Abstract
Eligibility for liver transplant is most commonly decided by measuring tumor size and number on radiographic imaging. However, this method often underestimates the extent of disease. Evaluation of tumor histology has been shown to improve risk stratification when compared with imaging-based transplant criteria, but the World Health Organization (WHO) guidelines for grading hepatocellular carcinoma (HCC) are imprecise and require subjective interpretation by the pathologist. We performed a retrospective analysis of 190 explanted livers containing HCC and correlated histologic features with posttransplant recurrence to formulate a three-tiered, point-based scoring system that categorizes tumors as having a low, intermediate, or high risk of recurrence. Our Recurrence Risk Assessment Score (RRAS) evaluates tumor architecture and specific cytologic features—nuclear pleomorphism, cytoplasmic amphophilia, and nuclear-to-cytoplasmic ratio—showing superior stratification of HCC recurrence risk compared with imaging criteria and grade assigned by WHO methodology. Stratifying tumors using RRAS criteria, the rate of recurrence after transplant was 0% among low-risk tumors (compared with 3% of well-differentiated tumors), 12% among intermediate-risk tumors (compared with 15% of moderately differentiated tumors), and 54% among high-risk tumors (compared with 29% of poorly differentiated tumors). Receiver operating characteristic analysis shows significantly improved performance of RRAS criteria in predicting HCC recurrence compared with WHO grade (area under curve of 0.841 and 0.671, respectively; P = 0.0061). Our results indicate that evaluation of tumor histology offers superior prediction of recurrence risk following liver transplantation compared with radiographic criteria, and that the RRAS system better stratifies recurrence risk compared with HCC grading by WHO methodology.
Keywords: HCC, hepatocellular carcinoma, grading, scoring, recurrence
Liver transplantation is an increasingly common therapeutic option for patients with hepatocellular carcinoma (HCC).1–5 Before 2002, fewer than 5% of liver transplants were performed for HCC; that number has now risen to 20%.6,7 The most widely used criteria to determine transplant eligibility rely on radiographic measurement of tumor size and number. This method of preoperative risk stratification was first validated by Mazzaferro et al,1 which established what became known as the Milan criteria. Since then, various modifications to the original Milan system have been proposed—examples include the University of California, San Francisco (UCSF) and “up-to-seven” criteria—but all commonly used selection methods share a fundamental reliance on radiographic measurements.3–5,8
In common practice, masses arising within a cirrhotic liver are presumed to be HCC when characteristic imaging findings are identified,9 and are treated accordingly without biopsy confirmation. However, a review of United Network for Organ Sharing (UNOS) data found that 31% of patients transplanted for a small HCC lesion (less than 1.9 cm) had been misdiagnosed on imaging and showed no evidence of viable or treated HCC in the explant.10 Furthermore, there is limited data evaluating recent imaging performance, as the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) have eliminated the requirement for tissue diagnosis in tumors that arise in cirrhotic livers and in the setting of chronic hepatitis B.11
Since the introduction of the Milan criteria, it has been known that the radiographic measures of tumor size and number are often discordant with measurements taken on gross evaluation. Multiple studies have reported that imaging-based selection criteria yield incorrect patient classification in 15% to 30% of transplant recipients, once preoperative tumor measurements are reconciled with gross examination of the explant.1,5,10,12,13 Small HCC lesions are frequently inapparent on preoperative imaging, and significant disagreement on tumor size is common.
Recently, multiple studies have found that histologic differentiation is a better predictor of tumor recurrence following transplantation when compared with radio-graphic methods of risk stratification.2,14–16 However, current World Health Organization (WHO) guidelines for grading HCC rely on a subjective interpretation of tumor characteristics without precisely defined histologic criteria,17 ultimately requiring the pathologist to integrate a variable number of features into a single impression. The absence of firm-grading parameters has led to a low level of agreement between pathologists.18
In this study, we analyzed specific histologic variables in HCC-containing liver explants and correlated these findings with tumor recurrence following transplantation. We then used these features to develop a point-based scoring system that better stratifies HCC recurrence risk compared with both imaging-based transplant criteria and current WHO tumor grading methodology.
MATERIALS AND METHODS
Patient Selection
Spanning 1997 to 2014, 1061 liver transplants were performed at University of California, San Francisco Medical Center. Three hundred fifty-one explanted livers contained HCC, including 5 cases of mixed HCC-cholangiocarcinoma that were excluded from our analysis. Of the remaining cases, 190 had adequate follow-up and accessible formalin-fixed paraffin-embedded tissue blocks for histologic analysis. Within this group, 184 had available preoperative imaging reports.
The median follow-up time was 6.9 years, ranging from 52 days to 19.7 years. Clinical and radiologic information were obtained from the electronic medical record. Collected data included imaging reports, progress notes, pathology reports, intrahepatic or metastatic tumor recurrence, and death.
Study Design
This study involved a retrospective review of both electronic medical records and microscopic evaluation of every case. In patients with preoperative imaging, the extent of disease was measured using computed tomography, magnetic resonance imaging, or ultrasound. Radiographic staging was determined by the last imaging study performed before liver transplant. In cases where ablation zones from bridging therapy—either transarterial chemoembolization or radio frequency ablation performed before transplantation—made determination of tumor size uncertain, the imaging study immediately before therapy was used. Tumor measurements from gross evaluation of the explant were obtained from the pathology report. Where indicated in reports, only measurements of viable tumor were used.
The primary outcome measured in this study was tumor recurrence, which included either intrahepatic tumor recurrence or metastatic spread of HCC following transplantation. All available slides and formalin-fixed paraffin-embedded tissue blocks from each case were gathered and reviewed. Hematoxylin and eosin (H&E), reticulin, and cytokeratin 19 (CK19) immunohistochemical stains were (prediluted clone RCK108; Dako/Agilent, Santa Clara, CA) were performed on the section of tumor with the most atypical cytoarchitectural features for each case.
The aim of this study was to correlate histologic characteristics with HCC recurrence and develop a clinically relevant scoring system. The following histologic parameters were evaluated in 109 explants: architectural pattern, nuclear-to-cytoplasmic ratio, nuclear pleomorphism, cytoplasmic amphophilia, presence of fatty change, macronucleoli, mitotic activity, cytoplasmic granularity, and CK19 labeling. After the initial evaluation, in which Recurrence Risk Assessment Score (RRAS) criteria were established, an additional 81 explants were evaluated; the performance of the RRAS in predicting tumor recurrence was compared with WHO grade in both the independent cohort and the cumulative total of 190 explants.
At our institution, a five-tiered grading system for HCC is used, which is derived from the WHO methodology. The WHO describes four histologic grades: well differentiated, moderately differentiated, poorly differentiated, and un-differentiated. At our institution, both poorly differentiated and undifferentiated patterns (using WHO definitions) are categorized as poorly differentiated. In addition, our system includes the following hybrid categories: well-to-moderately differentiated and moderately-to-poorly differentiated. As the WHO does not recognize these hybrid categories, our tumor classification was converted to the standard WHO grading system based on the worst histology present. Cases were evaluated independently and blinded to outcome by two pathologists: a subspecialty hepatobiliary pathologist (R.M.G.) and hepatobiliary pathology fellow (D.E.R.). A subset of 20 challenging cases, selected based on the severity of grading discrepancy, were additionally evaluated by a second subspecialty hepatobiliary pathologist (S.K.), and the degree of inter-rater agreement was assessed.
Radiographic transplant eligibility was established by either Milan or UCSF criteria. Milan criteria assignment required that the patient have either (1) a solitary tumor measuring 5 cm or less, or (2) no more than three tumors, each measuring 3 cm or less. Assignment to UCSF criteria required either (1) a solitary tumor measuring 6.5 cm or less, or (2) no more than three tumors, with the largest measuring 4.5 cm or less and the total tumor diameter measuring 8 cm or less.
Histologic Characteristics
Tumor Architecture
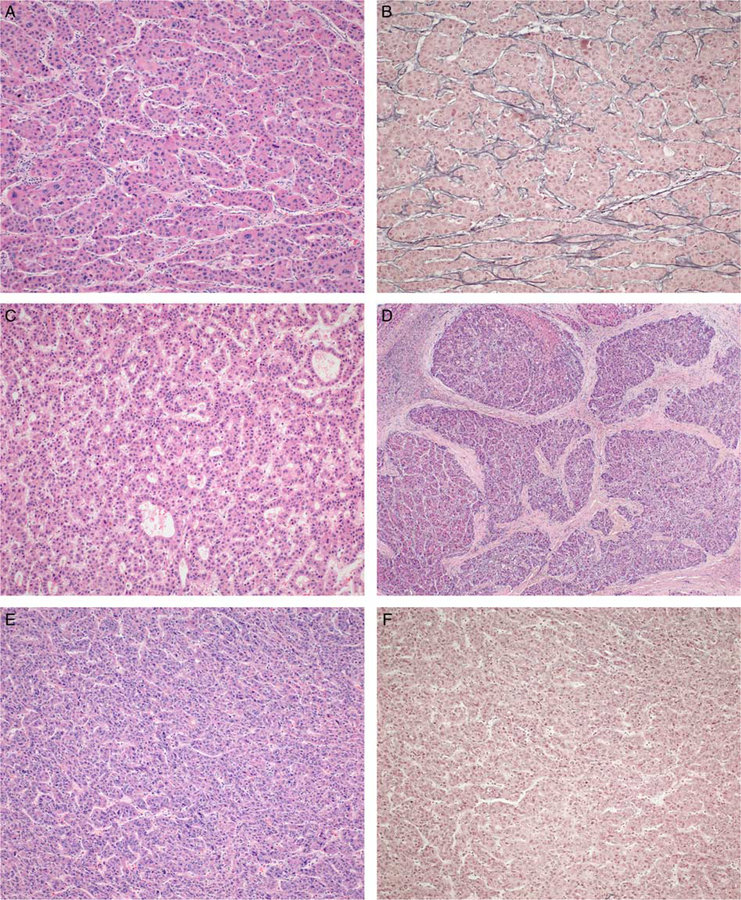
Architectural growth patterns were differentially associated with tumor recurrence (P = 0.0139; Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/PAS/A601). The WHO recognizes four architectural patterns for HCC, and our categorization was consistent with their established definitions. Briefly, a trabecular growth pattern was defined as variably thick cords of tumor cells separated by sinusoidal spaces (Figs. 1A, B). An acinar growth pattern describes gland-like structures within the tumor (Fig. 1C), typically formed by a monolayer of tumor cells and often admixed with trabecular architecture elsewhere. Neither trabecular nor acinar growth were significantly associated with tumor recurrence.
FIGURE 1.
Tumor architecture is classified into four growth patterns. Trabecular growth (A) shows cords of tumor cells separated by sinusoidal spaces, retaining some degree of reticulin framework (B) (H&E and reticulin). The acinar pattern (C) contains gland-like differentiation (H&E). A scirrhous growth pattern (D) shows thick and dense fibrous bands penetrating and separating the tumor into irregular nodules (H&E). Solid growth (E) denotes a pattern without apparent sinusoidal spaces, which can be confirmed if accompanied by near complete loss of reticulin framework (F) (H&E and reticulin).
However, two architectural patterns were associated with recurrence after transplantation: (1) scirrhous growth, which is characterized by the presence of marked and thick fibrous bands along intratumoral sinusoidal spaces, separating the tumor into small-to-medium size islands within a background of dense fibrosis (Fig. 1D), and (2) solid growth, which denotes a pattern in which sinusoidal spaces are inconspicuous (Figs. 1E, F). We found that distinguishing solid architecture from certain trabecular growth patterns on hematoxylin and eosin stain alone was not always possible. In unclear cases, a reticulin stain was used to help distinguish these patterns. Tumors with trabecular architecture show a disrupted-but-recognizable reticulin framework (Fig. 1B), especially along sinusoidal spaces, and we categorized a tumor as having solid architectural growth only if near complete loss of reticulin staining was observed in at least two 20× microscopic fields (0.95 mm2, Fig. 1F).
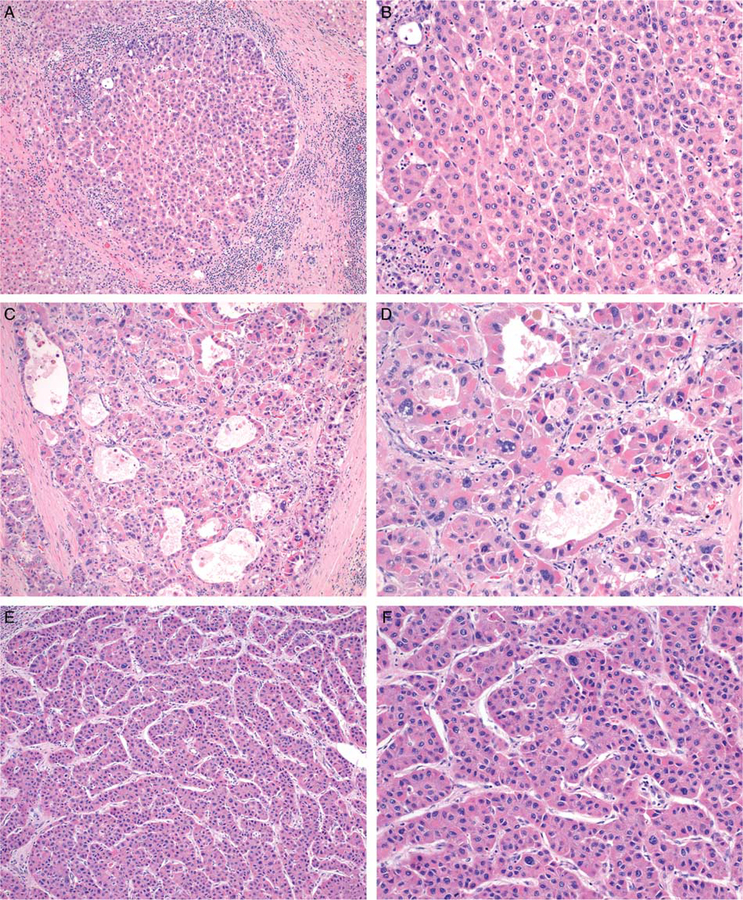
FIGURE 2.
The degree of nuclear pleomorphism falls into two categories. Tumors without significant pleomorphism (A, B) contain relatively uniform nuclei. Those with significant pleomorphism show nuclear size variation greater than 2× in at least two foci within a single 20× field. Some cases show diffuse nuclear pleomorphism (C, D) while this feature is only focally present in others (E, F) (H&E).
Nuclear Pleomorphism
A spectrum of nuclear atypia is found in HCC, which ranges from largely uniform nuclei, nearly indistinguishable from those of background hepatocytes, to markedly pleomorphic nuclei. The presence of nuclear pleomorphism was associated with tumor recurrence (P = 0.0010; Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/PAS/A601). We graded pleomorphism based primarily on the relative size variance between neighboring cell nuclei. Tumors without significant pleomorphism are permitted to show up to a 2× size variation in nearby cells (Figs. 2A, B). Some nuclear contour irregularity and wrinkling is acceptable, and variation in chromatin density is permitted. Tumors in which nuclear size variation exceeds 2× are classified as having significant pleomorphism. While some show obvious and diffuse pleomorphism (Figs. 2C, D), other cases with atypia are focal and, at times, more subtle (Figs. 2E, F). We considered a tumor to harbor nuclear pleomorphism if a cell with nuclear diameter more than twice that of its immediate neighbors was present in at least 2 distinct foci within a single 20× field (0.95 mm2).
Nuclear-to-Cytoplasmic Ratio
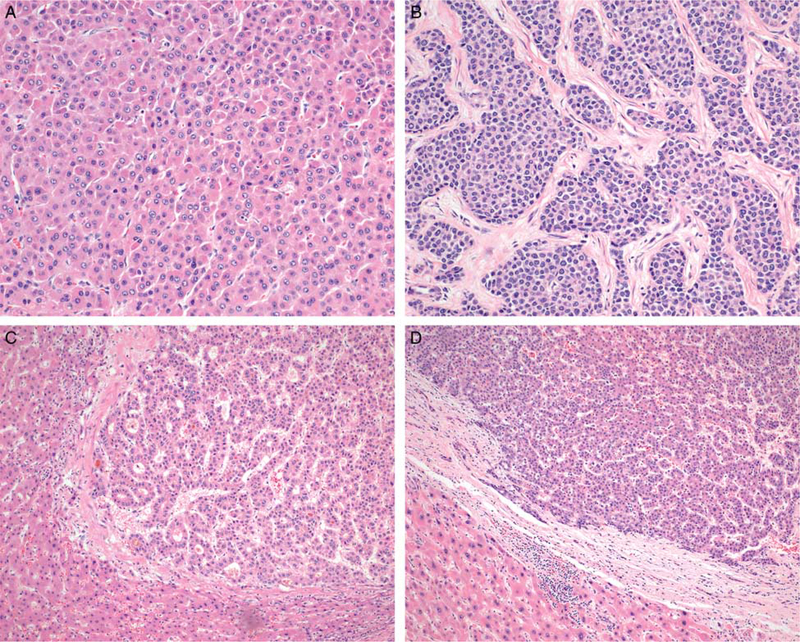
Nuclear-to-cytoplasmic ratio can be increased in HCC, sometimes imparting a striking appearance (Figs. 3A, B). Tumors in which multiple foci show a ratio of at least 50% are associated with a higher risk of recurrence (P = 0.0462; Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/PAS/A601). To determine this ratio, we quantified the relative volume of tumor nuclei and tumor cytoplasm within an area equivalent to one 40× microscopic field (0.24 mm2). We designated a tumor as having an increased nuclear-to-cytoplasmic ratio if the change was present in more than 50% of tumor cells in at least 2 separate 40× fields.
FIGURE 3.
Nuclear-to-cytoplasmic ratio is measured as either <50% (A) or ≥ 50% (B) (H&E). Cytoplasmic staining lies on a spectrum ranging from eosinophilic (C) to amphophilic (D) (H&E). Background hepatocytes can often act as a reference for the degree of eosinophilia within HCC.
Cytoplasmic Staining Characteristics
The cytoplasm in HCC ranges from eosinophilic, similar to the eosinophilia of background hepatocytes, to markedly amphophilic (Figs. 3C, D). Staining characteristics of tumor cell cytoplasm were significantly associated with recurrence (P = 0.0009; Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/PAS/A601). We classified a tumor as having amphophilic cytoplasm only if the finding was diffusely present in at least two contiguous 20× microscopic fields (1.9 mm2 total area).
Statistical Analysis
All statistical analyses were performed using Medcalc Statistical Software, version 17.0.4 (MedCalc Software bvba, Osten, Belgium), and the cutoff for statistical significance was P-value ≤ 0.05 in all cases. Statistical differences between categorical variables were determined by χ2 or Fisher exact tests. Multiple logistic regression was performed for multivariate analysis; variables were included in the regression if they had a P-value <0.1 on univariate analysis, and were introduced simultaneously into the model. Receiver operating characteristic (ROC) curves were calculated using logistic regression and evaluated to assess the accuracy, sensitivity, and specificity of histologic grading criteria. Inter-rater agreement was assessed by the weighted kappa statistic, using the methodology described in Fleiss et al.19
RESULTS
Tumor Characteristics
Radiologic and pathologic criteria for 190 explants are summarized in Table 1. Of these, 184 had preoperative imaging reports. One hundred sixty-one cases (87.5%) met Milan criteria, 17 (9.2%) exceeded Milan criteria but met UCSF criteria, and 6 cases (3.3%) exceeded UCSF criteria. Gross examination of the liver revealed discrepant transplant criteria assignment in 31.5% of cases, in which 79.3% were understaged on radiographic evaluation and 20.7% were overstaged. HCC in explants showing concordant radiologic and pathologic staging recurred at a rate of 8.7%, while cases understaged by imaging showed significantly worse outcomes, recurring at a rate of 19.6% (P = 0.0498).
TABLE 1.
Radiologic and Pathologic Characteristics of Liver Explants With HCC
| Recurrences | Total Cases | Percentage | Significance | |
|---|---|---|---|---|
| Total number of liver explants from 1997 to 2015 | 1061 | |||
| With HCC | 351 | |||
| With available paraffin-embedded tissue | 190 | |||
| With tissue and preoperative radiology reports | 184 | |||
| Transplant criteria on imaging | 0.1850 | |||
| Meets Milan criteria | 18 | 161 | 11.2 | |
| Meets UCSF criteria | 1 | 17 | 5.9 | |
| Exceeds Milan/UCSF criteria | 2 | 6 | 33.3 | |
| Transplant criteria on gross examination | 0.0179 | |||
| Meets Milan criteria | 10 | 128 | 7.8 | |
| Meets UCSF criteria | 5 | 35 | 14.3 | |
| Exceeds Milan/UCSF criteria | 6 | 21 | 28.6 | |
| Disagreement between imaging and gross examination | 0.4552 | |||
| Upstaged from Milan to UCSF criteria by gross examination | 4 | 26 | 15.4 | |
| Upstaged beyond Milan/UCSF criteria by gross examination | 5 | 20 | 25.0 | |
| Downstaged by gross examination | 1 | 12 | 8.3 | |
| Tumor differentiation | 0.0167 | |||
| Well differentiated | 2 | 74 | 2.7 | |
| Well-to-moderately differentiated | 4 | 21 | 19.0 | |
| Moderately differentiated | 11 | 81 | 13.6 | |
| Moderately-to-poorly differentiated | 1 | 5 | 20.0 | |
| Poorly differentiated | 3 | 9 | 33.3 | |
| Vascular invasion | < 0.0001 | |||
| Absent | 11 | 166 | 6.6 | |
| Present | 10 | 24 | 41.7 | |
| Number of masses on imaging | 0.0204 | |||
| 0 | 4 | 62 | 6.5 | |
| 1 | 13 | 74 | 17.6 | |
| 2 | 1 | 37 | 2.7 | |
| 3+ | 3 | 11 | 27.3 | |
| Size of largest mass on imaging (cm) | 0.4980 | |||
| 0–2 | 9 | 101 | 8.9 | |
| 2–4 | 9 | 63 | 14.3 | |
| ≥ 4 | 3 | 20 | 15.0 | |
| Aggregate size of masses on imaging (cm) | 0.2647 | |||
| 0–2 | 9 | 91 | 9.9 | |
| 2–4 | 7 | 51 | 13.7 | |
| 4–6 | 2 | 31 | 6.5 | |
| ≥6 | 3 | 11 | 27.3 | |
| Number of masses on gross examination | 0.4660 | |||
| 1 | 9 | 88 | 10.2 | |
| 2 | 6 | 52 | 11.5 | |
| 3 | 1 | 23 | 4.3 | |
| 4 | 2 | 15 | 13.3 | |
| 5+ | 3 | 12 | 25.0 | |
| Size of largest mass on gross examination (cm) | 0.0008 | |||
| 0–2 | 1 | 55 | 1.8 | |
| 2–4 | 9 | 92 | 9.8 | |
| ≥4 | 11 | 43 | 25.6 | |
| Aggregate size of masses on gross examination (cm) | 0.0408 | |||
| 0–2 | 0 | 39 | 0.0 | |
| 2–4 | 6 | 62 | 9.7 | |
| 4–6 | 6 | 40 | 15.0 | |
| ≥6 | 9 | 49 | 18.4 |
Overall, imaging tended to underestimate disease extent. In our dataset, preoperative imaging measured an aggregate tumor size of at least 6 cm in only 6% of patients. However, gross examination increased this value to 25%. Although measuring the size of the largest mass and the aggregate size of all masses was not predictive of outcome on radiographic examination, both were significantly associated with recurrence once the explant was examined grossly (P = 0.0008 and 0.0408, respectively).
The rate of HCC recurrence was 10.7% in cases that met either Milan or UCSF imaging criteria. Stratifying these cases by tumor differentiation using WHO methodology showed a significant association with recurrence in a stepwise manner (P = 0.0082): well-differentiated tumors recurred at a low rate (3%), while moderately differentiated tumors recurred in higher proportion (15%), and poorly differentiated tumors recurred with the highest frequency (29%) (Supplementary Fig. 1, Supplemental Digital Content 2, http://links.lww.com/PAS/A602).
Histologic Scoring Criteria
Tumors in 109 explants were evaluated for the presence of specific histologic parameters. These features included the architectural pattern (Fig. 1), presence or absence of nuclear pleomorphism (Fig. 2), nuclear-to-cytoplasmic ratio (Figs. 3A, B), degree of cytoplasmic amphophilia (Figs. 3C, D), presence of macronucleoli, fatty change, mitotic rate, cytoplasmic granularity, and immunohistochemical labeling of tumor cells by CK19.
Recurrence Risk Assessment Score
The presence of solid or scirrhous architectural patterns, high nuclear-to-cytoplasmic ratio, nuclear pleomorphism, cytoplasmic amphophilia, and high mitotic activity were significantly associated with tumor recurrence in univariate analysis (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/PAS/A601). The presence of fatty change, macronucleoli, granular cytoplasm, and positive immunohistochemical labeling for CK19 showed no significant association with recurrence.
Multiple logistic regression (Table 2) demonstrated strong correlation between tumor recurrence and nuclear pleomorphism, cytoplasmic amphophilia, solid and scirrhous architecture, and high nuclear-to-cytoplasmic ratio; however, mitotic activity showed only weak correlation and was excluded from our scoring system. The presence of both nuclear pleomorphism and vascular invasion were identified as independent predictors of HCC recurrence following transplantation (Supplementary Table 2, Supplemental Digital Content 3, http://links.lww.com/PAS/A603).
TABLE 2.
Multiple Logistic Regression Model for Histologic Features
| Variables | Coefficient (β) | SE | Wald (χ2) | Significance | Odds Ratio | 95% CI |
|---|---|---|---|---|---|---|
| Nuclear pleomorphism* | 1.449 | 0.602 | 5.791 | 0.016 | 4.260 | 1.309–13.871 |
| Cytoplasmic amphophilia | 1.395 | 0.832 | 2.809 | 0.094 | 0.248 | 0.049–1.267 |
| N:C ≥ 50% | 1.012 | 0.599 | 2.851 | 0.091 | 2.751 | 0.850–8.904 |
| Architecture: solid | 1.304 | 0.939 | 1.926 | 0.165 | 3.683 | 0.584–23.225 |
| Architecture: scirrhous | 1.021 | 0.713 | 2.054 | 0.152 | 2.777 | 0.687–11.222 |
| Architecture: acinar | 0.736 | 0.878 | 0.703 | 0.402 | 2.088 | 0.374–11.671 |
| Macronucleoli | 0.570 | 0.622 | 0.841 | 0.359 | 1.768 | 0.523–5.979 |
| Mitotic index | 0.337 | 0.596 | 0.320 | 0.571 | 1.401 | 0.436–4.507 |
| CK19 labeling | −1.229 | 1.350 | 0.828 | 0.363 | 0.293 | 0.021–4.127 |
| Intratumoral steatosis | −0.486 | 0.706 | 0.473 | 0.492 | 0.615 | 0.154–2.457 |
| Intercept | −3.477 | 0.750 |
Nuclear pleomorphism is a statistically significant independent predictor of tumor recurrence after transplant.
95% CI indicates 95% confidence interval for estimated odds ratio; intercept, mathematical constant;
N:C, nuclear-to-cytoplasmic ratio; Wald, Wald test statistic.
From these data, we developed a point-based risk stratification system, termed the RRAS, which assigns one point to each of four histologic features—tumor architecture, nuclear-to-cytoplasmic ratio, nuclear pleomorphism, and cytoplasmic amphophilia—yielding a composite score that can be evaluated to stratify the risk of tumor recurrence following liver transplantation (Table 3). We considered tumors containing all four histologic features to be high risk (RRAS of 4), the absence of all features to be low risk (RRAS of 0), and classified tumors in between as intermediate risk (RRAS of 1 to 3). Histologic characteristics were assessed across the entire tumor, and the reported RRAS category was based on the highest scoring discrete area. Even if the predominant pattern was low risk, the reported score reflected the area of tumor containing the most histologic features associated with recurrent disease. All areas were evaluated independently, and features were not combined across discrete foci. For example, if one focus contained nuclear pleomorphism without other adverse features (RRAS of 1), and a separate area contained all adverse features except nuclear pleomorphism (RRAS of 3), the reported category would be intermediate risk even though all four adverse features were identified across separate tumor foci.
TABLE 3.
Recurrence Risk Assessment Score
| Feature | Score |
|---|---|
| Architecture | |
| Trabecular or acinar | 0 |
| Scirrhous or solid | 1 |
| Nuclear-to-cytoplasmic ratio (%) | |
| < 50 | 0 |
| ≥ 50 | 1 |
| Nuclear pleomorphism | |
| Absent | 0 |
| Present | 1 |
| Cytoplasm | |
| Eosinophilic | 0 |
| Amphophilic | 1 |
| RRAS category | |
| Low risk | 0 points |
| Intermediate risk | 1–3 points |
| High risk | 4 points |
Point-based Scoring Better Predicts Tumor Recurrence
To evaluate performance of the RRAS system, we examined 81 additional explants and correlated our scoring methodology with recurrence status in this independent cohort, as well as in combination with the original 109 cases used to establish the RRAS.
When evaluating the most difficult cases in our study, WHO guidelines yielded only slight agreement between graders, while categorization using the RRAS criteria permitted moderate agreement within that same subset (Supplementary Fig. 2, Supplemental Digital Content 4, http://links.lww.com/PAS/A604). Tumors stratified by RRAS methodology do not merely recapitulate WHO categories, but instead include a mixture of differentiation grades. Of the 13 cases categorized as high risk, 3 showed poor differentiation, 8 were moderately differentiated, and 2 were well differentiated. One hundred seventeen cases were classified as intermediate risk, and these included 9 poorly differentiated, 74 moderately differentiated, and 34 well-differentiated tumors. Of the 60 low risk cases, 2 showed poor differentiation, 20 were moderately differentiated, and 38 were well differentiated.
A comparison of WHO and RRAS methodology found that a considerably higher proportion of patients who developed recurrent HCC were identified by high risk RRAS categorization (54%) compared with poor differentiation (29%) using WHO methodology (Table 4). In addition, a smaller proportion of recurrent HCC was identified among tumors categorized as low and intermediate risk by RRAS criteria, compared with well differentiated or moderately differentiated using WHO methods. Follow-up time was similar between these two groups. Of note, while we did not find an association between preoperative treatment status and either RRAS category or WHO grade, both systems were evaluated away from regions with obvious treatment effect wherever possible.
TABLE 4.
Posttransplant HCC Recurrence Stratified by RRAS and WHO Grade
| Recurrences | Total Cases | Percentage | |
|---|---|---|---|
| RRAS raw score | |||
| 0 | 0 | 60 | 0 |
| 1 | 3 | 52 | 5.8 |
| 2 | 5 | 43 | 11.6 |
| 3 | 6 | 22 | 27.3 |
| 4 | 7 | 13 | 53.9 |
| RRAS category | |||
| Low risk | 0 | 60 | 0 |
| Intermediate risk | 14 | 117 | 12.0 |
| High risk | 7 | 13 | 53.9 |
| WHO grade | |||
| Well differentiated | 2 | 74 | 2.7 |
| Moderately differentiated | 15 | 102 | 14.7 |
| Poorly differentiated | 4 | 14 | 28.6 |
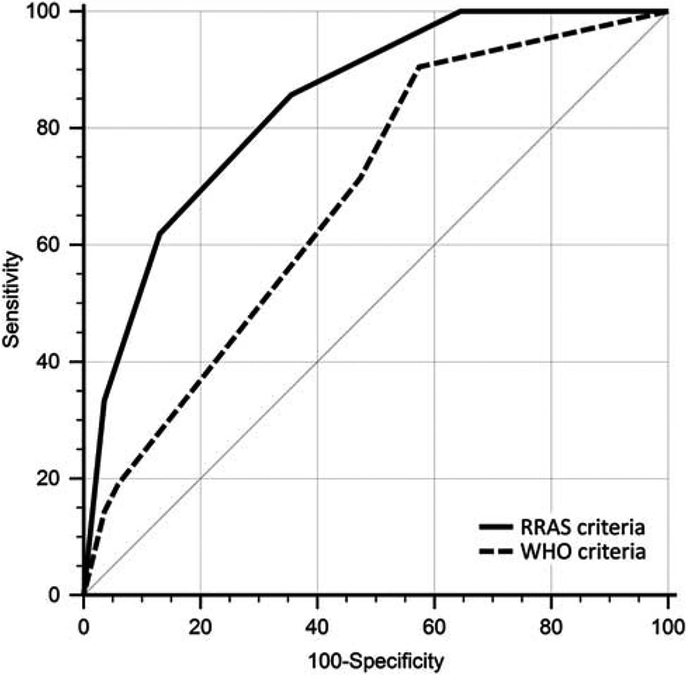
ROC analysis showed that WHO methodology has relatively low performance in predicting HCC recurrence (Fig. 4, area under curve = 0.617), whereas evaluating the same set of tumors using RRAS criteria yields significantly improved predictive performance (area under curve = 0.841; P = 0.0061). An analysis of 81 additional cases, which were not part of the initial cohort of explants used to establish the RRAS criteria, showed similar results (Supplementary Fig. 3, Supplemental Digital Content 5, http://links.lww.com/PAS/A605).
FIGURE 4.
ROC curves were analyzed to assess the performance of the WHO tumor grade and RRAS in predicting posttransplant HCC recurrence (area under curve = 0.617 and 0.841, respectively). RRAS allowed for a more accurate prediction of tumor recurrence following transplantation (P = 0.0061, n = 190).
Vascular invasion (both small and large vessel) is a well-established predictor of HCC recurrence and is routinely reported on resection specimens. However, the presence of small vessel invasion cannot be reliably excluded on biopsy due to limited sampling. In contrast, the cytologic and architectural components of the RRAS may be evaluated on either biopsy or resection specimens, and ROC curve analysis demonstrates that our point-based criteria better predicts tumor recurrence compared with vascular invasion alone (Supplementary Fig. 4, Supplemental Digital Content 6, http://links.lww.com/PAS/A606; P = 0.017). In practice, we would advocate reporting both RRAS and vascular involvement on resection specimens for optimal prediction of recurrence risk. For instance, small vessel invasion was seen in 17 of 117 (14.5%) cases categorized as intermediate risk by RRAS criteria, and 7 of these cases (41.2%) had tumor recurrence. Of the 14 patients with intermediate-risk HCC who had posttransplant recurrence, vascular invasion was present in half. Intermediate-risk HCC with vascular invasion may therefore represent a higher risk subgroup that warrants additional clinical scrutiny following transplantation.
DISCUSSION
The most commonly used method to determine eligibility for liver transplant is based on radiographic measurement of tumor extent, an approach that has remained largely unchanged over the past two decades. In the seminal paper by Mazzaferro et al,1 the rate of tumor recurrence in 48 patients meeting Milan criteria was 8.3%, and the expanded UCSF criteria proposed by Yao et al5 yielded a recurrence rate of 11.4% among 70 patients. These values are similar to what our institution has observed over the course of two decades.
However, it is well-established that, in a significant proportion of cases, radiographic tumor measurements differ from those found during pathologic examination to an extent that would modify the patient’s transplant eligibility.1,5,10,12,13,20 In our dataset, discrepancies between radiographic and gross measurements were sufficient to alter transplant criteria eligibility in 31.5% of cases. While radiographic measurement occasionally overestimated disease burden, the vast majority of discordant cases had been understaged by preoperative imaging and demonstrated a significantly higher recurrence rate compared with cases without measurement discrepancy. Complete reliance on radiographic tumor characteristics to determine transplant eligibility may therefore yield an unreliable prediction of a patient’s recurrence risk.
If the gross measurement of tumor size and number on explanted livers is considered the gold standard for assignment to Milan or UCSF criteria, then no improvement to imaging techniques will allow preoperative evaluation to exceed the accuracy of gross tumor measurements. The RRAS yielded a better prediction of tumor recurrence when compared with both radiographic and gross assignment to Milan or UCSF criteria (Supplementary Fig. 5, Supplemental Digital Content 7, http://links.lww.com/PAS/A607), and therefore better predicted recurrence than the actual and theoretical limit of imaging-based tumor measurements in this population.
Multiple recent studies have identified tumor histology as a powerful prognostic indicator following transplantation,21–25 and some medical centers have modified their liver transplant inclusion criteria to incorporate these findings. The University of Toronto, for instance, has reportedly performed a liver biopsy on the dominant lesion of patients who exceed the Milan criteria by imaging; within this subset, only patients with poorly differentiated HCC were considered ineligible, and tumors without poor differentiation on biopsy proceeded to transplant regardless of tumor size or number.4 Zhejiang University in Hangzhou, China has reportedly offered transplantation if preoperative biopsy confirmed the absence of poor differentiation and AFP levels were ≤ 400 ng/mL, no matter the aggregate tumor size on imaging.3
WHO methods are among the most widely used guidelines in classifying HCC, but these do not delineate precise histologic criteria to define degree of differentiation. Instead, the WHO describes a collection of architectural and cytologic features that typify tumors of a certain grade, relying on the pathologist to incorporate many observations into their diagnostic impression. This results in subjective weighting of individual characteristics and a low level of agreement between pathologists.18 The RRAS, in contrast, assigns a specific weight to distinct features, which allowed for moderate agreement even in cases for which the WHO grading method yielded only slight agreement among graders.
In our data, two cases graded as well differentiated were categorized as high risk, and two cases graded as poorly differentiated were categorized as low risk. Both cases designated as well differentiated had small foci containing the high-risk features identified in this study, although the majority of the tumor lacked high-risk histology (Supplementary Figs. 6A, 6B, Supplemental Digital Content 8, http://links.lww.com/PAS/A608). While the WHO does not indicate how intratumoral heterogeneity should be reported, our scoring system indicates that the RRAS is determined by the area of tumor with the most adverse histologic features. Two additional cases were categorized as low risk using RRAS criteria, but graded as poorly differentiated by WHO guidelines based largely on architectural and nuclear features not associated with HCC recurrence in our analysis. Both tumors contained large macronucleoli, varied chromatin density, acinar formation, and slightly irregular nuclear contours, but no significant nuclear size variance was present (Supplementary Figs. 6C, 6D, Supplemental Digital Content 8, http://links.lww.com/PAS/A608).
While some tumors arising in different organ systems are graded by histologic scoring criteria derived through studies that correlated specific features with survival or metastatic spread,26,27 no commonly used HCC classification system has been developed using similar methods. HCC metastasizes less frequently than other cancers28 and since the majority of HCC arises in a background of longstanding cirrhosis, mortality in patients with unresected HCC has historically been more closely linked to liver failure than with disseminated tumor burden. However, with the increased use of liver transplantation as a treatment option for HCC, a more precise method of stratifying posttransplant recurrence risk is needed.
Many recent studies have evaluated the impact of HCC “stem cell” derivation on tumor behavior.29–34 In addition to morphologic features, we assessed the significance of CK19 immunohistochemical staining. CK19 is a marker of hepatic progenitor cells, which is downregulated during differentiation into hepatocytes, but maintained in mature cholangiocytes. HCC with retained CK19 expression may have originated from hepatic progenitor cells rather than the mature hepatocyte, and multiple studies have identified its expression as predictive of tumor recurrence.30,34 However, in our analysis, CK19 was only expressed in 1.8% of tumors (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/PAS/A601) and did not show a significant association with tumor recurrence. Given the limited impact of CK19 expression in our dataset, we did not include CK19 status as part of the RRAS criteria. However, the prognostic value of hepatic “stemness” remains an area of active research; immunophenotypic and molecular evaluation may ultimately represent additional methods of risk stratification.
There is limited data comparing the concordance of tumor grade assigned on preoperative biopsy and the subsequent resection specimen. Colecchia et al35 evaluated 81 patients and found that the histologic grade on core biopsy (using Edmonson-Steiner criteria) concurred with the grade on resection in 91.4% of cases. The authors noted that concordance between histologic grade is high in smaller nodules, but decreases considerably in tumors larger than 6.5 cm. As HCC is a heterogenous cancer, this observation is expected. Larger tumors are known to harbor increased tumor heterogeneity and are more likely to contain areas of poor differentiation.36 However, this diagnostic limitation can be overcome with increased sampling on biopsy.37 As the RRAS criteria rely on architectural and cytologic features, the RRAS score could be used to evaluate both biopsy and resection specimens. If HCC were sampled before transplant, histologic evaluation may allow for improved stratification of a patient’s recurrence risk. In addition, tissue diagnosis in this setting would permit ongoing refinement of HCC imaging criteria as well as provide tumor samples for molecular testing and further study.11
More than 8000 patients annually now receive liver transplants in the United States (as reported by the Organ Procurement and Transplantation Network, February 2018), and this number has shown a steady increase. Despite adherence to radiographic criteria, HCC still recurs in 10% to 20% of patients following liver transplant,38–40 and tumor recurrence has become the most common cause of death among patients transplanted for HCC.40,41 More accurate quantification of a patient’s recurrence risk can help guide HCC surveillance strategies after transplant. Those with low-risk tumors would likely not benefit from posttransplant surveillance, given that no recurrences were observed in the 60 cases within our cohort over a median follow-up time of 7.9 years. Conversely, patients with intermediate-risk tumors should undergo posttransplant surveillance, and those with high-risk tumors may justify a longer surveillance period with shorter intervals between imaging. In addition, RRAS categorization could impact the choice of posttransplant immunosuppression. Although more study is needed, mammalian target of rapamycin-based immunosuppression could be useful for patients with high-risk tumors, as this therapy may have antineoplastic properties.42,43 High-risk patients may also benefit from clinical trial enrollment for adjuvant therapies given shortly after transplant to reduce recurrence risk. More broadly, stratification of tumors by recurrence risk may also prove useful in refining mandatory waiting periods for transplantation, though further study will be needed.
Our results demonstrate that histologic evaluation of HCC better predicts recurrence risk compared with the radiographic measurement of tumor size and number. By recognizing specific features associated with recurrence after transplant, we developed a histologic scoring system that better stratifies recurrence risk in HCC compared with tumor grade assigned using the WHO method.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the UCSF Liver Center for histology laboratory coordination.
Conflicts of Interest and Source of Funding:
The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.ajsp.com.
REFERENCES
- 1.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 2.Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Ann Surg. 1998;228:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726–1732. [DOI] [PubMed] [Google Scholar]
- 4.DuBay D, Sandroussi C, Sandhu L, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg. 2011;253:166–172. [DOI] [PubMed] [Google Scholar]
- 5.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. [DOI] [PubMed] [Google Scholar]
- 6.Halazun KJ, Patzer RE, Rana AA, et al. Standing the test of time: outcomes of a decade of prioritizing patients with hepatocellular carcinoma, results of the UNOS natural geographic experiment. Hepatology. 2014;60:1957–1962. [DOI] [PubMed] [Google Scholar]
- 7.Massie AB, Caffo B, Gentry SE, et al. MELD exceptions and rates of waiting list outcomes. Am J Transplant. 2011;11:2362–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. [DOI] [PubMed] [Google Scholar]
- 9.American College of Radiology Liver imaging reporting and data system, version 2014. Available at: www.acr.org/Quality-Safety/Resources/LIRADS. Accessed April 5, 2017.
- 10.Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127:S261–S267. [DOI] [PubMed] [Google Scholar]
- 11.Torbenson M, Schirmacher P. Liver cancer biopsy—back to the future?. Hepatology. 2015;61:431–433. [DOI] [PubMed] [Google Scholar]
- 12.Cillo U, Vitale A, Bassanello M, et al. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004;239:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasad KR, Young RS, Burra P, et al. Summary of candidate selection and expanded criteria for liver transplantation for hepatocellular carcinoma: a review and consensus statement. Liver Transpl. 2011;17(suppl 2):S81–S89. [DOI] [PubMed] [Google Scholar]
- 14.Osorio FM, Vidigal PV, Ferrari TC, et al. Histologic grade and mitotic index as predictors of microvascular invasion in hepatocellular carcinoma. Exp Clin Transplant. 2015;13:421–425. [DOI] [PubMed] [Google Scholar]
- 15.Tamura S, Kato T, Berho M, et al. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg. 2001;136:25–30; discussion 31. [PubMed] [Google Scholar]
- 16.Agopian VG, Harlander-Locke M, Zarrinpar A, et al. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416–427. [DOI] [PubMed] [Google Scholar]
- 17.Theise ND, Park YN, Curado MP, et al. Hepatocellular carcinoma In: Bosman FT, Carnerio F, Hruban RH, Theise ND, eds. WHO Classification of Tumours of the Digestive System, 4th ed Lyon: IARC; 2010:205–216. [Google Scholar]
- 18.Pirisi M, Leutner M, Pinato DJ, et al. Reliability and reproducibility of the edmondson grading of hepatocellular carcinoma using paired core biopsy and surgical resection specimens. Arch Pathol Lab Med. 2010;134:1818–1822. [DOI] [PubMed] [Google Scholar]
- 19.Fleiss JL, Levin L, Paik MC. Statistical Methods for Rates and Proportions, 3rd ed Hoboken, New Jersey: Wiley; 2003. [Google Scholar]
- 20.Freeman RB, Mithoefer A, Ruthazer R, et al. Optimizing staging for hepatocellular carcinoma before liver transplantation: a retrospective analysis of the UNOS/OPTN database. Liver Transpl. 2006;12:1504–1511. [DOI] [PubMed] [Google Scholar]
- 21.Herrero JI, Sangro B, Quiroga J, et al. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl. 2001;7:631–636. [DOI] [PubMed] [Google Scholar]
- 22.Esnaola NF, Lauwers GY, Mirza NQ, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. 2002;6:224–232; discussion 232. [DOI] [PubMed] [Google Scholar]
- 23.Molmenti EP, Klintmalm GB. Liver transplantation in association with hepatocellular carcinoma: an update of the International Tumor Registry. Liver Transpl. 2002;8:736–748. [DOI] [PubMed] [Google Scholar]
- 24.D’Amico F, Schwartz M, Vitale A, et al. Predicting recurrence after liver transplantation in patients with hepatocellular carcinoma exceeding the up-to-seven criteria. Liver Transpl. 2009;15:1278–1287. [DOI] [PubMed] [Google Scholar]
- 25.Harper AM, Edwards E, Washburn WK, et al. An early look at the Organ Procurement and Transplantation Network explant pathology form data. Liver Transpl. 2016;22:757–764. [DOI] [PubMed] [Google Scholar]
- 26.Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33:37–42. [DOI] [PubMed] [Google Scholar]
- 28.Budczies J, von Winterfeld M, Klauschen F, et al. The landscape of metastatic progression patterns across major human cancers. Oncotarget. 2015;6:570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung GE, Lee JH, Yoon JH, et al. Prognostic implications of tumor vascularity and its relationship to cytokeratin 19 expression in patients with hepatocellular carcinoma. Abdom Imaging. 2012;37:439–446. [DOI] [PubMed] [Google Scholar]
- 30.Durnez A, Verslype C, Nevens F, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006;49:138–151. [DOI] [PubMed] [Google Scholar]
- 31.Feng J, Zhu R, Chang C, et al. CK19 and glypican 3 expression profiling in the prognostic indication for patients with HCC after surgical resection. PLoS One. 2016;11:e0151501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JI, Lee JW, Kim JM, et al. Prognosis of hepatocellular carcinoma expressing cytokeratin 19: comparison with other liver cancers. World J Gastroenterol. 2012;18:4751–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthai SM, Ramakrishna B. Cancer stem cells in hepatocellular carcinoma—an immunohistochemical study with histopathological association. Indian J Med Res. 2015;142:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miltiadous O, Sia D, Hoshida Y, et al. Progenitor cell markers predict outcome of patients with hepatocellular carcinoma beyond Milan criteria undergoing liver transplantation. J Hepatol. 2015;63:1368–1377. [DOI] [PubMed] [Google Scholar]
- 35.Colecchia A, Scaioli E, Montrone L, et al. Pre-operative liver biopsy in cirrhotic patients with early hepatocellular carcinoma represents a safe and accurate diagnostic tool for tumour grading assessment. J Hepatol. 2011;54:300–305. [DOI] [PubMed] [Google Scholar]
- 36.Kenmochi K, Sugihara S, Kojiro M. Relationship of histologic grade of hepatocellular carcinoma (HCC) to tumor size, and demonstration of tumor cells of multiple different grades in single small HCC. Liver. 1987;7:18–26. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Wang J, Zhang X, et al. Diagnostic value of preoperative needle biopsy for tumor grading assessment in hepatocellular carcinoma. PLoS One. 2015;10:e0144216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma P, Welch K, Hussain H, et al. Incidence and risk factors of hepatocellular carcinoma recurrence after liver transplantation in the MELD era. Dig Dis Sci. 2012;57:806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao FY, Xiao L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587–2596. [DOI] [PubMed] [Google Scholar]
- 40.Zimmerman MA, Ghobrial RM, Tong MJ, et al. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143:182–188; discussion 188. [DOI] [PubMed] [Google Scholar]
- 41.Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geissler EK, Schnitzbauer AA, Zulke C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open-label phase 3 trial. Transplantation. 2016;100:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matter MS, Decaens T, Andersen JB, et al. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol. 2014;60:855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.