these antiretroviral drugs in HIV-infected human astrocytes.
Supplementary Material
Acknowledgments:
We gratefully acknowledge the support of the National Institutes of Health (NIH)-National Institute on Drug Abuse (NIDA) grants R01 DA036154; R01 DA036154-S1 Diversity Supplement in support to J.L; R21 DA041287 to N.E.H. We also acknowledge the financial support of Presidential Fellowship provided to C.R.O. by University Graduate School, Florida International University.
Footnotes
Conflict of Interest:
The authors declare that they have no conflict of interest.
References
- 1.2008. Life expectancy of individuals on combination antiretroviral therapy in high- income countries: a collaborative analysis of 14 cohort studies. Lancet (London, England) 372, 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akay C, Cooper M, Odeleye A, Jensen BK, White MG, Vassoler F, Gannon PJ, Mankowski J, Dorsey JL, Buch AM, Cross SA, Cook DR, Pena MM, Andersen ES, Christofidou-Solomidou M, Lindl KA, Zink MC, Clements J, Pierce RC, Kolson DL, Jordan-Sciutto KL, 2014. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. Journal of neurovirology 20, 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Hasani R, Bruchas MR, 2011. Molecular mechanisms of opioid receptor- dependent signaling and behavior. Anesthesiology 115, 1363–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alcami J, Lain de Lera T, Folgueira L, Pedraza MA, Jacque JM, Bachelerie F, Noriega AR, Hay RT, Harrich D, Gaynor RB, et al. , 1995. Absolute dependence on kappa B responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T lymphocytes. The EMBO journal 14, 1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreyev AY, Kushnareva YE, Starkov AA, 2005. Mitochondrial metabolism of reactive oxygen species. Biochemistry. Biokhimiia 70, 200–214. [DOI] [PubMed] [Google Scholar]
- 6.Apostolova N, Gomez-Sucerquia LJ, Gortat A, Blas-Garcia A, Esplugues JV, 2011. Compromising mitochondrial function with the antiretroviral drug efavirenz induces cell survival-promoting autophagy. Hepatology (Baltimore, Md.) 54, 1009–1019. [DOI] [PubMed] [Google Scholar]
- 7.Baloh RH, 2008. Mitochondrial dynamics and peripheral neuropathy. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry 14, 12–18. [DOI] [PubMed] [Google Scholar]
- 8.Bertrand L, Toborek M, 2015. Dysregulation of Endoplasmic Reticulum Stress and Autophagic Responses by the Antiretroviral Drug Efavirenz. Molecular pharmacology 88, 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blas-Garcia A, Apostolova N, Ballesteros D, Monleon D, Morales JM, Rocha M, Victor VM, Esplugues JV, 2010. Inhibition of mitochondrial function by efavirenz increases lipid content in hepatic cells. Hepatology (Baltimore, Md.) 52, 115–125. [DOI] [PubMed] [Google Scholar]
- 10.Bogoi RN, de Pablo A, Valencia E, Martin-Carbonero L, Moreno V, Vilchez- Rueda HH, Asensi V, Rodriguez R, Toledano V, Rodes B, 2018. Expression profiling of chromatin-modifying enzymes and global DNA methylation in CD4+ T cells from patients with chronic HIV infection at different HIV control and progression states. Clinical epigenetics 10, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boulanger MC, Liang C, Russell RS, Lin R, Bedford MT, Wainberg MA, Richard S, 2005. Methylation of Tat by PRMT6 regulates human immunodeficiency virus type 1 gene expression. Journal of virology 79, 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinkman K, Smeitink JA, Romijn JA, Reiss P, 1999. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet (London, England) 354, 1112–1115. [DOI] [PubMed] [Google Scholar]
- 13.Canizares S, Cherner M, Ellis RJ, 2014. HIV and aging: effects on the central nervous system. Seminars in neurology 34, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandra S, Mondal D, Agrawal KC, 2009. HIV-1 protease inhibitor induced oxidative stress suppresses glucose stimulated insulin release: protection with thymoquinone. Experimental biology and medicine (Maywood, N.J.) 234, 442–453. [DOI] [PubMed] [Google Scholar]
- 15.Chang Y, Levy D, Horton JR, Peng J, Zhang X, Gozani O, Cheng X, 2011. Structural basis of SETD6-mediated regulation of the NF-kB network via methyl-lysine signaling. Nucleic acids research 39, 6380–6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY, 2004. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. European journal of pharmacology 483, 175–186. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Jarujaron S, Wu X, Sun L, Zha W, Liang G, Wang X, Gurley EC, Studer EJ, Hylemon PB, Pandak WM Jr., Zhang L, Wang G, Li X, Dent P, Zhou H, 2009. HIV protease inhibitor lopinavir-induced TNF-alpha and IL-6 expression is coupled to the unfolded protein response and ERK signaling pathways in macrophages. Biochemical pharmacology 78, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chevalier MF, Julg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, Kwon DS, Rychert J, Lian J, Muller MI, Cutler S, McAndrew E, Jessen H, Pereyra F, Rosenberg ES, Altfeld M, Walker BD, Streeck H, 2011. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. Journal of virology 85, 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi WS, Palmiter RD, Xia Z, 2011. Loss of mitochondrial complex I activity potentiates dopamine neuron death induced by microtubule dysfunction in a Parkinson's disease model. The Journal of cell biology 192, 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen J, D'Agostino L, Wilson J, Tuzer F, Torres C, 2017. Astrocyte Senescence and Metabolic Changes in Response to HIV Antiretroviral Therapy Drugs. Frontiers in aging neuroscience 9, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decloedt EH, Rosenkranz B, Maartens G, Joska J, 2015. Central nervous system penetration of antiretroviral drugs: pharmacokinetic, pharmacodynamic and pharmacogenomic considerations. Clinical pharmacokinetics 54, 581–598. [DOI] [PubMed] [Google Scholar]
- 22.Dever SM, Rodriguez M, Lapierre J, Costin BN, El-Hage N, 2015. Differing roles of autophagy in HIV-associated neurocognitive impairment and encephalitis with implications for morphine co-exposure. Frontiers in microbiology 6, 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dever SM, Xu R, Fitting S, Knapp PE, Hauser KF, 2012. Differential expression and HIV-1 regulation of mu-opioid receptor splice variants across human central nervous system cell types. Journal of neurovirology 18, 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodson MW, Guo M, 2007. Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson's disease. Curr. Opin. Neurobiol. 17, 331–337. [DOI] [PubMed] [Google Scholar]
- 25.Du Y, Wooten MC, Wooten MW, 2009. Oxidative damage to the promoter region of SQSTM1/p62 is common to neurodegenerative disease. Neurobiology of disease 35, 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF, 2005. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL- 6 by astrocytes treated with opiates and HIV-1 Tat. Glia 50, 91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Hage N, Rodriguez M, Dever SM, Masvekar RR, Gewirtz DA, Shacka JJ, 2015. HIV-1 and morphine regulation of autophagy in microglia: limited interactions in the context of HIV-1 infection and opioid abuse. Journal of virology 89, 1024–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis R, Langford D, Masliah E, 2007. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nature reviews. Neuroscience 8, 33–44. [DOI] [PubMed] [Google Scholar]
- 29.Ene L, Duiculescu D, Ruta SM, 2011. How much do antiretroviral drugs penetrate into the central nervous system? Journal of medicine and life 4, 432–439. [PMC free article] [PubMed] [Google Scholar]
- 30.Fiume G, Vecchio E, De Laurentiis A, Trimboli F, Palmieri C, Pisano A, Falcone C, Pontoriero M, Rossi A, Scialdone A, Fasanella Masci F, Scala G, Quinto I, 2012. Human immunodeficiency virus-1 Tat activates NF-kappaB via physical interaction with IkappaB-alpha and p65. Nucleic acids research 40, 3548–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gangwani MR, Kumar A, 2015. Multiple Protein Kinases via Activation of Transcription Factors NF-kappaB, AP-1 and C/EBP-delta Regulate the IL-6/IL-8 Production by HIV-1 Vpr in Astrocytes. PLoS One 10, e0135633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giunta B, Ehrhart J, Obregon DF, Lam L, Le L, Jin J, Fernandez F, Tan J, Shytle RD, 2011. Antiretroviral medications disrupt microglial phagocytosis of beta- amyloid and increase its production by neurons: implications for HIV-associated neurocognitive disorders. Molecular brain 4, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, Hauser KF, 2001. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience 102, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B, 2011. Persistence of HIV- associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS (London, England) 25, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hauser KF, Knapp PE, 2014. Interactions of HIV and drugs of abuse: the importance of glia, neural progenitors, and host genetic factors. International review of neurobiology 118, 231–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hauser KF, Stiene-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC, 1996. mu-Opioid receptor-induced Ca2+ mobilization and astroglial development: morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca(2+)- dependent mechanism. Brain research 720, 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Invernizzi CF, Xie B, Richard S, Wainberg MA, 2006. PRMT6 diminishes HIV-1 Rev binding to and export of viral RNA. Retrovirology 3, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iribarne C, Berthou F, Carlhant D, Dreano Y, Picart D, Lohezic F, Riche C, 1998. Inhibition of methadone and buprenorphine N-dealkylations by three HIV-1 protease inhibitors. Drug metabolism and disposition: the biological fate of chemicals 26, 257–260. [PubMed] [Google Scholar]
- 39.Jackson WT, 2015. Viruses and the autophagy pathway. Virology 479–480, 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanazawa S, Okamoto T, Peterlin BM, 2000. Tat competes with CIITA for the binding to P-TEFb and blocks the expression of MHC class II genes in HIV infection. Immunity 12, 61–70. [DOI] [PubMed] [Google Scholar]
- 41.Kline ER, Bassit L, Hernandez-Santiago BI, Detorio MA, Liang B, Kleinhenz DJ, Walp ER, Dikalov S, Jones DP, Schinazi RF, Sutliff RL, 2009. Long-term exposure to AZT, but not d4T, increases endothelial cell oxidative stress and mitochondrial dysfunction. Cardiovascular toxicology 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kranick SM, Nath A, 2012. Neurologic complications of HIV-1 infection and its treatment in the era of antiretroviral therapy. Continuum (Minneapolis, Minn.) 18, 1319–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kravcik S, Gallicano K, Roth V, Cassol S, Hawley-Foss N, Badley A, Cameron DW, 1999. Cerebrospinal fluid HIV RNA and drug levels with combination ritonavir and saquinavir. Journal of acquired immune deficiency syndromes (1999) 21, 371–375. [PubMed] [Google Scholar]
- 44.Kumar A, Darcis G, Van Lint C, Herbein G, 2015. Epigenetic control of HIV-1 post integration latency: implications for therapy. Clin Epigenetics 7, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar GN, Rodrigues AD, Buko AM, Denissen JF, 1996. Cytochrome P450- mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. The Journal of pharmacology and experimental therapeutics 277, 423–431. [PubMed] [Google Scholar]
- 46.Kuusisto E, Salminen A, Alafuzoff I, 2001. Ubiquitin-binding protein p62 is present in neuronal and glial inclusions in human tauopathies and synucleinopathies. Neuroreport 12, 2085–2090. [DOI] [PubMed] [Google Scholar]
- 47.Kuusisto E, Salminen A, Alafuzoff I, 2002. Early accumulation of p62 in neurofibrillary tangles in Alzheimer's disease: possible role in tangle formation. Neuropathology and applied neurobiology 28, 228–237. [DOI] [PubMed] [Google Scholar]
- 48.Lagathu C, Eustace B, Prot M, Frantz D, Gu Y, Bastard JP, Maachi M, Azoulay S, Briggs M, Caron M, Capeau J, 2007. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antiviral therapy 12, 489–500. [PubMed] [Google Scholar]
- 49.Lahiri CD, Reed-Walker K, Sheth AN, Acosta EP, Vunnava A, Ofotokun I, 2016. Cerebrospinal fluid concentrations of tenofovir and emtricitabine in the setting of HIV-1 protease inhibitor-based regimens. Journal of clinical pharmacology 56, 492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lapierre J, Rodriguez M, Ojha CR, El-Hage N, 2018. Critical Role of Beclinl in HIV Tat and Morphine-Induced Inflammation and Calcium Release in Glial Cells from Autophagy Deficient Mouse. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levy D, Kuo AJ, Chang Y, Schaefer U, Kitson C, Cheung P, Espejo A, Zee BM, Liu CL, Tangsombatvisit S, Tennen RI, Kuo AY, Tanjing S, Cheung R, Chua KF, Utz PJ, Shi X, Prinjha RK, Lee K, Garcia BA, Bedford MT, Tarakhovsky A, Cheng X, Gozani O, 2011. Lysine methylation of the NF-kappaB subunit RelA by SETD6 couples activity of the histone methyltransferase GLP at chromatin to tonic repression of NF-kappaB signaling. Nature immunology 12, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, Vaeth M, Obel N, 2007. Survival of persons with and without HIV infection in Denmark, 1995–2005. Annals of internal medicine 146, 87–95. [DOI] [PubMed] [Google Scholar]
- 53.Manda KR, Banerjee A, Banks WA, Ercal N, 2011. Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood-brain barrier endothelial cells. Free radical biology & medicine 50, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maubert ME, Pirrone V, Rivera NT, Wigdahl B, Nonnemacher MR, 2015. Interaction between Tat and Drugs of Abuse during HIV-1 Infection and Central Nervous System Disease. Frontiers in microbiology 6, 1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melik Parsadaniantz S, Rivat C, Rostene W, Reaux-Le Goazigo A, 2015. Opioid and chemokine receptor crosstalk: a promising target for pain therapy? Nature reviews. Neuroscience 16, 69–78. [DOI] [PubMed] [Google Scholar]
- 56.Merlin JS, Bulls HW, Vucovich LA, Edelman EJ, Starrels JL, 2016. Pharmacologic and non-pharmacologic treatments for chronic pain in individuals with HIV: a systematic review. AIDS care 28, 1506–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreau K, Luo S, Rubinsztein DC, 2010. Cytoprotective roles for autophagy. Current opinion in cell biology 22, 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M, 1994. Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921. [DOI] [PubMed] [Google Scholar]
- 59.Murphy MP, 2009. How mitochondria produce reactive oxygen species. The Biochemical journal 417, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nooka S, Ghorpade A, 2017. HIV-1-associated inflammation and antiretroviral therapy regulate astrocyte endoplasmic reticulum stress responses. Cell death discovery 3, 17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Osborn L, Kunkel S, Nabel GJ, 1989. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc. Natl. Acad. Sci. U. S. A. 86, 2336–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, Kempf DJ, Mellors JW, Coffin JM, King MS, 2008. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A. 105, 3879–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papparella I, Ceolotto G, Berto L, Cavalli M, Bova S, Cargnelli G, Ruga E, Milanesi O, Franco L, Mazzoni M, Petrelli L, Nussdorfer GG, Semplicini A, 2007. Vitamin C prevents zidovudine-induced NAD(P)H oxidase activation and hypertension in the rat. Cardiovascular research 73, 432–438. [DOI] [PubMed] [Google Scholar]
- 64.Piccinini M, Rinaudo MT, Anselmino A, Buccinna B, Ramondetti C, Dematteis A, Ricotti E, Palmisano L, Mostert M, Tovo PA, 2005. The HIV protease inhibitors nelfinavir and saquinavir, but not a variety of HIV reverse transcriptase inhibitors, adversely affect human proteasome function. Antiviral therapy 10, 215–223. [PubMed] [Google Scholar]
- 65.Pitha PM, 2011. Innate antiviral response: role in HIV-1 infection. Viruses 3, 1179–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ran X, Ao Z, Trajtman A, Xu W, Kobinger G, Keynan Y, Yao X, 2017. HIV-1 envelope glycoprotein stimulates viral transcription and increases the infectivity of the progeny virus through the manipulation of cellular machinery. Scientific Reports 7, 9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rao VR, Ruiz AP, Prasad VR, 2014. Viral and cellular factors underlying neuropathogenesis in HIV associated neurocognitive disorders (HAND). AIDS research and therapy 11, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robertson K, Liner J, Meeker RB, 2012. Antiretroviral neurotoxicity. Journal of neurovirology 18, 388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robertson KR, Su Z, Margolis DM, Krambrink A, Havlir DV, Evans S, Skiest DJ, 2010. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology 74, 1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodriguez M, Lapierre J, Ojha CR, Estrada-Bueno H, Dever SM, Gewirtz DA, Kashanchi F, El-Hage N, 2017. Importance of Autophagy in Mediating Human Immunodeficiency Virus (HIV) and Morphine-Induced Metabolic Dysfunction and Inflammation in Human Astrocytes. Viruses 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosca A, Anton G, Ene L, Iancu I, Temereanca A, Achim CL, Ruta SM, 2017. Immunoassay and molecular methods to investigate DNA methylation changes in peripheral blood mononuclear cells in HIV infected patients on cART. Journal of immunoassay & immunochemistry 38, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, Burchell AN, Cohen M, Gebo KA, Gill MJ, Justice A, Kirk G, Klein MB, Korthuis PT, Martin J, Napravnik S, Rourke SB, Sterling TR, Silverberg MJ, Deeks S, Jacobson LP, Bosch RJ, Kitahata MM, Goedert JJ, Moore R, Gange SJ, 2013. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 8, e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, Wooten MW, 2004. Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol 24, 8055–8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shah A, Gangwani MR, Chaudhari NS, Glazyrin A, Bhat HK, Kumar A, 2016. Neurotoxicity in the Post-HAART Era: Caution for the Antiretroviral Therapeutics. Neurotoxicity research 30, 677–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Squitieri F, Cannella M, Sgarbi G, Maglione V, Falleni A, Lenzi P, Baracca A, Cislaghi G, Saft C, Ragona G, Russo MA, Thompson LM, Solaini G, Fornai F, 2006. Severe ultrastructural mitochondrial changes in lymphoblasts homozygous for Huntington disease mutation. Mechanisms of ageing and development 127, 217–220. [DOI] [PubMed] [Google Scholar]
- 76.Stiene-Martin A, Hauser KF, 1991. Glial growth is regulated by agonists selective for multiple opioid receptor types in vitro. Journal of neuroscience research 29, 538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swingler S, Morris A, Easton A, 1994. Tumour necrosis factor alpha and interleukin- 1 beta induce specific subunits of NFKB to bind the HIV-1 enhancer: characterisation of transcription factors controlling human immunodeficiency virus type 1 gene expression in neural cells. Biochemical and biophysical research communications 203, 623–630. [DOI] [PubMed] [Google Scholar]
- 78.Trejbalova K, Kovarova D, Blazkova J, Machala L, Jilich D, Weber J, Kucerova D, Vencalek O, Hirsch I, Hejnar J, 2016. Development of 5' LTR DNA methylation of latent HIV-1 provirus in cell line models and in long-term-infected individuals. Clinical epigenetics 8, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaidya NK, Ribeiro RM, Perelson AS, Kumar A, 2016. Modeling the Effects of Morphine on Simian Immunodeficiency Virus Dynamics. PLoS Comput. Biol. 12, e1005127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vallabhapurapu S, Karin M, 2009. Regulation and function of NF-kappaB transcription factors in the immune system. Annual review of immunology 27, 693–733. [DOI] [PubMed] [Google Scholar]
- 81.Van Duyne R, Easley R, Wu W, Berro R, Pedati C, Klase Z, Kehn-Hall K, Flynn EK, Symer DE, Kashanchi F, 2008. Lysine methylation of HIV-1 Tat regulates transcriptional activity of the viral LTR. Retrovirology 5, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X, Gao Y, Tan J, Devadas K, Ragupathy V, Takeda K, Zhao J, Hewlett I, 2012. HIV-1 and HIV-2 infections induce autophagy in Jurkat and CD4+ T cells. Cellular signalling 24, 1414–1419. [DOI] [PubMed] [Google Scholar]
- 83.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X, 2008. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl. Acad. Sci. U. S.A. 105, 19318–19323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang ZV, Hill JA, 2015. Protein quality control and metabolism: bidirectional control in the heart. Cell metabolism 21, 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie B, Invernizzi CF, Richard S, Wainberg MA, 2007. Arginine methylation of the human immunodeficiency virus type 1 Tat protein by PRMT6 negatively affects Tat Interactions with both cyclin T1 and the Tat transactivation region. Journal of virology 81, 4226–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X, Cao R, Liu R, Zhao R, Huang Y, Gurley EC, Hylemon PB, Pandak WM, Wang G, Zhang L, Li X, Zhou H, 2014. Reduction of the HIV protease inhibitor-induced ER stress and inflammatory response by raltegravir in macrophages. PLoS One 9, e90856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao L, Zhu Y, Wang D, Chen M, Gao P, Xiao W, Rao G, Wang X, Jin H, Xu L, Sui N, Chen Q, 2010. Morphine induces Beclin 1- and ATG5-dependent autophagy in human neuroblastoma SH-SY5Y cells and in the rat hippocampus. Autophagy 6, 386–394. [DOI] [PubMed] [Google Scholar]
- 88.Zhou D, Spector SA, 2008. Human immunodeficiency virus type-1 infection inhibits autophagy. AIDS (London, England) 22, 695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.